Abstract
Purpose:
We aimed to analyze and compare leukocyte telomere length (LTL) and age-dependent LTL attrition between childhood cancer survivors and non-cancer controls, and to evaluate the associations of LTL with treatment exposures, chronic health conditions (CHCs), and health behaviors among survivors.
Experimental Design:
We included 2,427 survivors and 293 non-cancer controls of European ancestry, drawn from the participants in St. Jude Lifetime Cohort Study (SJLIFE), a retrospective hospital-based study with prospective follow-up (2007-2016). Common non-neoplastic CHCs (59 types) and subsequent malignant neoplasms (5 types) were clinically assessed. LTL was measured with whole-genome sequencing data.
Results:
After adjusting for age at DNA sampling, gender, genetic risk score based on 9 SNPs known to be associated with telomere length, and eigenvectors, LTL among survivors was significantly shorter both overall (adjusted mean [AM]=6.20kb; SE=0.03kb) and across diagnoses than controls (AM=6.69kb; SE=0.07kb). Among survivors, specific treatment exposures associated with shorter LTL included chest or abdominal irradiation, glucocorticoid, and vincristine chemotherapies. Significant negative associations of LTL with 14 different CHCs, and a positive association with subsequent thyroid cancer occurring out of irradiation field were identified. Health behaviors were significantly associated with LTL among survivors aged 18-35 years (ptrend=0.03).
Conclusions:
LTL is significantly shorter among childhood cancer survivors than non-cancer controls, and is associated with CHCs and health behaviors, suggesting LTL as an aging biomarker may be a potential mechanistic target for future intervention studies designed to prevent or delay onset of CHCs in childhood cancer survivors.
Keywords: Leukocyte telomere length, chronic health condition, health behaviors, childhood cancer, cancer survivorship
Introduction
Telomeres, with DNA characteristically comprised of copies of TTAGGG motif, are nucleoprotein-DNA structures that cap both terminal ends of each chromosome and are designed to maintain genome integrity by preventing chromosome end-to-end fusions, nucleolytic erosion and homologous recombination(1). Inefficient end replication(2) and the fact that most somatic cells lack telomerase, the enzyme necessary for replenishing terminal telomere repeats lost during genome duplication, result in telomeres becoming shorter with each cell division in self-renewing tissues. When telomeres become critically short, a cell can no longer divide, triggering senescence or apoptosis(3). Telomere length is strongly correlated across different tissue types from the same individual, with similar rates of attrition over time(4).
In humans, leukocyte telomere length (LTL), generally several thousand base pairs long, is negatively correlated with chronological age and decreases by 22 to 45 base pairs per year(5). LTL reflects systematic influence on telomere maintenance in other tissues(4), serving as an excellent marker of aging at both cellular and organism levels. Not all LTL variability can be attributed directly to age, however. LTL is highly heritable(6-8), differs by gender(9), and is associated with health behaviors(10-14) including smoking, alcohol intake, diet, and physical activity, shared environmental factors in twins(15) and cancer treatment modalities and doses(16,17) including chemotherapy and radiation therapy (RT). LTL is also associated with age-related diseases(18,19), including cancers(20,21) and cardiovascular diseases(22,23).
The current population of childhood cancer survivors in the US is estimated to be over half a million (24). Adult survivors of childhood cancer may be a group particularly vulnerable to telomere attrition, because they have experienced biological damage to normal cellular mechanisms that is unlikely to completely recover after completion of cancer therapy. They are also at an increased risk for therapy-related late effects including many non-neoplastic chronic health conditions (CHCs) and subsequent neoplasms(25). Moreover, frailty in this population was reported to be strongly associated with risk of developing late effects including total mortality, suggesting accelerated aging in childhood cancer survivors(26). However, research focusing on the biological basis for premature aging in survivors of childhood cancer has been largely lagging as relatively few long-term cancer survivorship studies have assessed biomarkers of aging including telomeres(27). To address this gap, we employed St. Jude Lifetime Cohort Study (SJLIFE)(28,29) to compare LTL between long-term survivors of childhood cancer and community controls with no prior history of cancer, and examine LTL associations in multiple contexts, including childhood cancer treatment exposures, clinically-assessed CHCs including subsequent neoplasms, and modifiable health behaviors.
Materials and Methods
Study population
SJLIFE is a retrospective cohort with prospective clinical follow-up of 5+ year survivors of childhood cancer diagnosed and treated at St. Jude Children’s Research Hospital (SJCRH) between 1962 and 2012. Study design, and assessments of the SJLIFE cohort were described previously(30,31). Briefly, participants completed a battery of medical and laboratory assessments to characterize their health, and treatment information was abstracted from medical records. Participants also completed questionnaires covering health behaviors and demographic factors. Whole-genome sequencing (WGS) data were generated on the same sequencing platform (HiSeq X Ten System) for all study participants, as previously described (29). The average genome-wide coverage per sample was 36.8-fold, with the same read length (2x150) and was mapped with BWA using default settings. We considered additional filtering steps to ensure the following inclusion criteria were met for this analysis: 1) admixture coefficient for CEU (Utah residence with Northern and Western European ancestry) population ≥80% (European ancestry): admixture coefficients were estimated for each individual in a STRUCTURE analysis using 1000 Genomes Project Phase 3 version 5 data as reference populations which include CEU (n=99), JPT+CHB (Japanese and Chinese, n=207) and YRI (Yoruban in Nigeria, n=108). If an individual had ≥80% CEU, we designated European ancestry; 2) no excessive heterozygosity: more than three standard deviations from the mean; 3) no closely related pairs (first degree relatives) determined by identical-by-descent estimates; and 4) no Trisomy 21 (Down syndrome). This resulted in 2,427 survivors for the evaluation of LTL and associations with treatment exposures and CHCs (Supplementary Fig. S1).
CHCs were clinically-confirmed by medical records or identified by prospectively performed clinical assessments. For the comparison of survivors and controls, analysis was limited to those whose DNA extraction was completed with the same protocol in the Computational Biology Genomic Laboratory at SJCRH, resulting in 1,615 survivors and 293 controls of European ancestry. Controls in the SJLIFE study were primarily recruited from the communities where survivors live in and screened for eligibility criteria including: 1) 18 years of age or older; 2) not currently pregnant or lactating; and 3) matched to survivors based on age, sex, and race, as described in detail previously (25). Analysis of the association between LTL and health behaviors was restricted to 1,143 of the 1,615 survivors whose DNA sampling age was greater than the age at visit when health behaviors were gathered. The prospective clinical assessment for the SJLIFE cohort was activated in 2007, and the data used in this report reflects follow-up through December 2016. The SJLIFE genomic study was conducted in accordance with the Declaration of Helsinki and IRB-approved, and participants provided informed written consent.
Estimated telomere length using WGS
LTL was determined using a recently published software tool, TelSeq(32), which defines a sequence read as telomeric if it contains at least k occurrences of the motif (TTAGGG), where k defaults to 7. An estimate of LTL is computed by tkc/s where tk is the number of telomeric reads, c is a constant for genome length with GC content between 48% and 52% divided by number of telomere ends 46 (23 × 2), and s is the total number of reads with GC content between 48% and 52%. The TelSeq results have previously been shown to correlate (Pearson correlation coefficient ρ = 0.60) with Southern blot measurements based on 260 samples from the TwinsUK cohort(32). We also conducted Southern blot experiments to measure the LTL using 93 samples from the SJLIFE cohort and found good correlation with LTL estimates using WGS-based TelSeq method (ρ = 0.64).
Genetic risk score for telomere length
To represent the hereditary component of LTL, we constructed a weighted genetic risk score (GRS) based on nine single nucleotide polymorphisms (SNPs) known to be associated with telomere length [evidence of genome-wide significance (p<5×10−8)], and located within or near genes required for telomere maintenance. GRS was calculated by weighted summation of the number of alleles associated with longer telomere and published effect size for each telomere length associated allele across the nine SNPs (rs10936599, rs2736100, rs7675998, rs9420907, rs8105767, rs755017, rs11125529, rs6772228, and rs3027234)(33). There was no statistically significant difference in distribution of GRS between survivors of childhood cancer and controls.
Chronic health conditions
Chronic health conditions were defined by applying a modification of the Common Terminology Criteria for Adverse Events (CTCAE version 4.0)(34) to clinically validated/ascertained medical outcomes in 12 organ systems and for second cancers.(35) Outcomes were graded with possible ratings of 0 (no problem), 1 (mild), 2 (moderate), 3 (severe/disabling), 4 (life-threatening), or 5 (death) (35). We included 59 common (incidence count ≥20) non-neoplastic outcomes with grades 3-4, five subsequent neoplasms, and mortality in this analysis.
Treatment exposures
Treatment-related exposures included chemotherapy (chemotherapeutic agents received and cumulative doses) and radiotherapy (treatment fields and doses). Treatment information was abstracted from medical records for SJLIFE participants by trained research staff using a structured protocol as previously described(30), and radiation dosimetry was estimated from the primary radiation prescription records(36). Six major chemotherapy variables (anthracyclines, alkylating agents, glucocorticoids, vincristine, platinum agents, and epipodophyllotoxins) and three radiotherapy variables (brain-RT, chest-RT and abdomen/pelvic RT) were included in the analysis.
Health behaviors
Using data from self-report questionnaires, we defined five suboptimal health behaviors as the following: 1) <150 min/week of at least moderate physical activity;(37) 2) no participation in resistance training;(37) 3) current or former tobacco smoking (5 lifetime packs); 4) scoring in the lowest tertile on the healthy eating index based on the 2015 to 2020 Dietary Guideline for Americans;(38) and 5) either no or risky alcohol drinking defined as one episode of ≥5 (men) or ≥4 (women) drinks/day in the past year(38). Survivors were then grouped into three categories: 1) favorable (0 or 1 suboptimal health behavior); 2) intermediate (2 or 3 suboptimal health behaviors); and 3) unfavorable (4 or 5 suboptimal health behaviors).
Statistical analyses
To compare LTL between childhood cancer survivors and community controls, adjusted mean (AM) of least square of LTL and average difference in LTL by age were calculated for controls and survivors overall and by primary diagnosis group. The survivor-control analysis of LTL was assessed by linear regression adjusted for age at DNA sampling, gender, the telomere length GRS, and eigenvectors corresponding to top 10 principal components derived from the combined set of 1,615 survivors and 293 controls. The survivor-only analysis of LTL was additionally adjusted for cancer treatments and diagnosis of obesity established prior to measurement of LTL. A sensitivity analysis was performed additionally adjusted for health behaviors. The statistical difference of AM of LTL in different diagnosis groups was evaluated by Dunnett-Hsu test. The average difference in LTL by age in survivors and non-cancer controls was evaluated by t-test. Similarly, LTL was compared among survivors by prior cancer treatments (with or without each specific exposure) or health behaviors (favorable, intermediate or unfavorable group).
To evaluate the association of LTL with CHC, we used two approaches. First, a Cochran-Armitage trend test was carried out for each 3×2 cross-tabulation of each outcome by tertile of residual of LTL (adjusted for age at DNA sampling, DNA extraction method, and telomere length GRS). For this analysis, we examined CHCs present prior to or after DNA sampling. Second, we performed a time-to-event analysis using multivariable piecewise exponential regression models in which only CHCs diagnosed at DNA sampling or within 180 days of DNA sampling were considered. The associations between LTL tertile and newly occurrence of CHC were reported as relative rate (RR) and 95% confidence interval (CI). The event date was determined as the earliest date of clinical follow-up for late effects based on when the CHCs were diagnosed, or patient reports (e.g. headache), or the health-related metrics were measured (e.g. obesity). Since we assumed that there is no smooth linear relationship between the LTL and CHCs, we chose to use tertiles, which was the minimum number of groupings to show linear trend considering the number of survivors as well as the limited number of CHCs. The multivariable models were adjusted for age at primary diagnosis, sex, telomere GRS, eigenvectors, site specific radiation dose (0, <25Gy and ≥25Gy), tertiles of chemotherapy agents, health behaviors, and primary diagnosis. Follow up of survivors started at age of DNA sampling and was censored at age of their last-contact defined at the time of analysis or at death. Statistical significance was defined by two-sided p-value of 0.05. SAS program version 9.4 was used for all statistical analyses.
Results
Characteristics of study population
Median age at childhood cancer diagnosis, at DNA sampling, and at last follow-up of the 2,427 survivors included in this analysis were 7.0 years (range: 0-23.6), 31.8 years (range: 6.0-66.4), 35.7 years (range: 6.9-68.6), respectively. Survivors were 53.4% male, had leukemia (37.0%), sarcoma (12.7%), Hodgkin lymphoma (12.7%), central nervous system (CNS) tumors (11.0%), non-Hodgkin lymphoma (7.7%), and other cancers. Among survivors, 32.8% were exposed to brain RT, 27.2% chest RT, 22.0% abdominal/pelvic RT, 58.7% anthracyclines, 58.6% alkylating agents, 50.0% glucocorticoids, 70.4% vincristine, 11.6% platinum agents, and 36.2% epipodophyllotoxins. Over half of survivors (52.8%) reported participating in physical activity ≥150 minutes per week, and 39.2% in resistance training. Over half (54.5%) also reported moderate drinking, 75.9% had a normal health eating index, and 63.5% never smoked (Table 1).
Table 1.
Characteristics of participants in St. Jude Lifetime Cohort study
| Characteristics | Overall survivors | Restricted survivorsa | Non-cancer controlsa | |||
|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | |
| Total | 2,427 | (100.0) | 1,615 | (100.0) | 293 | (100.0) |
| Sex | ||||||
| Male | 1,295 | (53.4) | 855 | (52.9) | 141 | (48.1) |
| Female | 1,132 | (46.6) | 760 | (47.1) | 152 | (51.9) |
| Race | ||||||
| White | 2,425 | (99.9) | 1,613 | (99.9) | 289 | (98.6) |
| Other | 2 | (0.1) | 2 | (0.1) | 4 | (1.4) |
| Ethnic | ||||||
| Hispanic | 27 | (1.1) | 17 | (1.1) | 6 | (2.1) |
| Non-Hispanic | 2,400 | (98.9) | 1,598 | (99.0) | 287 | (98.0) |
| Diagnosis | ||||||
| Leukemia | 898 | (37.0) | 538 | (33.3) | - | - |
| CNS tumors | 266 | (11.0) | 218 | (13.5) | - | - |
| Sarcoma | 309 | (12.7) | 221 | (13.7) | - | - |
| Hodgkin lymphoma | 308 | (12.7) | 237 | (14.7) | - | - |
| Non-Hodgkin lymphoma | 186 | (7.7) | 116 | (7.2) | - | - |
| Neuroblastoma | 120 | (4.9) | 83 | (5.1) | - | - |
| Retinoblastoma | 67 | (2.8) | 34 | (2.1) | - | - |
| Wilm’s tumor | 154 | (6.4) | 96 | (5.9) | - | - |
| Other | 119 | (4.9) | 72 | (4.5) | - | - |
| Brain RT | ||||||
| No | 1,630 | (67.2) | 1,131 | (70.2) | - | - |
| ≤25 Gy | 481 | (19.8) | 249 | (15.4) | - | - |
| >25 Gy | 314 | (13.0) | 233 | (14.4) | - | - |
| Chest RT | ||||||
| No | 1,765 | (72.8) | 1,122 | (69.5) | - | - |
| ≤25 Gy | 284 | (11.7) | 206 | (12.8) | - | - |
| >25 Gy | 377 | (15.5) | 286 | (17.7) | - | - |
| Abdomen/Pelvic RT | ||||||
| No | 1,891 | (78.0) | 1,240 | (76.8) | - | - |
| ≤25 Gy | 257 | (10.6) | 182 | (11.3) | - | - |
| >25 Gy | 278 | (11.5) | 192 | (11.9) | - | - |
| Anthracycline | ||||||
| No | 1,002 | (41.3) | 639 | (39.6) | - | - |
| 1st tertile | 473 | (19.5) | 305 | (19.0) | - | - |
| 2nd tertile | 476 | (19.6) | 311 | (19.3) | - | - |
| 3rd tertile | 476 | (19.6) | 360 | (22.3) | - | - |
| Alkylating agent | ||||||
| No | 1,005 | (41.4) | 663 | (41.0) | - | - |
| 1st tertile | 473 | (19.5) | 324 | (20.1) | - | - |
| 2nd tertile | 474 | (19.5) | 307 | (19.0) | - | - |
| 3rd tertile | 474 | (19.5) | 321 | (19.9) | - | - |
| Glucocorticoids | ||||||
| No | 1,209 | (50.0) | 840 | (52.5) | - | - |
| 1st tertile | 402 | (16.7) | 231 | (14.4) | - | - |
| 2nd tertile | 379 | (15.7) | 251 | (15.7) | - | - |
| 3rd tertile | 423 | (17.5) | 279 | (17.4) | - | - |
| Vincristine | ||||||
| No | 712 | (29.6) | 520 | (32.4) | - | - |
| 1st tertile | 566 | (23.5) | 331 | (20.6) | - | - |
| 2nd tertile | 566 | (23.5) | 390 | (24.3) | - | - |
| 3rd tertile | 565 | (23.5) | 364 | (22.7) | - | - |
| Platinum | ||||||
| No | 2,139 | (88.4) | 1,390 | (86.4) | - | - |
| 1st tertile | 100 | (4.1) | 67 | (4.2) | - | - |
| 2nd tertile | 87 | (3.6) | 70 | (4.4) | - | - |
| 3rd tertile | 94 | (3.9) | 82 | (5.1) | - | - |
| Epipodophyllotoxins | ||||||
| No | 1,544 | (63.8) | 1,039 | (64.5) | - | - |
| 1st tertile | 292 | (12.1) | 199 | (12.3) | - | - |
| 2nd tertile | 294 | (12.1) | 198 | (12.3) | - | - |
| 3rd tertile | 292 | (12.1) | 176 | (10.9) | - | - |
| Physical activity | ||||||
| ≥150 minutes/week | 1,203 | (52.8) | 786 | (53.1) | 178 | (63.1) |
| <150 minutes/week | 1,075 | (47.2) | 694 | (46.9) | 104 | (36.9) |
| Strength | ||||||
| Normal | 888 | (39.2) | 572 | (38.7) | 148 | (52.9) |
| Abnormal | 1,378 | (60.8) | 905 | (61.3) | 132 | (47.1) |
| Alcohol intake | ||||||
| No or risky drinking | 927 | (45.5) | 600 | (45.8) | 141 | (49.3) |
| Moderate drinking | 1,110 | (54.5) | 710 | (54.2) | 145 | (50.7) |
| Healthy eating index | ||||||
| Normal | 1,697 | (75.9) | 1,118 | (76.5) | 235 | (83.0) |
| Abnormal | 538 | (24.1) | 343 | (23.5) | 48 | (17.0) |
| Smoking status | ||||||
| Never | 1,495 | (63.5) | 991 | (64.2) | 181 | (63.7) |
| Ever | 861 | (36.5) | 553 | (35.8) | 103 | (36.3) |
| Health behaviorsb | ||||||
| Favorable | 658 | (27.1) | 419 | (25.9) | 116 | (39.6) |
| Intermediate | 1,084 | (44.7) | 718 | (44.5) | 128 | (43.7) |
| Unfavorable | 343 | (14.1) | 210 | (13.0) | 30 | (10.2) |
| Unknown | 342 | (14.1) | 268 | (16.6) | 19 | (6.5) |
| Median | (Range) | Median | (Range) | Median | (Range) | |
| Age at diagnosis | 7.0 | (0-23.6) | 7.7 | (0-22.7) | - | - |
| Age at DNA sampling | 31.8 | (6.0-66.4) | 31.5 | (6.0-66.4) | 34.9 | (18.7-70.2) |
| Age at last follow up | 35.7 | (6.9-68.6) | 33.9 | (6.9-68.6) | 34.9 | (18.7-70.2) |
Abbreviations: CNS (central nervous system), Radiation therapy (RT)
DNA extraction was conducted by the same laboratory with the same protocol.
Suboptimal health behaviors included; 1) <150 min/week of at least moderate physical activity; 2) no participation in resistance training; 3) tobacco smoking; 4) scoring in the lowest tertile on the healthy eating index based on the 2015 to 2020 Dietary Guideline for Americans(38); and 5) either no or risky alcohol drinking.
The non-cancer controls were 48.1% male and the median age at DNA sampling was 34.9 (range: 18.7-70.2) years.
Telomere length in survivors and non-cancer controls (Figure 1a)
Figure 1.
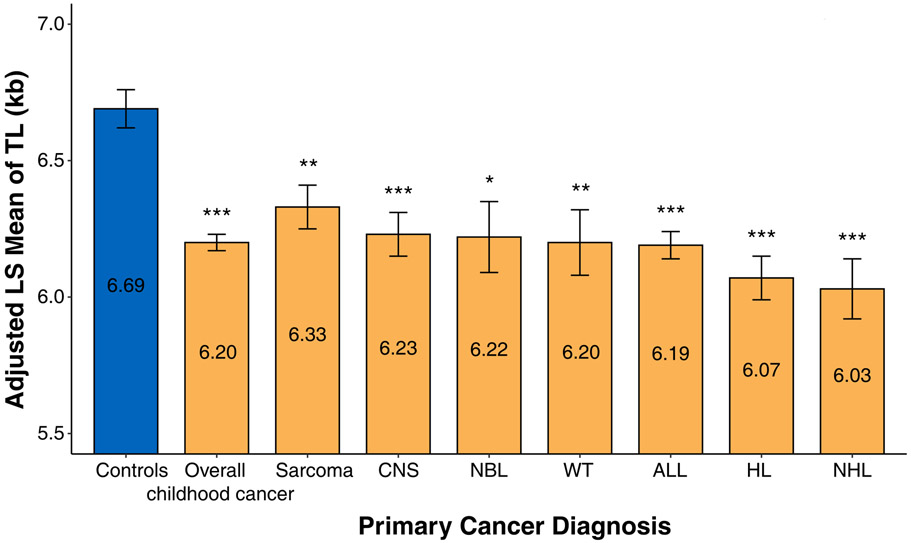
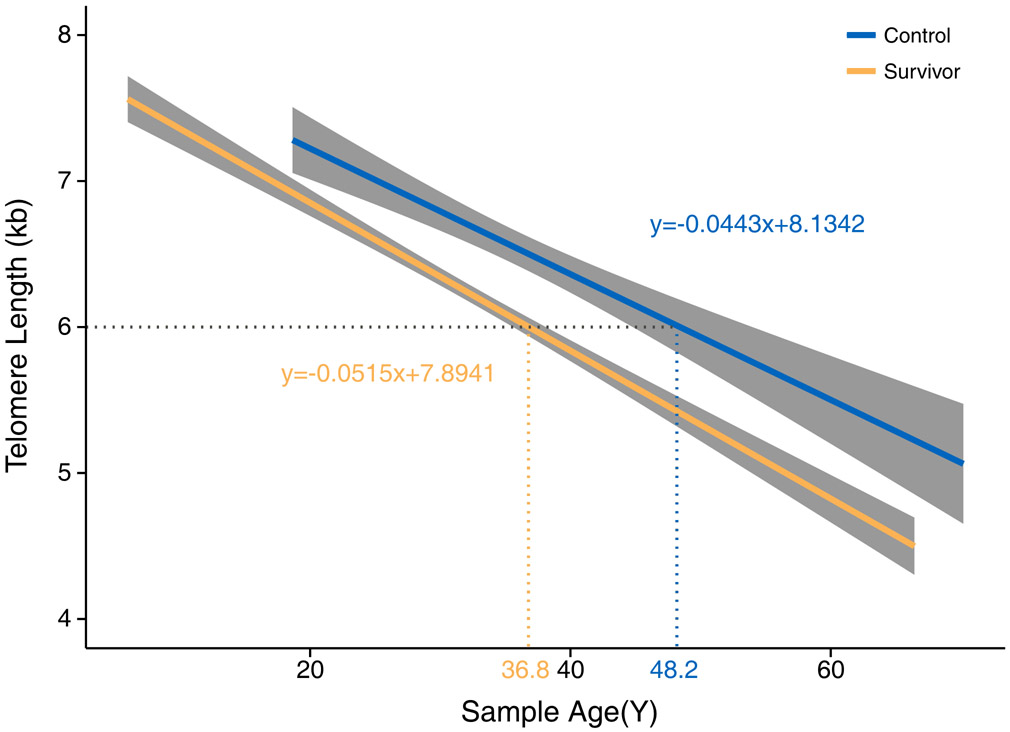
(a) Adjusted least square mean of LTL in controls and survivors overall and by their diagnoses. Statistical significance levels were depicted by *P<0.05, **P<0.01, and ***P<0.001. Abbreviations: CNS (Central Nervous System), ALL (Acute Lymphocytic leukemia), NBL (Neuroblastoma), WT (Wilms’ tumor), HL (Hodgkin lymphoma), NHL (non-Hodgkin lymphoma). (b) Linear regression lines of LTL by age at DNA sampling for controls and survivors.
LTL in survivors was shorter overall (AM=6.20kb, standard error (SE)=0.03kb) and across all diagnoses compared to community controls with no prior history of cancer (AM=6.69kb, SE=0.07kb) with high statistical significance (p<0.001). Compared with survivors of other diagnoses, sarcoma survivors had relatively longer LTL (AM=6.33kb), and survivors of Hodgkin lymphoma had relatively shorter LTL (AM=6.07kb) (Figure 1a). Similar results were observed when health behaviors were additionally adjusted for (Supplementary Table S1). Figure 1b illustrates that the average LTL of 6kb corresponds to age of 36.8-years among survivors and age of 48.2-years among community controls, suggesting an acceleration of aging by 11.4 years (dotted line). However, the average difference in LTL by age (i.e., age slope of the linear regression line) was only modestly greater among survivors (52bp/year) than among non-cancer community controls (44bp/year) with no statistically significant difference (p=0.95).
Telomere length and cancer treatment exposures (Figure 2)
Figure 2. Associations of LTL with cancer treatment exposures.
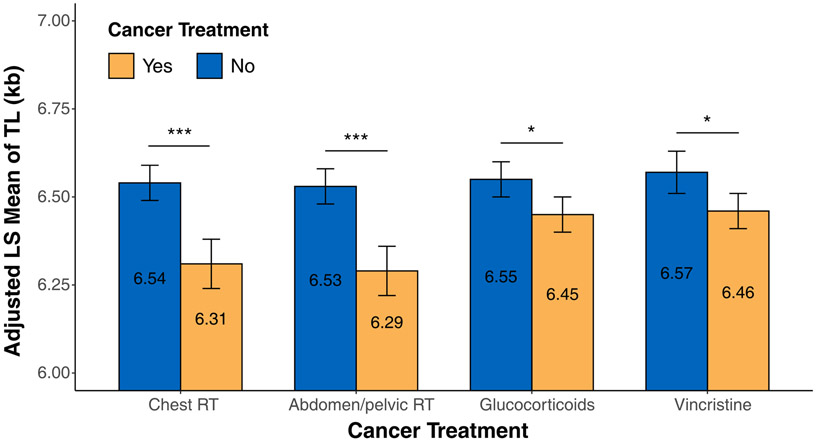
Statistical significance levels were depicted by *P<0.05, **P<0.01, and ***P<0.001.
When we compared LTL between survivors exposed and not exposed to each RT and chemotherapy respectively, survivors exposed to chest RT (p<0.001) and abdomen/pelvic RT (p<0.001) had significantly shorter LTL than non-exposed survivors (Figure 2). However, there was no statistically significant association between LTL and brain RT (Supplementary Table S2). Survivors exposed to glucocorticoids (p=0.04) and vincristine (p=0.03) also had shorter LTL than non-exposed survivors (Figure 2), but no associations between alkylating agents, anthracyclines, epipodophyllotoxin, or platinum and LTL were detected (Supplementary Table S2). Similar results were observed when health behaviors were additionally adjusted for (Supplementary Table S3). In multivariable model including all four treatments that were individually significantly associated with LTL (Supplementary Table S4), only two treatments remained statistically significant including abdomen/pelvic RT (p=0.05) and glucocorticoids (p=0.02) due to a very strong positive correlation between chest RT and abdomen/pelvic RT (Phi Coefficient=0.81) and a strong positive correlation between glucocorticoids and vincristine (Phi Coefficient=0.46).
Telomere length and CHCs (Table 2)
Table 2.
Associations of LTL with non-neoplastic CHCs and subsequent malignant neoplasms with statistical significance
| Total (N=2,427, 100%) |
1st Tertile (N=809, 100%) |
2nd Tertile (N=809, 100%) |
3rd Tertile (N=809, 100%) |
Ptrend | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | N | (%) | ||
| Non-neoplastic CHCs | |||||||||
| Cardiomyopathy | 102 | (4.2) | 44 | (5.4) | 32 | (4.0) | 26 | (3.2) | 0.013 |
| Cholecystitis | 198 | (8.2) | 76 | (9.4) | 66 | (8.2) | 56 | (6.9) | 0.035 |
| Chronic Hepatitis C | 55 | (2.3) | 22 | (2.7) | 21 | (2.6) | 12 | (1.5) | 0.047 |
| Hypercholesterolemia | 23 | (0.9) | 9 | (1.1) | 11 | (1.4) | 3 | (0.4) | 0.036 |
| Hypertriglyceridemia | 52 | (2.1) | 26 | (3.2) | 15 | (1.9) | 11 | (1.4) | 0.005 |
| Fibrosis/Cirrhosis | 73 | (3.0) | 28 | (3.5) | 30 | (3.7) | 15 | (1.9) | 0.029 |
| Gastritis/Duodenitis | 50 | (2.1) | 25 | (3.1) | 17 | (2.1) | 8 | (1.0) | 0.001 |
| Gastrointestinal ulcer | 30 | (1.2) | 15 | (1.9) | 8 | (1.0) | 7 | (0.9) | 0.036 |
| Headaches | 89 | (3.7) | 38 | (4.7) | 28 | (3.5) | 23 | (2.8) | 0.024 |
| Hypertension | 170 | (7.0) | 66 | (8.2) | 56 | (6.9) | 48 | (5.9) | 0.040 |
| Lymphatic infection | 23 | (0.9) | 11 | (1.4) | 7 | (0.9) | 5 | (0.6) | 0.039 |
| Obesity | 962 | (39.6) | 346 | (42.8) | 326 | (40.3) | 290 | (35.9) | 0.002 |
| Obstructive pulmonary deficit | 97 | (4.0) | 43 | (5.3) | 30 | (3.7) | 24 | (3.0) | 0.008 |
| Restrictive pulmonary deficit | 52 | (2.1) | 27 | (3.3) | 14 | (1.7) | 11 | (1.4) | 0.003 |
| Subsequent neoplasms | |||||||||
| Thyroid Cancer | 15 | (0.6) | 2 | (0.2) | 4 | (0.5) | 9 | (1.1) | 0.013 |
Abbreviations: LTL (leukocyte telomere length), CHC (chronic health condition), and GRS (genetic risk score).
There was a negative association between tertile of LTL residual and 14 common non-neoplastic CHCs, including cardiomyopathy (p=0.013), cholecystitis (p=0.035), chronic hepatitis C (p=0.047), hypercholesterolemia (p=0.036), hypertriglyceridemia (p=0.005), fibrosis/cirrhosis (p=0.029), gastritis/duodenitis (p=0.001), gastrointestinal ulcer (p=0.036), headaches (p=0.024), hypertension (p=0.040), lymphatic infection (p=0.039), obesity (p=0.002), obstructive pulmonary deficit (p=0.008), and restrictive pulmonary deficit (p=0.003). In contrast, the tertile of LTL residuals was positively associated with the occurrence of a secondary thyroid cancer (p=0.013). The association between LTL and overall mortality did not reach statistical significance (p=0.08). Additional results examining associations between LTL and CHC are presented in Supplementary Table S5. When we modeled the number of any CHCs for each survivor, we observed that survivors with shorter telomere length were more likely to have multiple CHCs (p<0.05) (Supplementary Table S6). Time to event analysis indicated negative associations of LTL (3rd tertile vs. 1st tertile of LTL residual) with restrictive pulmonary deficit (RR=0.43, 95% CI=0.20-0.88, ptrend=0.02) and showed the marginally significant associations of LTL with hypertriglyceridemia (ptrend=0.06) and obstructive pulmonary deficit (ptrend=0.06) (Supplementary Table S7). We also observed a negative association between tertiles of LTL residual and 13 common non-neoplastic CHCs diagnosed before DNA sampling (Supplementary Table S8).
Telomere length and health behaviors (Figure 3)
Figure 3. Associations of LTL with health behaviors by age groups.
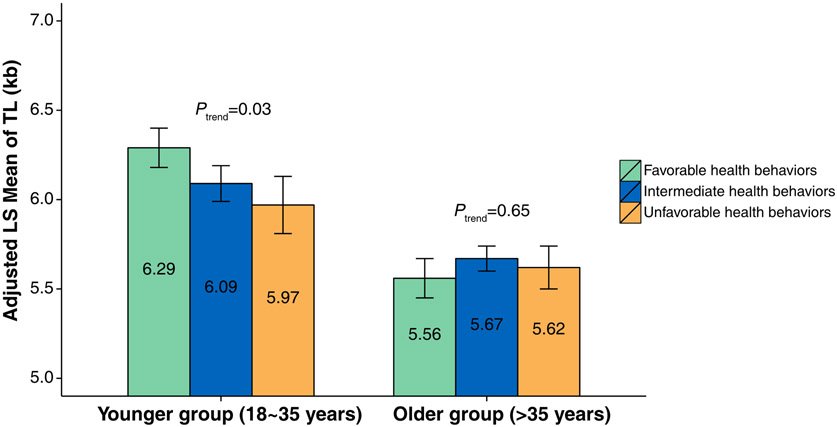
Among survivors ages 18 to 35 years (the younger group), those with favorable, intermediate and unfavorable health behaviors were 38.9%, 49.6% and 11.5%, respectively. Among survivors >35 years of age (the older group), the proportion in the favorable health category was lower (21.9%, 62.3% and 15.8%). For the younger survivors, LTL tended to be shorter among survivors with unfavorable health behaviors (ALSM of LTL=5.97kb; 95% CI=5.65kb-6.29kb) compared with those with favorable health behaviors (ALSM of LTL=6.29kb; 95% CI=6.06kb-6.51kb) with statistical significance for trend across three groups of health behaviors (p=0.03). In contrast, LTL is comparable among survivors in the older group with different health behavior categories. When we compared LTL according to individual component of health behaviors, no statistically significant associations were observed (Supplementary Table S9).
Discussion
The St. Jude Lifetime Cohort Study provided a unique opportunity to investigate LTL in childhood cancer survivors and non-cancer community controls. This comparison demonstrated that survivors have reduced telomere reserve when compared to their age-matched peers but a similar age-dependent telomere attrition. In combination with the findings of associations between specific diagnoses and treatment exposures with LTL, these data suggest that cellular damage related either to cancer and/or its treatment in children is a discrete, early event rather than an acceleration of expected aging processes, suggesting an aging acceleration model (Supplementary Fig. S2) different from the one previously proposed(39). In addition, our analysis found an association between early telomere attrition with the occurrence of CHCs. It is well-established that survivors of childhood cancer experience age-related diseases much earlier than individuals in the general population(25), but it is not clear if LTL attrition is causally related or simply associated with the same treatment exposures that are causally associated with the occurrence of CHCs. Importantly, with comprehensive modeling of telomere length in survivors (Supplementary Fig. S3), we were able to demonstrate an association between poor health behaviors and shorter LTL in younger adult survivors, which suggests that LTL may serve as a potential biomarker for future studies of evaluating the effectiveness of lifestyle-related interventions.
Radiation and chemotherapeutic agents cause acute cellular damage and are biologically well-known to induce telomere dysfunction(40,41). Exposing T-lymphocytes and fibroblasts to doxorubicin, etoposide, or radiation results in telomere shortening, down-regulation of telomerase activity, and diminished expression of telomerase reverse transcriptase (TERT) and telomere binding proteins(16,42). However, a systematic review of 25 epidemiological studies on effects of RT and chemotherapy on telomere length did not result in any definitive conclusions among studies(17). Two longitudinal studies of childhood cancer that reported shorter telomere length following cancer treatment were limited by small sample sizes (N=24 and 25)(43,44). Our findings provided strong epidemiological evidence for telomere shortening following cancer treatments, especially chest RT, abdomen/pelvic RT, glucocorticoids, and vincristine. However, it is possible that associations between specific treatment exposures and LTL are partly mediated by biological differences in LTL profiles that exist across cancer histologies, which were not adjusted in the analysis due to the collinearity with specific RT or chemotherapy.
Our examination of associations between LTL and non-neoplastic CHCs were consistent with findings from studies using the general population, in which shorter LTL has been associated with chronic diseases resulting from restricted cell proliferation. For example, negative associations between LTL and obesity, hypertension or cardiomyopathy have been reported in the general population (10,45,46). Telomere shortening could cause chronic inflammation mediated by apoptosis and cellular senescence. Aging-related dysregulation of telomerase could independently cause mitochondrial dysfunction that could lead to increased oxidative stress. Inflammation and oxidative stress are considered underlying preclinical process of chronic disease that results in biological aging. (47) Specifically, telomere shortening was demonstrated to possibly contribute to metabolic dysfunction including abdominal fat and metabolic abnormalities in mice model (48) and its involvement in the mammalian target of rapamycin (mTOR) pathway links telomere shortening with cardiovascular diseases.(49) In addition, it is evident that accelerated aging caused by inflammation and oxidative stress was related to development or progression of several chronic diseases, specifically in liver diseases,(50) headaches,(51) chronic hepatitis C infection, (52) pulmonary disease. (53)
In contrast, longer LTL has been shown to be mostly associated with cancers characterized by enhanced cellular proliferation(20). Interestingly, we also found a positive association between LTL and subsequent thyroid cancer developing out of the field of prior irradiation treatment. However, a previous study reported survivors of childhood cancer with shorter telomeres were at increased risk for development of subsequent thyroid cancer (54). Furthermore, a recent study by the same group reported that genetically-inferred telomere length using telomere length-associated genetic variants was not associated with risk for subsequent thyroid cancer (55). Both studies were based on genetic and clinical data from the Childhood Cancer Survivor Study with substantial overlap in survivors with subsequent thyroid cancer. Data showing telomere length and cancer associations are also weak in prospective studies, suggesting that telomere shortening largely occurs after diagnosis and therefore may not be of value in predicting cancer incidence(56).
Favorable health behaviors are thought to exert influences over time by reducing oxidative stress and inflammation, thus promoting telomere health.(10-14) In our study, the inverse association between the longer telomere and favorable health behaviors was limited to younger adult survivors of childhood cancer. We hypothesize that this finding result from alteration of health behaviors among older survivors after development of chronic health conditions when telomere damage has already occurred. We observed no statistically significant associations in the analyses of associations between individual component of health behaviors and telomere length. Given that health behaviors typically occur in clusters, we evaluated associations between combined effects (grouped) of health behaviors and telomere length.(57,58) However, reverse causality between favorable health behaviors and longer telomere length may exist where survivors with longer telomere length and healthier physiological state preferentially select favorable health behaviors. Rigorous investigations examining how health behaviors influence telomere length and resulting CHCs, which will require a longitudinal multi-time point study design to: (1) study telomere dynamics and other informative aging biomarkers over time; (2) evaluate associations between telomere health and future chronic disease incidence; and, (3) investigate the impact of health behaviors and psychosocial factors on telomere length and other biomarkers, i.e. mechanistic pathways to disease will certainly advance the field.
Although comprehensive analyses and modeling of telomere lengths for this study produced some intriguing and promising leads, there are limitations to be considered. First, because we analyzed correlative rather than causative associations between LTL and CHCs, potential reverse casual effects of CHCs on the telomere length can confound the associations. To circumvent this problem, this analysis was conducted considering time-to-event where we only considered those late effects that occurred after DNA sampling age for LTL measurement for each survivor in order to explain temporal sequence between LTL and late effects. Second, our sample size is small considering the number of survivors with specific chronic health condition including subsequent neoplasms. Third, our cohort is still quite young (median attained age=36 years), therefore further follow up is needed. Fourth, since the specific chemotherapies and radiation dosages have changed over time, results may be not be applicable to survivors treated more recently. Lastly, we did not examine LTL by types of leukocytes (T or B cells).
Our findings suggest that LTL could be a promising biomarker of aging and aging-related CHCs among childhood cancer survivors. Knowledge gained from this study provides a potential mechanistic pathway responsible for accelerated aging and supports additional research to determine the possible clinical translation of LTL as an aging biomarker in childhood cancer survivors. Use of telomere length has been proposed in personalized medicine and prevention (59), which may help to guide future pharmaceutical discovery and inform non-pharmacologic intervention strategies for health promotion and disease prevention in childhood cancer survivors who are more vulnerable to developing aging-related CHCs.
Supplementary Material
Translational Relevance.
Leukocyte telomere length (LTL) has not been extensively evaluated among childhood cancer survivors, even though it is widely believed that survivors are vulnerable to telomere attrition following exposures to cytotoxic cancer treatments. In this current study, LTL associated with various treatment exposures is significantly shorter among survivors than controls with no history of cancer. LTL is inversely associated with prevalence of 14 different chronic health conditions, whereas favorable health behaviors are associated with longer LTL among younger survivors. Our findings suggest that LTL as a promising aging biomarker associated with prevalence of chronic health conditions may be a mechanistic target for interventions based on health behaviors among survivors of childhood cancer. In the modern era of precision medicine, telomere length may inform strategies for health promotion and disease prevention in childhood cancer survivors who are most vulnerable to developing aging-related chronic health conditions.
Acknowledgements:
This research was supported by funding from the American Lebanese Syrian Associated Charities to St. Jude Children’s Research Hospital and by grants (5R01CA174851 to K.K. Ness, 5P30CA021765 to Charles Roberts and 5U01CA195547 to M.M. Hudson and L.L. Robison) from the National Institutes of Health to St. Jude Children’s Research Hospital.
Footnotes
Conflict of Interest:
The authors declare no potential conflicts of interest.
References
- 1.O’Sullivan RJ, Karlseder J. Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol 2010;11(3):171–81 doi 10.1038/nrm2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levy MZ, Allsopp RC, Futcher AB, Greider CW, Harley CB. Telomere end-replication problem and cell aging. J Mol Biol 1992;225(4):951–60. [DOI] [PubMed] [Google Scholar]
- 3.Artandi SE, Attardi LD. Pathways connecting telomeres and p53 in senescence, apoptosis, and cancer. Biochem Biophys Res Commun 2005;331(3):881–90 doi 10.1016/j.bbrc.2005.03.211. [DOI] [PubMed] [Google Scholar]
- 4.Daniali L, Benetos A, Susser E, Kark JD, Labat C, Kimura M, et al. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun 2013;4:1597 doi 10.1038/ncomms2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muezzinler A, Zaineddin AK, Brenner H. A systematic review of leukocyte telomere length and age in adults. Ageing Res Rev 2013;12(2):509–19 doi 10.1016/j.arr.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Mangino M, Hwang SJ, Spector TD, Hunt SC, Kimura M, Fitzpatrick AL, et al. Genome-wide meta-analysis points to CTC1 and ZNF676 as genes regulating telomere homeostasis in humans. Hum Mol Genet 2012;21(24):5385–94 doi 10.1093/hmg/dds382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Codd V, Nelson CP, Albrecht E, Mangino M, Deelen J, Buxton JL, et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet 2013;45(4):422–7, 7e1-2 doi 10.1038/ng.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pooley KA, Bojesen SE, Weischer M, Nielsen SF, Thompson D, Amin Al Olama A, et al. A genome-wide association scan (GWAS) for mean telomere length within the COGS project: identified loci show little association with hormone-related cancer risk. Hum Mol Genet 2013;22(24):5056–64 doi 10.1093/hmg/ddt355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardner M, Bann D, Wiley L, Cooper R, Hardy R, Nitsch D, et al. Gender and telomere length: systematic review and meta-analysis. Exp Gerontol 2014;51:15–27 doi 10.1016/j.exger.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mundstock E, Sarria EE, Zatti H, Mattos Louzada F, Kich Grun L, Herbert Jones M, et al. Effect of obesity on telomere length: Systematic review and meta-analysis. Obesity (Silver Spring) 2015;23(11):2165–74 doi 10.1002/oby.21183. [DOI] [PubMed] [Google Scholar]
- 11.Arsenis NC, You T, Ogawa EF, Tinsley GM, Zuo L. Physical activity and telomere length: Impact of aging and potential mechanisms of action. Oncotarget 2017;8(27):45008–19 doi 10.18632/oncotarget.16726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gielen M, Hageman GJ, Antoniou EE, Nordfjall K, Mangino M, Balasubramanyam M, et al. Body mass index is negatively associated with telomere length: a collaborative cross-sectional meta-analysis of 87 observational studies. Am J Clin Nutr 2018;108(3):453–75 doi 10.1093/ajcn/nqy107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davinelli S, Trichopoulou A, Corbi G, De Vivo I, Scapagnini G. The potential nutrigeroprotective role of Mediterranean diet and its functional components on telomere length dynamics. Ageing Res Rev 2019;49:1–10 doi 10.1016/j.arr.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Nomikos NN, Nikolaidis PT, Sousa CV, Papalois AE, Rosemann T, Knechtle B. Exercise, Telomeres, and Cancer: “The Exercise-Telomere Hypothesis”. Front Physiol 2018;9:1798 doi 10.3389/fphys.2018.01798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huda N, Tanaka H, Herbert BS, Reed T, Gilley D. Shared environmental factors associated with telomere length maintenance in elderly male twins. Aging Cell 2007;6(5):709–13 doi 10.1111/j.1474-9726.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 16.Li P, Hou M, Lou F, Bjorkholm M, Xu D. Telomere dysfunction induced by chemotherapeutic agents and radiation in normal human cells. Int J Biochem Cell Biol 2012;44(9):1531–40 doi 10.1016/j.biocel.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 17.Gallicchio L, Gadalla SM, Murphy JD, Simonds NI. The Effect of Cancer Treatments on Telomere Length: A Systematic Review of the Literature. J Natl Cancer Inst 2018;110(10):1048–58 doi 10.1093/jnci/djy189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrett JH, Iles MM, Dunning AM, Pooley KA. Telomere length and common disease: study design and analytical challenges. Hum Genet 2015;134(7):679–89 doi 10.1007/s00439-015-1563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Opresko PL, Shay JW. Telomere-associated aging disorders. Ageing Res Rev 2017;33:52–66 doi 10.1016/j.arr.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Zhao Q, Zhu W, Liu T, Xie SH, Zhong LX, et al. The Association of Telomere Length in Peripheral Blood Cells with Cancer Risk: A Systematic Review and Meta-analysis of Prospective Studies. Cancer Epidemiol Biomarkers Prev 2017;26(9):1381–90 doi 10.1158/1055-9965.EPI-16-0968. [DOI] [PubMed] [Google Scholar]
- 21.Walsh KM, Whitehead TP, de Smith AJ, Smirnov IV, Park M, Endicott AA, et al. Common genetic variants associated with telomere length confer risk for neuroblastoma and other childhood cancers. Carcinogenesis 2016;37(6):576–82 doi 10.1093/carcin/bgw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 2014;349:g4227 doi 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheller Madrid A, Rode L, Nordestgaard BG, Bojesen SE. Short Telomere Length and Ischemic Heart Disease: Observational and Genetic Studies in 290 022 Individuals. Clin Chem 2016;62(8):1140–9 doi 10.1373/clinchem.2016.258566. [DOI] [PubMed] [Google Scholar]
- 24.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer 2014;14(1):61–70 doi 10.1038/nrc3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhakta N, Liu Q, Ness KK, Baassiri M, Eissa H, Yeo F, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet 2017;390(10112):2569–82 doi 10.1016/S0140-6736(17)31610-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ness KK, Krull KR, Jones KE, Mulrooney DA, Armstrong GT, Green DM, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime cohort study. J Clin Oncol 2013;31(36):4496–503 doi 10.1200/JCO.2013.52.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cupit-Link MC, Kirkland JL, Ness KK, Armstrong GT, Tchkonia T, LeBrasseur NK, et al. Biology of premature ageing in survivors of cancer. ESMO Open 2017;2(5):e000250 doi 10.1136/esmoopen-2017-000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA 2013;309(22):2371–81 doi 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Wilson CL, Easton J, Thrasher A, Mulder H, Liu Q, et al. Genetic Risk for Subsequent Neoplasms Among Long-Term Survivors of Childhood Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2018;36(20):2078–87 doi 10.1200/JCO.2018.77.8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hudson MM, Ness KK, Nolan VG, Armstrong GT, Green DM, Morris EB, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatric blood & cancer 2011;56(5):825–36 doi 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hudson MM, Ehrhardt MJ, Bhakta N, Baassiri M, Eissa H, Chemaitilly W, et al. Approach for Classification and Severity Grading of Long-term and Late-Onset Health Events among Childhood Cancer Survivors in the St. Jude Lifetime Cohort. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2017;26(5):666–74 doi 10.1158/1055-9965.EPI-16-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding Z, Mangino M, Aviv A, Spector T, Durbin R, Consortium UK. Estimating telomere length from whole genome sequence data. Nucleic Acids Res 2014;42(9):e75 doi 10.1093/nar/gku181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Machiela MJ, Lan Q, Slager SL, Vermeulen RC, Teras LR, Camp NJ, et al. Genetically predicted longer telomere length is associated with increased risk of B-cell lymphoma subtypes. Hum Mol Genet 2016;25(8):1663–76 doi 10.1093/hmg/ddw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. March 25, 2019. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. <http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf>. March 25, 2019.
- 35.Hudson MM, Ehrhardt MJ, Bhakta N, Baassiri M, Eissa H, Chemaitilly W, et al. Approach for Classification and Severity Grading of Long-term and Late-Onset Health Events among Childhood Cancer Survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomarkers Prev 2017;26(5):666–74 doi 10.1158/1055-9965.EPI-16-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stovall M, Weathers R, Kasper C, Smith SA, Travis L, Ron E, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res 2006;166(1 Pt 2):141–57 doi 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 37.Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, et al. The Physical Activity Guidelines for AmericansPhysical Activity Guidelines for AmericansPhysical Activity Guidelines for Americans. JAMA 2018;320(19):2020–8 doi 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dietary Guidelines for Americans 2015-2020, Eighth edition. U.S. Department of Health and Human Services and U.S. Department of Agriculture; 2015. [Google Scholar]
- 39.Ness KK, Kirkland JL, Gramatges MM, Wang Z, Kundu M, McCastlain K, et al. Premature Physiologic Aging as a Paradigm for Understanding Increased Risk of Adverse Health Across the Lifespan of Survivors of Childhood Cancer. J Clin Oncol 2018;36(21):2206–15 doi 10.1200/JCO.2017.76.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu Y, Leong W, Guerin O, Gilson E, Ye J. Telomeric impact of conventional chemotherapy. Front Med 2013;7(4):411–7 doi 10.1007/s11684-013-0293-z. [DOI] [PubMed] [Google Scholar]
- 41.Shim G, Ricoul M, Hempel WM, Azzam EI, Sabatier L. Crosstalk between telomere maintenance and radiation effects: A key player in the process of radiation-induced carcinogenesis. Mutat Res Rev Mutat Res 2014. doi 10.1016/j.mrrev.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fumagalli M, Rossiello F, Clerici M, Barozzi S, Cittaro D, Kaplunov JM, et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat Cell Biol 2012;14(4):355–65 doi 10.1038/ncb2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franco S, Ozkaynak MF, Sandoval C, Tugal O, Jayabose S, Engelhardt M, et al. Telomere dynamics in childhood leukemia and solid tumors: a follow-up study. Leukemia 2003;17(2):401–10 doi 10.1038/sj.leu.2402815. [DOI] [PubMed] [Google Scholar]
- 44.Engelhardt M, Ozkaynak MF, Drullinsky P, Sandoval C, Tugal O, Jayabose S, et al. Telomerase activity and telomere length in pediatric patients with malignancies undergoing chemotherapy. Leukemia 1998;12(1):13–24. [DOI] [PubMed] [Google Scholar]
- 45.Tellechea ML, Pirola CJ. The impact of hypertension on leukocyte telomere length: a systematic review and meta-analysis of human studies. J Hum Hypertens 2017;31(2):99–105 doi 10.1038/jhh.2016.45. [DOI] [PubMed] [Google Scholar]
- 46.Chatterjee S, de Gonzalo-Calvo D, Derda AA, Schimmel K, Sonnenschein K, Bavendiek U, et al. Leukocyte telomere length correlates with hypertrophic cardiomyopathy severity. Sci Rep 2018;8(1):11227 doi 10.1038/s41598-018-29072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J, Rane G, Dai X, Shanmugam MK, Arfuso F, Samy RP, et al. Ageing and the telomere connection: An intimate relationship with inflammation. Ageing Research Reviews 2016;25:55–69 doi 10.1016/j.arr.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 2011;470(7334):359–65 doi 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Z, Ming XF. mTOR signalling: the molecular interface connecting metabolic stress, aging and cardiovascular diseases. Obesity reviews : an official journal of the International Association for the Study of Obesity 2012;13 Suppl 2:58–68 doi 10.1111/j.1467-789X.2012.01038.x. [DOI] [PubMed] [Google Scholar]
- 50.Urabe Y, Nouso K, Higashi T, Nakatsukasa H, Hino N, Ashida K, et al. Telomere length in human liver diseases. Liver 1996;16(5):293–7 doi 10.1111/j.1600-0676.1996.tb00748.x. [DOI] [PubMed] [Google Scholar]
- 51.Ren H, Collins V, Fernandez F, Quinlan S, Griffiths L, Choo KH. Shorter telomere length in peripheral blood cells associated with migraine in women. Headache 2010;50(6):965–72 doi 10.1111/j.1526-4610.2010.01693.x. [DOI] [PubMed] [Google Scholar]
- 52.Hoare M, Gelson WT, Das A, Fletcher JM, Davies SE, Curran MD, et al. CD4+ T-lymphocyte telomere length is related to fibrosis stage, clinical outcome and treatment response in chronic hepatitis C virus infection. Journal of hepatology 2010;53(2):252–60 doi 10.1016/j.jhep.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mui TS, Man JM, McElhaney JE, Sandford AJ, Coxson HO, Birmingham CL, et al. Telomere length and chronic obstructive pulmonary disease: evidence of accelerated aging. Journal of the American Geriatrics Society 2009;57(12):2372–4 doi 10.1111/j.1532-5415.2009.02589.x. [DOI] [PubMed] [Google Scholar]
- 54.Gramatges MM, Liu Q, Yasui Y, Okcu MF, Neglia JP, Strong LC, et al. Telomere content and risk of second malignant neoplasm in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Clin Cancer Res 2014;20(4):904–11 doi 10.1158/1078-0432.CCR-13-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gramatges MM, Morton LM, Yasui Y, Arnold MA, Neglia JP, Leisenring WM, et al. Telomere Length-Associated Genetic Variants and the Risk of Thyroid Cancer in Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study (CCSS). Cancer Epidemiol Biomarkers Prev 2019;28(2):417–9 doi 10.1158/1055-9965.EPI-18-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pooley KA, Sandhu MS, Tyrer J, Shah M, Driver KE, Luben RN, et al. Telomere length in prospective and retrospective cancer case-control studies. Cancer Res 2010;70(8):3170–6 doi 10.1158/0008-5472.CAN-09-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khera AV, Emdin CA, Drake I, Natarajan P, Bick AG, Cook NR, et al. Genetic Risk, Adherence to a Healthy Lifestyle, and Coronary Disease. The New England journal of medicine 2016;375(24):2349–58 doi 10.1056/NEJMoa1605086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whayne TF Jr., Saha SP. Genetic Risk, Adherence to a Healthy Lifestyle, and Ischemic Heart Disease. Current cardiology reports 2019;21(1):1 doi 10.1007/s11886-019-1086-z. [DOI] [PubMed] [Google Scholar]
- 59.Gorenjak V, Akbar S, Stathopoulou MG, Visvikis-Siest S. The future of telomere length in personalized medicine. Front Biosci (Landmark Ed) 2018;23:1628–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


