Abstract
Indole-3-acetamide (IAM) was the first confirmed auxin biosynthetic intermediate in some plant pathogenic bacteria. Exogenously applied IAM or production of IAM by overexpressing the bacterial iaaM gene in Arabidopsis causes auxin overproduction phenotypes. However, it is still inconclusive whether plants use IAM as a key precursor for auxin biosynthesis. Herein, we report the isolation IAM hydrolase 1 (IAMH1) gene in Arabidopsis from a forward genetic screen for IAM-insensitive mutants that display normal auxin sensitivities. IAMH1 has a close homolog named IAMH2 that is located right next to IAMH1 on chromosome IV in Arabidopsis. We generated iamh1 iamh2 double mutants using our CRISPR/Cas9 gene editing technology. We show that disruption of the IAMH genes rendered Arabidopsis plants resistant to IAM treatments and also suppressed the iaaM overexpression phenotypes, suggesting that IAMH1 and IAMH2 are the main enzymes responsible for converting IAM into IAA in Arabidopsis. The iamh double mutants did not display obvious developmental defects, indicating that IAM does not play a major role in auxin biosynthesis under normal growth conditions. Our findings provide a solid foundation for clarifying the roles of IAM in auxin biosynthesis and plant development.
Keywords: Auxin, auxin biosynthesis, Indole-3-acetamide, Arabidopsis, CRISPR, IAMH1
1. Introduction
Auxin plays essential roles in many aspects of plant growth and development (Zhao, 2018, 2014). Auxin concentrations in plant cells need to be tightly controlled so that plants can grow properly in response to developmental and environmental signals. Plants have evolved a complex network to effectively modulate auxin concentrations. Auxin biosynthesis, degradation, and transport all contribute to establishing proper auxin concentrations in cells (Cheng et al., 2007; Davies et al., 1999; Galweiler et al., 1998; Leznicki and Bandurski, 1988; Staswick et al., 2005; Stepanova et al., 2008; Strader and Bartel, 2009; Tao et al., 2008; Zhao et al., 2013; Zheng et al., 2016). Recent studies have shown that spatially and temporally regulated auxin biosynthesis is involved in determining almost all of the major developmental processes including embryogenesis, seedling growth, vascular pattern formation, and flower development (Chen et al., 2016; Chen et al., 2014; Cheng et al., 2006; Zheng et al., 2013). Understanding the molecular mechanisms of auxin biosynthesis provides the necessary tools for effectively modulating auxin levels in plants, thus allowing us to improve agriculturally important traits such as branching and root architecture.
Auxin is generally believed to be synthesized through both tryptophan (Trp)-dependent and Trp-independent pathways (Zhao, 2014). Very little is known about the Trp-independent pathway. But a recent report demonstrates that the cytosolic indole synthase (INS) is a key enzyme in the elusive Trp-independent pathway and that mutants defective in INS functions display phenotypes during early embryogenesis (Wang et al., 2015). But the roles of INS in auxin biosynthesis remain controversial (Nonhebel, 2015). Trp-dependent pathways have also not been fully elucidated. It has been proposed that Trp may be converted to IAA, the main natural auxin in plants, through several routes (Zhao, 2014). Trp can be metabolized into tryptamine (TAM) and indole-3-pyruvate (IPA) by PLP-dependent decarboxylases and aminotransferases, respectively. It is also known that Trp can be converted into indole-3-acetaldoxime (IAOx) by CYP79B2 and CYP79B3 P450 monooxygenases (Zhao et al., 2002). Moreover, plants also produce indole-3-acetamide (IAM) and indole-3-acetonitrile (IAN) from Trp (Sugawara et al., 2009). All of the aforementioned Trp metabolites including TAM, IPA, IAOx, and IAM have been proposed as intermediates for auxin biosynthesis in plants. However, so far only IPA has been firmly established as an important auxin biosynthetic intermediate in plants (Mashiguchi et al., 2011; Stepanova et al., 2011; Won et al., 2011). Disruption of either IPA biosynthesis or metabolism in Arabidopsis, maize, and rice leads to dramatic developmental defects (Cheng et al., 2006; Gallavotti et al., 2008; Phillips et al., 2011; Yamamoto et al., 2007; Zhang et al., 2018). It has been shown that Trp is converted into IAA using IPA as the intermediate in two steps in the so-called TAA/YUC pathway (Dai et al., 2013). Trp is first metabolized into IPA by the TAA family of aminotransferases and subsequently the YUC family of monooxygenases catalyzes the conversion of IPA into IAA (Zhao, 2012). The TAA/YUC pathway is evolutionary conserved among plant species and it is required for all of the major developmental processes in Arabidopsis. Therefore, the TAA/YUC pathway has been recognized as a major auxin biosynthesis pathway.
The roles of the other Trp metabolites in auxin biosynthesis and plant development have not been fully resolved. IAOx has long been recognized as a potential auxin biosynthesis precursor. Over accumulation of IAOx in Arabidopsis by either overexpressing the biosynthetic enzyme CYP79B2 or by inactivating IAOx metabolizing enzymes such as SUR1 and SUR2 leads to auxin overproduction (Boerjan et al., 1995; Delarue et al., 1998; Zhao et al., 2002). Although IAOx can be metabolized into IAA in Arabidopsis, the exact mechanisms by which IAOx is converted into IAA are not understood at present. It is generally accepted that IAOx probably is not a major intermediate for auxin biosynthesis for two reasons. First, complete elimination of IAOx production in Arabidopsis by knocking out both CYP79B2 and B3 does not lead to dramatic developmental defects. Second, IAOx is only produced in a limited number of plant species that produce indolic glucosinolates (Kasahara, 2016).
The biosynthetic route for IAOx is well understood, but the reactions from IAOx to IAA have not been elucidated. In contrast, enzymes responsible for converting some Trp metabolites such as IAN into IAA are known (Bartel and Fink, 1994), but the biosynthetic route for IAN and IAM are not well understood. IAN can be converted into IAA in plants when added to plant growth media. It was shown more than two-decade ago that IAN is converted into IAA by a family of nitrilases (Bartel and Fink, 1994). Mutations in nitrilase 1 (nit1) in Arabidopsis render the mutant resistant to exogenous IAN (Bartel and Fink, 1994). Under normal growth conditions, nit1 mutants do not display obvious developmental defects probably because of the compensatory effects provided by NIT1 homologs in Arabidopsis. It is still an outstanding question whether nitrilases and IAN play an important role in auxin biosynthesis and plant development.
IAM was the first definitively identified intermediate used in Trp-dependent auxin biosynthesis pathways in bacteria. Plant pathogens such as agrobacterium and pseudomonas synthesize auxin from Trp when they infect plants (Comai and Kosuge, 1982; Yamada et al., 1985). The bacteria-produced auxin alters the growth and developmental patterns of the infected plant cells so that the pathogens can use the plant cells to produce carbon- and nitrogen- rich compounds for their growth. The pathogens convert Trp into IAM using the bacterial iaaM Trp-2-monooxygenase and subsequently the pathogen-encoded hydrolase iaaH converts IAM to IAA (Comai and Kosuge, 1982; Yamada et al., 1985). Arabidopsis and other plants produce IAM in the absence of a bacterial infection, suggesting that plants may use IAM as an auxin biosynthetic intermediate as well (Sugawara et al., 2009). Furthermore, IAM was proposed as an intermediate in a route that converts IAOx into IAA (Sugawara et al., 2009). It is well known that Arabidopsis and other plants have the capacity to convert IAM into IAA. Overexpression of iaaM in Arabidopsis, petunia, and tobacco led to auxin overproduction phenotypes (Eklof et al., 2000; Mezzetti et al., 2004; Romano et al., 1995). It is hypothesized that plant hydrolases can convert IAM produced by the iaaM transgene to generate IAA. Bioinformatics analyses have identified a small family of IAM hydrolases named as amidases that share significant homology to the bacterial iaaH proteins (Pollmann et al., 2003). Arabidopsis amidase I has been shown to have the capacity to hydrolyze IAM into IAA in vitro and in Arabidopsis (Pollmann et al., 2003). However, the amidase mutants do not display much reduced sensitivity to exogenous IAM (Pollmann et al., 2003), suggesting that plants probably also use other unidentified hydrolases to convert IAM to IAA. Identification of additional enzymes that are responsible for converting IAM to IAA will help us to unambiguously determine whether IAM is a key auxin biosynthetic intermediate in plants and whether IAM-derived auxin plays an important role in plant growth and development. Understanding of how IAM is converted into IAA in plants will also clarify whether IAM is an important intermediate in metabolizing IAOx into IAA.
In this study, we present the identification of two homologous genes that encode Indole-3-acetamide hydrolases (IAMHs) responsible for converting IAM to IAA in Arabidopsis. Arabidopsis plants that lacked the IAMH activities were resistant to exogenous IAM. Mutations in the IAMH genes suppressed the auxin overproduction phenotypes caused by overexpression of iaaM, the bacterial auxin biosynthetic gene. The iamh mutants did not display any obvious growth and developmental defects under normal laboratory conditions, suggesting that the IAM-derived auxin probably is not the main source of auxin required for Arabidopsis development under normal growth conditions. This work identified the main enzymes for hydrolyzing IAM to IAA in Arabidopsis and clarified the roles of IAM in auxin biosynthesis and plant development.
2. Results
2.1. IAM promotes plant growth and activates the auxin reporter DR5-GUS
IAM is the key intermediate used by some plant pathogenic bacteria to synthesize auxin (Fig. 1A) (Comai and Kosuge, 1982; Yamada et al., 1985). The two-step pathway catalyzed by the bacterial iaaM and iaaH effectively converts Trp into IAA (Fig. 1A). The iaaM gene has been widely used to genetically modulate auxin levels in plants (Cheng et al., 2006; Eklof et al., 2000; Romano et al., 1995). Arabidopsis seedlings grown on IAM-containing media in light had much elongated hypocotyls and developed epinastic cotyledons (Fig. 1B). IAM also slightly inhibited the elongation of primary roots (Fig. 1B). IAM-treated plants resembled closely to the well-characterized Arabidopsis auxin overproduction mutants such as YUC overexpression lines and sur1 (Boerjan et al., 1995; Zhao et al., 2001), suggesting that IAM either activates an auxin signaling pathway directly or IAM is converted into IAA, the active natural auxin. Interestingly, seedlings grown on IAA-containing media did not display long hypocotyls and epinastic cotyledon (Fig. 1B). Rather IAA mainly inhibited primary root elongation and stimulated lateral root initiation and elongation (Fig. 1B).
Fig 1.
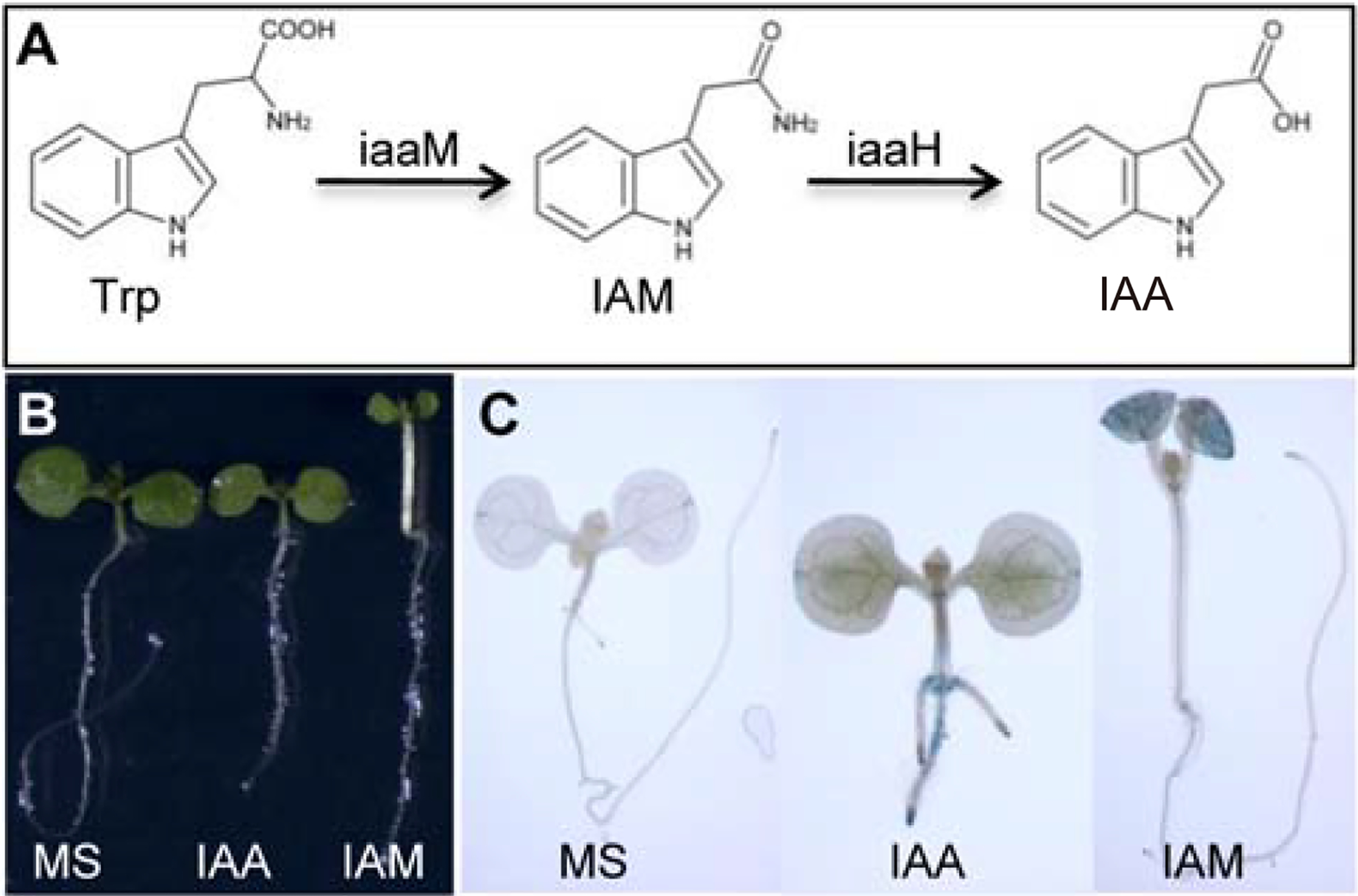
Indole-3-acetamide (IAM) is a potential auxin biosynthetic intermediate in plants and IAM treatments affect plant growth and activate the auxin reporter DR5-GUS. A: A proposed Trp-dependent auxin biosynthetic pathway using IAM as the intermediate. This pathway is used by plant pathogenic bacteria such as Agrobacteria, which uses the iaaM and iaaH genes to convert Trp to IAA. The roles of IAM in plant auxin biosynthesis are not clear. B: Five-day old Arabidopsis seedlings grown on MS media and media containing 20 μM IAA or IAM. Note that IAA inhibits primary root elongation and IAM stimulates hypocotyl growth. Note that the seedling above the label IAA and IAM refer to seedlings grown on IAA and IAM-containing media, respectively. C: Activation of DR5-GUS expression by IAA and IAM. Interestingly, IAM mainly activates DR5-GUS expression in aerial tissue whereas IAA increases DR5-GUS signal in the root.
We investigated whether IAM activated the expression of the auxin reporter DR5-GUS. As shown in Fig. 1C, seedlings grown on IAM-containing media had much elevated expression levels of DR5-GUS in the cotyledons and true leaves compared to seedlings grown on regular media. Activation of DR5-GUS expression in aerial part by IAM is consistent with the observation that IAM mainly stimulated hypocotyl elongation and changed the shape of cotyledons (Fig. 1B). In contrast, IAA activated DR5-GUS expression in the roots (Fig. 1C). Our results indicated that IAM and IAA caused different developmental phenotypes in Arabidopsis seedlings (Fig. 1B & C). The observed differences were probably caused by differences in uptake and transport of the two compounds. It is very clear that IAM treatment could activate the auxin reporter and caused phenotypes related to elevated auxin levels.
2.2. A genetic screen for mutants resistant to IAM
Arabidopsis seedlings grown on 20 μM IAM phenocopied the YUC overexpression plants, which produce elevated levels of auxin (Cheng et al., 2006; Zhao et al., 2001). Because auxin overproduction mutants display phenotypes different from those caused by IAA treatments and because previous genetic screens for auxin resistant mutants were mainly conducted using exogenous IAA or synthetic auxin 2,4-D, we initially hypothesized that a genetic screen for mutants that can suppress YUC overexpression lines would uncover novel auxin genes. We hypothesized that such a genetic screen might be able to identify genes that are important for auxin biosynthesis, conjugation, degradation, transport or auxin signaling. Unfortunately, the YUC overexpression lines were not stable and the strong lines were completely sterile. Therefore, genetic screens for suppressors/enhancers of YUC overexpression lines were not productive. Because of the strong phenotypic similarities between IAM-treated plants and the YUC overexpression lines, we believed that genetic screens for IAM resistant mutants would mimic the screens for suppressors of YUC overexpression lines.
We mutagenized Arabidopsis seeds using EMS and conducted the genetic screen using 7- to 9-day old seedlings grown on 20 μM IAM under light. The putative mutants should have short hypocotyls and normal cotyledon shapes. We screened M2 seeds from 1000 individual M1 plants and identified more than 100 putative IAM-resistant mutants, which were subsequently transplanted to soil. Among the putative mutants, many were dwarf with dark-green leaves, which are very similar to the brassinolide (BR) biosynthesis and signaling mutants. Such obvious BR mutants were discarded. We conducted second round screens with M3 seeds and confirmed 24 IAM-resistant mutants. One of the mutants, #483, was almost insensitive to IAM. Light grown mutant #483 had a short hypocotyl and flat cotyledons (Fig. 2A) when grown on 20 μM IAM, but the mutant was very similar to WT when grown on MS media (Fig. S1A). Activation of DR5-GUS expression by IAM was also suppressed in the mutant #483 (Figs. 2B and S2B). We backcrossed the mutant to WT Col and out-crossed it to WT Ler. About 25% seedlings from either F2 populations displayed the IAM resistant phenotype, suggesting that the phenotype was caused by a recessive mutation of a single locus.
Fig 2.
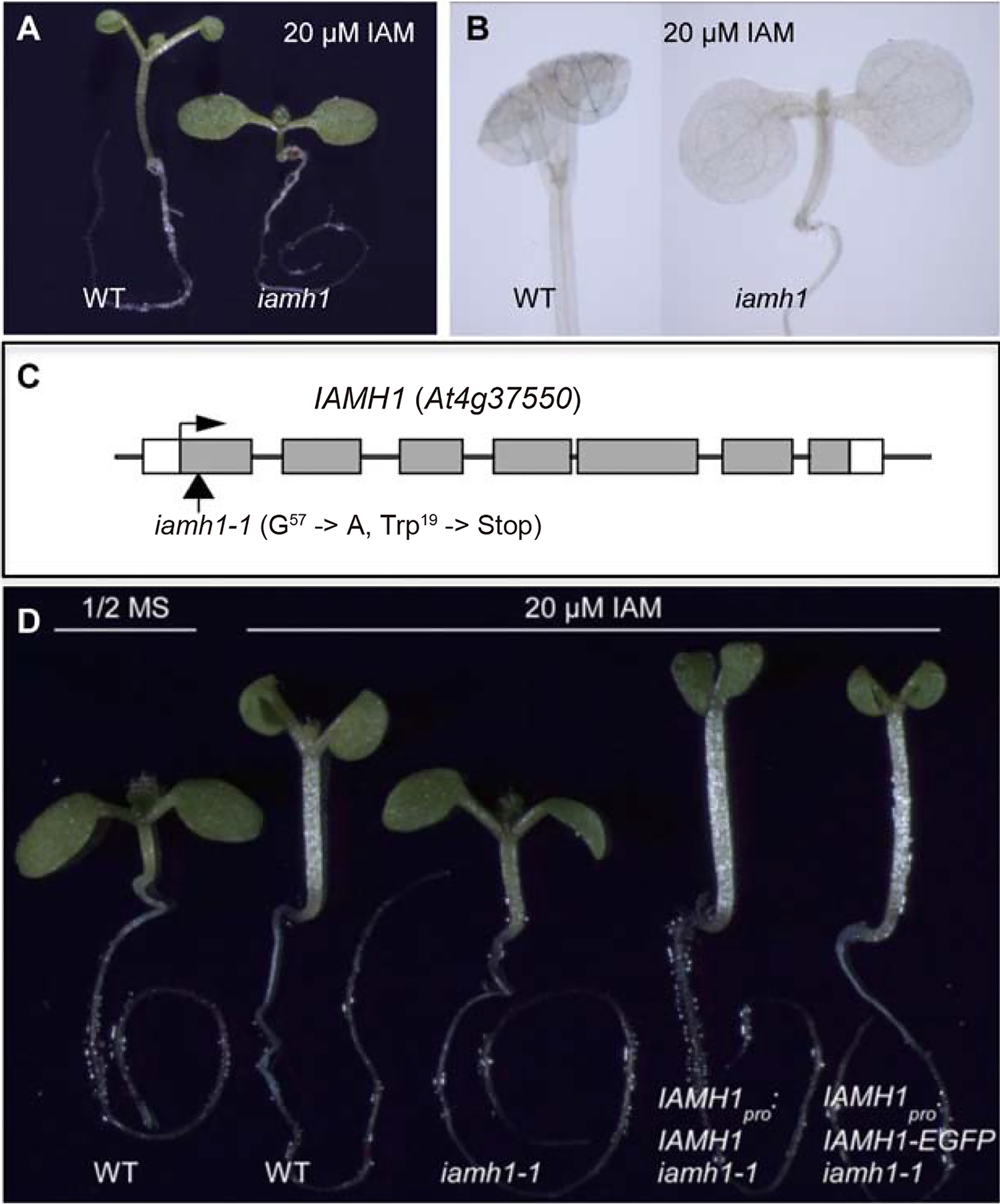
Isolation and cloning of an IAM resistant mutant (iamh1). A: Isolation of an IAM-resistant mutant, which does not have elongated hypocotyl and does not display epinastic cotyledons when grown on 20 μM IAM-containing media. B: The expression of DR5-GUS auxin reporter is not induced by IAM treatments in the iamh1 mutant. C: The iamh1 mutation was identified by map-based positional cloning. The IAMH1 gene is At4g37550. The iamh1–1 mutant harbors a G to A mutation in At4g37550 that results in a premature stop codon. D: The iamh1 phenotypes are rescued by wild type IAMH1 genomic DNA or IAMH1 genomic DNA fused with GFP driven by the IAMH1 promoter.
2.3. Cloning of the IAMH1 gene
We mapped the mutation in the #483 mutant to the bottom of chromosome IV and narrowed the mapping interval down to a 330 Kb region. Among the ORFs in the mapping interval, At4g37550 and At4g37560 encode putative Acetamidase/Formamidases, which potentially have the hydrolase activities that can break an amide bond. We hypothesized that a mutation in At4g37550 or At4g37560 probably would abolish the conversion of IAM into IAA in Arabidopsis, thus causing the IAM-insensitive phenotypes. We sequenced the genomic DNA of At4g37550 and At4g37560 from the mutant #483 and identified a G to A conversion in the first exon of At4g37550 (Fig. 2). The mutation converted a Trp codon to a premature stop codon (Fig. 2C), suggesting that the mutant is likely a null allele.
To confirm that the identified mutation in the At4g37550 gene caused the observed IAM insensitive phenotype, we obtained a T-DNA insertion mutant of At4g37550 from the ABRC stock center. The T-DNA mutant was also resistant to IAM treatment, demonstrating that #483 mutant phenotypes were caused by the disruption of At4g37550. We renamed #483 mutant iamh1–1 (IAM hydrolase 1) and At4g37550 gene IAMH1. The T-DNA allele was named iamh1–2.
To further demonstrate that we had identified the causal mutation in iamh1–1, we transformed iamh1–1 plants with a construct that harbored the At4g37550 genomic fragment including its own promoter. As shown in Fig. 2D, the IAMH1 genomic fragment fully restored the IAM sensitivity of the iamh1–1 mutant. We also expressed an IAMH1-GFP fusion under the control of the IAMH1 promoter in the iamh1–1 background. The GFP fusion could also fully rescue the iamh1–1 phenotypes. Interestingly, the complementation transgenic lines appeared to have longer hypocotyls than wild type plants grown under the same conditions (Fig. 2D). The differences were probably caused by a slight overexpression of the transgenes. Such an observation further supports the hypothesis that IAMH1 is involved in converting IAM to IAA in Arabidopsis.
IAMH1-like genes have been identified in all of the plant genomes including Chlamydomonas reinhardtii and Physcomitrella patens. A phylogenetic analysis of the IAMH1proteins across representative plant species was shown in Fig. S2. IAMH1 is highly conserved throughout the plant kingdom, as shown in the multiple sequence alignment of IAMH1 proteins from various plant species (Fig. S3). For example, the Chlamydomonas reinhardtii IAMH1 protein share 75% amino acid sequence identity with the Arabidopsis IAMH1.
2.4. IAMH1 is broadly expressed and is not localized in the nucleus.
We expressed the GUS reporter under the control of the IAMH1 promoter in Arabidopsis. At seedling stage, the GUS expression was broadly distributed in cotyledons, true leaves, and root tips (Fig. 3). At reproductive stage, GUS expression was observed in young flowers, gynoecia, and in inflorescences (Fig. 3). Expression of IAMH1-GFP fusion driven by IAMH1 promoter showed that IAMH1 was clearly not expressed in the nucleus (Fig. 3)
Fig 3.
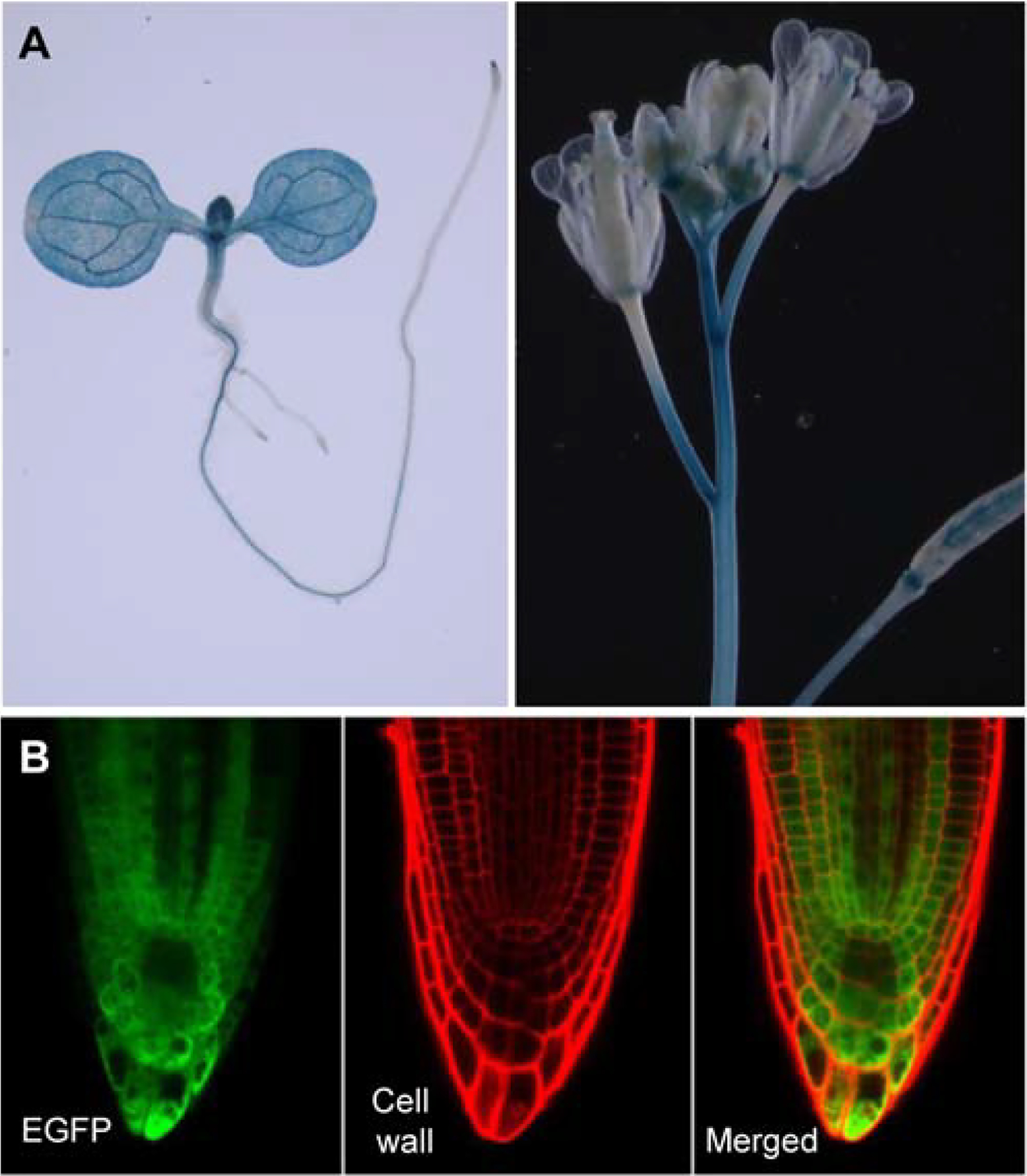
Expression pattern of iamH1 and sub-cellular localization of IAMH1 protein. A: The GUS expression patterns of IAMH1pro:GUS Transgenic lines. Note that the reporter has a broad expression pattern. B: Expression of the IAMH1-EGFP fusion in Arabidopsis roots driven by the IAMH1 promoter. IAMH1 appears to be located in the cytosol.
2.5. Arabidopsis genome contains two copies of IAMH genes
Neither iamh1–1 nor iamh1–2 showed obvious developmental defects under normal growth conditions, despite that both mutant alleles were resistant to IAM. Blastp analysis using IAMH1 protein as query identified At4g37560 as a close homolog of IAMH1 in the Arabidopsis genome (Fig. 4). We named At4g37560 IAMH2. IAMH2 and IAMH1 share 90% amino acid sequence identity. Because of the high sequence homology, we hypothesized that IAMH2 might also play an important role in converting IAM into IAA in Arabidopsis. Functional redundancy between IAMH1 and IAMH2 may explain our observation that iamh1–1 and iamh1–2 did not show obvious developmental defects.
Fig 4.
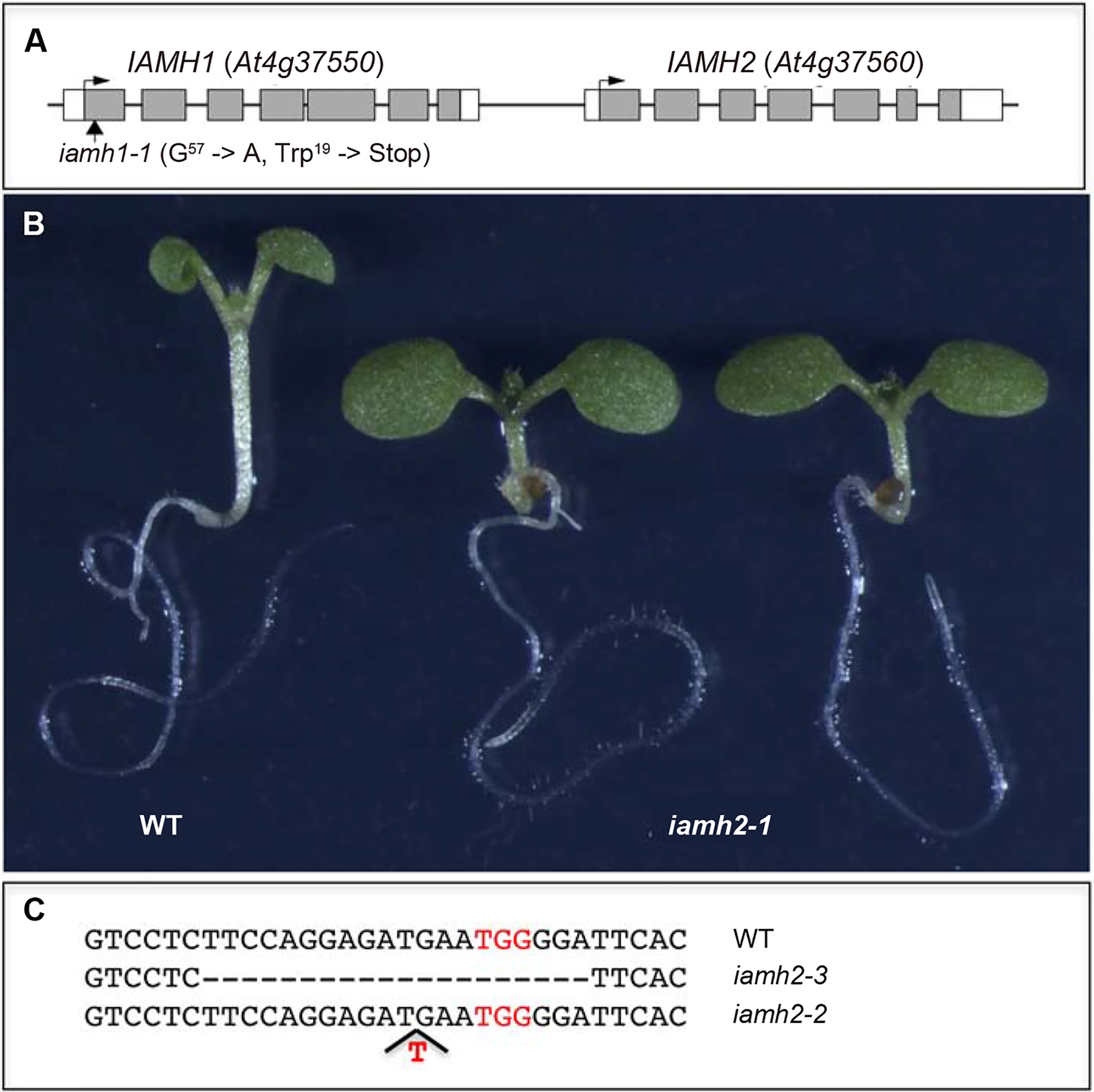
The IAMH2 gene is also involved in converting IAM into IAA. A: IAMH1 has a close homolog, IAMH2. The two genes are tandem repeats located on Chromosome IV. B: A T-DNA insertion in IAMH2 also caused resistance to exogenous IAM. C: Generation of iamh2 alleles in iamh1–1 by CRISPR/Cas9 gene editing technology. TGG in red is the PAM site for CRISPR/Cas9. The iamh2–2 allele harbors one T insertion and iamh2–3 contains a 20 bp deletion.
We obtained a T-DNA insertion mutant from the ABRC stock center to test whether iamh2 was also resistant to IAM. As shown in Fig. 4B, iamh2–1 had short hypocotyls and normal cotyledons when grown on 20 μM IAM whereas wild type plants developed long hypocotyls and epinastic cotyledons, demonstrating that disruption of IAMH2 also led to IAM resistance. These data suggest that IAMH2 likely has overlapping functions with IAMH1.
2.6. Construction of iamh1 iamh2 double mutants
In order to assess the roles of the IAMH genes in auxin biosynthesis and Arabidopsis development, we need to inactivate both IAMH genes simultaneously. The two IAMH genes are located at Chromosome IV as tandem repeats (Fig. 4A). It is virtually impossible to generate iamh1 iamh2 double mutants by crossing two single mutants together because of the extremely tight linkage between the two genes. We employed our recently developed ribozyme-based CRISPR technology (Gao et al., 2016; Gao et al., 2015; Gao and Zhao, 2014) to generate iamh2 mutations in the iamh1–1 background. We obtained two potential loss-of-function iamh2 alleles (Fig 4C): iamh2–2 and iamh2–3. The iamh2–2 contained a single bp insertion after the nucleotide 330 from the ATG start codon, which generated an immediate stop codon (Fig. 4C). Therefore, iamh2–2 is likely a null allele. The iamh2–3 allele harbored a 20 bp deletion from nucleotide 322 to 342 (A in the ATG start codon counts as the first nucleotide). Such a large deletion in iamh2–3 was also likely to completely abolish IAMH2 function. The mutations were confirmed by DNA sequencing (Fig. S4).
We backcrossed both iamh2 alleles to wild type Col plants to segregate out the CRISPR/Cas9. Both iamh1–1 iamh2–2 and iamh1–1 iamh2–3 double mutants were viable and fertile (Fig. S5). In fact, we did not observe any obvious developmental defects in the iamh double mutants under normal plant growth conditions. Our data suggest that auxin-derived from IAM probably is not required for Arabidopsis growth and development under laboratory growth conditions.
2.7. Suppression of the iaaM overexpression phenotypes by disrupting the IAMH genes
The bacterial auxin biosynthetic gene iaaM encodes a Trp monooxygenase, which converts Trp to IAM (Fig. 1). Overexpression iaaM alone is sufficient to cause auxin overproduction phenotypes (Cheng et al., 2006; Romano et al., 1995). The iaaM overexpression lines display long hypocotyls and epinastic cotyledons at seedling stage (Fig. 5A). The overexpression lines also produce more upright and narrower true leaves with longer petioles at young adult stages (Fig. 5B). When we introduced the same construct into iamh1–1 iamh2–3, the auxin overproduction phenotypes disappeared (Fig. 5A and B), suggesting that IAMH enzymes are needed to convert endogenously produced IAM into IAA. Both WT and the iamh1–1 iamh2–3 double mutants grew normally without the iaaM gene (Fig. S5).
Fig 5.
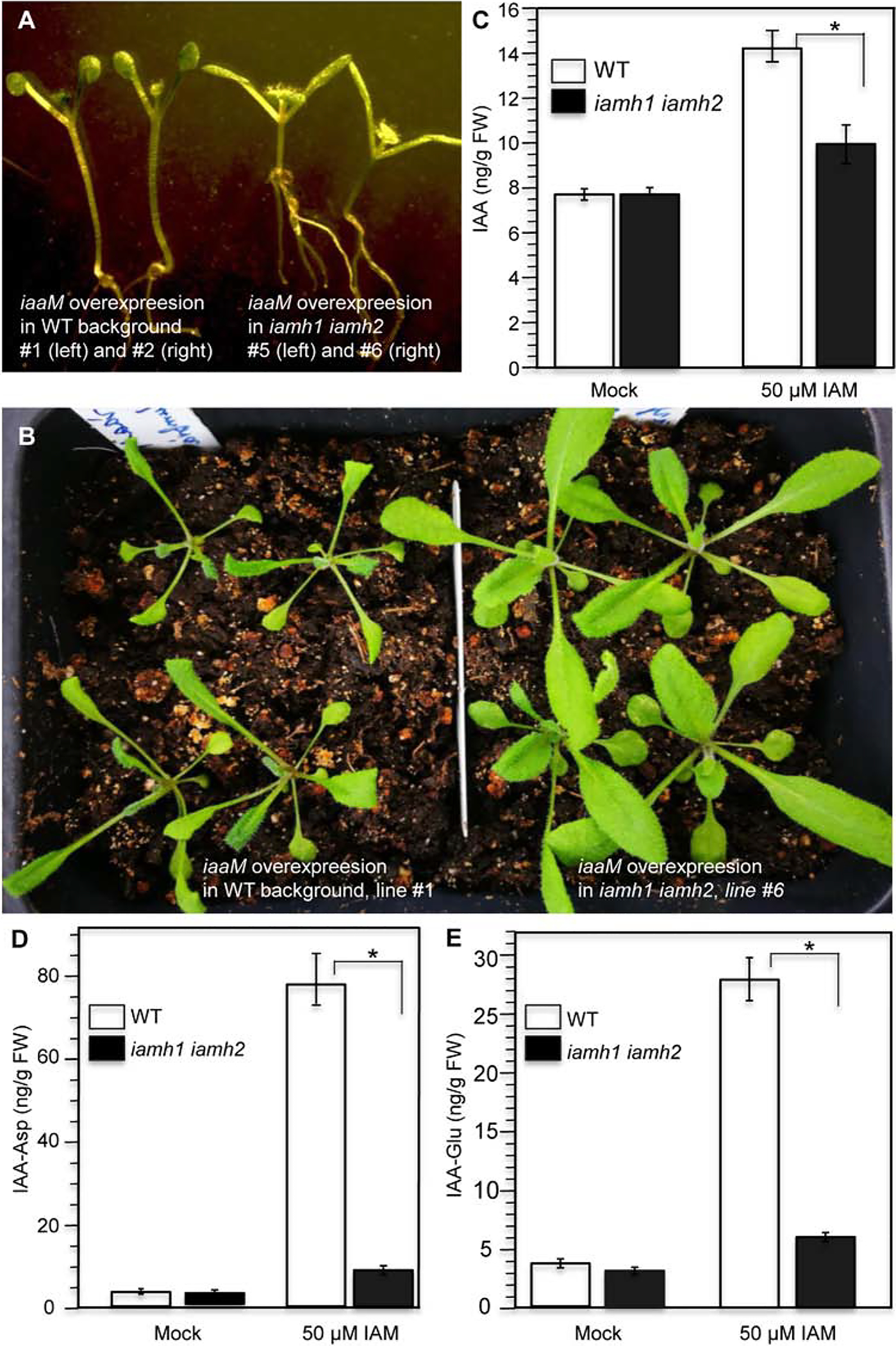
The IAMH genes are major contributors to hydrolysis of IAM in Arabidopsis. A: Disruption of the IAMH genes suppressed the iaaM overexpression phenotypes. Note that overexpression of the bacterial iaaM gene led to long hypocotyls and epinastic cotyledons (left, two independent T1 plants), which are characteristic auxin overproduction phenotypes. B: Young adult independent T1 plants of iaaM overexpression lines in WT (left) and in the iamh1 iamh2 −3 background (right). C: WT and the double mutants contained similar levels of IAA (t-value = −0.31235; P-value = 0.385191.). However, IAM treatment caused a dramatic increase of IAA in WT plants, but much less increase in the iamh1–1 iamh2–3 double mutants background (t-value = 6.55; P-value = 0.001404). D: IAM treatment caused about 80-fold increase of IAA-Asp conjugate in wild type, but only a slight increase in the iamh1–1 iamh2–3 double mutants (t-value = 19.95751; P-value = 0.000019). E: IAM treatment led to an increase in IAA-Glu synthesis in WT, but the increase was very small in the iamh1–1 iamh2–3 double mutants (t-value = 13.28318; P-value = 0.000093). * indicates statistically significant.
We analyzed auxin concentrations in IAM treated plants, which had obvious auxin overproduction phenotypes (Fig. 1). The IAA concentrations in iamh1 iamh2 double mutants were very similar to those observed in WT plants (Fig. 5C). WT plants grown on IAM-containing media had much elevated IAA concentrations (Fig. 5C). However, only a slight increase of IAA in the iamh1 iamh2 double mutants was observed (Fig. 5C).
One of the effective mechanisms for plants to maintain auxin homeostasis is to conjugate IAA to amino acids (Staswick et al., 2005). We observed a dramatic increase of IAA-Asp and IAA-Glu in the IAM treated WT plants (Fig. 5D and E). In contrast, IAM treatment in iamh1 iamh2 double mutants did not induce a substantial increase in IAA-Asp and IAA-Glu conjugates (Fig. 5D and E), further supporting that IAM was not adequately hydrolyzed into IAA in the iamh1 iamh2 double mutants.
3. Discussion
Although IAM was the first confirmed Trp metabolite that could serve as an auxin precursor (Comai and Kosuge, 1982), its role in auxin biosynthesis in plants has never been clarified. In this paper, we uncovered two IAMH genes that encode hydrolases that are capable of converting IAM into IAA in Arabidopsis. We showed that Arabidopsis plants lacked the two IAMH genes were resistant to IAM treatments. Disruption of the IAMH genes also suppressed the developmental abnormalities caused by overexpression of the iaaM gene, suggesting that the IAMHs are the main enzymes in converting IAM into IAA in Arabidopsis. Together with previous findings that Arabidopsis plants contained measurable amount IAM (Sugawara et al., 2009), we conclude that IAM is a viable candidate for auxin biosynthesis in plants.
The iamh1 iamh2 double mutants did not display obvious developmental defects under our normal growth conditions, suggesting that auxin synthesized from the IAM pathway may not play critical roles in Arabidopsis development. However, we noticed that iamh1 iamh2 double mutants were not entirely insensitive to IAM (Fig. 5). IAM treatments still slightly increased IAA concentrations in the double mutants (Fig. 5). We also observed that both IAA-Asp and IAA-Glu were increased in the double mutants (Fig. 5), although the amplitudes of the increases were much smaller compared to those of WT treated with IAM. Our results suggest that Arabidopsis plants have other hydrolases that are capable of converting IAM to IAA. An obvious candidate is the Amidase1, which has been studied extensively for almost two decades (Pollmann et al., 2003). It will be interesting to further knock out the amidase family of genes in the iamh1 iamh2 double mutant background. Analysis of the higher order mutants in terms of IAM sensitivity and developmental phenotypes will provide a more definitive answer to the roles of IAM in auxin biosynthesis and plant development.
In summary, we have identified two IAM hydrolase genes that are responsible for converting IAM into IAA in Arabidopsis. We showed that the IAMH genes are required for the auxin overproduction phenotypes caused by IAM treatments or by overexpressing the bacterial iaaM gene. Our findings provide a foundation for determining the roles of IAM in auxin biosynthesis and in plant development. Moreover, our findings will also help resolve the molecular mechanisms by which IAOx is converted into IAA.
4. Materials and Methods
4.1. Plant materials and growth conditions
The iamh1–2 and iamh2–1 T-DNA mutants were obtained from the Arabidopsis stock center. Plants were grown under long-day conditions (16-h light and 8-h dark) at 22 °C. Seeds were surfaced sterilized by 70% ethanol and air-dried on filter papers in the hood before placed on plates containing Murashige and Skoog (MS) media (supplemented with IAA or IAM when indicated). The plates with seeds were then incubated at 4 °C for 2 days before placed in the growth chamber. Seedlings were grown on the plates in the growth chamber until 7–9 days old, and then transferred to soil if needed.
4.2. Screen for Arabidopsis mutants resistant to IAM
Wild type Columbia Arabidopsis seeds were mutagenized using Ethyl methanesulfonate (EMS) treatments. Seeds from each mutagenized plant were harvested individually and grown on MS media containing 20 μM IAM. WT plants have obviously elongated hypocotyls. IAM- resistant seedlings with reduced hypocotyl length were selected and further characterized. The iamh1–1 mutant was backcrossed 2 times to WT Col to remove background mutations.
4.3. Plant transformation
Arabidopsis plants with different genetic backgrounds were transformed using corresponding T-DNA constructs in Agrobacteria GV3101 using the floral dipping method described previously (Clough and Bent, 1998).
4.4. Constructs and transgenic plants
The IAMH1 promoter was amplified from Arabidopsis genomic DNA. The 2.8 Kb IAMH1promoter was cloned into pBI101.3 plasmid to drive the expression of the GUS reporter (Beta-glucuronidase). The IAMH1pro:GUS construct was transformed into WT Col plants and T1 plants were selected on MS plates containing 50 μg/mL kanamycin.
The IAMH1pro:IAMH1 construct was made using pART27 as the backbone with the entire 5.5 Kb IAMH1 genomic fragment, which includes the 2.8 Kb promoter region upstream of the start codon, the 2.0 Kb region coding region, and the 657 bp region after the stop codon. The iamh1–1 plants were transformed with the IAMH1pro:IAMH1 construct and T1 plants were selected on MS plates containing 20 μM IAM. Plants showing restored IAM sensitivity were selected as complemented lines.
IAMH1pro:IAMH1-EGFP construct was made using pART27 as the plasmid backbone. We fused the 4.9 Kb IAMH1genomic fragment, which included the 2.8 Kb promoter region and the 2.0 Kb region from the start codon to just before the stop codon, with the EGFP gene.
CRISPR construct targeting the IAMH2 gene was generated using our ribozyme- based guide RNA CRISPR system described previously (Gao et al., 2015; Gao and Zhao, 2014). The CRISPR target site chosen for IAMH2 gene was GTCCTCTTCCAGGAGATGAATGG, which is in the second exon and the 314 to 336 bp region counting from the ATG start codon in the cDNA. The iamh1–1 plants were transformed with our CRISPR constructs and T1 plants were selected on MS plates containing 16 μg/mL hygromycin.
4.5. Genotyping the iamh1–1 mutant
Plants with iamh1–1 mutation were genotyped using primers 5’- GATGACGCC- AAGCGTGTAAGC −3’ and 5’- CTGGGAATTCAGAGGTAAGCAC −3’ to amplify the genomic DNA and then digested with NcoI. PCR products from WT plants would be cut into two fragments (0.6 Kb + 0.9 Kb) while the PCR products from the iamh1–1 mutant would appear as a single band (1.5 Kb).
4.6. Beta-glucuronidase (GUS) staining
GUS staining of IAMH1pro:GUS plants and DR5-GUS plants were performed according to the previously described protocol (Zhao et al., 2001).
4.7. Confocal imaging of root tips
Root tips of IAMH1pro:IAMH1-EGFP plants were stained using Propidium Iodide and visualized using a confocal microscope. The cell contour appeared as red florescence signal and IAMH1-EGFP fusion proteins appeared as green florescence signal.
4.8. Analysis of auxin metabolites
Plant materials were first frozen in liquid nitrogen and stored at −80°C. We homogenized about 30 mg frozen plant tissues in 0.3 mL of extraction buffer using a Tissue Lyser (Qiagen) with the 3 mm zirconia beads for 3 min. The extraction buffer (80% acetonitrile/1% acetic acid aqueous solution) contained [phenyl-13C6] IAA, [13 C4, 15N]IAA-Asp and [13C5, 15N]IAA-Glu, which served as internal standards. Extracts were centrifuged at 13,000 × g for 3 min at 4°C, and the supernatant was collected. We repeated the extraction twice, but did not add additional internal standards. We combined the extracts and added 2 mL of 1% acetic acid/H2O. Then the total volume was reduced to <1 mL by evaporation using a Speed Vac from Thermo Fisher Scientific. We then loaded the concentrated extracts onto a 1 mL Oasis HLB column (Waters), and washed the column with 1 mL 1% acetic acid/H2O. We eluted IAA, IAA-Asp, and IAA-Glu with 2 mL 80% acetonitrile/1% acetic acid. The elute was then reduced to <1 mL by evaporation using a Speed Vac. We loaded the fraction containing IAA, IAA-Asp, and IAA-Glu onto a 1mL Oasis WAX column. After washing with 1 mL 1% acetic acid/H2O and subsequent 2 mL 80% acetonitrile/H2O, we eluted IAA with 2 mL 80% acetonitrile/1% acetic acid. IAA-Asp and IAA-Glu were eluted with 2 mL 80% acetonitrile/0.8% formic acid. We used a Speed Vac to dry each fraction through evaporation. We re-dissolved each fraction in 30 μL 1% acetic acid/H2O, and injected it into an Agilent 6420 Triple Quad system (Agilent) with a ZORBAX Eclipse XDB-C18 column (1.8 mm, 2.1×50 mm). IAA separation by HPLC followed the following conditions: We used solvent A (0.05% acetic acid/H2O) and solvent B (acetonitrile/0.05% acetic acid) to generate certain solvent gradients. We first ran 3% solvent B for 3 min and then a gradient from 3% to 15% of solvent B over 20 min at a flow rate of 0.2 mL min−1 with column temperature set at 40°C. The MS/MS analysis conditions for IAA and [phenyl-13C6]IAA (positive ion mode) were conducted as previously described (Sugawara et al., 2015).
Conditions for HPLC separation of IAA-Asp and IAA-Glu were the following: Solvent A (0.1% formic acid/H2O) and Solvent B (acetonitrile/0.05% formic acid were used for running the column. We used a gradient of 3% to 8% of solvent B for 2 min, a gradient of 8% to 11% of solvent B for 7min, and a gradient of 11% to 15% of solvent B over 15 min at a flow rate of 0.2 mL min–1 at 30°C. The following conditions were used the MS/MS analysis of IAA-Asp and [13C4, 15N]IAA-Asp: Capillary = 4,000 V, fragmentor voltage = 95 V, collision energy = 19 V, dwell time = 250 ms, and MS/MS transition (m/z) = 291/130 for unlabeled IAA-Asp and 296/130 for [13C4, 15N]IAA-Asp. We used the following conditions for MS/MS analysis of IAA-Glu and [13C5, 15N]IAA-Glu: capillary = 4,000 V, fragmentor voltage = 95 V, collision energy = 20 V, dwell time = 250 ms and MS/MS transition (m/z) = 305/130 for unlabeled IAA-Glu and 311/130 for [13C5, 15N]IAA-Glu.
4.9. Statistical analysis.
The developmental differences among iamh mutants, transgenic lines, and wild type plants were obvious and qualitative, rendering statistical analysis unnecessary. For auxin and auxin metabolite analysis, we conducted Student’s t-test to determine whether the differences between samples were statistically significant. The actual t-value and p-value were provided in the text and in the figure legends.
Supplementary Material
Acknowledgment
This research was supported by the NIH grant R01GM114660 to YZ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartel B, Fink GR, 1994. Differential regulation of an auxin-producing nitrilase gene family in Arabidopsis thaliana. Proc Natl Acad Sci U S A 91, 6649–6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerjan W, Cervera MT, Delarue M, Beeckman T, Dewitte W, Bellini C, Caboche M, Van Onckelen H, Van Montagu M, Inze D, 1995. Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell 7, 1405–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Tong J, Xiao L, Ruan Y, Liu J, Zeng M, Huang H, Wang JW, Xu L, 2016. YUCCA-mediated auxin biogenesis is required for cell fate transition occurring during de novo root organogenesis in Arabidopsis. J Exp Bot 67, 4273–4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Dai X, De-Paoli H, Cheng Y, Takebayashi Y, Kasahara H, Kamiya Y, Zhao Y, 2014. Auxin overproduction in shoots cannot rescue auxin deficiencies in Arabidopsis roots. Plant Cell Physiol 55, 1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Dai X, Zhao Y, 2006. Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev 20, 1790–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Dai X, Zhao Y, 2007. Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis. Plant Cell 19, 2430–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF, 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Comai L, Kosuge T, 1982. Cloning characterization of iaaM, a virulence determinant of Pseudomonas savastanoi. J Bacteriol 149, 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Mashiguchi K, Chen Q, Kasahara H, Kamiya Y, Ojha S, DuBois J, Ballou D, Zhao Y, 2013. The biochemical mechanism of auxin biosynthesis by an Arabidopsis YUCCA flavin-containing monooxygenase. J Biol Chem 288, 1448–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies RT, Goetz DH, Lasswell J, Anderson MN, Bartel B, 1999. IAR3 encodes an auxin conjugate hydrolase from Arabidopsis. Plant Cell 11, 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delarue M, Prinsen E, Onckelen HV, Caboche M, Bellini C, 1998. Sur2 mutations of Arabidopsis thaliana define a new locus involved in the control of auxin homeostasis. Plant J 14, 603–611. [DOI] [PubMed] [Google Scholar]
- Eklof S, Astot C, Sitbon F, Moritz T, Olsson O, Sandberg G, 2000. Transgenic tobacco plants co-expressing Agrobacterium iaa and ipt genes have wild-type hormone levels but display both auxin-and cytokinin-overproducing phenotypes. Plant J 23, 279–284. [DOI] [PubMed] [Google Scholar]
- Gallavotti A, Barazesh S, Malcomber S, Hall D, Jackson D, Schmidt RJ, McSteen P, 2008. sparse inflorescence1 encodes a monocot-specific YUCCA-like gene required for vegetative and reproductive development in maize. Proc Natl Acad Sci U S A 105, 15196–15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galweiler L, Guan C, Muller A, Wisman E, Mendgen K, Yephremov A, Palme K, 1998. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282, 2226–2230. [DOI] [PubMed] [Google Scholar]
- Gao X, Chen J, Dai X, Zhang D, Zhao Y, 2016. An Effective Strategy for Reliably Isolating Heritable and Cas9-Free Arabidopsis Mutants Generated by CRISPR/Cas9-Mediated Genome Editing. Plant Physiol 171, 1794–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Zhang Y, Zhang D, Dai X, Estelle M, Zhao Y, 2015. Auxin binding protein 1 (ABP1) is not required for either auxin signaling or Arabidopsis development. Proc Natl Acad Sci U S A 112, 2275–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Zhao Y, 2014. Self-processing of ribozyme-flanked RNAs into guide RNAs in vitro and in vivo for CRISPR-mediated genome editing. J Integr Plant Biol 56, 343–349. [DOI] [PubMed] [Google Scholar]
- Kasahara H, 2016. Current aspects of auxin biosynthesis in plants. Biosci Biotechnol Biochem 80, 34–42. [DOI] [PubMed] [Google Scholar]
- Leznicki AJ, Bandurski RS, 1988. Enzymic synthesis of indole-3-acetyl-1-O-beta-d-glucose. II. Metabolic characteristics of the enzyme. Plant Physiol 88, 1481–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiguchi K, Tanaka K, Sakai T, Sugawara S, Kawaide H, Natsume M, Hanada A, Yaeno T, Shirasu K, Yao H, McSteen P, Zhao Y, Hayashi K, Kamiya Y, Kasahara H, 2011. The main auxin biosynthesis pathway in Arabidopsis. Proc Natl Acad Sci U S A 108, 18512–18517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzetti B, Landi L, Pandolfini T, Spena A, 2004. The defH9-iaaM auxin-synthesizing gene increases plant fecundity and fruit production in strawberry and raspberry. BMC Biotechnol 4, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonhebel HM, 2015. Tryptophan-Independent Indole-3-Acetic Acid Synthesis: Critical Evaluation of the Evidence. Plant Physiol 169, 1001–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Skirpan AL, Liu X, Christensen A, Slewinski TL, Hudson C, Barazesh S, Cohen JD, Malcomber S, McSteen P, 2011. vanishing tassel2 encodes a grass-specific tryptophan aminotransferase required for vegetative and reproductive development in maize. Plant Cell 23, 550–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollmann S, Neu D, Weiler EW, 2003. Molecular cloning and characterization of an amidase from Arabidopsis thaliana capable of converting indole-3-acetamide into the plant growth hormone, indole-3-acetic acid. Phytochemistry 62, 293–300. [DOI] [PubMed] [Google Scholar]
- Romano CP, Robson PR, Smith H, Estelle M, Klee H, 1995. Transgene-mediated auxin overproduction in Arabidopsis: hypocotyl elongation phenotype and interactions with the hy6–1 hypocotyl elongation and axr1 auxin-resistant mutants. Plant Mol Biol 27, 1071–1083. [DOI] [PubMed] [Google Scholar]
- Staswick PE, Serban B, Rowe M, Tiryaki I, Maldonado MT, Maldonado MC, Suza W, 2005. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 17, 616–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie DY, Dolezal K, Schlereth A, Jurgens G, Alonso JM, 2008. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133, 177–191. [DOI] [PubMed] [Google Scholar]
- Stepanova AN, Yun J, Robles LM, Novak O, He W, Guo H, Ljung K, Alonso JM, 2011. The Arabidopsis YUCCA1 flavin monooxygenase functions in the indole-3-pyruvic acid branch of auxin biosynthesis. Plant Cell 23, 3961–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader LC, Bartel B, 2009. The Arabidopsis PLEIOTROPIC DRUG RESISTANCE8/ABCG36 ATP binding cassette transporter modulates sensitivity to the auxin precursor indole-3-butyric acid. Plant Cell 21, 1992–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara S, Hishiyama S, Jikumaru Y, Hanada A, Nishimura T, Koshiba T, Zhao Y, Kamiya Y, Kasahara H, 2009. Biochemical analyses of indole-3-acetaldoxime-dependent auxin biosynthesis in Arabidopsis. Proc Natl Acad Sci U S A 106, 5430–5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Ferrer JL, Ljung K, Pojer F, Hong F, Long JA, Li L, Moreno JE, Bowman ME, Ivans LJ, Cheng Y, Lim J, Zhao Y, Ballare CL, Sandberg G, Noel JP, Chory J, 2008. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133, 164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Chu J, Yu T, Xu Q, Sun X, Yuan J, Xiong G, Wang G, Wang Y, Li J, 2015. Tryptophan-independent auxin biosynthesis contributes to early embryogenesis in Arabidopsis. Proc Natl Acad Sci U S A 112, 4821–4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won C, Shen X, Mashiguchi K, Zheng Z, Dai X, Cheng Y, Kasahara H, Kamiya Y, Chory J, Zhao Y, 2011. Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis. Proc Natl Acad Sci U S A 108, 18518–18523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Palm CJ, Brooks B, Kosuge T, 1985. Nucleotide sequences of the Pseudomonas savastanoi indoleacetic acid genes show homology with Agrobacterium tumefaciens T-DNA. Proc Natl Acad Sci U S A 82, 6522–6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Kamiya N, Morinaka Y, Matsuoka M, Sazuka T, 2007. Auxin biosynthesis by the YUCCA genes in rice. Plant Physiol 143, 1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Li R, Xing J, Yan L, Wang R, Zhao Y, 2018. The YUCCA-Auxin-WOX11 Module Controls Crown Root Development in Rice. Front Plant Sci 9, 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, 2012. Auxin biosynthesis: a simple two-step pathway converts tryptophan to indole-3-acetic acid in plants. Mol Plant 5, 334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, 2014. Auxin biosynthesis. Arabidopsis Book 12, e0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, 2018. Essential Roles of Local Auxin Biosynthesis in Plant Development and in Adaptation to Environmental Changes. Annu Rev Plant Biol 69, 417–435. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, Weigel D, Chory J, 2001. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291, 306–309. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Hull AK, Gupta NR, Goss KA, Alonso J, Ecker JR, Normanly J, Chory J, Celenza JL, 2002. Trp-dependent auxin biosynthesis in Arabidopsis: involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes Dev 16, 3100–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Zhang Y, Liu X, Zhang X, Liu S, Yu X, Ren Y, Zheng X, Zhou K, Jiang L, Guo X, Gai Y, Wu C, Zhai H, Wang H, Wan J, 2013. A role for a dioxygenase in auxin metabolism and reproductive development in rice. Dev Cell 27, 113–122. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Guo Y, Novak O, Chen W, Ljung K, Noel JP, Chory J, 2016. Local auxin metabolism regulates environment-induced hypocotyl elongation. Nat Plants 2, 16025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Guo Y, Novak O, Dai X, Zhao Y, Ljung K, Noel JP, Chory J, 2013. Coordination of auxin and ethylene biosynthesis by the aminotransferase VAS1. Nat Chem Biol 9, 244–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


