Abstract
Background:
We aimed to detect IL-17, MMP-9 and CD23 in serum of patients with colorectal cancer to provide some proper references for diagnosis and treatment of this disease.
Methods:
Overall, 287 patients with colorectal cancer were collected in the Digestive Surgery Department of Chinese PLA General Hospital, Beijing, China from January 2017 to November 2018 and were used as the study group, meanwhile, 200 people who took physical examination in the same period were used as the control group. They were retrospectively analyzed. The concentrations of IL-17, MMP-9 and CD23 in serum were detected by ELISA 10 d before and after treatment and 30 d after treatment. The relationship between IL-17, MMP-9 and CD23 concentration and clinicopathology was analyzed.
Results:
The concentrations of CD23, IL-17 and MMP-9 in peripheral blood of the patients in the study group were significantly higher than those in the control group (P<0.001). IL-17, MMP-9 and CD23 were negatively correlated with treatment time and pathological features in the study group (P<0.001).
Conclusion:
The concentrations of IL-17, MMP-9 and CD23 obviously increased in peripheral blood of patients with colorectal cancer, the three were negatively correlated with treatment time and were significantly correlated with TNM staging and differentiation degree of colorectal cancer. It is expected to estimate the illness.
Keywords: Clinicopathology, Colorectal cancer, Interleukin-17
Introduction
Colorectal cancer, a malignant tumor originated from mucosal epithelium of large intestine, is one of the most common malignant tumors in digestive tract (1, 2). As one of the common tumors in clinic, colorectal cancer has an extremely high morbidity and mortality (3). There were about 1.4 million new cases in 2012, among which there were 700,000 deaths (4). At present, the treatment method of colorectal cancer is mainly surgery or chemoradiotherapy (5), but colorectal cancer has no obvious indications in early period, so it is easily ignored by patients. Once the patient is diagnosed, colorectal cancer has already been in advanced period, during this time, cancer cells have generally spread and metastasized, which makes it difficult to treat colorectal cancer by resection (6, 7) and is one of the reasons why the prognosis of colorectal cancer is poor.
The pathogenesis of colorectal cancer is not yet clear. The main function of matrix metalloproteinase-9 (MMP-9) is degrading and remodeling the dynamic balance of extracellular matrix (8). At present, MMP-9 has been abnormally expressed in tumors and participate in metastasis and invasion of tumors (9, 10). IL-17 has obvious changes in treatment of rectal cancer, but the specific mechanism has not been clear (11, 12). CD23 is a low-affinity IgE receptor expressed in dendritic cells, monocytes and B cells (13). Some scholars believe that CD23 can be produced in nasopharyngeal cancer tissue infected by EBV, the main mechanism is that the nuclear antigen of EBV nuclear antigen II, which is produced during EBV incubation period, is a necessity of B lymphocyte’s transformation, during which c-myc gene is regulated and CD23 is produced (14).
At present, the functions of IL-17, MMP-9 and CD23 in colorectal cancer have not been confirmed. However, IL-17, MMP-9 and CD23 in serum of patients with colorectal cancer were detected by experiments in this paper to provide some proper references for diagnosis and treatment of colorectal cancer in clinic in the future.
Methods
General data
overall, 287 patients with colorectal cancer, who were admitted to the Digestive Surgery Department of the First Affiliated Hospital of Nanjing Medical University, Beijing, China from January 2017 to November 2018, were collected and were used as the study objects. Meanwhile, 200 people who took physical examination were used as the control group, they were retrospectively analyzed.
This experiment was approved by the Ethics Committee of The First Affiliated Hospital of Nanjing Medical University, all the objects signed the informed consent form.
Inclusion criteria and exclusion criteria
Inclusion criteria: patients conformed to clinical symptoms of colorectal cancer (15); patients were diagnosed with colorectal cancer by the pathology biopsy of The First Affiliated Hospital of Nanjing Medical University; patients received follow-up treatment in the First Affiliated Hospital of Nanjing Medical Universityl after they were diagnosed; patients had complete case data; patients were willing to cooperate with the medical workers of the hospital; patients were from 30 to 70 yr old.
Exclusion criteria: patients had other tumors, cardiovascular and cerebrovascular diseases, organ failure, liver dysfunction and kidney dysfunction, mental illness, physical disability, history of familial genetic diseases; patients could-n’t take care of themselves; patients were bedridden for long time; patients had drug allergy; patients transferred to other hospital halfway. JK-(a)-5931, the detection was carried out strictly in accordance with the instructions of the kit. The other part of the serum was used to detect CD23, Ficoll density gradient centrifugation was used to separate peripheral blood mononuclear cells (PBMC), then 5 mL of lymphocyte separation solution (Shanghai Yuanmu Biotechnology Co., Ltd., item number: YS-6131) was added into the centrifuge tube, next, anticoagulated venous blood and sterile PBS were added into it, the ratio was 1:1, lastly, the mixture was mixed equably. A pipette was used to add liquid drop-wise until to the layered liquid level, then the mixture was centrifuged horizontally at 400 g×30 min. The upper liquid was discarded, then the pipette was inserted into cloud layer to collect monocytes and transferred them into another tube. PBS with a volume of 5 times was added into the tube, then the mixture was centrifuged at 300 g×10 min, the cells were rinsed twice, the supernatant was discarded, next, red blood cell lysate was added into the mixture, it was incubated for 2 minutes at room temperature, next, PBS was added into the mixture, the mixture was rinsed twice. Finally, the supernatant was discarded, RPMI-1640 medium with 10% of PBS (Wuhan Chundu Biotechnology Co., Ltd., item number: CDLG-5404) was added into the mixture. The resuspended cells were counted by Cellqutst software.
Observation indicators
The concentrations of IL-17, MMP-9 and CD23 in serum of the patients in the study group were detected 10 d before and after treatment and 30 d after treatment. The correlation among IL-17, MMP-9, CD23 and treatment time was analyzed. The relationship between the concentrations of IL-17, MMP-9, CD23 in the study group and clinicopathology was analyzed.
Statistical methods
All the experiment results were calculated by SPSS24.0 statistical software (Shanghai Yuchuang Network Technology Co., Ltd.). All the graphs were drawn by Graphpad8 software (Shenzhen Softhead Technology Co., Ltd.) and the results were checked twice. The measurement data were expressed in the form of rate, chi-square test was used in the comparison between groups. The enumeration data were expressed in the form of mean value ± standard deviation, t-test was used in the comparison between groups. Variance analysis of repeated measure was used in the comparison in groups at different time points. Correlation analysis was performed by Spearman correlation analysis. P<0.050 was considered to be statistically significant.
Results
The comparison of the general data
The general data of the patients in two groups were compared, including age, BMI, platelet, red blood cell, white blood cell count, gender, nationality, residence, marriage, smoking, drinking, exercise habits (Table 1).
Table 1:
The comparison of the clinical data of the patients in two groups [n (%)]
| Variable | The study group (n=287) | The control group (n=200) | t/2 | P |
|---|---|---|---|---|
| Age (yr) | 47.82±10.54 | 46.33±9.84 | 1.577 | 0.116 |
| BMI (kG/m2) | 22.84±4.2 | 22.17±5.04 | 1.592 | 0.112 |
| Platelet (×109cells/L) | 228.21±50.14 | 220.42±57.21 | 1.591 | 0.112 |
| Red blood cell (×1012cells/L) | 4.66±0.64 | 4.72±0.51 | 1.104 | 0.270 |
| White blood cell (×109cells/L) | 8.15±1.17 | 7.96±1.34 | 1.660 | 0.098 |
| Gender: Male | 184 (64.11) | 142 (71.00) | 2.527 | 0.112 |
| Female | 103 (35.89) | 58 (29.00) | ||
| Nationality: Han nationality | 282 (98.26) | 198 (99.00) | 0.458 | 0.498 |
| Minority | 5 (1.74) | 2 (1.00) | ||
| Residence: City | 235 (81.88) | 172 (86.00) | 1.456 | 0.228 |
| Countryside | 52 (18.12) | 28 (14.00) | ||
| Marriage: Married | 261 (90.94) | 175 (87.50) | 1.488 | 0.223 |
| Unmarried | 26 (9.06) | 25 (12.50) | ||
| Smoking: Yes | 197 (68.64) | 150 (75.00) | ||
| No | 90 (31.36) | 50 (25.00) | ||
| Drinking: Yes | 212 (73.87) | 142 (71.00) | 0.488 | 0.485 |
| No | 75 (26.13) | 58 (29.00) | ||
| Exercise: Yes | 31 (10.80) | 26 (13.00) | 0.551 | 0.458 |
| No | 256 (89.20) | 174 (87.00) | ||
| TNM staging*: I∼II | 168 (58.54) | |||
| III∼IV | 119 (41.46) | |||
| Differentiation degree | ||||
| Middle differentiation and low differentiation | 157 (54.70) | |||
| High differentiation | 130 (45.30) | |||
| Lymph node metastasis | ||||
| Yes | 108 (37.63) | |||
| No | 179 (62.37) | |||
| Infiltration degree | ||||
| Infiltrating to serosa | 139 (48.43) | |||
| Not infiltrating to serosa | 148 (51.57) | |||
| The type of tumor | ||||
| Villous adenoma | 125 (43.55) | |||
| Villous adenoma | 125 (43.55) | |||
Note: TNM staging criteria refer to AJCC cancer staging manual (14)
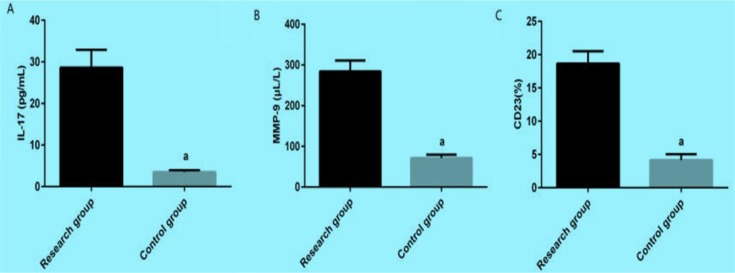
The concentrations of IL-17, MMP-9 and CD23 in serum of the study group before treatment were significantly higher than those in the control group, P<0.001 (Fig. 1).
Fig. 1:
The comparison of the concentrations of IL-17, MMP-9 and CD23 between two groups.
A: The comparison of the concentrations of IL-17 between two groups. After detected by ELISA, the concentration of IL-17 in the study group is significantly higher than that in the control group. a means compared with the concentration of IL-17 in the study group, P<0.001. B: The comparison of the concentrations of MMP-9 between two groups. After detected by ELISA, the concentration of MMP-9 in the study group is significantly higher than that in the control group. a means compared with the concentration of MMP-9 in the study group, P<0.001. C: The comparison of the concentrations of CD23 between two groups. After detected by ELISA, the concentration of CD23 in the study group is significantly higher than that in the control group. a means compared with the concentration of CD23 in the study group, P<0.001
The changes of IL-17, MMP-9 and CD23 in the study group before and after treatment
The levels of IL-17, MMP-9 and CD23 in the study group 10 d after treatment were significantly lower than those before treatment (P<0.001). The levels of IL-17, MMP-9 and CD23 30 d after treatment were significantly lower than those 10 d after treatment (P<0.001). IL-17, MMP-9, and CD23 were negatively correlated with the treatment time of the study group (r=−0.757, −0.847, −0.851, P<0.001) (Table 2, Fig. 2–4).
Table 2:
The changes of IL-17, MMP-9 and CD23 in the study group before and after treatment
| Variable | IL-17 (pg/m) | MMP-9 (μL/) | CD23 (%) |
|---|---|---|---|
| Before treatment | 28.63±4.26 | 284.62±26.21 | 18.66±1.86 |
| 10 d after treatment | 24.66±3.05a | 256.72±15.05a | 15.57±2.06a |
| 30 d after treatment | 19.15±2.42ab | 212.83±10.52ab | 11.94±1.57ab |
| F | 2982.107 | 7172.243 | 2950.184 |
| P | < 0.001 | < 0.001 | < 0.001 |
Note: a means compared with IL-17, MMP-9, CD23 in the same group before treatment, P<0.001; b means compared with IL-17, MMP-9, CD23 in the same group 10 d after treatment, P<0.001
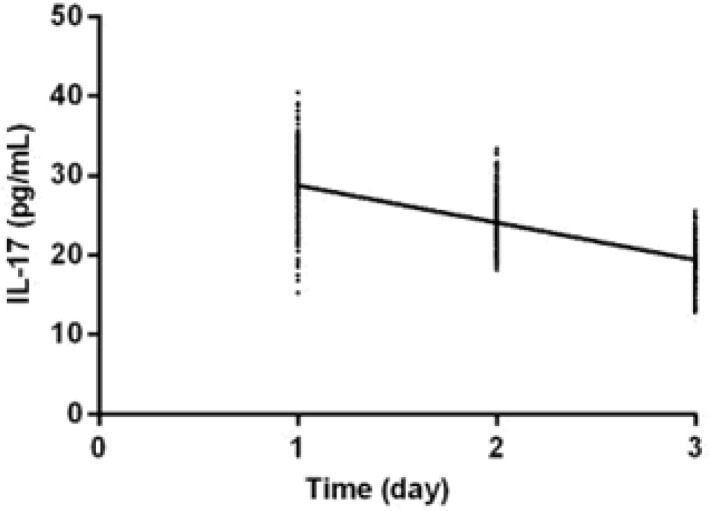
Fig. 2:
The correlation analysis between IL-17 and treatment time. IL-17 was negatively correlated with treatment time in the study group (r=−0.757, P<0.001)
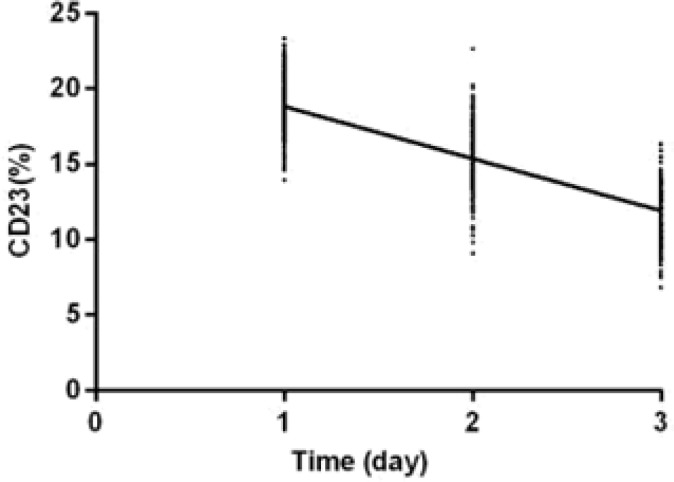
Fig. 4.
The correlation analysis between CD23 and treatment time. CD23 was negatively correlated with treatment time in the study group (r=−0.851, P<0.001)
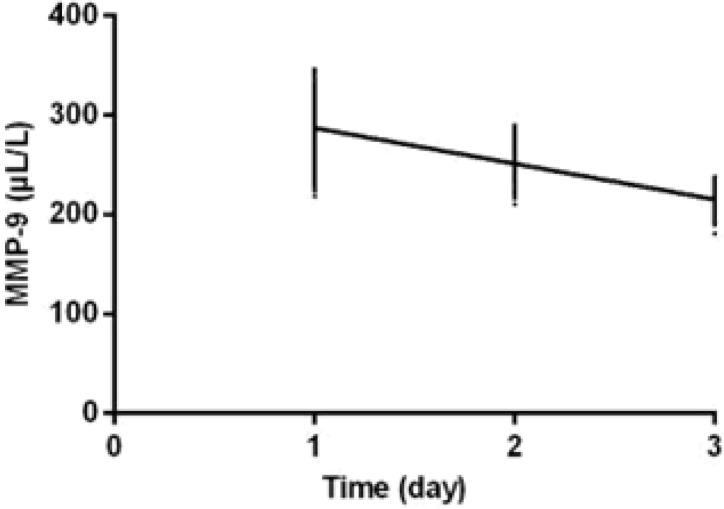
Fig. 3:
The correlation analysis between MMP-9 and treatment time. MMP-9 was negatively correlated with treatment time in the study group (r=−0.847, P<0.001)
The relationship between the concentrations of IL-17, MMP-9, CD23 in the study group and clinicopathology
IL-17, MMP-9 and CD23 was related to TNM staging, differentiation degree, lymph node metastasis, and infiltration degree (P<0.001) (Table 3–5).
Table 3:
The relationship between the concentration of IL-17 and clinicopathology in the study group (pg/mL)
| Variable | n | Concentration | t | P |
|---|---|---|---|---|
| Age (yr) > 60 | 164 | 27.94±4.56 | 0.328 | 0.743 |
| ≤60 | 123 | 28.11±4.05 | ||
| BMI (KG/m2): > 26 | 189 | 28.04±4.15 | 0.205 | 0.838 |
| ≤26 | 98 | 28.15±4.62 | ||
| Gender : Male | 184 | 28.36±4.04 | 0.820 | 0.413 |
| Female | 103 | 27.95±4.11 | ||
| Nationality : Han nationality | 282 | 27.86±4.12 | 0.108 | 0.915 |
| Minority | 5 | 28.06±4.32 | ||
| Residence : City | 235 | 28.11±4.15 | 0.251 | 0.802 |
| Countryside | 52 | 27.95±4.25 | ||
| Marriage: Married | 261 | 28.65±4.62 | 0.467 | 0.641 |
| Unmarried | 26 | 28.21±4.22 | ||
| Smoking :Yes | 197 | 27.86±4.15 | 0.247 | 0.805 |
| No | 90 | 28.07±4.03 | ||
| Drinking :Yes | 212 | 28.16±4.03 | 0.365 | 0.716 |
| No | 75 | 28.36±4.22 | ||
| Exercise : Yes | 31 | 27.68±4.62 | 0.112 | 0.911 |
| No | 256 | 27.58±4.72 | ||
| TNM staging : I∼II | 168 | 24.51±3.62 | 11.312 | < 0.001 |
| III∼IV | 119 | 30.17±4.86 | ||
| Differentiation degree | 13.783 | < 0.001 | ||
| Middle differentiation and low differentiation | 157 | 31.87±2.57 | ||
| High differentiation | 130 | 26.63±3.84 | ||
| Lymph node metastasis : Yes | 108 | 31.52±3.84 | 11.142 | < 0.001 |
| No | 179 | 25.66±4.58 | ||
| Infiltration degree | 17.582 | < 0.001 | ||
| Infiltrating to serosa | 139 | 31.84±2.66 | ||
| Not infiltrating to serosa | 148 | 24.83±3.59 | ||
| The type of tumor | 0.147 | 0.883 | ||
| Villous adenoma | 125 | 28.24±4.15 | ||
| Adenoma | 162 | 28.17±3.86 |
Table 5:
The relationship between the concentration of CD23 and clinicopathology in the study group (%)
| Variable | n | Concentration | t | P | |
|---|---|---|---|---|---|
| Age (yr) | > 60 | 164 | 17.89±1.85 | 0.723 | 0.470 |
| ≤60 | 123 | 18.04±1.58 | |||
| BMI (KG/m2) | > 26 | 189 | 18.44±1.46 | 1.525 | 0.128 |
| ≤26 | 98 | 18.15±1.65 | |||
| Gender | Male | 184 | 17.68±1.15 | 1.134 | 0.258 |
| Female | 103 | 17.84±1.14 | |||
| Nationality | Han nationality | 282 | 18.16±1.15 | 0.019 | 0.985 |
| Minority | 5 | 18.15±1.84 | |||
| Residence | City | 235 | 17.15±1.89 | 1.095 | 0.275 |
| Countryside | 52 | 17.46±1.64 | |||
| Marriage | Married | 261 | 18.07±1.52 | 0.659 | 0.511 |
| Unmarried | 26 | 17.86±1.84 | |||
| Smoking | Yes | 197 | 18.48±1.68 | 1.628 | 0.105 |
| No | 90 | 18.84±1.86 | |||
| Drinking | Yes | 212 | 17.48±1.16 | 1.086 | 0.278 |
| No | 75 | 17.65±1.18 | |||
| Exercise | Yes | 31 | 17.75±1.81 | 1.235 | 0.218 |
| No | 256 | 18.15±1.69 | |||
| TNM staging | I∼II | 168 | 16.89±1.54 | 13.912 | < 0.001 |
| III∼IV | 119 | 19.66±1.82 | |||
| Differentiation degree | Middle differentiation and low differentiation | 157 | 20.59±2.89 | 12.763 | < 0.001 |
| High differentiation | 130 | 17.15±1.15 | |||
| Lymph node metastasis | Yes | 108 | 18.56±1.15 | 1.910 | 0.057 |
| No | 179 | 18.27±1.30 | |||
| Infiltration degree | Infiltrating to serosa | 139 | 21.55±2.56 | 20.733 | < 0.001 |
| Not infiltrating to serosa | 148 | 16.15±1.81 | |||
| The type of tumor | Villous adenoma | 125 | 18.65±1.15 | 1.138 | 0.256 |
| Adenoma | 162 | 18.48±1.33 |
Table 4:
The relationship between the concentration of MMP-9 and clinicopathology in the study group (μL/L)
| Variable | n | Concentration | t | P | |
|---|---|---|---|---|---|
| Age (yr) | > 60 | 164 | 280.17±28.11 | 0.409 | 0.683 |
| ≤60 | 123 | 281.51±26.57 | |||
| BMI (KG/m2) | > 26 | 189 | 287.15±25.16 | 0.884 | 0.378 |
| ≤26 | 98 | 284.51±21.56 | |||
| Gender | Male | 184 | 279.56±26.15 | 1.286 | 0.200 |
| Female | 103 | 275.46±25.48 | |||
| Nationality | Han nationality | 282 | 289.56±29.15 | 0.308 | 0.759 |
| Minority | 5 | 285.51±30.45 | |||
| Residence | City | 235 | 284.11±30.84 | 0.444 | 0.657 |
| Countryside | 52 | 286.15±25.64 | |||
| Marriage | Married | 261 | 281.51±25.26 | 1.462 | 0.145 |
| Unmarried | 26 | 289.14±26.51 | |||
| Smoking | Yes | 197 | 287.15±26.48 | 0.593 | 0.553 |
| No | 90 | 289.15±26.52 | |||
| Drinking | Yes | 212 | 294.85±48.56 | 0.544 | 0.587 |
| No | 75 | 298.45±51.15 | |||
| Exercise | Yes | 31 | 281.06±25.61 | 1.759 | 0.080 |
| No | 256 | 289.84±26.32 | |||
| TNM staging | I∼II | 168 | 248.41±35.51 | 13.982 | < 0.001 |
| III∼IV | 119 | 308.51±36.41 | |||
| Differentiation degree | Middle differentiation and low differentiation | 157 | 311.51±26.56 | 12.501 | < 0.001 |
| High differentiation | 130 | 265.16±36.15 | |||
| Lymph node metastasis | Yes | 108 | 298.86±28.52 | 10.468 | < 0.001 |
| No | 179 | 260.85±30.55 | |||
| Infiltration degree | Infiltrating to serosa | 139 | 278.15±35.44 | 0.733 | 0.464 |
| Not infiltrating to serosa | 148 | 280.85±26.55 | |||
| The type of tumor | Villous adenoma | 125 | 287.41±27.56 | 0.231 | 0.818 |
| Adenoma | 162 | 288.15±26.51 |
Discussion
In recent years, with the change of dietary habits, the incidence of various digestive tract tumors has been increasing (16–18). Many factors and genes may be involved in the occurrence and development of colorectal cancer (19–22), but the functions of IL-17, MMP-9 and CD23 in colorectal cancer have not yet been clear. ROP-γt ubiquitination inhibits the occurrence of colonic inflammation mediated by IL-17 and tumors (23). As an inactivated form of 10 kDa propeptide in non-small cell lung cancer, MMP-9 can facilitate secretion of cells (24).
Due to the biological function of MMP-9, like cutting gelatin, it can be activated by other MMP or tissue plasminogen activator (tPA)-plasmin system, also, it can facilitate the movement of malignant cells. CD23 is involved in the occurrence and developmentof small cell lymphoma (23–25). This study analyzed the correlation between the three and colorectal cancer as well as the relationship between the three and clinicopathology to verify the functions of IL-17, MMP-9 and CD23 in colorectal cancer.
The results of this experiment showed that the concentrations of IL-17, MMP-9 and CD23 in peripheral blood of patients with colorectal cancer were significantly increased, suggesting that IL-17, MMP-9 and CD23 might be involved in occurrence and development of colorectal cancer. IL-17, MMP-9 and CD23 were negatively correlated with treatment time, suggesting that the recovery of patients with colorectal cancer can be estimated by detecting the concentrations of IL-17, MMP-9 and CD23 in peripheral blood of patients. IL-17, MMP-9 and CD23 were obviously related to TNM staging and differentiation degree of colorectal cancer, suggesting that the severity of colorectal cancer can be estimated by detecting the concentrations of IL-17, MMP-9 and CD23 in peripheral blood of patients in clinic.
In this study, the concentration of IL-17 obviously increased before the patients with colorectal cancer were treated, but it obviously decreased after treatment. This result is consistent with other studies (26–28), where the concentration of IL-17 also obviously increased and it was related to susceptibility of gastric cancer, which could support the results of this experiment.
It is speculated that the mechanism of IL-17, which involves in development of colorectal cancer, is inhibiting proliferation and activation of NK cells, lymphocytes’ ability to secrete cytokines, and proliferation of T cells and facilitating angiogenesis of tumors, as well as inducing metastasis and infiltration of the nidus of colorectal cancer through cytokines (29). MMP-9 is a zinc ion proteolytic enzyme that can trigger a variety of inflammations (30), suggesting that the mechanism that MMP-9 involves in colorectal cancer is also associated with inflammation. CD23 can facilitate synthesis and secretion of IgE, mediate adhesion between cells, and help basophils to release histamine. CD23 facilitates synthesis of IgE when the concentration of IgE decreases and inhibits synthesis of IgE when the concentration of IgE increases, which is often used as an activation marker of B cells (31). The concentration of CD23 increases in serum of patients with colorectal cancer, indicating dysfunction of B cells, imbalance of cell subsets, and destruction of microbial environment in patients with colorectal cancer.
There are some limitations in this study. For example, the mechanism of IL-17, MMP-9 and CD23 in colorectal cancer needs to be further investigated to verify our results. At present, there are few studies about the functions of IL-17, MMP-9 and CD23 in colorectal cancer at home and abroad, so it is hard to cite a number of other experiment results to carry out comparison and discussion. The difference between the results of this experiment and the results of other experiments cannot be excluded. Moreover, the experiment period was short, so it was difficult to estimate the effects of IL-17, MMP-9 and CD23 on prognosis of colorectal cancer.
Conclusion
The concentrations of IL-17, MMP-9 and CD23 obviously increase in peripheral blood of patients with colorectal cancer, they are negatively correlated with treatment time and are significantly correlated with TNM staging and differentiation degree of colorectal cancer. It is expected to estimate illness progression and treatment of patients with colorectal cancer by detecting IL-17, MMP-9 and CD23 in the future.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
No funding was received in this study.
Footnotes
Conflict of interests
The authors declare that there is no conflict of interest.
References
- 1.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. (2008). Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med, 149: 627–637. [DOI] [PubMed] [Google Scholar]
- 2.Keegan TH, Ries LA, Barr RD, et al. (2016). Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer, 122: 1009–1016. [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Siegel RL, Ward EM, Jemal A. (2016). Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev, 25: 16–27. [DOI] [PubMed] [Google Scholar]
- 4.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. (2017). Global patterns and trends in colorectal cancer incidence and mortality. Gut, 66: 683–691. [DOI] [PubMed] [Google Scholar]
- 5.Miller KD, Siegel RL, Lin CC, et al. (2016). Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin, 66: 271–289. [DOI] [PubMed] [Google Scholar]
- 6.Seligmann JF, Fisher D, Smith CG, et al. (2017). Investigating the poor outcomes of BRAF-mutant advanced colorectal cancer: analysis from 2530 patients in randomised clinical trials. Ann Oncol, 28: 562–568. [DOI] [PubMed] [Google Scholar]
- 7.Venook AP, Niedzwiecki D, Lenz HJ, et al. (2017). Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA, 317: 2392–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vafadari B, Salamian A, Kaczmarek L. (2016). MMP-9 in translation: from molecule to brain physiology, pathology, and therapy. J Neurochem, 139 Suppl 2: 91–114. [DOI] [PubMed] [Google Scholar]
- 9.Burlaka AP, Ganusevich II, Gafurov MR, Lukin SM, Sidorik EP. (2016). Stomach Cancer: Interconnection between the Redox State, Activity of MMP-2, MMP-9 and Stage of Tumor Growth. Cancer Microenviron, 9: 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawicki S, Zajkowska M, Glazewska EK, Bedkowska GE, Szmitkowski M. (2016). Plasma levels and diagnostic utility of VEGF, MMP-9, and TIMP-1 in the diagnosis of patients with breast cancer. Onco Targets Ther, 9: 911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papotto PH, Ribot JC, Silva-Santos B. (2017). IL-17 (+) gammadelta T cells as kick-starters of inflammation. Nat Immunol, 18: 604–611. [DOI] [PubMed] [Google Scholar]
- 12.Wang K, Karin M. (2015). The IL-23 to IL-17 cascade inflammation-related cancers. Clin Exp Rheumatol, 33: S87–90. [PubMed] [Google Scholar]
- 13.Selb R, Eckl-Dorna J, Neunkirchner A, et al. (2017). CD23 surface density on B cells is associated with IgE levels and determines IgE-facilitated allergen uptake, as well as activation of allergen-specific T cells. J Allergy Clin Immunol, 139: 290–299.e294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raphael J, Valent A, Hanna C, et al. (2014). Myeloid sarcoma of the nasopharynx mimicking an aggressive lymphoma. Head Neck Pathol, 8: 234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amin MB, Greene FL, Edge SB, et al. (2017). The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin, 67: 93–99. [DOI] [PubMed] [Google Scholar]
- 16.Otsuki T, Matsuzaki H, Lee S, et al. (2016). Environmental factors and human health: fibrous and particulate substance-induced immuno-logical disorders and construction of a health-promoting living environment. Environ Health Prev Med, 21: 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derakhshan MH, Arnold M, Brewster DH, et al. (2016). Worldwide Inverse Association between Gastric Cancer and Esophageal Adenocarcinoma Suggesting a Common Environmental Factor Exerting Opposing Effects. Am J Gastroenterol, 111: 228–239. [DOI] [PubMed] [Google Scholar]
- 18.Bishehsari F, Mahdavinia M, Vacca M, Malekzadeh R, Mariani-Costantini R. (2014). Epidemiological transition of colorectal cancer in developing countries: environmental factors, molecular pathways, and opportunities for prevention. World J Gastroenterol, 20: 6055–6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drewes JL, Housseau F, Sears CL. (2016). Sporadic colorectal cancer: microbial contributors to disease prevention, development and therapy. Br J Cancer, 115: 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie H, Ren X, Xin S, et al. (2016). Emerging roles of circRNA_001569 targeting miR-145 in the proliferation and invasion of colorectal cancer. Oncotarget, 7: 26680–26691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mir R, Pradhan SJ, Patil P, Mulherkar R, Galande S. (2016). Wnt/beta-catenin signaling regulated SATB1 promotes colorectal cancer tumor-igenesis and progression. Oncogene,35 (13):1679–91. [DOI] [PubMed] [Google Scholar]
- 22.Zhao L, Yu H, Yi S, et al. (2016). The tumor suppressor miR-138-5p targets PD-L1 in colorectal cancer. Oncotarget, 7: 45370–45384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kathania M, Khare P, Zeng M, et al. (2016). Itch inhibits IL-17-mediated colon inflammation and tumorigenesis by ROR-gammat ubiquitination. Nat Immunol, 17: 997–1004. [DOI] [PubMed] [Google Scholar]
- 24.Gong L, Wu D, Zou J, et al. (2016). Prognostic impact of serum and tissue MMP-9 in non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget, 7: 18458–18468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez-Matas M, Rodriguez-Justo M, Morilla R, Catovsky D, Matutes E. (2000). Quantitative expression of CD23 and its ligand CD21 in chronic lymphocytic leukemia. Haematologica, 85: 1140–1145. [PubMed] [Google Scholar]
- 26.Wu X, Yang T, Liu X, et al. (2016). IL-17 promotes tumor angiogenesis through Stat3 pathway mediated upregulation of VEGF in gastric cancer. Tumour Biol, 37: 5493–5501. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q, Liu S, Parajuli KR, et al. (2017). Inter-leukin-17 promotes prostate cancer via MMP7-induced epithelial-to-mesenchymal transition. Oncogene, 36: 687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao YW, Xu M, Xu Y, Li D, Zhou S. (2015). Effect of three common IL-17 single nucleotide polymorphisms on the risk of developing gastric cancer. Oncol Lett, 9: 1398–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaffen SL, Jain R, Garg AV, Cua DJ. (2014). The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol, 14: 585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jablonska-Trypuc A, Matejczyk M, Rosochacki S. (2016). Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anti-cancer drugs. J Enzyme Inhib Med Chem, 31: 177–183. [DOI] [PubMed] [Google Scholar]
- 31.Yildiz B, Cetin N, Kural N, Colak O. (2013). CD19 + CD23+ B cells, CD4 + CD25+ T cells, E-selectin and interleukin-12 levels in children with steroid sensitive nephrotic syndrome. Ital J Pediatr, 39: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]