Abstract
Background:
This study aimed to identify the prevalence and potential factors associated with Entamoeba gingivalis in adolescents in the city of Kerman, southeastern Iran, 2017.
Methods:
In this descriptive cross-sectional study, 315 adolescents (mean age; 15 yr) consisting of 189 males and 126 females were randomly selected. For each adolescent, two specimens were collected for culturing and examination by polymerase chain reaction (PCR). Univariate and multivariate logistic regression models were performed to explore any association with demographic and clinical variables.
Results:
The prevalence of E. gingivalis was 11.7%. Totally, 30 (15.9%) males and 7 (5.6%) females were infected with E. gingivalis. The rate of infection in males was 2.8 times higher than that in females (P<0.001). Statistical analysis identified 4 major factors including sex (OR=4.12, P<0.001), gingival index with severe inflammation (OR = 50, P<0.001), Candida spp. infection (OR=4.41, P<0.001) and decay-missing-filled teeth [DMFT (OR=3.27, P<0.001)]. In contrast to the aforementioned factors, adolescents with history of antibiotic consumption were significantly protected from E. gingivalis infection (OR= 3.24, P<0.001). Culture media detected 9.2% (n= 29), whilst PCR identified 11.4% (n= 36) of infection.
Conclusion:
The present findings clearly demonstrate a positive association between E. gingivalis and distinct demographic and clinical risk determinants. Therefore, dental practitioners and health surveillance personnel should be aware of these confounding factors to rigorously detect and critically manage oral health issues in school-age children in order to prevent or at least minimize the eventual periodontal complications in later life.
Keywords: Entamoeba gingivalis, Prevalence, Adolescents, Associated factors, Iran
Introduction
Entamoeba gingivalis is a cosmopolitan anaerobic amoebic protozoan wildly distributed in oral cavity of humans (1). Trophozoite has variable size range of 10–35μm. The amoeba has no cyst in its life cycle; therefore, it is transmitted either directly via direct contacts, mainly by kissing or indirectly through trophozoite-contaminated food, toothpicks, gum or other utensils (2). This opportunistic organism inhabits the gums and in the area surrounding the teeth including the spaces between teeth cavities, along the gingival fringes of gums, dental tartar, necrotic mucosa around the teeth and also in the gingival pockets (3).
E. gingivalis, aside from its contribution to bad smelling of the mouth, could be an indicator of oral health status and it is generally considered as an oral commensal but reports show that it displays a pathogenic potential associated with gingivitis (4–6) in children and eventually with periodontal diseases in later life, especially in minority groups such as immunocompromised patients (7, 8). The majority of the periodontal disturbances in higher age groups commonly have their stem earlier in childhood life (9–11).
Epidemiological reports demonstrated variable prevalence rates of E. gingivalis infection among the children (8, 14). Its occurrence may vary according to age, presence of gingivitis, periodontitis and immunosuppressive conditions (7, 14, 15). School children are extremely susceptible to a broad range of gingival infections such as bacteria and protozoa (9, 10, 16). Gingivitis with varying degrees of severity is a universal finding and a common phenomenon in children. Obviously, there are controversies concerning its pathogenicity characteristic as this amoeba is commonly found in healthy children and with gingivitis as well (17).
Therefore, the present study aimed to explore the prevalence and associated-factors for E. gingivalis in a group of adolescents in southeastern of Iran by culture and PCR in 2017.
Materials and Methods
Ethical statement
This project was granted approval by the Ethics Committees of Kerman University of Medical Sciences (project no. 95000230, Ethics no. IR.KMU.REC1395.409).
All studied school children, authorities and teachers and if required their parents, were well informed of the aims and procedures and those willingly interested to participate. Those who signed the written informed consent form were included in the study.
Design and population
This study was performed as a cross-sectional and analytical survey between January and June 2018 at School of Medicine, Kerman University of Medical Sciences, Kerman, eastern Iran. The adolescents were randomly selected from four elementary schools consisting of two males and two females. Each adolescent was subjected to a thorough examination of the oral cavity. For each individual, a structured questionnaire was completed and all demographic and clinical characteristics were recorded.
Inclusion and exclusion criteria
Overall 382 school children were examined and of which 67 individuals were excluded due to various reasons. Of the children examined, 315 were deemed eligible for enrolment in the study.
Sample collection
For each adolescent, two specimens were collected by scraping debris along the gingival line using a sterile periodontal curette (3, 18, 19). One sample was inoculated into a tube containing sterile physiological saline solution and stored at −20 °C for further PCR examination. The other specimen was cultured in a biphasic medium tube containing horse serum slant overlaid, 6 ml Ringer’s solution (HSr Medium), 200 μl of rice starch (5 mg/ml) and 2% penicillin/ streptomycin (20). The inoculated tubes were labeled and promptly transported to the Medical Parasitology Diagnostic Laboratory at School of Medicine. The cultured tubes were incubated at 35.5 °C in digital incubator and examined two times at 48 h intervals by a light microscope.
Diagnosis of the isolates
The detection of E. gingivalis was performed following growth of the trophozoite in the culture media. A clean slide was prepared and small drop of liquid medium alongside the solid phase was placed on the slide and the wet smear preparation was thoroughly examined visually by a light microscope (×400 magnifications). In addition, for some isolates, portion of the samples were transferred into polyvinyl alcohol fixative and stained by trichrome staining technique and by Giemsa (21) (Fig. 1 A&B).
Fig. 1:
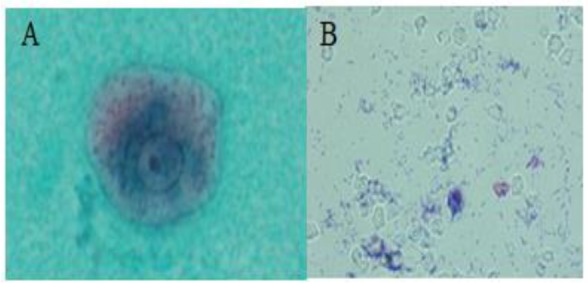
Entamoeba gingivalis, trichrome-stained (A) and Trichomonas tenax, Giemsa-stained (B)
Molecular identification DNA extraction
DNA was directly extracted from E. gingivalis trophozoite obtained from the culture media. Briefly the organism was washed three times in physiological saline solution (3000 rpm for 5 min, pH; 7.2). DNA was extracted by proteinase K using the High Pure Template Purification Kit (Roche, Germany) based on the Kit’s instruction.
Polymerase chain reaction (PCR)
The extracted DNA was used for amplification of the ribosomal RNA of E. gingivalis and Trichomonas tenax by polymerase chain reaction (PCR) with self-designed specific primers based on the previous records of ribosomal DNA in nucleotide database of NCBI. A 454-bp fragment of the SrRNA gene, amplified by specific forward (5′-GCGCATTTCGAACAGGAATGTAGA-3′) and reverse (5′-CAAAGCCTTTTCAATAGTATCTTCATTCA-3′) primers for E. gingivalis was used. Also, a 496-bp fragment of 18S ribosomal RNA gene and precise forward (5′- ATGACCAG-TTCCATCGATGCCATTC -3′) and reverse (5′- CTCCAAAGATTCTGCCACTAACAAG -3′) primers for T. tenax. All primers were designed with primer 3 online program (22) and checked for specific amplification with Primer-BLAST tools (23) and confirmed with positive known isolates. PCR was performed in 50μl using 3.5 mM MgCl2, 250 mM of each of the dNTPs, 40 pmol of each primer, 2 U Taq polymerase, and 4 mL (20–100 ng/mL) of the DNA template, under the following thermal profile: 5 min at 94 °C as an initial denaturation step, followed by 35 cycles of 30 sec at 94 °C, 30 sec at 58 °C, 60 sec at 72 °C, and a final extension step of 10 min at 72 °C. The amplicons were electrophoresed on 1% (w/v) agarose gel. DNA of reference strains of E. gingivalis and T. tenax were used as positive and distilled water as negative controls. The DNA ladder was Gene Ruler 100 bp (Roche, Germany).
Statistical analysis
Data were entered into SPSS ver. 20 (Chicago, IL, USA) and the significance of statistical difference between proportion (χ2) was determined. In this study, the associated factors which might have roles with amoebic infection as defined by odds ratios were calculated. An odds ratio (OR) is a measure of association between causative agent (E. gingivalis) and demographic or clinical characteristics compared to the odds of those with no infection (control group). Initially, the univariate logistic regression model was used to evaluate each variable individually. Then those variables with P-value <0.2 were analyzed by multivariate logistic regression method by backward stepwise manner to exclude the effect of confounding factors. The significance level was considered at P<0.05.
Results
Demographic and Clinical characteristics
Overall, 315 children in four adolescent schools consisting of two males (n=189) and two females (n=126) (Table 1) were randomly selected (mean age; 15 and range; 13–16 yr old).
Table 1:
Baseline characteristics and infection rate of Entamoeba gingivalis in adolescents in suburban areas of Kerman city by culture and PCR, 2017
| Characteristics | Total | Infected | ||
|---|---|---|---|---|
| Variable | No. | % | No. | % |
| Male | 189 | 60.0 | 30 | 15.9* |
| Female | 126 | 40.0 | 7 | 5.6 |
| Total | 315 | 100 | 37 | 11.7 |
P<0.001
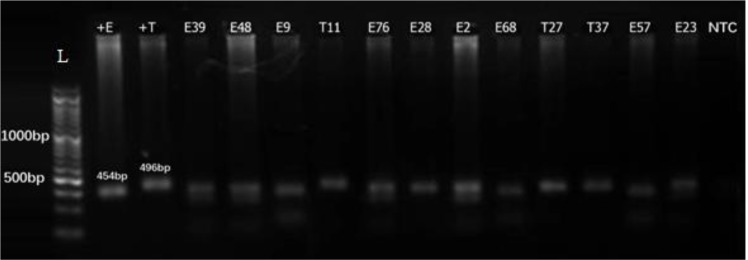
They were interviewed and clinically examined for oral health status and for E. gingivalis and T. tenax infection by direct smear microscopy, culture media and molecular identification using PCR technique. In general, 11.7% (n=37) of the adolescents were infected with E. gingivalis including 30 (15.9%) males and 7 (5.6%) females. The proportion of the infection rate in males was 2.8 times higher than that in female children (P<0.001). In the present investigation 7 cases (2.2%) of T. tenax were also identified. Due to the limited number of infections and consequent inconclusive analysis, this protozoan was not included in the study. Culture media detected 9.2% (n=29), whilst PCR identified 11.4% (n= 36) (Fig. 2).
Fig. 2:
Agarose gel electrophoresis of the representative Entamoeba gingivalis and Trichmonas tenax isolates obtained from adolescents in suburban areas of Kerman city by culture and PCR, 2017. L: DNA size marker, 100 bp; NTC: negative control (distilled water); +E: positive control (Entamoeba gingivalis, 454 bp); +T: positive control (Trichomonas tenax, 496 bp); E39, 48, 9, 76, 28, 2, 68, 57 and 23 E. gingivalis; T11, 27, 37 T. tenax (clinical isolates).
Associated-factors analysis
The univariate logistic regression analysis demonstrated 5 main associated-factors (Table 2) including; namely sex (OR=4.12, CI=1.8–9.6, P<0.001), history of antibiotic consumption (OR=3.243, CI=1.68–6.27, P<0.001), gingival index with category of severe inflammation (OR=50, CI= 4.53–551.41, P<0.001), Candida species co-infection (OR= 4.41, CI = 2.04–9.26, P<0.001) and DMFT (OR=3.26, CI=2.35–4.5, P≤0.001) were significantly associated with E. gingivalis infection rate in adolescents. In contrast to the aforementioned factors, adolescents with history of antibiotic consumption were significantly protected from the E. gingivalis infection (OR=3.24, CI=1.68–6.27, P<0.001) as detected by only univariate model. The multivariate model confirmed only 3 major risk determinants (Table 3) consisting of gingival index with severe inflammatory reaction (OR=30.14, CI=1.10–823.31, P<0.04), Candida spp. co-infection (OR= 3.74, CI=1.32–10.56, P<0.013) and DMFT (OR= 3.27, CI= 2.30–4.66, P<0.001). Factors which showed a negative association with infection by univariate and multivariate model included parents’ occupation and history of systemic diseases (Table 4).
Table 2:
Associated-factors for Entamoeba gingivalis in adolescents analyzed by univariate logistic model in suburban areas of Kerman city by culture and PCR methods, 2017
| P value | 95% CI | OR | Infected No (%) | Total | Characteristic | |
|---|---|---|---|---|---|---|
| < 0.001 | 1.8–9.6 | 4.12 | 30 (81.1) | 189 | Male | Sex |
| 1 | 7 (18.9) | 126 | Female | |||
| 1 | 16 (43.2) | 133 | Yes | History of antibiotic use | ||
| < 0.001 | 1.68–6.27 | 3.243 | 21 (56.8) | 182 | No | |
| 1 | 1 (2.7) | 23 | No inflammation | |||
| 0.12–10.29 | 1.12 | 5 (13.5) | 72 | Mild inflammation | ||
| 0.29–17.77 | 2.26 | 21 (56.8) | 207 | Moderate inflammation | Gingival Index | |
| < 0.001 | 4.53–551.41 | 50 | 10 (27.0) | 13 | Sever inflammation | |
| < 0.001 | 2.04–9.26 | 4.41 | 29 (78.4) | 263 | Yes | Candida spp. |
| 1 | 8 (21.6) | 52 | No | |||
| < 0.001 | 2.37–4.5 | 3.26 | 315 | Yes | DMFT* | |
| 0 | No |
Decay-missing and filled teeth
Table 3:
Associated-factors for Entamoeba gingivalis in adolescents analyzed by multivariate model in suburban areas of Kerman city by culture and PCR methods, 2017
| P value | 95% CI | OR | Infected No (%) | Total | Characteristic | |
|---|---|---|---|---|---|---|
| 1 | 1 (2.7) | 17 | No inflammation | |||
| 0.92–30.06 | 1.66 | 5 (13.5) | 87 | Mild inflammation | Gingival Index | |
| 0.3–52.17 | 3.46 | 21 (56.8) | 196 | Moderate inflammation | ||
| <0.04 | 1.10–823.31 | 30.14 | 10 (27) | 15 | Sever inflammation | |
| <0.013 | 1.328–10.56 | 3.744 | 29 (78.4) | 171 | Yes | Candida spp. |
| 1 | 8 (21.6) | 144 | No | |||
| <0.001 | 2.30–4.66 | 3.27 | 37 (100) | 315 | Yes | DMFT* |
| 1 | 0 | No |
Decay-missing and filled teeth
Table 4:
Non-associated factors for Entamoeba gingivalis in adolescents in suburban areas of Kerman city by culture and PCR methods, 2017
| Characteristic | Infected No (%) | Total | OR | 95% CI | P value | |
|---|---|---|---|---|---|---|
| Fathers’ job | Unemployed | 1 (5.5) | 23 | 0 | 0 | 1 |
| Free job | 27 (63.8) | 195 | 1.28 | 0.63–2.61 | 0.5 | |
| Employee | 9 (30.6) | 97 | 1 | |||
| Mothers’ job | Unemployed | 0 (0) | 15 | 1.06 | 0.30–3.76 | 0.92 |
| Free job | 34 (91.9) | 214 | 0 | 0 | 1 | |
| Employee | 3 (8.1) | 86 | 1 | |||
| History of systemic diseases | Yes | 2 (5.4) | 33 | 1.13 | 0.88 | |
| No | 35 (94.6) | 282 | 1 | 0.24–5.26 |
Discussion
In this study, the prevalence rate of E. gingivalis was fairly high. This findings might be negatively related to how school children perceive their oral health affairs. Oral hygiene is a key component of overall health status. The impact of the parasitic agents in periodontium pathology is still scarce and controversial (24). However, not only bacteria remain as the etiological agents of the periodontal diseases, but also parasites could be the cause (4–6). Periodontal diseases including gingivitis and periodontitis are the impact of disruption between the host-oral microbiome involvements. These diseases are caused by diverse and complex multiple factors (6, 24–26). The infection rate was significantly higher in male than female group. One possible explanation for male elementary school children showed higher rate of infection might be related to the epidemiological and behavioral differences. On the other hand, females are more disciplined and sensitive to oral health issues compared with male school children. The exact association between the sex and the risk of amoeba is complex and requires further investigation (27).
According to our data overall 11.7% of the adolescents showed infection with E. gingivalis, whilst a prevalence rate of 18% was reported from Shiraz (17). In comparison with our findings, an infection rate of 41.7% with E. gingivalis was reported in patient referred to the Faculty of Dentistry in Tehran, demonstrating a significantly higher prevalence rate compared with the data found by the present study (28). In contrast, in Ahvaz, only 0.5% of the examined individuals were infected with this amoeba, performing a lower frequency relative to our finding (29). On the other hand, in Ahvaz, 15.7% of the age range 6–14 yr old were infected with E. gingivalis and simultaneously they had also gingivitis (30). Due to presence of various factors including age, gender, socioeconomic characteristics, poor oral health conditions, such comparisons are inconclusive (6). Moreover, several other associated-factors seem to have some roles in prevalence of infection with E. gingivalis entails periodontal tissue damage, poor oral health condition, tooth loss, dental caries, low educational level, cigarette smoking, drugs and food (5, 31).
The amoeba possesses the capacity to be involved in sever gingival inflammatory reactions. Overall, the infection rate of E. gingivalis in adolescent school children was higher among those with severe gingival index compared to the healthy control group. This result is consistent with that found by Luszczak et al (5). E. gingivalis is able to alter the leukocyte metabolism by excessive production of elastase, a mechanism by which enabling the amoeba to induce gingival bleeding and severe inflammation. In addition, the role of this amoeba in pathophysiological changes of periodontium has been reported (25, 26). This amoeba is capable of producing substances to optimize the anaerobic condition for their long survival. Moreover, the high infection rate of this protozoan in the mouth cavity is well-associated with poor oral hygiene and concluded this amoeba is common among subjects with low standard of living conditions (32) and also in patients with human immunodeficiency virus type-1 (HIV-1) (7, 15, 33). Other demographic and clinical factors including age, gender, cigarette smoking and presence of periodontal diseases could play a role in prevalence of this amoeba.
The use of broad-spectrum drugs likewise has potential effect in providing fertile grounds for Candida species co-infection in this study. C. albicans is concurrently found to be enhanced in association with numerous microorganisms including bacteria (34). The interactions involved between the organisms in co-infection are the complexity and diversity of this mutual relationship. One of the main reasons for little attention to this issue would be difficult to understand such association.
Decay- missing- filled teeth (DMFT) in children particularly in adolescent schools is an important oral and public health issue. The US Dentists for Disease Control and Prevention have reported that tooth decay is the most prevalent chronic childhood disease, over 5 times more frequent than asthma in children, 5–17 yr old (35). One of the main reasons for subjects who have DMFT is the creation of a suitable space for dwelling and replication of the microorganisms (15, 36). This organism tends to be involved in cases of poor oral hygiene and poor periodontal health (14, 16, 37).
The present study was carried out during the cold months of the year when common cold was a prevalent event in aggregated school-aged children. Therefore, the consumption of local or systemic drugs such as antibiotics (amoxicillin and tetracycline) and other anti-infective agents with broad spectrum of action against various parasitic and microbial organisms is common phenomenon (38). Metronidazole is the drug of choice for treatment of all intestinal parasites including amoebae, Giardia and trichomonads and also for wide ranges of anaerobic bacteria (39). The use of such broad action drugs could have residual or major impact on this amoeba and related bacterial microflora (3, 6, 19).
The diagnosis of parasitic infections in oral cavity is still based on direct microscopic examination of debris and tissue scrapings. However, this method has low sensitivity. Culturing of the organism along with DNA-based PCR methods (29 vs. 36 cases, respectively) are highly accurate with higher sensitivity power (6); although, in this study comparison of the methods was not the purpose.
Conclusion
The present findings clearly demonstrated a positive association between E. gingivalis and demographic and clinical risk determinants. Knowledge of the major risk factors for E. gingivalis infection is essential in improving public and oral health strategies. Therefore, dental practitioners and health surveillance personnel should be aware of these confounding factors to rigorously detect and critically manage oral health issues in school-age children in order to prevent or at least minimize the eventual periodontal complications in later life.
Ethical considerations
Ethical issues (including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
We would like to thank the adolescent school children and their teachers for their cooperation in sample collection. We would also like to thank the Department of Medical Parasitology and Mycology at School of Medicine for their assistance in performing the PCR and culturing methods. This project was supported by Kerman University of Medical Sciences.
Footnotes
Conflict of interests
The authors declare that there is no conflict of interest.
References
- 1.Bhutta ZA, Black RE. (2013). Global maternal, newborn, and child health so near and yet so far. N Engl J Med, 369 (23):2226–35. [DOI] [PubMed] [Google Scholar]
- 2.Bogitsh BJ, Carter CE, Oeltmann TN. (2013). General characteristics of the Trematoda. Human parasitology, 4th edn Academic Press, Waltham, 153–178. [Google Scholar]
- 3.Ghapanchi J, Kamali F, Moattari A, et al. (2015). In vitro comparison of cytotoxic and antibacterial effects of 16 commercial toothpastes. J Int Oral Health, 7(3): 39–43. [PMC free article] [PubMed] [Google Scholar]
- 4.El-Dardiry MA. (2016). Detection of Entamoeba gingivalis trophozoites in patients suffering from gingivitis versus healthy. Adv Environ Biol, 10(12): 222–226. [Google Scholar]
- 5.Luszczak J, Bartosik M, Rzymowska J, et al. (2016). The occurrence of Entamoeba gingivalis among patients with periodontal disease. Pharm Med Sci, 29 (2):86–9. [Google Scholar]
- 6.Trim RD, Skinner MA, Farone MB, et al. (2011). Use of PCR to detect Entamoeba gingivalis in diseased gingival pockets and demonstrate its absence in healthy gingival sites. Parasitol Res. 109 (3):857–64. [DOI] [PubMed] [Google Scholar]
- 7.Cembranelli SB, Souto FO, Ferreira-Paim K, et al. (2013). First evidence of genetic intraspecific variability and occurrence of Entamoeba gingivalis in HIV (+)/AIDS. PloS One, 8 (12):e82864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kashefi P, Amighi A, Khalifesoltani M. (2015). Prevalence of hemodynamic, acid and base, coagulation, electrolytes, and blood glucose disturbances and requiring inotropic support in patients with brain death on arrival at hospital intensive care unit. J Isfahan Med Sch, 33 (2):337. [Google Scholar]
- 9.Burt B. (2005). Epidemiology of periodontal Diseases. J Periodontol, 76(8): 1406–1419. [DOI] [PubMed] [Google Scholar]
- 10.Halfon N, Larson K, Slusser W. (2013). Associations between obesity and comorbid mental health, developmental, and physical health conditions in a nationally representative sample of US children aged 10 to 17. Acad pediatr, 13 (1):6–13. [DOI] [PubMed] [Google Scholar]
- 11.Kumar PS, Leys EJ, Bryk JM, et al. (2006). Changes in periodontal health status are associated with bacterial community shifts as assessed by quantitative 16S cloning and sequencing. J Clin Microbiol, 44 (10):3665–3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-hamiar AK, Kezar MY, Al-Khafaji YA. (2011). Prevalence of Oral Protozoa in Periodontitis and Gingivitis Patients Whose Attended to Clinics Periodontics, Dentistry College\Babylon Univ. Al-Kufa J Biol, 3 (1):223–225. [Google Scholar]
- 13.Bhaijee F, Bell D. (2011). Entamoeba gingivalis in Acute Osteomyelitis of the Mandible. Case Rep Med, 2011: 357301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrews JH, Hecht EP, Bashirian S. (1982). Association between the fungus Acremonium curvulum and Eurasian water milfoil, Myriophyllum spicatum. Canadian Journal of Botany, 60 (7):1216–21. [Google Scholar]
- 15.Sharma K, Kaur H. (2015). Oral health in relation to nutritional status, age and sex among 14–18 years children of Naraingarh, Haryana. Braz Dent Sci, 18(3):68–76. [Google Scholar]
- 16.PAri A, Ilango P, Subbareddy V, Katamreddy V, Parthasarthy H. (2014). Gingival diseases in childhood–a review. J Clin Diagn Res, 8(10): ZE01–ZE04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghabanchi J, Zibaei M, Afkar MD, Sarbazie A. (2010). Prevalence of oral Entamoeba gingivalis and Trichomonas tenax in patients with periodontal disease and healthy population in Shiraz, southern Iran. Indian J Dent Res, 21(1):89–91. [DOI] [PubMed] [Google Scholar]
- 18.Elith J, Graham CH, Anderson RP, et al. (2006). Novel methods improve prediction of species’ distributions from occurrence data. Ecography, 29(2):129–51. [Google Scholar]
- 19.Schuster FL, Sullivan JJ. (2002). Cultivation of clinically significant hemoflagellates. Clin Microbiol Rev, 15(3):374–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark CG, Diamond LS. (2002). Methods for cultivation of luminal parasitic protists of clinical importance. Clin Microbiol Rev, 15(3):329–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McHardy IH, Wu M, Shimizu-Cohen R, et al. (2014). Detection of intestinal protozoa in the clinical laboratory. J Clin Microbiol, 52(3):712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Untergasser A, Cutcutache I, Koressaar T, et al. (2012). Primer3—new capabilities and interfaces. Nucleic Acids Res, 40(15):e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye J, Coulouris G, Zaretskaya I, et al. (2012). Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics, 13(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker JL, Bor B, Agnello M, Shi W, He X. (2017). Ecology of the oral microbiome: beyond bacteria. Trends Microbiol, 25(5):362–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marty M, Lemaitre M, Kemoun P, Morrier J-j, Monsarrat P. (2017). Trichomonas tenax and periodontal diseases: a concise review. Parasitology, 144(11):1417–25. [DOI] [PubMed] [Google Scholar]
- 26.Chomicz L, Padzik M, Szaflik JP, et al. (2014). Monitoring of in vitro dynamics of Acanthamoeba strains isolated from infected eyes as a useful tool in keratitis management. Exp parasitol, 145(3):S73–S77. [DOI] [PubMed] [Google Scholar]
- 27.Vaccarino V, Badimon L, Corti R, et al. (2011). Ischaemic heart disease in women: are there sex differences in pathophysiology and risk factors? Position paper from the working group on coronary pathophysiology and microcirculation of the European Society of Cardiology. Cardiovasc Res, 90 (1):9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gharavi M, Hekmat S, Ebrahimi A, Jahani M. (2006). Buccal cavity protozoa in patients referred to the faculty of dentistry in Tehran, Iran. Iran J Parasitol, 1 (1):43–6. [Google Scholar]
- 29.Rahdar M, Maraghi S, Azizi A, Mousanejad F, Vazirianzadeh B. (2013). A study on the frequency of buccal cavity protozoa in patients with periodontitis and gingivitis in Ahvaz, southwest of Iran in 2009. Jundishapur J Health Sci, 4(4):85–89. [Google Scholar]
- 30.Shahrzad S SS, ApS M-JK. (2017). The prevalence of Trichomonas tenax and Entamoeba gingivalis in mental disabilities children at Ahvaz welfare centers, Iran. Arvand J Health Med Sci, 2(2):71–4. [Google Scholar]
- 31.Onyido A, Amadi E, Olofin I, Onwumma A, Okoh I, Chikwendu C. (2011). Prevalence of Entamoeba gingivalis and Trichomonas tenax among dental patients attending Federal School of Dental Technology and Therapy clinic, Enugu, Nigeria. Oral Dis, 11(9): 350–358. [Google Scholar]
- 32.Bergquist R. (2009). Parasitic infections affecting the oral cavity. Periodontol 2000, 49 (1):96–105. [DOI] [PubMed] [Google Scholar]
- 33.Lucht E, Albert J, Linde A, et al. (1993). Human immunodeficiency virus type 1 and cytomegalovirus in saliva. J Med Virol, 39 (2):156–62. [DOI] [PubMed] [Google Scholar]
- 34.Swidergall M, Solis NV, Lionakis MS, Filler SG. (2018). EphA2 is an epithelial cell pattern recognition receptor for fungal β-glucans. Nat Microbiol, 3(1):53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beltrán-Aguilar ED1, Barker LK, Canto MT, et al. (2005). Surveillance for dental caries, dental sealants, tooth retention, edentulism, and enamel fluorosis: United States, 1988–1994 and 1999–2002. MMWR Surveill Summ, 54(3);1–43. [PubMed] [Google Scholar]
- 36.Mielnik-Błaszczak M, Rzymowska J, Michałowski A, Skawińska-Bednarczyk A, Błaszczak J. (2018). Entamoeba gingivalis– prevalence and correlation with dental caries in children from rural and urban regions of Lublin Province, Eastern Poland. Ann Agric Environ Med, 25(4): 656–658. [DOI] [PubMed] [Google Scholar]
- 37.Rowan-Legg A. (2013). Oral health care for children–a call for action. Paediatr Child Health, 18 (1):37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winkelhoff AJV, Rams TE, Slots J. (1996). Systemic antibiotic therapy in periodontics. Periodontol 2000, 10:45–78. [DOI] [PubMed] [Google Scholar]
- 39.Guerrero A, Griffiths GS, Nibali L, et al. (2005). Adjunctive benefits of systemic amoxicillin and metronidazole in non surgical treatment of generalized aggressive periodontitis: a randomized placebocontrolled clinical trial. J Clin Periodontol, 32(10):1096–107. [DOI] [PubMed] [Google Scholar]