Abstract
Background
Therapeutic lateral neck dissection (LND) is recommended in papillary thyroid carcinoma (PTC) patients with clinically lateral lymph node metastasis (LLNM), whether underwent level V LND remains controversial for lacking of sensitive predicting system. BRAFV600E mutation is associated with aggressive tumor behavior, recurrence, and disease-specific mortality of PTC. However, the relationship between BRAFV600E mutation and level V LNM is unclear.
Methods
Univariate and multivariate analyses were retrospectively conducted on the potential predictive factors of 252 PTC patients who underwent initial treatment of neck lymph node dissection from September 2015 to October 2018 in our institute. BRAFV600E mutation and the clinicopathological characteristics of the two groups were compared.
Results
LLNM was presented in 208 (82.5%) patients and level II–V LNM was present in 42.8%, 71.2%, 85.1%, 17.8% patients, respectively. BRAFV600E mutation was observed in 188 (74.6%) patients and was significantly associated with patients’ age, lymphocytic thyroiditis, capsule invasion, bilateral central lymph node metastasis (CLNM) and level V LNM in PTC. Univariate analysis revealed that lymphocytic thyroiditis, tumor size, number of CLNM, Level II LNM, Level III LNM, simultaneous Level II+III, simultaneous Level III+IV and simultaneous Level II+III+IV were significantly correlated with Level V LNM. In addition, multivariate analysis revealed that tumor size ≥2.5 cm, number of CLNM≥3, level II metastases and BRAFV600E mutation were independent Level V LNM predictors (odds ratio 3.910, 3.660, 8.410, 0.439; 95% CI 1.737–10.135, 1.054–12.713, 1.233–57.355, 0.280–0.827, respectively).
Conclusion
In summary, we presented several independent predictive factors for level V LNM in PTC patients. We constructed a risk prediction model consisting of tumor size ≥2.5 cm, number of CLNM≥3 and level II metastases and BRAFV600E mutation that may guide surgeons to evaluate the nodal status in PTC and perform tailored therapeutic LND.
Keywords: papillary thyroid carcinoma, BRAFV600E mutation, level V lymph nodes metastasis, pathological features
Introduction
Thyroid cancer is a common endocrine malignancy, and has become the fourth most common malignant cancer in women in China.1 Papillary thyroid carcinoma (PTC) is the most common subtype of thyroid cancer, influencing the health and quality of life of people worldwide.2,3 Cervical lymph node metastasis (LNM), a common clinical phenomenon in PTC, is an independent risk factor for local recurrence and PTC-specific mortality.4,5 Therefore, therapeutic lateral neck dissection (LND) is generally recommended in patients with LNM. In general, the extent of therapeutic LND includes level II–V. However, whether routine level V lymphadenectomy in patients with PTC with clinically lateral lymph node metastasis (LLNM) remains controversial.6 An increasing number of recent studies have shown that the incidence of level V lymph node metastasis is significantly lower than the incidence of level II–IV lymph node metastasis.7 In addition, level V lymphadenectomy may cause postoperative complications such as shoulder dysfunction, supraclavicular numbness, neuralgia, and sternocleidomastoid muscle atrophy.8 Thus, an effective therapeutic LND is critical to postoperative outcome. A majority of previous studies had explored some predictors for level V LNM in PTC patients based on clinical and sonographic characteristic. However, not taking the genetic background into consideration makes predicting the evidence of level V LNM less accurate that we clinicians are confused.
The BRAFV600E mutation has been well known to be the most common oncogenic mutation of PTC, occurring in approximately 45% of patients cases on average.9 These are well-established BRAFV600E mutation constitutes to aggressive tumor behavior as well as poor clinical outcomes, including recurrence of PTC, and PTC-specific mortality.10,11 However, BRAFV600E mutation related to level V LNM is unclear. Emerging evidence has shown a potential value of BRAFV600E mutation in predicting central cervical lymph node metastasis of PTC.12,13 Given these data, in this retrospective study, we tested our hypothesis that BRAFV600E mutation might constitute a genetic background conferring LNM and that BRAF status could thus differentiate the prognostic risk of level V LNM in PTC.
Methods
Study Population
This work obtained approval to retrospectively review the medical records of patients from the Ethics Committee of Xiangya Hospital, Central South University (approval number, 2019030440), and conducted in accordance with the Declaration of Helsinki. Written informed consent for the evaluation of BRAFV600E mutation status was obtained from every participant patient prior to thyroidectomy. In addition to approving the study protocol, the Ethics Committee required neither patient approval nor informed consent for the review of records. Furthermore, we confirmed that the data related to this manuscript were anonymized.
We retrospectively reviewed the medical records of consecutive patients with PTC who underwent simultaneous total thyroidectomy (TT), bilateral central neck dissection (CND), and LND (at least from levels II to V) at the department of General Surgery, Xiangya Hospital, Central South University between September 2015 and October 2018. Patients were excluded if they had (a) a previous history of thyroidectomy or (b) refusal of BRAFV600E mutation analysis or (c) absent or insufficient ultrasonography image or (d) other subtypes of thyroid carcinoma. As a result, a total of 252 patients were enrolled in this study. Almost all patients were from central China, and most of them were from Hunan Province.
Before operation, all patients underwent preoperative physical examination, high-quality thyroid ultrasonography (US), and US-guided fine-needle aspiration biopsy (USgFNAB) of the primary tumor. The final diagnosis of primary tumors and cervical LNM was based on pathological examination of surgical specimens by two separate pathologists. In this study, lateral neck nodes were classified into neck levels (II to V) based on the criteria of the American Head and Neck Society.14 In addition, BRAFV600E mutation status was determined after surgical and medical treatments in all patients and did not affect decision making regarding treatments. We isolated genomic DNA from primary PTC tumors and analysed the sequence of exon 15 of BRAF gene for V600E mutation according to published studies.15
Statistical Analysis
Risk factors including BRAFV600E mutation status, sex, age, thyroid-stimulating hormone (TSH) level, lymphocytic thyroiditis, tumor size, capsular invasion, ultrasound findings (including location, solid component, shape, margin, echogenicity, calcifications), and the extent of LNM were obtained and analyzed in this study.
In univariate analysis, categorical variables were analyzed using Pearson’s chi-square test and continuous variables were analyzed using the Student’s t-test or the Wilcoxon rank-sum test. Besides, we performed receiver-operating characteristic (ROC) curve analysis to determine the optimal cut-off points for patient age, tumor size, and number of central lymph node metastasis (CLNM) as well as to test the accuracy of those continuous variables in predicting level V lymph node metastasis. The area under the curve (AUC) > 0.700 was considered to be meaningful. At last, a binary logistic regression model was used to evaluate the risk factors for level V lymph node metastasis in PTC. SPSS software (version 22.0; SPSS, Chicago, IL) was used for these analyses. All p-values were two sided, and a value ≤0.05 was considered significant.
Results
Demographic Variables
As summarized in (Table 1), a total of 252 patients with PTC, of whom 69.8% (176) were women and 30.2% (76) were men, were included in the study, with a median age of 39.6±11.9 years (range, 12 to 72) at diagnosis of PTC and 229 (90.9%) were younger than 55 years. There were 43 patients with thyroid dysfunction (6 hyperthyroidisms and 37 hypothyroidisms, respectively) and 89 patients with lymphocytic thyroiditis. BRAFV600E mutation was observed in 188 (74.6%) patients. Suspicious ultrasonography features including solid component, echogenicity, calcification, and irregular/lobulated margins were examined, calcification was observed in 233 (92.5%) patients.
Table 1.
Demographics and Clinical Characteristics of 252 Solitary Papillary Thyroid Carcinoma Patients
| Characteristics | Results (%) |
|---|---|
| No. of patients | 252 |
| Sex | |
| Male | 76(30.2) |
| Female | 176(69.8) |
| Age (mean and range) | 39.6 ± 11.9 (12 to 72) |
| ≥55 | 23(9.1) |
| ˂55 | 229(90.9) |
| BRAFV600E mutation | 188(74.6) |
| TSH levels | |
| Low | 6(2.4) |
| Normal | 209(82.9) |
| High | 37(14.7) |
| Lymphocytic thyroiditis | 89(35.3) |
| Tumor size (cm, mean) | 1.981 ± 1.246 |
| ≥1.0 | 212(84.1) |
| ˂1.0 | 40(15.9) |
| Calcification of the tumor on neck US | 233(92.5) |
| Multifocality | |
| Yes/No | 125(49.6)/127(50.4) |
| Bilaterally | |
| Yes/No | 91(36.1)/161(63.9) |
| Capsule invasion | 69(27.4) |
| CLNM (mean) | 205(81.3)/(4.286 ± 4.524) |
| Ipsilateral | 204(80.9) |
| Contralateral | 24(9.5) |
| Bilateral | 55(21.8) |
| LLNM | 208(82.5) |
| Level II | 89(42.8) |
| Level III | 148(71.2) |
| Level IV | 177(85.1) |
| Level V | 37(17.8) |
Abbreviations: TSH, thyroid-stimulating hormone; US, ultrasonography; CLNM, central lymph node metastases; LLNM, lateral lymph node metastasis.
In our study, LNM was histologically confirmed to involve the central compartment (CLNM) in 205 patients (81.3%) and the lateral compartment (LLNM) in 208 patients (82.5%). Out of the 205 patients with CLNM, the mean ± SD (Standard Deviation) number of metastatic lymph nodes was 4.286±4.524 and 204 patients (80.9%) had LNM in the central compartment ipsilateral to the primary tumor. 24 (9.5%) had LNM in the contralateral central compartment and 55 (21.8%) had LNM in the bilateral central compartments. Out of 208 patients with LLNM, Level IV metastases were most common (177/208; 85.1%), followed by level III (148/208; 71.2%), level II (89/208; 42.8%), and level V (37/208; 17.8%) metastases.
BRAFV600E Mutation Is Associated with Multiple Clinicopathological Features Including Level V Lymph Node Metastasis
To understand the relationship between BRAFV600E mutation and clinicopathological features, univariate analysis was conducted. We found that BRAFV600E mutation was associated with age, lymphocytic thyroiditis, capsule invasion, LNM in the bilateral central compartments and Level V lymph node metastasis (p <0.05). The mean ± SD of age in BRAFV600E mutation group was significantly higher than in wild group (40.755±11.407 vs 36.031±12.520, p <0.01). Similarly, capsule invasion was easier to happen in BRAFV600E mutation group (which) was significantly higher than in wild group (31.4% vs 15.6%, p <0.01). Moreover, patients with lymphocytic thyroiditis were more common in BRAF wild group than in BRAFV600E mutation group (51.6% vs 29.8%, p <0.01). Also, we found that patients with wild BRAF status were observed with higher risk of bilateral CLNM (31.3% vs 18.6%, p <0.01) and Level V lymph node metastasis (25.0% vs 11.2%, p <0.01). That is to say, BRAFV600E mutation could be a protective factor for Level V LNM in PTC patients. Detailed information is shown in (Table 2).
Table 2.
Associations Between BRAFV600E Mutation and Clinicopathological Characteristics in PTC
| Parameter | BRAFV600E Mutation Status | P-value | |
|---|---|---|---|
| Mutation, n (%) | Wild, n (%) | ||
| Total | 188(74.6) | 64(25.4) | |
| Female | 133(70.7) | 43(67.2) | 0.592 |
| Age (Mean ± SD) | 40.755±11.407 | 36.031±12.520 | 0.006a |
| ≥55 | 17(9.0) | 6(9.4) | 0.936 |
| ˂55 | 171(91.0) | 58(90.6) | |
| TSH levels | |||
| Low | 3(1.6) | 3(4.7) | 0.110 |
| Normal | 161(85.6) | 48(75.0) | |
| High | 24(12.8) | 13(20.3) | |
| Lymphocytic thyroiditis | 56(29.8) | 33(51.6) | 0.002b |
| Tumor size (Mean ± SD, cm) | 1.964±1.288 | 2.031±1.123 | 0.711 |
| >1.0 | 58(30.9) | 25(39.1) | 0.227 |
| ≤1.0 | 130(69.1) | 39(60.9) | |
| Location | |||
| Left lobe | 58(30.9%) | 13(20.3%) | 0.056 |
| Right lobe | 59(31.3%) | 32(50.0%) | |
| Isthmus | 9(4.8%) | 1(1.6%) | |
| Bilateral | 62(33.0%) | 18(28.1%) | |
| Solid component on neck US | |||
| Pure solid | 183(97.3) | 61(95.3) | 0.424 |
| Echogenicity of the tumor on neck US | |||
| Hypoechoic | 166(88.3) | 57(89.1) | 0.868 |
| Margin of the tumor on neck US | |||
| Smooth | 54(28.7) | 16(25.0) | 0.566 |
| Ill-defined margin | 134(71.3) | 48(75.0) | |
| Calcification of the tumor on neck US | 172(91.5) | 61(95.3) | 0.317 |
| Multifocality | 96(51.1) | 29(45.3) | 0.427 |
| Capsule invasion | 59(31.4) | 10(15.6) | 0.015b |
| Bilateral tumor | 71(37.8) | 20(31.3) | 0.349 |
| CLNM | 149(79.3) | 56(87.5) | 0.144 |
| Ipsilateral | 148 (78.7) | 56 (87.5) | 0.143 |
| Contralateral | 16(8.5) | 8(12.5) | 0.348 |
| Bilateral | 35(18.6) | 20(31.3) | 0.035b |
| CLNM number (Mean ± SD) | 3.894±3.893 | 5.438±5.896 | 0.054 |
| LLNM | 155(82.4) | 53(82.8) | 0.947 |
| Level II | 62(33.0) | 27(42.2) | 0.183 |
| Level III | 106(56.4) | 42(65.6) | 0.195 |
| Level IV | 130(69.1) | 47(73.4) | 0.517 |
| Level V | 21(11.2) | 16(25.0) | 0.007b |
Notes: Variables with statistical significance were shown in bold. aThe Student’s t-test was adopted. bThe Wilcoxon rank-sum test was adopted.
Abbreviations: PTC, papillary thyroid carcinoma; SD, Standard Deviation; TSH, thyroid-stimulating hormone; US, ultrasonography; CLNM, central lymph node metastases; LLNM, lateral lymph node metastasis.
Distribution of Level V Lymph Node Metastasis Among PTC Patients with Different Clinicopathological and Ultrasonography Features
As shown in (Table 3), we did not find any significant association between level V LNM and sex, age, TSH levels, ultrasonography features. However, patients with lymphocytic thyroiditis appeared to have a higher prevalence of level V LNM than those without (51.4% vs 32.6%, p <0.05) and the mean size of the primary tumor in patients with level V LNM was larger than that in patients without level V LNM (mean ± SD: 2.695±1.185 vs 1.859±1.217, p <0.05). Since in ROC analysis, we found the optimal cutoff tumor size between the two groups was 2.45 cm, so, we took 2.5 cm as the cut-off value of tumor size in the following Univariate analysis and Multivariate analysis. We found patients with tumor size >2.5 have a higher prevalence of level V LNM (64.9% vs 35.1%, p <0.001).
Table 3.
Univariate Analysis of Risk Factors Related to Level V Lymph Node Metastasis in PTC
| Parameter | Level V Lymph Node Metastases | P-value | |
|---|---|---|---|
| Present, n (%) | Absent, n (%) | ||
| Total | 37(14.7) | 215(85.3) | |
| Sex | |||
| Male | 14(37.8) | 62(28.8) | 0.271 |
| Female | 23(62.2) | 153(71.2) | |
| Age (Mean ± SD) | 37.811±14.966 | 39.856±11.248 | 0.432a |
| ≥55 | 4(10.8) | 19(8.8) | 0.700 |
| ˂55 | 33(89.2) | 196(91.2) | |
| TSH levels | |||
| Low | 2(5.4) | 4(1.9) | 0.052 |
| Normal | 25(67.6) | 184(85.6) | |
| High | 10(27.0) | 27(12.6) | |
| Lymphocytic thyroiditis | 19(51.4) | 70(32.6) | 0.027b |
| Tumor size (Mean ± SD, cm) | 2.695±1.185 | 1.859±1.217 | ˂0.001a |
| ≤2.5 | 13(35.1) | 156(72.6) | ˂0.001b |
| >2.5 | 24(64.9) | 59(27.4) | |
| Pure solid on neck US | 35(94.6) | 209(97.2) | 0.402 |
| Echogenicity of the tumor on neck US | |||
| Hypoechoic | 31(83.8) | 192(89.3) | 0.331 |
| Smooth margin of the tumor on US | 31(83.8) | 151(70.2) | 0.089 |
| Calcification of the tumor on neck US | 37(100) | 196(91.2) | 0.060 |
| Multifocality | 21(56.8) | 104(48.4) | 0.346 |
| Capsule invasion | 11(29.7) | 58(27.0) | 0.729 |
| Bilateral tumor | 15(40.5) | 76(35.3) | 0.544 |
| CLNM | 35(94.6) | 170(79.1) | 0.025b |
| Ipsilateral | 34 (79.1) | 170 (91.9) | 0.067 |
| Contralateral | 8(21.6) | 16(7.4) | 0.007b |
| Bilateral | 15(40.5) | 40(18.6) | 0.003b |
| Number of CLNM (Mean ± SD) | 7.649±6.845 | 3.707±3.713 | 0.002a |
| LLNM | 37(100) | 171(79.5) | 0.002b |
| Level II | 25(67.6) | 64(29.8) | ˂0.001b |
| Level III | 32(86.5) | 116(54.0) | ˂0.001b |
| Level IV | 29(78.4) | 148(68.8) | 0.241 |
| Level II+III | 21(56.8) | 52(24.2) | ˂0.001b |
| Level III+IV | 26(70.3) | 92(42.8) | 0.002b |
| Level II+III+IV | 17(45.9) | 44(20.5) | 0.001b |
Notes: Variables with statistical significance were shown in bold. aThe Student’s t-test was adopted; bThe Wilcoxon rank-sum test was adopted.
Abbreviations: PTC, papillary thyroid carcinoma; SD, standard deviation; TSH, thyroid-stimulating hormone; US, ultrasonography; CLNM, central lymph node metastases; LLNM, lateral lymph node metastasis.
CLNM was observed significantly related to the prevalence of level V LNM, especially in patients with contralateral CLNM and bilateral CLNM (p <0.01, both). Besides, the number of CLNM in patients with level V LNM was significantly bigger than those without level V LNM (p <0.01). Univariate analysis also showed that the presence of level V LNM was significantly associated with Level II, Level III, and simultaneous Level II+III, Level III+IV and Level II+III+IV lymph node metastases.
Multivariate Logistic Analysis for Level V LNM of PTC
To define the predictors of level V LNM of PTC, we performed binary logistic regression analyses with BRAFV600E mutation status and clinicopathologic features (Table 4). In our result, we found that BRAFV600E mutation was statistically significant and associated with level V LNM (OR =0.439, 95% CI, 0.280–0.827, p =0.027). However, tumor size ≥2.5 cm (OR =3.910, 95% CI, 1.737–10.135, p =0.001), the number of CLNM≥3 (OR =3.660, 95% CI, 1.054–12.713, p =0.041) and level II LNM (OR =8.410, 95% CI, 1.233–57.355, p =0.030) turned out to be independent risk factors associated with level V LNM. Besides, coexisting lymphocytic thyroiditis, presence of CLNM, contralateral CLNM, bilateral CLNM, Level III metastasis, simultaneous Level II+III metastases, simultaneous Level III+IV metastases and simultaneous Level II+III+IV metastases were not found to be associated with level V LNM.
Table 4.
Multivariate Analysis of Risk Factors Related to Level V Lymph Node Metastasis in PTC
| Variables | OR | 95% CI | P-value |
|---|---|---|---|
| BRAFV600E mutation | 0.439 | 0.280–0.827 | 0.027 |
| Lymphocytic thyroiditis | 0.032 | 0.878–4.703 | 0.098 |
| Tumor size ≥2.5 cm | 3.910 | 1.737–10.135 | 0.001 |
| Present of CLNM | 0.923 | 0.136–6.278 | 0.934 |
| Number of CLNM≥3 | 3.660 | 1.054–12.713 | 0.041 |
| Contralateral CLNM | 2.395 | 0.776–7.391 | 0.129 |
| Bilateral CLNM | 0.892 | 0.298–2.665 | 0.837 |
| Level II metastasis | 8.410 | 1.233–57.355 | 0.030 |
| Level III metastasis | 7.648 | 0.785–63.832 | 0.060 |
| Level II+III metastases | 0.752 | 0.053–10.634 | 0.832 |
| Level III+IV metastases | 1.210 | 0.193–7.592 | 0.839 |
| Level II+III+IV metastases | 0.162 | 0.015–1.734 | 0.132 |
Note: Variables with statistical significance were shown in bold.
Abbreviations: PTC, papillary thyroid carcinoma; OR, odds ratio; 95% CI, 95% confidence interval; CLNM, central lymph node metastases;
Association Between Risk Factors and Level V Lymph Node Metastasis in the Score System
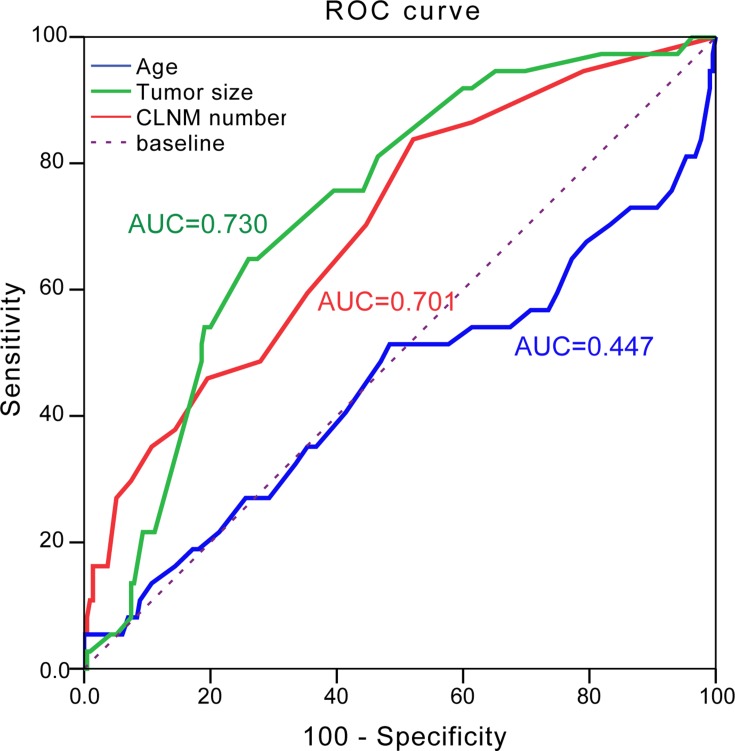
Finally, we computed a risk score for each patient based on age, tumor size and the number of CLNM and constructed a ROC curve using the risk score. In our result, the area under the curve of tumor size and CLNM number were 0.730 and 0.701, respectively, which implied to be well predictors of level V LNM. Moreover, when set cut-off point of 2.45cm of tumor size and cut-off point of 2.5 of CLNM number resulted in a sensitivity of 64.9%, specificity of 74.0% (positive predictive value (PPV)=30.0%, negative predictive value (NPV)=92.4%, and accuracy =72.6%) and cut-off point of 2.5 of CLNM number in a sensitivity of 83.8% and a specificity of 47.9% (PPV=21.7%, NPV=94.5%, and accuracy =53.2%) for predicted level V LNM, respectively. However, no significant relationship was observed between patient age and level V LNM (Figure 1).
Figure 1.
Receiver operating characteristic (ROC) curve for age (blue line), tumor size (green line) and CLNM number (red line) in the prediction of level V LNM in PTC.
Abbreviations: LNM, lymph node metastases; CLNM, central lymph node metastasis; AUC, area under curve.
Discussion
This study is the first study evaluating both clinicopathological features and genetic background for predicting level V LNM in PTC patients. We identified four suspicious features significantly associated with level V LNM of PTC: BRAFV600E mutation, tumor size ≥2.5 cm, number of CLNM≥3 and level II metastasis. According to our results, among those predictors, we firstly observed that BRAFV600E mutation carriers (OR =0.439, 95% CI, 0.280–0.827, p =0.027) were less likely to present level V LNM. While, as the number of other predictors (tumor size ≥2.5 cm, number of CLNM≥3 and level II metastases) increased, the possibility of level V LNM of PTC also significantly increased. According to published studies, the ability to detect BRAFV600E mutation in FNAB cytologic specimens is not inferior to that in postoperative pathologic specimens,16 which means BRAFV600E mutation would be considered as a preoperative predictive factor for occult level V LNM of PTC patients.
Nowadays PTC belongs to the low-risk cancer with rarely life-threatening; however, the presence of LNM significantly increases the risk of locoregional recurrence.17 Emerging evidences have demonstrated decreased disease-free survival rate and increased mortality associated with regional LNM.18 Even though lots of novel methods were emerging to target cancer cells and LNM of PTC,19 LND is generally recommended in PTC patients with LNM. However, the extensive postoperative complications caused by LND should not be ignored which will reduce the quality of life of patients. Therefore, determining a rational extent of therapeutic LND is vital. Whether level V should be included in therapeutic LND continues to be controversial. Therefore, we analyzed the frequency and the risk factors for level V LNM in PTC with clinically LLNM to determine the rational extent of therapeutic LND.
Currently, the methods used to diagnose LNM include computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound imaging. In our study, the preoperative US characteristics we collected were not statistically related to level V LNM, which is consistent with the previous studies by Yang et al.6 Therefore, preoperative US cannot effectively predict the presence of level V LNM. The new technology Ultrasound-guided fine needle aspiration cytology (USgFNAC) is recommended as the gold standard used in the diagnosis of PTC lymph node metastasis.20 Although USgFNAC was showed to be the most specific and accurate imaging modality to detect cervical LNM, the latest research reported that the false-negative rate of USgFNAC could be as high as 45–52%, which has a relatively lower sensitivity than US.21 In addition, given the closer relationship of the node to the surrounding vascular structures, routine preoperative USgFNAC is not done to guide LND at most institutions including ours. Besides, there were studies reported the association between clinicopathological and ultrasonography features and the risk of having positive LLNM or level V LNM,6 however, seldom their genetic backgrounds are taken into consideration. Like other cancers, PTC is a genetically driven disease and mutation of the BRAF gene is common in PTC. In this study, patients with BRAFV600E mutation accounted for 74.6% of PTC patients, consistent with the previous reports (occurring more than 45% of patient cases).15 Over the last decade, the relationship between BRAFV600E mutation and clinicopathological characteristics in PTC has been well studied. BRAFV600E mutation could lead to an increase in tumor recurrence and cancer-related mortality.22,23 As expected, BRAFV600E mutation was confirmed to be significantly related to patients’ age, lymphocytic thyroiditis, capsule invasion, bilateral CLNM and level V LNM in PTC in our study but the phenomenon we observed that BRAFV600E mutation seems to be a protection factor of level V LNM needs further investigations.
Several previous studies have indicated that, in patients with PTC and clinical LNM, most LLNM were levels II, III, and IV. Almost consistent with previous studies, we found that LLNM mainly occurred at levels II, III, and IV with frequencies of 35.3%, 58.7%, and 70.2%, respectively. We also found the number of CLNM and level II metastasis in PTC are indications for increased risk level V LNM. In addition, we observed that tumor size≥2.5 cm presented a 3.91-fold increased risk of level V LNM in PTC patients. Consistent to previous studies, Zhou et al found that tumor size>7 mm was a risk factor of CLNM and another report also demonstrated that larger tumor size of PTC enhanced tumor aggressiveness and worsened survival of patients.24,25
There are some potential limitations to our study. Because this was a retrospective observational study and there might be selection bias. Moreover, the 252 patients of this study are all Chinese, and whether the identified factors can predict level V LNM in other races needs further investigation. Furthermore, several risk factors for level V LNM such as family history, behavior and Tg level have not been investigated in our study.
Conclusion
In summary, we presented several independent predictive factors for level V LNM in PTC patients. We constructed a risk prediction model consisting of tumor size ≥2.5 cm, number of CLNM≥3 and level II metastasis and BRAFV600E mutation that may guide surgeons to evaluate the nodal status in PTC and perform tailored therapeutic LND.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (81974423, 81902729), the Key Research and Development Programme of Hunan Province (2019SK2031) and the Natural Science Foundation of Hunan Province of China (2017JJ2393).
Abbreviations
PTC, papillary thyroid carcinoma; LNM, lymph node metastasis; LND, lateral neck dissection; LLNM, lateral lymph node metastasis; CND, central neck dissection; TT, total thyroidectomy; US, ultrasonography; USgFNAB, US-guided fine-needle aspiration biopsy; TSH, thyroid-stimulating hormone; ROC, receiver operating characteristic; CLNM, central lymph node metastasis; AUC, area under the curve; SD, Standard Deviation; CT, computed tomography; MRI, magnetic resonance imaging; USgFNAC, Ultrasound-guided fine needle aspiration cytology; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value; OR, odds ratio.
Disclosure
The authors declare that they have no competing interests in this work.
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 2.Ho AS, Luu M, Barrios L, et al. Incidence and mortality risk spectrum across aggressive variants of papillary thyroid carcinoma. JAMA Oncol. 2020. doi: 10.1001/jamaoncol.2019.6851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powers AE, Marcadis AR, Lee M, Morris LGT, Marti JL. Changes in trends in thyroid cancer incidence in the united states, 1992 to 2016. JAMA. 2019;322(24):2440–2441. doi: 10.1001/jama.2019.18528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin S, Bao W, Yang YT, Bai T, Bai Y. Establishing a prediction model for lateral neck lymph node metastasis in patients with papillary thyroid carcinoma. Sci Rep. 2018;8(1):17355. doi: 10.1038/s41598-018-35551-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang TS, Sosa JA. Thyroid surgery for differentiated thyroid cancer - recent advances and future directions. Nat Rev Endocrinol. 2018;14(11):670–683. doi: 10.1038/s41574-018-0080-7 [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Gong Y, Yan S, Zhu J, Li Z, Gong R. Risk factors for level V lymph node metastases in solitary papillary thyroid carcinoma with clinically lateral lymph node metastases. Cancer Med. 2016;5(8):2161–2168. doi: 10.1002/cam4.792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim YC, Choi EC, Yoon YH, Koo BS. Occult lymph node metastases in neck level V in papillary thyroid carcinoma. Surgery. 2010;147(2):241–245. doi: 10.1016/j.surg.2009.09.002 [DOI] [PubMed] [Google Scholar]
- 8.Miyauchi A, Ito Y, Oda H. Insights into the management of papillary microcarcinoma of the thyroid. Thyroid. 2018;28(1):23–31. doi: 10.1089/thy.2017.0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao J, Liu P, Yu Y, et al. Comparison of diagnostic methods for the detection of a BRAF mutation in papillary thyroid cancer. Oncol Lett. 2019;17(5):4661–4666. doi: 10.3892/ol.2019.10131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28(7):742–762. doi: 10.1210/er.2007-0007 [DOI] [PubMed] [Google Scholar]
- 11.Wang F, Zhao S, Shen X, et al. BRAF V600E confers male sex disease-specific mortality risk in patients with papillary thyroid cancer. J Clin Oncol. 2018;36(27):2787–2795. doi: 10.1200/JCO.2018.78.5097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Li XL, Zhao CK, et al. Conventional ultrasound, immunohistochemical factors and BRAF(V600E) mutation in predicting central cervical lymph node metastasis of papillary thyroid carcinoma. Ultrasound Med Biol. 2018;44(11):2296–2306. doi: 10.1016/j.ultrasmedbio.2018.06.020 [DOI] [PubMed] [Google Scholar]
- 13.Kim SK, Lee JH, Woo JW, et al. BRAF V600E mutation: differential impact on central lymph node metastasis by tumor size in papillary thyroid carcinoma. Head Neck. 2016;38(Suppl 1):E1203–1209. doi: 10.1002/hed.24192 [DOI] [PubMed] [Google Scholar]
- 14.American Thyroid Association Surgery Working G, American Association of Endocrine S, American Academy of O-H, et al.Consensus statement on the terminology and classification of central neck dissection for thyroid cancer.Thyroid.2009;19(11):1153–1158. doi: 10.1089/thy.2009.0159 [DOI] [PubMed] [Google Scholar]
- 15.Smith RA, Salajegheh A, Weinstein S, Nassiri M, Lam AK. Correlation between BRAF mutation and the clinicopathological parameters in papillary thyroid carcinoma with particular reference to follicular variant. Hum Pathol. 2011;42(4):500–506. doi: 10.1016/j.humpath.2009.09.023 [DOI] [PubMed] [Google Scholar]
- 16.Kwak JY, Kim EK, Chung WY, Moon HJ, Kim MJ, Choi JR. Association of BRAFV600E mutation with poor clinical prognostic factors and US features in Korean patients with papillary thyroid microcarcinoma. Radiology. 2009;253(3):854–860. doi: 10.1148/radiol.2533090471 [DOI] [PubMed] [Google Scholar]
- 17.Zhang K, Li C, Liu J, Li Z, Ma C. Down-regulation of APTR and it’s diagnostic value in papillary and anaplastic thyroid cancer. Pathol Oncol Res. 2018. [DOI] [PubMed] [Google Scholar]
- 18.Xiang Y, Lin K, Dong S, Qiao LI, He Q, Zhang X. Prediction of central lymph node metastasis in 392 patients with cervical lymph node-negative papillary thyroid carcinoma in Eastern China. Oncol Lett. 2015;10(4):2559–2564. doi: 10.3892/ol.2015.3544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Long M, Huang P, et al. Emerging integrated nanoclay-facilitated drug delivery system for papillary thyroid cancer therapy. Sci Rep. 2016;6:33335. doi: 10.1038/srep33335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sawka AM, Carty SE, Haugen BR, et al. American thyroid association guidelines and statements: past, present, and future. Thyroid. 2018;28(6):692–706. doi: 10.1089/thy.2018.0070 [DOI] [PubMed] [Google Scholar]
- 21.Jun HH, Kim SM, Kim BW, Lee YS, Chang HS, Park CS. Overcoming the limitations of fine needle aspiration biopsy: detection of lateral neck node metastasis in papillary thyroid carcinoma. Yonsei Med J. 2015;56(1):182–188. doi: 10.3349/ymj.2015.56.1.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SK, Park I, Woo JW, et al. Predicting factors for bilaterality in papillary thyroid carcinoma with tumor size <4 cm. Thyroid. 2017;27(2):207–214. doi: 10.1089/thy.2016.0190 [DOI] [PubMed] [Google Scholar]
- 23.Celik M, Bulbul BY, Ayturk S, et al. The relation between BRAFV600E mutation and clinicopathological characteristics of papillary thyroid cancer. Med Glas. 2020;17(1). [DOI] [PubMed] [Google Scholar]
- 24.Zhou YL, Gao EL, Zhang W, et al. Factors predictive of papillary thyroid micro-carcinoma with bilateral involvement and central lymph node metastasis: a retrospective study. World J Surg Oncol. 2012;10:67. doi: 10.1186/1477-7819-10-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roti E, Rossi R, Trasforini G, et al. Clinical and histological characteristics of papillary thyroid microcarcinoma: results of a retrospective study in 243 patients. J Clin Endocrinol Metab. 2006;91(6):2171–2178. doi: 10.1210/jc.2005-2372 [DOI] [PubMed] [Google Scholar]