Abstract
Background
Insulin-resistant individuals are known to have dyslipidemia and are predicted to be at high risk of cardiovascular events. Vitamin D deficiency was shown to be associated with dyslipidemia; however, the type of dyslipidemia associated with vitamin D deficiency in insulin-resistant individuals is not determined. Furthermore, there is evidence linking insulin resistance with low-grade inflammation suggesting levels of pro-inflammatory cytokines to be increased in insulin-resistant states.
Objective
This study was performed to evaluate the impact of vitamin D deficiency, defined as serum level of 25(OH)D below 20 ng/mL, on lipid profile and inflammatory markers such as interleukin (IL-6) and IL-8, as well as soluble thrombomodulin (TM) in the serum of insulin-resistant individuals.
Methods
A total of 4114 individuals had simultaneous serum 25(OH)D, insulin, and lipid panel testing during 2013 as part of the United Arab Emirates National Diabetes and Lifestyle (UAEDIAB) study. Multivariate logistic regression analysis was used to assess the association between serum level of 25(OH)D and lipid profile in insulin-sensitive versus -resistant individuals. The lipid panel was stratified into high total cholesterol (TC: >6.2 mmol/L), high low-density lipoprotein-cholesterol (LDL-C: >2.59 mmol/L), high triglycerides (TG: >2.3 mmol/L), and low high-density lipoprotein-cholesterol (HDL-C: <1.55 mmol/L) dyslipidemia. Furthermore, the immunomodulatory and vasculoprotective effects of 25(OH)D were assessed by measuring the levels of IL-6, IL-8, and soluble TM in serum using ELISA.
Results
More than half of the 4114 individuals were insulin resistant (n=2760, 67%) and around one-fifth of them were vitamin D-deficient (n=796, 19%). After adjusting for age, gender, body mass index, smoking, ethnicity, and educational level, the only dyslipidemia associated with vitamin D-deficient-insulin-resistant individuals (OR 2.09 [95]; P=0.009) was lower HDL-C. Furthermore, deficient 25(OH)D individuals with low HDL-C levels had higher circulatory IL-6 and IL-8 levels, and higher serum soluble TM compared to individuals with sufficient 25(OH)D and normal lipid profiles (median, IL-6 pg/mL 0.82 vs 1.71, P=0.001; median, IL-8 pg/mL 51.31 vs 145.6, P=0.003; and median, soluble TM ng/mL 5.19 vs 7.38, P<0.0001; in sufficient vs deficient groups, respectively).
Conclusion
The results of our study showed that in insulin-resistant individuals, vitamin D deficiency status is associated with HDL-C dyslipidemia and higher serum inflammatory and endothelial damage markers.
Keywords: vitamin D-deficient, 25(OH)D, insulin-resistant, HDL-C dyslipidemia, IL-6, IL-8, thrombomodulin, lipid panels, UAEDIAB
Introduction
Recent studies suggest that serum level of 25-hydroxyvitamin D [25(OH)D] lower than 20 ng/mL or a vitamin D-deficient status, is associated with increased risk of cardiovascular diseases (CVD),1 including hypertension,2 heart failure,3 and ischemic heart disease4–6. Several reports also indicated that severe vitamin D deficiency defined as 25(OH)D <10 ng/mL is a predictor for sudden cardiac arrest in individuals with pre-existing CVD.7 Vitamin D is believed to impact CVD through various mechanisms including impaired glucose and lipid metabolism pathways.8 Many of these pathways are relevant to insulin resistance as well, given that insulin resistance is a risk factor for dyslipidemia and low levels of serum 25(OH)D.9
Although the association between 25(OH)D and serum lipid profile has been reported in numerous studies,10,11 this association may vary by insulin resistance status. Epidemiological studies suggest an inverse association between 25(OH)D and serum lipid profile12 although it is highly controversial which lipid may be affected by increased serum 25(OH)D levels. Randomized clinical trials (RCTs) have yielded conflicting results.13,14 Of note, these studies were limited by confounding effects of vitamin D with added calcium supplementation; and study designs that were not precisely aimed at vitamin D-deficient status or did not use a sufficient dose of vitamin D to achieve optimal serum level for 25(OH)D (>30ng/mL).15
There are evidence suggesting a correlation between low-grade inflammation and insulin-resistant states, as some studies have revealed a clear association between the chronic activation of pro-inflammatory pathways such as nuclear-κB (NF- κB) and decreased insulin sensitivity.16 Elevated levels of tumor necrosis factor-α (TNF), interleukin-6 (IL-6) and interleukin (IL-8) have all been reported in insulin-resistant states.17 Chronic inflammation in these individuals plays an important role in the development of atherosclerosis leading to increased CVD risk.18 In addition, endothelial dysfunction may be associated with the pathogenesis of insulin resistance syndrome. Plasma thrombomodulin (TM) levels also reflect endothelial damage in the state of insulin resistance.19
In the absence of definitive evidence, there is a need to further investigate how changes in serum 25(OH)D level are related to changes in atherogenic lipid levels and circulatory inflammatory markers in insulin-resistant individuals who are at higher risk of cardiovascular events.20 Therefore, in this cross-sectional approach, we studied the association between serum 25(OH)D level and the lipid profile as well as the circulatory inflammatory markers in a large population derived from the Dubai and Northern Emirates of the United Arab Emirates (UAE).
Methods
Ethical Considerations
Ethical approval for the United Arab Emirates National Diabetes and Lifestyle (UAEDIAB) study was obtained from the University of Sharjah Research Ethics Committee and UAE Ministry of Health (Al-Qassimi Hospital) Research Ethics Committee. Written informed consent was obtained from all study participants.
Study Population
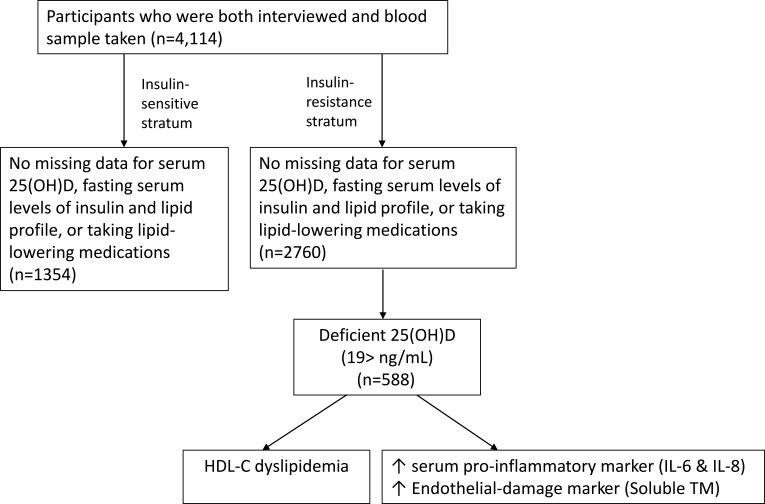
The UAEDIAB study had 5020 individuals across the UAE.21,22 We selected the data from 4114 adult individuals that included simultaneous serum 25(OH)D level, lipid panels, and fasting serum insulin tests during 2013 for analysis (Figure 1). These individuals met the following inclusion criteria: were 18 to 80 years of age and had simultaneous 25(OH)D, lipid panel, and fasting serum insulin tests inclusive. The exclusion criteria were pregnant and those who were on lipid-lowering medications.
Figure 1.
Flow diagram of UAEDIAB cohort and analytic sample.
Serum 25(OH)D Concentration
Serum 25(OH)D level was measured using chemiluminescent immunoassay (DiaSorin, LIAISON 25®Vitamin D TOTAL Assay), according to the manufacturer’s instructions. The coefficient of variation for the instrument was between 10% and 15%, and the sensitivity for the assay was 4 ng/mL.23 We stratified 25(OH)D results into consensus clinical strata:24 deficient (<20 ng/mL; n=796), sufficient (20–50 ng/mL; n=2502), and high serum 25(OH)D concentration (>50 ng/mL; n=461). For multiple regression analysis, vitamin D status was reported as sufficient (20–50 ng/mL) or deficient (<20 ng/mL).
Lipid Panels
The lipid panel results including total cholesterol (TC), low-density lipoprotein-cholesterol (LDL)-C, triglycerides (TG), and high-density lipoprotein-cholesterol (HDL-C) were stratified into high or low levels of dyslipidemia based on consensus clinical strata:25,26 high TC (> 6.2 mmol/L; n=516), high LDL-C (>2.59 mmol/L; n=3130), high TG (>2.3 mmol/L; n=708), and low HDL-C (<1.55 mmol/L; n=3501) dyslipidemia.27 Total cholesterol and triglyceride were measured enzymatically. High-density lipoprotein cholesterol (HDL-C) and LDL-C were measured by a homogeneous direct method (Roche Diagnostics, Mannheim, Germany).
HOMA-Estimated Insulin Resistance Score
Fasting serum insulin and serum glucose test results were used to calculate the HOMA-IR used to assess insulin resistance score using the following formula HOMA-IR= (glucose in mmol/L × insulin in mIU/mL)/22.5.28 Those with HOMA-IR >1.8 were considered to be insulin resistant.29,30
ELISA Analysis
Levels of IL-6, IL-8, and soluble TM in serum samples were measured using ELISA by applying a biotin-streptavidin-peroxidase detection system with the respective Abcam kits (IL-6, Cat # ab46042; IL-8, Cat # ab46032; and CD141, Cat # ab46508 Cambridge, MA, USA) according to the manufacturer’s instructions. Each sample was assayed in duplicate and values were expressed as the mean of 2 measures per sample.
Statistical Analysis
Univariate Analysis
Participants’ independent variables consisted of demographic variables, ethnic subgroups, educational level, BMI, waist circumference, smoking status, serum 25(OH)D status, and lipid profile. In Table 1 and Supplementary Table 1, continuous variables with normal distribution were presented as mean and standard deviation (SD); non-normal variables were reported as median and interquartile range (IQR). Categorical variables were reported as counts and percentages. For two-way analysis, variables were compared across groups using χ2 test for categorical data and Student’s t-test or Mann–Whitey U-test for continuous data depending on skewness of the data.
Table 1.
Characteristics of Participants Stratified by Insulin-Resistant and Insulin-Sensitive Status
| Characteristics | Insulin-Resistant n=2760 | Insulin-Sensitive n=1354 | P-value |
|---|---|---|---|
| Age (SD), years | 40 (12) | 38 (12) | <0.001 |
| Sex (%) | |||
| Male | 1848 (74) | 862 (70) | 0.025 |
| Female | 641 (26) | 357 (30) | |
| Race/Ethnicity | |||
| Arabs | 1052 (45) | 598 (53) | <0.001 |
| Asian | 1221 (53) | 503 (44) | |
| Othersa | 49 (2) | 35 (3) | |
| Education | |||
| Less than high school | 406 (16) | 188 (15) | 0.015 |
| High school diploma | 1117 (45) | 588 (48) | |
| University bachelor’s degree | 748 (30) | 372 (31) | |
| Postgraduate degree | 201 (8) | 66 (5) | |
| BMI Categories | |||
| Underweight | 18 (1) | 40 (4) | <0.001 |
| Normal | 562 (25) | 525 (48) | |
| Overweight | 1020 (45) | 388 (35) | |
| Obese | 683 (30) | 149 (13) | |
| Waist Circumference | |||
| Abdominally obese | 1012 (41) | 292 (24) | <0.001 |
| Non-abdominally obese | 1447 (59) | 914 (76) | |
| Average level of physical activity per day | 1.01 ± 2.0 | 1.15 ± 2.1 | 0.067 |
| Smoking Status | |||
| Current smoker | 463 (19) | 222 (18) | 0.927 |
| Former smoker | 106 (4) | 51 (4) | |
| Never smoker | 1874 (77) | 929 (77) | |
| Serum 25(OH)D, median (IQR), ng/mL | 28.50 (18.5) | 31.20 (21.4) | <0.001 |
| Serum D [25(OH)D], Status (%) | |||
| High concentration (>50 ng/mL) | 283 (11) | 178 (16) | <0.001 |
| Sufficiency (20–50 ng/mL) | 1751 (67) | 751 (66) | |
| Deficiency (<20 ng/mL) | 588 (22) | 208 (18) | |
| Lipid Profile | |||
| HDL (<1.55 mmol/L) | 2531 (92) | 970 (72) | <0.001 |
| LDL (>2.59 mmol/L) | 2242 (81) | 888 (66) | <0.001 |
| TG (>2.3 mmol/L) | 614 (22) | 94 (7) | <0.001 |
| Total cholesterol (>6.2 mmol/L) | 402 (15) | 114 (8) | <0.001 |
Notes: aOthers were defined as Westerners and Africans. Statistical significance: P ≤ 0.05.
Multivariate Analysis and Model Development
The first aim of the study was to identify the association between serum 25(OH)D level and risk of dyslipidemia using a logistic regression model, adjusted for age, sex, ethnicity, educational level, BMI, and smoking status. These cofounding variables were selected for logistic regression model according to their clinical31 and statistical significance.32 To avoid any strong correlation among the variables in the model, they were evaluated for multicollinearity by measuring the variance inflation factors and magnitude of standard errors.
Comparison of Serum Levels of IL-6, IL-8, and Soluble TM in 25(OH)D- Deficient Compared to 25(OH)D-Sufficient Insulin-Resistant Individuals
The second aim of this study was to compare the median IL-6, IL-8, and soluble TM serum levels between 25(OH)D-deficient and 25(OH)D-sufficient insulin-resistant individuals. Mann–Whitey U-test was used for the two-way analysis between these variables.
All analysis was two-sided, with a P-value of <0.05 considered statistically significant. The analysis was performed using SPSS Version 26 (IBM Corporation, Chicago, USA) and Graphpad Prism 7 (GraphPad Software Inc., San Diego, USA).
Results
Table 1 demonstrates the characteristics of the participants from the UAEDIAB study (n=4114) categorized by insulin resistance status. Insulin-resistant individuals were more obese (30% vs. 13%, P<0.001) and had higher fasting serum lipid-profiles (HDL-C dyslipidemia [92% vs. 72%, P<0.001]; LDL-C dyslipidemia [81% vs. 66%, P<0.001]; TG dyslipidemia [22% vs. 7%, P<0.001]; TC dyslipidemia [15% vs. 8%, P<0.001]) compared with insulin-sensitive individuals. The proportion of 25(OH)D deficiency was higher for insulin-resistant individuals as well (22% vs. 18%, P<0.001). Participants' characteristics according to serum 25(OH)D status categories are shown in Supplementary Table 1.
Association of 25(OH)D Deficiency with HDL-C Dyslipidemia in Insulin-Resistant Individuals
Results from the multiple logistic regression models adjusted for age, sex, ethnicity, educational level, BMI, and smoking status are presented in Table 2. Insulin-resistant individuals with deficient 25(OH)D had a significantly increased risk of HDL-C dyslipidemia compared with the reference group (OR 2.09 [95% CI, 1.18-3.39]; P=0.009), but there was no significant association between other dyslipidemias and 25(OH)D deficiency in insulin-resistant subjects.
Table 2.
Odds Ratios (ORs) for the Association Between Serum 25(OH)D Levels and Insulin-Resistance Status Stratified by Lipid-Profile
| Lipid Profile | 25(OH)D Statusa | Insulin-Sensitive | Insulin-Resistant | ||
|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | ||
| HDL (<1.55 mmol/L) | Sufficient | 1 | 1 | ||
| Deficient | 1.00 (0.57–1.74) | 0.998 | 2.09 (1.18–3.39) | 0.009 | |
| LDL (>2.59 mmol/L) | Sufficient | 1 | 1 | ||
| Deficient | 1.27 (0.75–2.13) | 0.360 | 1.09 (0.79–1.52) | 0.935 | |
| TG (>2.3 mmol/L) | Sufficient | 1 | 1 | ||
| Deficient | 1.53 (0.74–3.15) | 0.242 | 1.24 (0.94–1.64) | 0.130 | |
| Total cholesterol (>6.2 mmol/L) | Sufficient | 1 | 1 | ||
| Deficient | 0.93 (0.46–1.87) | 0.844 | 1.35 (0.99–1.84) | 0.055 | |
Notes: aAdjusted for age, sex, ethnicity, educational level, BMI, and smoking status. Statistical significance: P ≤ 0.05.
Serum 25(OH)D-Deficient Insulin-Resistant Individuals Had Higher Serum IL-6, IL-8, and Soluble TM Levels
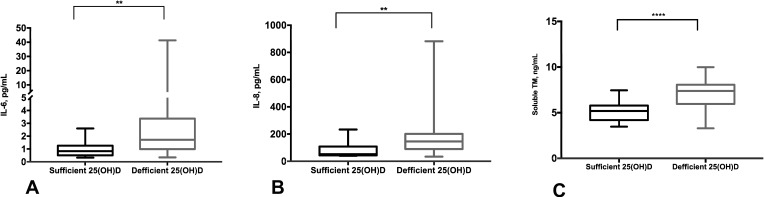
We found that insulin-resistant individuals who had 25(OH)D deficiency had higher serum IL-6, IL-8, and soluble TM levels compared to those with 25(OH)D sufficiency and normal-lipid profiles (median, IL-6 pg/mL 0.82 vs 1.71, P=0.001; median, IL-8 pg/mL 51.31 vs 145.6, P=0.003; and median, solubleTM ng/mL 5.19 vs 7.38, P<0.0001; in serum 25(OH)D-sufficient vs serum 25(OH)D-deficient groups, respectively) (Figure 2A–C). Also, there was an inverse correlation between serum 25(OH)D and solubleTM levels in insulin-resistant individuals (r=−0.342, P=0.010) (Supplementary Figure 1).
Figure 2.
Elevated serum of IL-6, IL-8, and soluble TM levels in 25(OH)D-deficient insulin-resistant individuals. Representative data showing an increase in the serum levels of IL-6 (A), IL-8 (B), and soluble TM (C) in 25(OH)D-deficient compared to 25(OH)D-sufficient insulin-resistant individuals. The cytokine levels were estimated using human ELISA assays. **P<0.01, ****P<0.0001.
Discussion
In this study, by recruiting 4114 individuals with different ethnicities living across UAE, we were able to determine that low serum levels of HDL-C is the characteristic dyslipidemia seen in 25(OH) D-deficient insulin-resistant subjects. Moreover, we found increased levels of circulating pro-inflammatory markers such as IL-6 and IL-8, and endothelial damage marker,33 thrombomodulin, in 25(OH)D-deficient insulin-resistant individuals (Figure 1). Since numerous studies have identified HDL-C dyslipidemia as an independent predictor of CV events34–37, our results imply that links between serum level of 25(OH)D lower than 20 ng/mL and CV events might be through HDL-C and serum levels of IL-6, IL-8, and soluble TM in insulin-resistant individuals who have proven to be at significant increased CV disease risk.38
Although it is a general agreement that high serum 25(OH)D levels are associated with a favorable serum lipid profile; it is highly controversial which lipids may be affected by increased serum 25(OH)D levels. The association between deficient 25(OH)D and HDL-C dyslipidemia found in this study is consistent with prior studies by Faridi et al10 and Lupton et al.11 Ponda et al13 that aimed to determine the association between serum 25(OH)D and lipid profile reported that raising serum 25(OH)D levels from 20 to 30 ng/mL in 6260 subjects was associated with a mean increase in TC and HDL-C, but, nonsignificant changes in LDL-C and TG. By taking the insulin variable in consideration, and adjusting for demographic, lifestyle, and use of lipid-lowering medications, our study was able to show that low serum 25(OH)D levels is associated with HDL-C dyslipidemia in insulin-resistant individuals.
Notably, in this study, we have seen that in our multi-ethnic cohort, insulin-resistant state was associated with unfavorable lipid-profiles, particularly increased LDL-C, TC, and TG and decreased HDL-C as compared with insulin-sensitive state. Previous studies have mainly linked TG and HDL-C with insulin-resistant state, or used the TG-HDL-C ratio as a surrogate of insulin resistance;29,30 However, the relation between the TG-HDL-C ratio with insulin-resistant state might differ by ethnicity as there is a report that the TG-HDL-C ratio are not a marker of insulin resistance in black African-Americans population.39
Association of serum 25(OH)D deficiency with circulating pro-inflammatory cytokines such as IL-640,41 and IL-842 is in line with numerous reports43 but our study is novel in linking low serum 25(OH)D to higher circulatory IL-6 and IL-8 in insulin-resistant individuals, and to elevated serum TM levels, in particular. Thrombomodulin, is a glycoprotein that is found both membrane-bound in the vascular endothelium and in a soluble form in the plasma. The membrane-bound TM is dissociated from the endothelium during disorders involving vascular damage, such as various infections, sepsis, and inflammation, presumably cleaved from endothelial cells by neutrophil-delivered enzymes, and becomes soluble in systemic circulation.44 Taken together, soluble TM is considered as a marker of endothelial damage and has been reported as such in various studies.33,45
Moreover, the role of TM in inflammation has also been reported and thrombomodulin expression in monocytes triggers lipopolysaccharide-induced inflammatory response,46 resulting in higher circulatory IL-6. Another report showed that the vascular endothelial expression of vitamin D receptor and 1-alpha hydroxylase, the 25(OH)D to 1,25-dihydroxyvitamin D converting enzyme, was lower in 25(OH)D-deficient versus -sufficient middle-aged adults, suggesting that endothelial damage can mediate nuclear-κB signaling, resulting in increased synthesis of pro-inflammatory cytokine IL-6 in vitamin D-deficient versus sufficient subject.47 Furthermore, another study also related the anti-inflammatory effect of vitamin D to downregulating NF-κB signaling.48 In our study, compared with sufficient 25(OH)D individuals, deficient 25(OH)D had higher serum levels of downstream nuclear-κB related inflammatory markers such as IL-6 and IL-8. Although soluble TM is not included in the subsets of NF-κB target genes, its endothelial expression has been reported to be mediated through TNF-alpha signaling that is part of NF-κB pathway.49 However, the exact molecular mechanisms underlying the protective effect of vitamin D on reducing inflammation-induced vascular endothelial damage and its relation to serum levels of soluble TM is not well elucidated and requires further research.
Moreover, in this study insulin-resistant individuals had lower median serum 25(OH)D levels as compared to insulin-sensitive individuals. Low 25(OH)D serum levels in these individuals were also associated with HDL-C dyslipidemia, increased serum levels of inflammatory markers, including IL-6 and IL-8, and enhanced levels of soluble TM, as a marker of endothelial damage. Given that insulin resistance is proven to be at risk to develop CV disease,38 vitamin D supplementation could reduce endothelial dysfunction leading to CVD in insulin-resistant individuals who have proven to be at significant increased CVD risk.38
There are a few limitations in our study that must be acknowledged. Firstly, although our study features a large, nationally representative sample that improves the generalizability of our results, a limitation is its cross-sectional nature. Information and blood samples were taken from study participants at the same point in time and longitudinal follow-up data is not available. Secondly, we were unable to assess the longitudinal effects of changes in serum 25(OH)D and its impact on serum lipid profiles. However, in line with our result that HDL-C dyslipidemia is associated with serum 25(OH)D deficiency, a cohort study with longitudinal follow-up design has shown that raising serum 25(OH)D levels from <20 ng/mL to >30 ng/mL was associated with a mean increase in HDL-C levels but nonsignificant changes in serum LDL-C and TGs levels.13 Thirdly, in this study, the inverse relationship between elevated serum TM levels, marker of inflammation-induced endothelial damage,33 and low serum 25(OH)D (<20 ng/mL) in insulin-resistant individuals was based on small sample sizes. Hence, we recommend future studies to evaluate its validity and applicability in a larger cohort.
In conclusion, our study has found that serum levels of 25(OH)D lower than 20 ng/mL is associated with HDL-C dyslipidemia in insulin-resistant individuals with increased serum levels of inflammatory markers, including IL-6 and IL-8, and enhanced levels of soluble TM, as a marker of endothelial damage.
Acknowledgments
In this study we kindly acknowledge Dr. Hisham Siddeq from Rashid Centre for Diabetes and Research who was responsible for conducting all the blood tests and quality control. This study was under the auspices of the University of Sharjah and was partially supported by the Ministry of Health, Sanofi and Baker IDI Heart and Diabetes Institute (Melbourne, Australia). Sanofi had no role in study design, data collection, data analysis, or writing of the manuscript. We are grateful to the Diabetes Steering Committee for facilitating and contributing to the original study, to our research staff who facilitated the original study and we are grateful to all the participants for their time and efforts.
Funding Statement
N.S. is funded by Al-Jalila Foundation (Grant Code: AJF150326), Diabetes and Metabolic Syndrome Research Group (University of Sharjah code 150306), and National Diabetes and Lifestyle Survey (MOH Code 120301).
Abbreviations
25(OH)D, 25-hydroxyvitamin D; CVD, cardiovascular diseases; LDL-C, low density lipoprotein-cholesterol; TG, triglycerides; TC, total cholesterol; HDL-C, high-density lipoprotein-cholesterol; TM, thrombomodulin; UAEDIAB, United Arab Emirates National Diabetes and Lifestyle.
Data Sharing Statement
The UAEDIAB data used for this study are accessible upon request from the corresponding author, Nabil Sulaiman.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–511. doi: 10.1161/CIRCULATIONAHA.107.706127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Resnick LM, MÜLler FB, Laragh JH. Calcium-regulating hormones in essential hypertension: relation to plasma renin activity and sodium metabolism. Ann Intern Med. 1986;105(5):649–654. doi: 10.7326/0003-4819-105-5-649 [DOI] [PubMed] [Google Scholar]
- 3.Zittermann A, Schleithoff SS, Tenderich G, Berthold HK, Körfer R, Stehle P. Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol. 2003;41(1):105–112. doi: 10.1016/S0735-1097(02)02624-4 [DOI] [PubMed] [Google Scholar]
- 4.Watson KE, Abrolat ML, Malone LL, et al. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997;96(6):1755–1760. doi: 10.1161/01.CIR.96.6.1755 [DOI] [PubMed] [Google Scholar]
- 5.Scragg R, Jackson R, Holdaway IM, Lim T, Beaglehole R. Myocardial infarction is inversely associated with plasma 25-hydroxyvitamin D3 levels: a community-based study. Int J Epidemiol. 1990;19(3):559–563. doi: 10.1093/ije/19.3.559 [DOI] [PubMed] [Google Scholar]
- 6.Poole KES, Loveridge N, Barker PJ, et al. Reduced vitamin D in acute stroke. Stroke. 2006;37(1):243–245. doi: 10.1161/01.STR.0000195184.24297.c1 [DOI] [PubMed] [Google Scholar]
- 7.Pilz S, März W, Wellnitz B, et al. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab. 2008;93(10):3927–3935. doi: 10.1210/jc.2008-0784 [DOI] [PubMed] [Google Scholar]
- 8.Lind L, Hänni A, Lithell H, Hvarfner A, Sörensen OH, Ljunghall S. Vitamin D is related to blood pressure and other cardiovascular risk factors in middle-aged men. Am J Hypertens. 1995;8(9):894–901. doi: 10.1016/0895-7061(95)00154-H [DOI] [PubMed] [Google Scholar]
- 9.Imga NN, Karci AC, Oztas D, Berker D, Guler S. Effects of vitamin D supplementation on insulin resistance and dyslipidemia in overweight and obese premenopausal women. Arch Med Sci. 2019;15(3):598. doi: 10.5114/aoms.2018.75864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faridi KF, Zhao D, Martin SS, et al. Serum vitamin D and change in lipid levels over 5 y: the atherosclerosis risk in communities study. Nutrition. 2017;38:85–93. doi: 10.1016/j.nut.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lupton JR, Faridi KF, Martin SS, et al. Deficient serum 25-hydroxyvitamin D is associated with an atherogenic lipid profile: the Very Large Database of Lipids (VLDL-3) study. J Clin Lipidol. 2016;10(1):72–81. doi: 10.1016/j.jacl.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jorde R, Figenschau Y, Hutchinson M, Emaus N, Grimnes G. High serum 25-hydroxyvitamin D concentrations are associated with a favorable serum lipid profile. Eur J Clin Nutr. 2010;64(12):1457. doi: 10.1038/ejcn.2010.176 [DOI] [PubMed] [Google Scholar]
- 13.Ponda MP, Huang X, Odeh MA, Breslow JL, Kaufman HW. Vitamin D may not improve lipid levels: a serial clinical laboratory data study. Circulation. 2012;126(3):270–277. doi: 10.1161/CIRCULATIONAHA.111.077875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Xia N, Yang Y, Peng D-Q. Influence of vitamin D supplementation on plasma lipid profiles: a meta-analysis of randomized controlled trials. Lipids Health Dis. 2012;11(1):42. doi: 10.1186/1476-511X-11-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jorde R, Grimnes G. Vitamin D and Lipids: Do We Really Need More Studies? Am Heart Assoc; 2012. [Google Scholar]
- 16.Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med. 2008;14(3–4):222–231. doi: 10.2119/2007-00119.Tilg [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-α, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem. 2003;278(46):45777–45784. doi: 10.1074/jbc.M301977200 [DOI] [PubMed] [Google Scholar]
- 18.Roifman I, Beck PL, Anderson TJ, Eisenberg MJ, Genest J. Chronic inflammatory diseases and cardiovascular risk: a systematic review. Can J Cardiol. 2011;27(2):174–182. doi: 10.1016/j.cjca.2010.12.040 [DOI] [PubMed] [Google Scholar]
- 19.Aso Y, Fujiwara Y, Tayama K, Takanashi K, Inukai T, Takemura Y. Relationship between plasma soluble thrombomodulin levels and insulin resistance syndrome in type 2 diabetes: a comparison with von Willebrand factor. Exp Clin Endocrinol Diabetes. 2001;109(04):210–216. doi: 10.1055/s-2001-15108 [DOI] [PubMed] [Google Scholar]
- 20.Després J-P, Lamarche B, Mauriège P, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med. 1996;334(15):952–958. doi: 10.1056/NEJM199604113341504 [DOI] [PubMed] [Google Scholar]
- 21.Sulaiman N, Albadawi S, Abusnana S, et al. High prevalence of diabetes among migrants in the United Arab Emirates using a cross-sectional survey. Sci Rep. 2018;8(1):1–8. doi: 10.1038/s41598-018-24312-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamoudi R, Sharif-Askari NS, Sharif-Askari FS, et al. Prediabetes and diabetes prevalence and risk factors comparison between ethnic groups in the United Arab Emirates. Sci Rep. 2019;9(1):1–7. doi: 10.1038/s41598-019-53505-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ersfeld DL, Rao DS, Body -J-J, et al. Analytical and clinical validation of the 25 OH vitamin D assay for the LIAISON® automated analyzer. Clin Biochem. 2004;37(10):867–874. doi: 10.1016/j.clinbiochem.2004.06.006 [DOI] [PubMed] [Google Scholar]
- 24.Rusińska A, Płudowski P, Walczak M, et al. Vitamin D supplementation guidelines for general population and groups at risk of vitamin D deficiency in Poland—recommendations of the polish society of pediatric endocrinology and diabetes and the expert panel with participation of national specialist consultants and representatives of scientific societies—2018 update. Front Endocrinol (Lausanne). 2018;9:246. doi: 10.3389/fendo.2018.00420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al Sayed N, Al Waili K, Alawadi F, et al. Consensus clinical recommendations for the management of plasma lipid disorders in the Middle East. Int J Cardiol. 2016;225:268–283. doi: 10.1016/j.ijcard.2016.09.081 [DOI] [PubMed] [Google Scholar]
- 26.Reiner Ž, Catapano AL, De Backer G, et al. ESC/EAS guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32(14):1769–1818. doi: 10.1093/eurheartj/ehr158 [DOI] [PubMed] [Google Scholar]
- 27.Hamer M, O’Donovan G, Stamatakis E. High-density lipoprotein cholesterol and mortality: too much of a good thing? Arterioscler Thromb Vasc Biol. 2018;38(3):669–672. doi: 10.1161/ATVBAHA.117.310587 [DOI] [PubMed] [Google Scholar]
- 28.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 29.Esteghamati A, Ashraf H, Khalilzadeh O, et al. Optimal cut-off of homeostasis model assessment of insulin resistance (HOMA-IR) for the diagnosis of metabolic syndrome: third national surveillance of risk factors of non-communicable diseases in Iran (SuRFNCD-2007). Nutr Metab. 2010;7(1):26. doi: 10.1186/1743-7075-7-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gayoso-Diz P, Otero-González A, Rodriguez-Alvarez MX, et al. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: effect of gender and age: EPIRCE cross-sectional study. BMC Endocr Disord. 2013;13(1):47. doi: 10.1186/1472-6823-13-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kabadi SM, Lee BK, Liu L. Joint effects of obesity and vitamin D insufficiency on insulin resistance and type 2 diabetes: results from the NHANES 2001–2006. Diabetes Care. 2012;35(10):2048–2054. doi: 10.2337/dc12-0235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steyerberg EW, Eijkemans MJC, Harrell FE Jr, Habbema JDF. Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med. 2000;19(8):1059–1079. doi: [DOI] [PubMed] [Google Scholar]
- 33.Boffa MC, Karochkine M, Berard M. Plasma thrombomodulin as a marker of endothelium damage. Nouv Rev Fr Hematol. 1991;33(6):529–530. [PubMed] [Google Scholar]
- 34.Barter P, Gotto AM, LaRosa JC, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357(13):1301–1310. doi: 10.1056/NEJMoa064278 [DOI] [PubMed] [Google Scholar]
- 35.Assmann G, Schulte H, von Eckardstein A, Huang Y. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis. 1996;124:S11–S20. doi: 10.1016/0021-9150(96)05852-2 [DOI] [PubMed] [Google Scholar]
- 36.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease: the Framingham Study. Am J Med. 1977;62(5):707–714. doi: 10.1016/0002-9343(77)90874-9 [DOI] [PubMed] [Google Scholar]
- 37.Curb JD, Abbott RD, Rodriguez BL, et al. A prospective study of HDL-C and cholesteryl ester transfer protein gene mutations and the risk of coronary heart disease in the elderly. J Lipid Res. 2004;45(5):948–953. doi: 10.1194/jlr.M300520-JLR200 [DOI] [PubMed] [Google Scholar]
- 38.Lakka H-M, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288(21):2709–2716. doi: 10.1001/jama.288.21.2709 [DOI] [PubMed] [Google Scholar]
- 39.Sumner AE, Finley KB, Genovese DJ, Criqui MH, Boston RC. Fasting triglyceride and the triglyceride–HDL cholesterol ratio are not markers of insulin resistance in African Americans. Arch Intern Med. 2005;165(12):1395–1400. doi: 10.1001/archinte.165.12.1395 [DOI] [PubMed] [Google Scholar]
- 40.Blondon M, Cushman M, Jenny N, et al. Associations of serum 25-hydroxyvitamin D with hemostatic and inflammatory biomarkers in the multi-ethnic study of atherosclerosis. J Clin Endocrinol Metab. 2016;101(6):2348–2357. doi: 10.1210/jc.2016-1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Q, Zhu Z, Liu Y, Tu X, He J. Relationship between serum vitamin D levels and inflammatory markers in acute stroke patients. Brain Behav. 2018;8(2):e00885. doi: 10.1002/brb3.885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dauletbaev N, Herscovitch K, Das M, et al. Down‐regulation of IL‐8 by high‐dose vitamin D is specific to hyperinflammatory macrophages and involves mechanisms beyond up‐regulation of DUSP1. Br J Pharmacol. 2015;172(19):4757–4771. doi: 10.1111/bph.13249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adegoke SA, Smith OS, Adekile AD, Figueiredo MS. Relationship between serum 25-hydroxyvitamin D and inflammatory cytokines in paediatric sickle cell disease. Cytokine. 2017;96:87–93. doi: 10.1016/j.cyto.2017.03.010 [DOI] [PubMed] [Google Scholar]
- 44.Esmon CT. The roles of protein C and thrombomodulin in the regulation of blood coagulation. J Biol Chem. 1989;264(9):4743–4746. [PubMed] [Google Scholar]
- 45.Remková A, Kováčová E, Príkazská M, Kratochvíl’ová H. Thrombomodulin as a marker of endothelium damage in some clinical conditions. Eur J Intern Med. 2000;11(2):79–84. doi: 10.1016/S0953-6205(00)00066-2 [DOI] [PubMed] [Google Scholar]
- 46.Wang M, Gao P, Wu X, et al. Impaired anti-inflammatory action of glucocorticoid in neutrophil from patients with steroid-resistant asthma. Respir Res. 2016;17(1):153. doi: 10.1186/s12931-016-0462-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. 2011;57(1):63–69. doi: 10.1161/HYPERTENSIONAHA.110.160929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Janjetovic Z, Zmijewski MA, Tuckey RC, et al. 20-hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-κB activity by increasing IκBα levels in human keratinocytes. PLoS One. 2009;4(6):e5988. doi: 10.1371/journal.pone.0005988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sohn RH, Deming CB, Johns DC, et al. Regulation of endothelial thrombomodulin expression by inflammatory cytokines is mediated by activation of nuclear factor-kappa B. Blood. 2005;105(10):3910–3917. doi: 10.1182/blood-2004-03-0928 [DOI] [PubMed] [Google Scholar]