Abstract
Aim
The primary objective of this review is to develop practice-based expert group opinions on the cardiovascular (CV) safety and utility of modern sulfonylureas (SUs) in cardiovascular outcome trials (CVOTs).
Background
The United States Food and Drug Administration issued new guidance to the pharmaceutical industry in 2008 regarding the development of new antihyperglycemic drugs. The guidance expanded the scope for the approval of novel antihyperglycemic drugs by mandating CVOTs for safety. A few long-term CVOTs on dipeptidyl peptidase 4 inhibitors, glucagon-like peptide 1 receptor agonists, and sodium-glucose cotransporter 2 inhibitors have been completed, while others are ongoing. SUs, which constitute one of the key antihyperglycemic agents used for the management of type 2 diabetes mellitus (T2DM), have been used as comparator agents in several CVOTs. However, the need for CVOTs on modern SUs remains debatable. In this context, a multinational group of endocrinologists convened for a meeting and discussed the need for CVOTs of modern SUs to evaluate their utility in the management of patients with T2DM. At the meeting, CVOTs of modern SUs conducted to date and the hypotheses derived from the results of these trials were discussed.
Review results
The expert group analyzed the key trials emphasizing the CV safety of modern SUs and also reviewed the results of various CVOTs in which modern SUs were used as comparators. Based on literature evidence and individual clinical insights, the expert group opined that modern SUs are cardiosafe and that since they have been used as comparators in other CVOTs, CVOTs of SUs are not required.
Conclusion
Modern SUs can be considered a cardiosafe option for the management of patients with diabetes mellitus and CV disease; thus CVOTs among individuals with T2DM are not required.
Keywords: Cardiosafe, Modern sulfonylureas, Cardiovascular outcome trials
1. Introduction
Cardiovascular diseases (CVDs) are the preeminent cause of mortality in persons with type 2 diabetes mellitus (T2DM). Therefore, evaluating the effect of antidiabetic therapy on cardiovascular (CV) outcomes and glycemic control is very important in the management of T2DM. At the same time, it is important that antidiabetic medications themselves do not increase the CV risk in persons with T2DM.1
Several major clinical trials of intensive vs. less stringent glycemic control failed to demonstrate that intensive glucose lowering significantly reduces the CV risk. The long-term noninterventional follow-up of the Diabetes Control and Complications Trial (DCCT), conducted among individuals with type 1 diabetes, and the UK Prospective Diabetes Study (UKPDS) and Veterans Affairs Diabetes Trial, conducted among individuals with type 2 diabetes, were suggestive of a “legacy” macrovascular benefit associated with intensive glycemic control, although not as apparent as the effect on microvascular complications2. In the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation(ADVANCE)trial, intensive glucose control was associated with a 10% relative reduction in the combined outcomes of major macrovascular and microvascular events, primarily as a consequence of 21% relative reduction in nephropathy. 3.In the ADVANCE-ON study, which was a follow-up of the ADVANCE trial, 8494 patients with T2DM were followed up for a period of 5.4 years for glucose control comparison and 5.9 years for blood pressure–lowering comparison. There was no evidence of long-term benefits with respect to death and macrovascular events with intensive glucose control. 4.
In 2008, the Food and Drug Administration (FDA) issued guidance to the pharmaceutical industry to specifically focus on the CV safety of novel antihyperglycemic agents5,6.
The concern regarding adverse CV outcomes in trials related to antihyperglycemic drugs arose following the 2005 muraglitazar and 2007 rosiglitazone saga 5,6.
Sulfonylureas (SUs) continue to play a key role in the treatment armamentarium for T2DM, despite the introduction of several novel antihyperglycemic agents. In the University Group Diabetes Program (UGDP) era, concerns were raised about the CV safety of SUs. Nevertheless, the evolving evidence base suggests that early CV concerns with the SUs in the UGDP era could be attributed to deleterious effects of first- and second-generation SUs on ischemic preconditioning. Modern SUs do not inhibit the mitochondrial adenosine triphosphate–sensitive potassium (KATP) channel opening in cardiac myocytes and, thereby, preserve myocardial ischemic preconditioning. 7. Evidence suggests that these agents are associated with a reduced risk of CV mortality and exhibit a low risk of myocardial infarction (MI) and hospitalization, associated with acute coronary syndrome (ACS) 8 9,10.
Glimepiride has been used as a comparator agent in several cardiovascular outcome trials (CVOTs). Analyses of glimepiride CVOTs to date have proved the CV safety of modern SUs. A subject that remains debatable is whether modern SUs, which are cardiosafe and used as comparators in CVOTs, themselves require CVOTs to evaluate their utility in T2DM management.
In this regard, at an international meeting held in India, experts reviewed available literature evidence on the CV safety of modern SUs and also discussed glimepiride CVOTs conducted to date.
2. Methodology
During a two-day international meeting held at Delhi, India, experts reviewed available literature evidence and discussed the importance of CVOTs and their benefits—in terms of clinical guidance for the use of antihyperglycemic agents in the management of T2DM. At the meeting, evidence suggestive of a decreased CV risk and mortality associated with second- and third-generation SUs was analyzed. Experts discussed CVOTs such as Thiazolidinediones or SUs Cardiovascular Accidents Intervention Trial (TOSCA.IT), Cardiovascular and Renal Microvascular Outcome Study with Linagliptin (CARMELINA), and Cardiovascular Outcome Study of Linagliptin Versus Glimepiride in Patients with Type 2 Diabetes (CAROLINA) that had established the CV safety of modern SUs. The key discussion points of the experts covering the cardiosafety of modern SUs and whether CVOTs are required for use of modern SUs in the management of T2DM are summarized under “Panel recommendations.”
3. Results
3.1. Difference between CVOTs and conventional glycemic efficacy trials
Conventional glycemic efficacy trials differ from CVOTs with regard to objectives, the number of patients involved, duration of the trial, comparator drugs, and inclusion and exclusion criteria (Table 1). Conventional glycemic efficacy trials include subjects with diabetes on the basis of certain inclusion criteria, such as glycosylated hemoglobin (HbA1c) levels, baseline drugs, age, and body mass index. Subjects are then randomized to an investigational drug or a comparator/placebo. Trial outcomes are assessed periodically, and subjects are followed for a specific timeframe 11.
Table 1.
Differences between conventional glycemic trial and CVOTs 11.
| Conventional glycemic efficacy trials | CVOTs | |
|---|---|---|
| Objective | Efficacy of drug compared with a placebo or comparator | Compare CV outcomes |
| Number of patients | 300–600 (based on sample size calculation) | In thousands |
| Duration | 26–104 weeks | Many years or event driven |
| Background glycemic therapies | Limited to rescue therapies and dose changes for hypoglycemia | More flexibility for investigator |
| Comparator | Placebo or active comparator | Usually placebo |
| Inclusion/exclusion | Mainly low-risk patients or minimal CV risk | Patients with high CV risk factors, known atherosclerotic vascular disease, recent CV event |
CVOT: cardiovascular outcome trial; CV:cardiovascular.
On the contrary, CVOTs are trials designed to find out how a drug performs in comparison with standard care, in terms of predefined CV end points. In these studies, subjects with diabetes and high CV event rates are randomized to an investigational drug or a comparator to accrue the required number of CV events in the limited period of the trial. The outcome assessed includes a composite of major adverse CV events (MACE), such as nonfatal MI, nonfatal stroke, CV death, hospitalization for angina, hospitalization for heart failure (HF), urgent revascularization for unstable angina, and death from any cause 11.
The first drug to be approved following the introduction of the new FDA guidance was bromocriptine. This led to other drugs being evaluated in a similar manner. Bromocriptine mesylate was approved by the United States FDA (US FDA) in 2009 12.
3.1.1. Advantages of CVOTs
Cardiovascular safety outcome trials offer certain advantages that are clinically relevant:
-
•
The hard CV end points in subjects at high risk of CV events are prespecified and universally decided. Hence, there is uniformity of reporting and event capture across multiple sites across geographical areas. Before the 2008 FDA guidance, CV end points were recorded as prespecified serious adverse events that were not universally decided. As a result, the pooling of data from multiple studies might not be the same as in CVOTs 11.
-
•
A few adverse effects of investigational drug may emerge only after significant patient-years of exposure. As a result of large patient numbers in CVOTs, it is possible to identify such late-emerging adverse events 11.
CVOTs can help to recognize specific off-target actions that may be different from actions specific to the class of molecules 11.
3.2. Glycemic equipoise and CVOTs
Equipoise is defined as a “state of equilibrium.” According to the glycemic equipoise hypothesis, in CVOTs of any antidiabetic drug, the two opposing arms should maintain and achieve similar glycemic levels during and at the end of the trial. Glycemic equipoise aims to assess whether the drug can achieve CV safety or benefit, independent of its glucose-lowering efficacy. However, modern CVOTs, which are designed based as per US FDA guidance, do not consider glycemic equipoise as an important CVOT outcome. Modern CVOTs do not aim at demonstrating glucose-lowering efficacy 13.
3.2.1. Cardiac safety of SUs
3.2.1.1. Reassurance from landmark trials on cardiac safety of SUs
The publication of the UGDP trial in 1970 raised concerns for the first time on the safety of SUs. The trial reported that tolbutamide, a first-generation SU, might be associated with an increased risk of CV death. Subsequently, it was proposed that first- and second-generation SUs have less selective binding affinity for SU receptors on cardiac myocytes, thereby contributing to adverse effects on cardiac tissue 14,15.
The DCCT was the first trial that established an association between the intensity of glucose control and the development of early vascular complications. This multicenter study included 1441 subjects with type 1 diabetes and demonstrated that a glycated hemoglobin difference of approximately 2% maintained over a median of 6.5 years was effective in reducing the risk of microvascular complications by over 50% [Nathan DM,1993]14. The UKPDS, published in 1998, further confirmed the benefits of intensified glycemic control with the use of SUs (mostly glibenclamide and chlorpropamide) in relation to similar microvascular outcomes as in the DCCT but among subjects with newly diagnosed T2DM 14,16,17. Analysis of a small substudy of the UKPDS demonstrated that the addition of metformin (MET) to a SU was associated with an increased risk of all-cause mortality compared with SU monotherapy 14,16,17
3.2.1.2. Modern SUs and CV safety: clinical evidence
Clinical evidence suggests that modern SUs are associated with reduced risk of CV morbidity, lower risk of MI, and lower risk for hospitalization for ACS, compared with conventional SUs.
3.2.1.2.1. Modern SUs: associated with reduced risk of cardiovascular mortality
Simpson et al 8 conducted a network meta-analysis to assess the relative risk of mortality and adverse CV events associated with SUs. A meta-analysis of 18 studies including 167,327 patients showed that gliclazide and glimepiride were associated with a lower risk of all-cause and CV-related mortality compared with glibenclamide.
The clinical implications of SU and MET, as monotherapies and as combination therapy—in relation to CV mortality—was assessed by Ioacara et al. 18 The study reported a significant beneficial effect on all-cause mortality for SUs added to initial MET monotherapy and similarly when MET was added to SUs among patients with T2DM (n = 11,374). However, there was a significant increase in all-cause mortality for both SU replacing MET and MET replacing SU. The study concluded that in patients with T2DM who had failed monotherapy, initiation of combination therapy should be encouraged, compared with replacement of SU with MET or MET with SUs.
An observational study was conducted to evaluate the correlation between selectivity for beta-cells among various SUs and CV mortality among patients with T2DM . The study assessed three-year mortality in 696 patients with T2DM receiving insulin secretagogues and MET. Patients treated with combinations of SU and biguanides at enrollment had significantly higher mortality when compared with the rest of the sample (5.2 vs. 6.4% yearly; p < 0.05). Mortality was significantly higher among patients receiving repaglinide and gliclazide compared with patients receiving glimepiride. The study concluded that SUs with greater selectivity for beta-cells, such as glimepiride, are associated with lower mortality when used in combination with MET, compared with other SUs such as glibenclamide 19.
3.2.1.2.2. SUs have low risk for hospitalization for ACS and urgent revascularization procedure: clinical evidence
Zeller et al 10 conducted a study to evaluate the impact of SUs on in-hospital outcomes in patients with MI. The study assessed whether outcomes differed between patients with MI vs. patients with diabetes, who did not receive SUs. The incidence of in-hospital complications, specifically in-hospital death, was higher in the insulin group vs. the SU group. The study noted that the incidence of in-hospital complications was similar in patients treated with gliclazide or glimepiride. A subgroup analysis also confirmed that mortality was lower among patients on gliclazide or glimepiride. The study findings suggested that hospital mortality among patients admitted with acute MI and who received SUs before admission was lower than that among patients who did not receive such treatment.
The relationship between individual SUs and the risk of overall mortality was assessed in a study conducted among a large cohort of patients with type 2 diabetes with coronary artery disease CAD. A total of 1921 mortality events in the entire cohort (n = 11,141) and 322 in the subgroup with a history of documented CAD (n = 1505) was reported. The subanalysis on patients with documented CAD revealed a trend toward an increased overall mortality risk with glyburide vs. glimepiride (1.36 [0.96–1.91]) and glipizide vs. glimepiride (1.39 [0.99–1.96]) 20.
Although the overall mortality was not substantially influenced by the choice of SU, a subgroup analysis of patients with documented CAD showed a trend toward an increased overall mortality risk with glyburide vs. glimepiride and glipizide vs. glimepiride. Hence, glimepiride may be the preferred SU in those with underlying CAD 20.
3.2.1.2.3. Recommendations on use of SUs in individuals with diabetes mellitus and CV risk
Several international guidelines recommend the use of modern SUs in patients with T2DM with CV risk.
3.2.1.2.4. American Diabetes Association and International Diabetes federation recommendation on SUs for CV safety
The ADA21 and IDF22 consider modern SUs as CV safe and recommend the use of modern SUs in patients with T2DM with CV risk.
3.2.1.2.5. South Asia Consensus Recommendations
According to the consensus recommendations on SU and SU combinations for the management of T2DM 23:
-
•
There is insufficient evidence to suggest that modern SUs increase CV risk. Modern SUs are preferred over conventional SUs in patients with diabetes and CV disease.
-
•
Among SUs, short-acting drugs, especially those metabolized in the liver (glipizide), should be preferred in patients with moderate/severe renal impairment. For mild/moderate renal impairment, modern SUs may also be used but preferably at lower doses.
3.2.1.2.6. SUs and CVOTs
SUs have been used extensively to ensure glycemic control in most CVOTs. SUs have been used as comparators in major CVOTs. Key CVOTs in which SUs were used as comparators include Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes Mellitus (EMPA-REG), Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Event (DECLARE-TIMI), Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care (EXAMINE), and Trial Evaluating Cardiovascular Outcomes with Sitagliptin. Table 2 details the percentage of participants receiving SUs in these trials.
Table 2.
Number of patients on SU in CVOTs control and treatment groups.
| Name of the CVOT | Number of patients on SU in control group (% of patients) | Number of patients on SU in treatment group |
|---|---|---|
| LEADER [28] | 2363 (50.6) | 2370 (50.8) |
| ELIXA [29] | 1016 (33.5) | 988 (32.6) |
| HARMONY [30] | 1379 (29) | 1346 (28) |
| ORIGIN [31] | 1810 (28.9) | 1901 (30.3) |
| DECLARE-TIMI 58[25] | 3707 (43.2) | 3615 (42.1) |
| EMPA-REG [24] | 220 (39.1) | 440 (37.4) |
| TECOS [26] | 3299 (45.0) | 3346 (45.6) |
| EXAMINE [27] | 1237 (46.2) | 1266 (46.9) |
| CARMELINA33,34 | 1140 (32.7) | 1102 (31.5) |
LEADER: Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Trials; ELIXA: Evaluation of Lixisenatide in Acute Coronary Syndrome; HARMONY: Effect of Albiglutide, When Added to Standard Blood Glucose Lowering Therapies, on Major Cardiovascular Events in Subjects With Type 2 Diabetes Mellitus; ORIGIN: Outcome Reduction with Initial Glargine Intervention; DECLARE-TIMI 58: Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Event; ESRD: End-stage renal disease; EMPA-REG: Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus; TECOS: Trial Evaluating Cardiovascular Outcomes with Sitagliptin; EXAMINE: Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care; CARMELINA: Cardiovascular and Renal Microvascular Outcome Study With Linagliptin in Patients With Type 2 Diabetes Mellitus.
The EMPA-REG trial assessed the effects of empagliflozin, a sodium-glucose cotransporter 2 inhibitor, in addition, to standard care, on CV morbidity and mortality in patients with T2DM at high CV risk. The primary outcome occurred in 490 of 4687 patients (10.5%) in the pooled empagliflozin group and in 282 of 2333 patients (12.1%) in the placebo group (hazard ratio [HR] in the empagliflozin group, 0.86; 95.02% confidence interval [CI], 0.74 to 0.99; P = 0.04 for superiority).The EMPA-REG outcome trial concluded that patients with T2DM at high risk for CV events who received empagliflozin, as compared with the placebo, had a lower rate of the primary composite CV outcome and of death from any cause when the study drug was added to standard care 24.
The DECLARE-TIMI trial assessed CV outcomes among patients with T2DM at an increased risk for atherosclerotic CV disease and treated with dapagliflozin. In the study, 17,160 patients were randomized to receive either dapagliflozin or placebo. The study observed that dapagliflozin did not result in a lower rate of MACE (8.8% in the dapagliflozin group and 9.4% in the placebo group; HR: 0.93; 95% CI: 0.84 to 1.03; p = 0.17) but did result in a lower rate of CV death or hospitalization for HF. The study concluded that in patients with T2DM who had or were at risk for atherosclerotic CV disease, treatment with dapagliflozin did not result in a higher or lower rate of MACE vs. placebo but resulted in a lower rate of CV death or hospitalization for HF. This finding reflects a lower rate of hospitalization for HF 25.
The Trial Evaluating Cardiovascular Outcomes with Sitagliptin study assessed the long-term effect of adding sitagliptin, a DPP4i, to usual care in patients with T2DM with CV disease. Sitagliptin was noninferior to placebo for the primary composite CV outcome (HR: 0.98; 95% CI: 0.88 to 1.09; p < 0.001). The study concluded that, among patients with T2DM with established CV disease, adding sitagliptin to usual care does not increase the risk of major adverse CV events, hospitalization for HF, or other adverse events 26.
In the EXAMINE study, the CV safety of alogliptin was assessed among patients with T2DM with high CV risk. Patients with T2DM and an ACS event in the previous 15–90 days were randomly assigned to alogliptin or placebo plus standard treatment for diabetes and CV disease prevention. In the study, 5380 patients were assigned to alogliptin (n = 2701) or placebo (n = 2679) and followed up for a median of 533 days. The exploratory extended MACE end point was seen in 433 (16·0%) patients assigned to alogliptin and in 441 patients (16·5%) assigned to placebo (HR: 0·98, 95% CI: 0.86–1.12). Hospital admission for HF was the first event in the 85 (3·1%) patients taking alogliptin vs. the 79 (2·9%) taking placebo (HR: 1·07, 95% CI: 0.79–1.46). The study concluded that in patients with T2DM with ACS, alogliptin does not increase the risk of HF 27.
3.2.1.2.7. Glimepiride CVOTs conducted to date
A few important CVOTs in which glimepiride was used as a comparator include: TOSCA.IT, CARMELINA, and CAROLINA.
3.2.1.2.8. Thiazolidinediones or SUs Cardiovascular Accidents Intervention Trial
TOSCA.IT was a multicenter, randomized, pragmatic clinical trial. In this trial, patients aged 50–75 years with type 2 diabetes (n = 4956) who were inadequately controlled with MET monotherapy (2–3 g per day) were randomly assigned to add-on pioglitazone (15–45 mg) or an SU (5–15 mg of glibenclamide, 2–6 mg of glimepiride, or 30–120 mg of gliclazide, in accordance with local practice) 32.
The primary outcome was a composite of the first occurrence of all-cause death, nonfatal MI (including silent MI), nonfatal stroke, or urgent coronary revascularization. The key secondary outcome was a composite of ischemic CV disease that included first occurrence of sudden death, fatal and nonfatal MI (including silent MI), fatal and nonfatal stroke, leg amputation above the ankle, and any revascularization of the coronary, leg, or carotid arteries 32.
In the MET plus SUs group, 24 (2%) patients were given glibenclamide, 723 (48%) glimepiride, and 745 (50%) gliclazide. Based on a futility analysis, the study was stopped when the median follow-up was 57.3 months. There were no significant between-group differences in the composite primary outcome (HR: 0.96, 95% CI: 0.74–1.26, p = 0.79) or in its components. The proportion of patients who had the expanded CV outcomes was also similar in the two study groups (HR: 1.03, 0.82–1.28, p = 0.81). The primary CV composite outcome occurred in 105 patients treated with MET and pioglitazone and in 108 patients treated with MET and SU (Table 3).
Table 3.
Primary CV composite outcome in study groups.
| Study group | Number of patients with primary CV outcome |
|---|---|
| Metformin + pioglitazone | 105 |
| Metformin + sulfonylurea | 108 |
CV:cardiovascular.
The study findings suggested that the incidence of total CV events with SUs (mostly glimepiride and gliclazide) as add-on to MET in patients with type 2 diabetes inadequately controlled with MET alone was similar to that with pioglitazone 32.
3.2.1.2.9. Cardiovascular and Renal Microvascular Outcome Study with Linagliptin
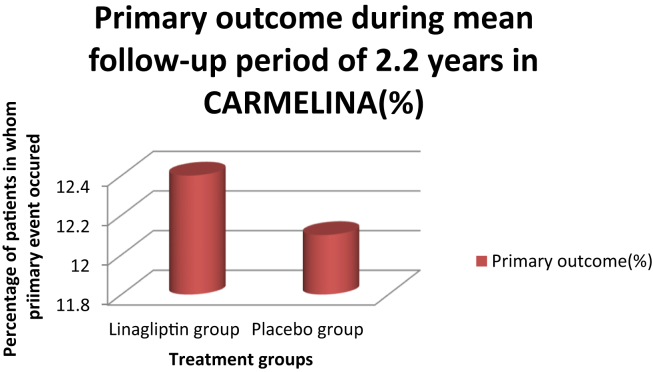
The CARMELINA trial evaluated the effect of linagliptin on CV outcomes and kidney outcomes in patients with T2DM at high risk of CV and kidney events. The CARMELINA study was a randomized, placebo-controlled, multicenter noninferiority trial. In the trial, patients were randomized to receive 5 mg of linagliptin once daily (n = 3494) or placebo once daily (n = 3485). The primary outcome evaluated during the study was the time to the first occurrence of the composite of CV death, nonfatal MI, or nonfatal stroke. Of the 6991 patients, 6979 received at least one dose of the study medication and 98.7% completed the study. During a follow-up period of 2.2 years, the primary outcome occurred in 434 of 3494 patients (12.4%) and 420 of 3485 patients (12.1%) in the linagliptin and placebo groups, respectively (Fig. 1).33,34
Fig. 1.
Primary outcome in study groups during the follow-up period.
The study concluded that among adults with T2DM and high CV and renal risk, linagliptin added to usual care, compared with placebo added to usual care, leads to a noninferior risk of composite CV outcome over a median of 2.2 years.33,34
3.2.1.2.10. Cardiovascular Outcome Study of Linagliptin Versus Glimepiride in Patients With Type 2 Diabetes
The CAROLINA trial investigated the long-term impact on CV morbidity and mortality, relevant efficacy parameters (e.g. glycemic parameters), and safety (e.g. weight and hypoglycemia) of linagliptin in patients with type 2 diabetes at elevated CV risk receiving usual care and compared the outcomes against glimepiride.33,34
The CAROLINA study involved 6033 individuals with type 2 diabetes from 607 sites in 43 countries and is the longest CV trial to date with a median follow-up period of 6.3 years. Subjects underwent a two- to four-week, open-label, placebo run-in period, during which glucose-lowering therapy previously taken by subjects was continued unchanged. After the run-in period, patients who met the inclusion criteria were randomly assigned 1:1 to receive 5 mg of linagliptin or 1–4 mg of glimepiride once daily in addition to their existing antidiabetic therapy. After a starting dose of 1 mg/day, glimepiride was up-titrated at four-week intervals during the first 16 weeks to a maximum dose of 4 mg/day. The dose of glimepiride was increased if fasting self-monitored blood glucose values were >110 mg/dL (6.1 mmol/L), unless the investigator considered it would place the patient at an increased risk of hypoglycemia.33,34
The primary outcome was time to the first occurrence of any of the following adjudicated components of the primary composite end point: CV death (including fatal stroke and fatal MI), nonfatal MI (excluding silent MI), or nonfatal stroke.
The three-point MACE occurred in 11.8% of the 3023 subjects receiving linagliptin compared with 12% of the 3010 subjects receiving glimepiride. In addition, nonsignificant differences were observed between linagliptin and glimepiride for each component of CV death (HR: 1.00; 5.6% vs. 5.6%; p = 0.9863), nonfatal MI (HR: 1.01; 4.8% vs. 4.7%; p = 0.9060), and nonfatal stroke (HR: 0.87; 3.0% vs. 3.5%; p = 0.3352).
The CAROLINA trial indicates the CV safety of glimepiride and resolves the decades long CV safety controversy stoked by the UGDP trial. Cardiologists and endocrinologists alike should be reassured about the CV safety of glimepiride. Cardiovascular safety should no longer be a parameter when deciding to use SUs to treat a patient with T2DM.33,34
It is important to note that the baseline participant characteristics widely varied between CARMELINA and CAROLINA trials. In CARMELINA, nearly 26% of patients on placebo had history of HF; however, in the CAROLINA trial only 5% of patients on glimepiride had history of HF. Also occurrences of hypoglycemia are more common even with new-generation SU when compared with the placebo. In moribund CV patients, incidence of hypoglycemia and other related CV events will be comparatively higher. Therefore, the CAROLINA trial results cannot be extrapolated to the CARMELINA trial results.
3.2.1.2.11. Panel recommendations
-
➢
Good glycemic control can positively influence the long-term development of CVD and mortality.
-
➢
CV safety outcome trials offer certain advantages that are clinically relevant. These include uniformity of reporting and recognition of specific off-target actions of molecules.
-
➢
Second- and third-generation SUs are not associated with an increased risk of all-cause mortality, CV mortality, MI, or stroke.
-
➢
Modern SUs are preferred over conventional SUs in patients with diabetes and CV disease.
-
➢
Guidelines consider modern SUs to be cardiosafe.
-
➢
Results of the CAROLINA trial underline the CV safety of modern SUs.
-
➢
The panel opined that modern SUs are cardiosafe and preferable over conventional SUs in patients with diabetes mellitus and CV disease. Because SUs have been used as comparators in other CVOTs, CVOTs of SUs are not required.
4. Conclusion
SUs could be considered an integral pharmacotherapeutic agent for the management of T2DM. Evidence suggests that modern SUs—in addition to having well-established glycemic efficacy, safety, and tolerability—are cardiosafe. The modern SU glimepiride has been used as a comparator or standard care in a few completed and ongoing CVOTs to evaluate the CV safety of other antidiabetic agents; therefore, CVOTs of SUs are not required to evaluate their utility in the management of T2DM.
Compliance with ethics guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Disclosure/acknowledgments
All authors had full access to the articles reviewed in this manuscript and take complete responsibility for the integrity and accuracy of this manuscript. The content published herein solely represents the views and opinions of the authors. The details published herein are intended for informational, educational, academic, and/or research purposes and are not intended to substitute for professional medical advice, diagnosis, or treatment.
Medical writing support was provided by Dr. Rajshri Mallabadi and Dr. Kavitha Ganesha of BioQuest Solutions Pvt. Ltd and paid for by Sanofi India. Sanofi India helped in organization and logistic support for this expert forum meeting.
Funding
This expert opinion initiative was supported by Sanofi India.
Authorship
All named authors met the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Conflict of interest
All authors have none to declare.
References
- 1.Nissen S.E., Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 2.Cefalu W.T., Kaul S., Gerstein H.C. Cardiovascular outcomes trials in type 2 diabetes: where do we go from here? Reflections from a diabetes care. Expert Forum Diabetes Care. 2018;41(1):14–31. doi: 10.2337/dci17-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 4.Zoungas S., Chalmers J., Neal B. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014;371(15):1392–1406. doi: 10.1056/NEJMoa1407963. [DOI] [PubMed] [Google Scholar]
- 5.Nissen S.E., Wolski K., Topol E.J. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. J Am Med Assoc. 2005;294(20):2581–2586. doi: 10.1001/jama.294.20.joc50147. [DOI] [PubMed] [Google Scholar]
- 6.FDA . Silver Spring: FDA; 2008. Guidance for Industry Diabetes Mellitus—Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes. [Google Scholar]
- 7.Kalra S., Aamir A.H., Raza A. Place of sulfonylureas in the management of type 2 diabetes mellitus in South Asia: a consensus statement. Indian J Endocrinol Metab. 2015;19(5):577–596. doi: 10.4103/2230-8210.163171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simpson S.H., Lee J., Choi S. Mortality risk among sulfonylureas: a systematic review and network meta-analysis. Lancet Diabetes Endocrinol. 2015;3(1):43–51. doi: 10.1016/S2213-8587(14)70213-X. [DOI] [PubMed] [Google Scholar]
- 9.Hayward R.A., Reaven P.D., Wiitala W.L. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372(23):2197–2206. doi: 10.1056/NEJMoa1414266. [DOI] [PubMed] [Google Scholar]
- 10.Zeller M., Danchin N., Simon D. Impact of type of preadmission sulfonylureas on mortality and cardiovascular outcomes in diabetic patients with acute myocardial infarction. J Clin Endocrinol Metab. 2010 Nov;95(11):4993–5002. doi: 10.1210/jc.2010-0449. [DOI] [PubMed] [Google Scholar]
- 11.John M., Unnikrishnan A.G., Kalra S. Cardiovascular outcome trials for anti-diabetes medication: a holy grail of drug development? Indian Heart J. 2016;68(4):564–571. doi: 10.1016/j.ihj.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahajan R. Bromocriptine mesylate: FDA-approved novel treatment for type-2 diabetes. Indian J Pharmacol. 2009;41(4):197–198. doi: 10.4103/0253-7613.56070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalra S., John M., Unnikrishnan A.G. Glycemic equipoise. Indian J Endocrinol Metab. 2017;21(1):18–20. doi: 10.4103/2230-8210.194361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Webb D.R., Davies M., Khunti K. Sulfonylureas: historic to contemporary role in the management of type 2 diabetes. 2018. https://www.medicographia.com/2018/12/sulfonylureas-historic-to-contemporary-role-in-the-management-of-type-2-diabetes/ Available at:
- 15.Goldner M.G., Knatterud G.L., Prout T.E. Effects of hypoglycemic agents on vas- cular complications in patients with adult-onset diabetes. 3. Clinical implications of UGDP results. J Am Med Assoc. 1971;218(9):1400–1410. [PubMed] [Google Scholar]
- 16.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 17.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352(9131):854–865. [PubMed] [Google Scholar]
- 18.Ioacara S., Guja C., Reghina A. All-cause and cardiovascular mortality associated with sulphonylurea and metformin therapy in type 2 diabetes. Endocr Res. 2018;43(2):97–105. doi: 10.1080/07435800.2017.1422745. [DOI] [PubMed] [Google Scholar]
- 19.Monami M., Luzzi C., Lamanna C. Three-year mortality in diabetic patients treated with different combinations of insulin secretagogues and metformin. Diabetes Metab Res Rev. 2006;22(6):477–482. doi: 10.1002/dmrr.642. [DOI] [PubMed] [Google Scholar]
- 20.Pantalone K.M., Kattan M.W., Yu C. The risk of overall mortality in patients with type 2 diabetes receiving glipizide, glyburide, or glimepiride monotherapy: a retrospective analysis. Diabetes Care. 2010;33(6):1224–1229. doi: 10.2337/dc10-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ADA Standards of medical care in diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S90–S102. doi: 10.2337/dc19-S009. 2019. [DOI] [PubMed] [Google Scholar]
- 22.IDF . 2017. Clinical Practice Recommendations for Managing Type 2 Diabetes in Primary Care –.https://www.idf.org/e-library/guidelines/128-idf-clinical-practice-recommendations-for-managing-type-2-diabetes-in-primary-care.html Available at: [DOI] [PubMed] [Google Scholar]
- 23.Kalra S., Bahendeka S., Sahay R. Consensus recommendations on sulfonylurea and sulfonylurea combinations in the management of type 2 diabetes mellitus–International task force. Indian J Endocr Metab. 2018;22:132–157. doi: 10.4103/ijem.IJEM_556_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zinman B., Wanner C., Lachin J.M. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 25.Wiviott S.D., Raz I., Bonaca M.P. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 26.Green J.B., Bethel M.A., Armstrong P.W. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 27.Zannad F., Cannon C.P., Cushman W.C. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385(9982):2067–2076. doi: 10.1016/S0140-6736(14)62225-X. [DOI] [PubMed] [Google Scholar]
- 28.Marso S.P., Daniels G.H., Brown-Frandsen K. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfeffer M.A., Claggett B., Diaz R. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 30.Green J.B., Hernandez A.F., D'Agostino R.B. Harmony outcomes: a randomized, double-blind, placebo-controlled trial of the effect of albiglutide on major cardiovascular events in patients with type 2 diabetes mellitus-Rationale, design, and baseline characteristics. Am Heart J. 2018;203:30–38. doi: 10.1016/j.ahj.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 31.Gerstein H.C., Bosch J., Dagenais G.R. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367(4):319–328. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 32.Vaccaro O. Effects on the incidence of CV events of the addition of pioglitazone vs. sulfonylureas in patients with type 2 diabetes inadequately controlled with metformin (TOSCA.IT): a randomised, multicentre trial. Lancet Diabetes Endocrinol. 2017;5:887–897. doi: 10.1016/S2213-8587(17)30317-0. [DOI] [PubMed] [Google Scholar]
- 33.Rosenstock J., Perkovic V., Johansen O.E. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA Randomized Clinical Trial. J Am Med Assoc. 2019;321(1):69–79. doi: 10.1001/jama.2018.18269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenstock J., Kahn S.E., Johansen O.E. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes the CAROLINA randomized clinical trial. J Am Med Assoc. 2019;322(12):1155–1166. doi: 10.1001/jama.2019.13772. [DOI] [PMC free article] [PubMed] [Google Scholar]