Abstract
NADPH oxidase-derived reactive oxygen species (ROS) regulates platelet function and thrombosis. It remains controversial regarding NOX2’s role in platelet function. As a regulatory subunit for NOX2, whether p47phox regulates platelet function remains unclear. Our study intends to evaluate p47phox’s role in platelet function. Platelets were isolated from wild-type or p47phox-/- mice followed by analysis of platelet aggregation, granule secretion, surface receptors expression, spreading, clot retraction and ROS generation. Additionally, in vivo hemostasis, arterial and venous thrombosis was assessed. Moreover, human platelets were treated with PR-39 to inhibit p47phox activity followed by analysis of platelet function. p47phox deficiency significantly prolonged tail-bleeding time, delayed arterial and venous thrombus formation in vivo as well as reduced platelet aggregation, ATP release and αIIbβ3 activation. In addition, p47phox-/- platelets presented impaired spreading on fibrinogen or collagen and defective clot retraction concomitant with decreased phosphorylation of Syk and PLCγ2. Moreover, CRP or thrombin-stimulated p47phox-/- platelets displayed reduced intracellular ROS generation which was further decreased after inhibition of NOX1. Meanwhile, p47phox deficiency increased VASP phosphorylation and decreased phosphorylation of ERK1/2, p38, ERK5 and JNK without affecting AKT and c-PLA2 phosphorylation. Furthermore, p47phox translocates to membrane to interact with both NOX1 and NOX2 after stimulation with CRP or thrombin. Finally, inhibition of p47phox activity by PR-39 reduced ROS generation, platelet aggregation and clot retraction in human platelets. In conclusion, p47phox regulates platelet function, arterial and venous thrombus formation and ROS generation, indicating that p47phox might be a novel therapeutic target for treating thrombotic or cardiovascular diseases.
Keywords: p47phox, NOX1, NOX2, Platelet function, Thrombosis
Graphical abstract
Highlights
-
•
p47phox deficiency impaired hemostasis, delayed arterial and venous thrombosis.
-
•
Reduced platelet aggregation, spreading and clot retraction in p47phox-/- platelet.
-
•
Decreased ROS production and elevated VASP phosphorylation in p47phox-/- platelet.
-
•
p47phox deficiency decreased phosphorylation of ERK1/2, p38 MAPK, ERK5 and JNK.
-
•
p47phox translocates to membrane to interact with both NOX1 and NOX2 after stimulation.
1. Introduction
At sites of vascular injury, circulating platelets adhere to damaged blood vessel wall through recognition of exposed collagen/von Willebrand factor(VWF) by the primary platelet-specific surface receptors glycoprotein (GP)VI (binds collagen and fibrin) and GPIbα (binds VWF) [1,2]. Engagement of these platelets receptors initiates transduction of intra-platelet signaling pathway, leading to activation of platelet integrin αIIbβ3 which shifts from a low-to a high-affinity state (inside-out signaling) and mediates platelet aggregation and thrombus formation through binding to VWF or fibrinogen [3]. Meanwhile, ligand binding to αIIbβ3 also induces αIIbβ3 outside-in signaling [4], resulting in tyrosine phosphorylation of signaling proteins [[5], [6], [7]], including c-Src, spleen tyrosine kinase (Syk) and phospholipase Cγ2 (PLCγ2) which mediates downstream platelet responses, such as granule secretion, platelet spreading and clot retraction [8,9].
Reactive oxygen species (ROS) are natural by-products of aerobic metabolism and consisted of radical and non-radical oxygen species formed by the partial reduction of oxygen, and regulate several cellular signaling pathways under both physiological and pathological conditions [10,11]. Recently, ROS has been shown to regulate platelet function and thrombus formation [2] and antioxidants have been investigated for the prevention and treatment of thrombotic or cardiovascular diseases [12,13]. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) is a major enzyme responsible for generating ROS in the vasculature [14]. Four NOX family members have been identified in intravascular cells including NOX1, NOX2, NOX4 and NOX5. In response to stimulation, NOX2 (also known gp91phox) forms a complex with p22phox, p47phox, p67phox and the small GTPase Rac, whereas, NOX1 interacts with p22phox, NOXO1 (similar to p47phox), NOXA1 (similar to p67phox) and Rac [15]. NOX1 and NOX2 have been found to be expressed in platelets and are a major source of ROS production in platelets as genetic deficiency of either NOX1 or NOX2 reduces ROS generation and inhibits platelet function in mice [16]. In addition, platelets from patients with chronic granulomatous disease (X-linked disease caused by genetic deficiency of NOX2) [17] showed diminished agonist-induced ROS generation [18]. However, it remains controversial regarding the role of NOX2 in ROS generation and platelet function as demonstrated by some studies showing no difference of ROS generation and platelet activation in NOX2 deficient mice compared to wild type mice [19,20]. Although p47phox is generally believed to be the regulatory subunit of NOX2 and NOXO1 is the regulatory subunit for NOX1 [15], in smooth muscle cells (SMCs), p47phox-/- SMCs presented diminished superoxide production and reduced proliferative response to growth factors compared with wild-type cells. However, no difference was observed between gp91phox-/- (NOX2-/-) SMCs and wild-type SMCs [21], indicating that p47phox and NOX2 plays different roles in SMCs after stimulation. Considering the conflicting results in terms of the NOX2’s role in ROS generation and platelet function, whether p47phox regulates ROS production and platelet function has not been extensively studied although there is only one study showing no difference of collagen-induced platelet aggregation in p47phox deficient platelets [22]. However, the collagen concentration used in this study was very high (30 μg/ml), which might overcome the functional defect of p47phox-/- platelets.
In the present study, we characterized the role of p47phox in platelet function and thrombus formation using p47phox deficient mice and demonstrated that p47phox involves in platelet function, intracellular ROS generation, hemostasis, arterial and venous thrombus formation.
2. Materials and methods
2.1. Animals
p47phox-/- mice [23] were purchased from The Jackson Laboratory and C57BL/6NJ mice were used as a control. p47phox-/- mice were genotyped according to the protocol (Standard PCR Assay) in The Jackson Laboratory website. All the experimental procedures were approved by the Ethnic Committee of Xuzhou Medical University.
2.2. Platelet preparation
Platelets were isolated from mouse or human blood as previously described [24,25]. Mouse blood was drawn into ACD anti-coagulated tube followed by differential centrifugation to isolate platelets which were resuspended in Tyrode’s buffer. For human platelets, ACD-anti-coagulated venous blood was centrifuged to obtain platelet-rich plasma (PRP) which was centrifuged, washed and resuspended in Tyrode’s buffer. Platelets were allowed to rest for 1 h at room temperature before experimental use. All procedures involving collection of mouse and human blood were approved by the Ethic Committee of Xuzhou Medical University. Informed consent was obtained from all participants and experimentation with human blood was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). All animal experiments were complied with the ARRIVE guidelines and carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
2.3. Quantitative real-time PCR
The expression of GPIbα and GPVI mRNA was detected by quantitative real-time PCR as described previously [25]. Briefly, RNA was isolated from platelets for cDNA synthesis followed by PCR amplification on a LightCycler® R480 II (Roche Life Science). The primers sequences for GPIbα and GPVI were: GPIbα Forward primer: 5’-AGTTCATACTACCCACTGGAGCC-3’, Reverse primer: 5’-GTGGGTTTATGAGTTGGAGGC-3’; GPVI Forward primer: 5’-AGGAGACCTTCCATCTTACCCA-3’, Reverse primer: 5’ GAGCAAAACCAAATGGAGGG-3’. The expression of GPIbα and GPVI mRNA was calculated using 2−ΔΔCt method and normalized to internal control (β-actin).
2.4. Electron microscopy
Platelets were fixed in 3% glutaraldehyde, dehydrated and embedded using Epon812. Then, ultrathin section was made using LKB-V ultramicrotome and stained with lead citrate and uranyl acetate. Results were observed under a transmission electron microscope (JEOL-1200EX) and images were obtained using a Morada G2 digital camera.
2.5. Platelet aggregation, granule release and αIIbβ3 expression
Mouse platelet aggregation after stimulated with thrombin (0.03 U/ml) or CRP (0.1 μg/ml) was detected in a Lumi-Aggregometer Model 700 (Chrono-log Corporation, Havertown, PA, USA) at 37 °C with stirring (1000 rpm). ATP release was monitored in parallel with platelet aggregation using luciferin/luciferase reagent (Chrono-log Corporation). Platelet α-granule release as presented by P-selectin expression was measured by flow cytometry using PE-conjugated anti-P-selectin antibody as described previously [26]. The platelet αIIbβ3 integrin expression was measured using FITC-conjugated mouse anti-human CD41a antibody (αIIb) by flow cytometry.
2.6. Platelet spreading and clot retraction
Platelets were placed on glass coverslips which were pre-coated with fibrinogen (10 μg/ml) or collagen (10 μg/ml) for 90 min at 37 °C followed by staining with Alexa Fluor-546-labelled phalloidin. Spreading was observed under a fluorescence microscopy (Nikon-80i) (X100 oil objective). The coverage was quantified using Image J software. Clot retraction was performed after addition of thrombin (1 U/ml) in the presence of 2 mM Ca2+ and 0.5 mg/ml fibrinogen as described previously [24,25].
2.7. Tail bleeding assay and FeCl3-induced arterial thrombosis
Tail bleeding assay was performed as described previously [24,25]. For analysis of arterial thrombosis, platelets (1 × 108) were labelled with calcein and injected into mice via tail vein injection followed by treating the mesenteric arterioles with 10% w/v FeCl3 to induce thrombus formation which was dynamically observed under a fluorescence microscopy (Olympus IX53) [24,25].
2.8. Deep vein thrombosis
After anesthesia with isoflurane-oxygen mixture, an incision was made in the midline of the abdomen to expose the intestines which were soaked in warm saline to prevent drying out. The inferior vena cava (IVC) without side branches was ligated with a 2-0 nonabsorbable suture using a stenosis method. At 24 h following IVC ligation, thrombi were excised for measurement of weight and length.
2.9. In vitro thrombosis under arterial flow conditions
Mouse blood was collected, labelled with mepacrine (100 μM) and then perfused through Bioflux plates which were pre-coated with fibrillar collagen (100 μg/ml) in a microfluidic whole-blood perfusion assay (Bioflux-200 system) under an arterial shear conditions for 5 min at a shear force of 40 dyn/cm2. Thrombus formation was dynamically monitored under an inverted fluorescence microscopy (Olympus IX53). The platelet-covered area was quantified using Bioflux software (Fluxion).
2.10. Measurement of ROS generation
After stimulation with CRP or thrombin, intracellular ROS generation was measured using 2’,7’-dichlorofluorescein (H2DCF-DA) as described previously [27,28]. For some experiments, platelets were pretreated with ML171 (5 μM) (NOX1 inhibitor) (MedChemExpress) and/or gp91ds-at (50 μM) (NOX2 inhibitor) (AnaSpec) or scrambled gp91 ds-tat (AnaSpec) for 10 min prior to stimulation.
2.11. Isolation of platelet membrane and cytosol
After stimulation, platelets were centrifuged at 15,000 g for 5 min to collect the supernatant which was then centrifuged at 100,000 g for 2 h. The supernatant was considered as the cytosol and the insoluble pellet was defined as the membrane fraction followed by immunoblotting analysis of p47phox expression.
2.12. Immunoprecipitation
After stimulation with CRP (5 μg/ml) for 30s or thrombin (1 U/ml) for 60s, platelets were lysed in NP-40 lysis buffer (containing PMSF, protease and phosphatase inhibitor) on ice for 15 min followed by centrifugation at 10,000 g for 20 min at 4 °C to collect the supernatant which was pre-cleared with protein A/G agarose at 4 °C for 3 h and centrifuged to collect the supernatant. The supernatant was then incubated with antibody against p47phox or mouse IgG (control) for 1 h at 4 °C followed by addition of protein A/G agarose for overnight incubation at 4 °C on a rocker. The agarose beads were harvested after centrifugation at 10,000 g for 30 s and rinsed three times with NP-40 lysis buffer followed by addition of loading buffer for subsequent immunoblotting.
2.13. Immunoblotting
Immunoblotting assay involves antibodies against p47phox (Santa Cruz Biotechnology); NOX1 and NOX2 (Proteintech); p67phox (Abcam); NOXO1 (ABclonal Technology); Rac (Santa Cruz Biotechnology); Syk (anti-Tyr-525 and pan-Syk, Bioworld Technology); PLCγ2 (anti-Tyr-1217 and pan-PLCγ2; Bioworld Technology); VASP (anti-Ser157, Affinity Biosciences; anti-Ser239 and pan-VASP, Cell Signaling Technology); ERK1/2 (anti-Thr202/Tyr204 and pan-ERK1/2, Cell Signaling Technology); p38 MAPK (anti-Thr180/Tyr182, Cell Signaling Technology); ERK5 (anti-Thr218/Tyr220, Cell Signaling Technology; pan-ERK5, Proteintech); JNK (anti-Thr183/Tyr185, Cell Signaling Technology; pan-JNK, Affinity Biosciences); AKT (anti-Thr308, Cell Signaling Technology; pan-AKT, Affinity Biosciences); c-PLA2 (anti-Ser505 and pan-c-PLA2, Affinity Biosciences); NOX1 (Novus Biologicals); NOX2 (Abcam).
2.14. Statistical analysis
Data are represented as mean ± standard deviation (SD) or standard error (SE) where indicated and analyzed by student t-test, one-way or two-way ANOVA. p < 0.05 indicates a significant difference.
3. Results
3.1. p47phox deficiency impairs in vivo hemostasis and thrombus formation
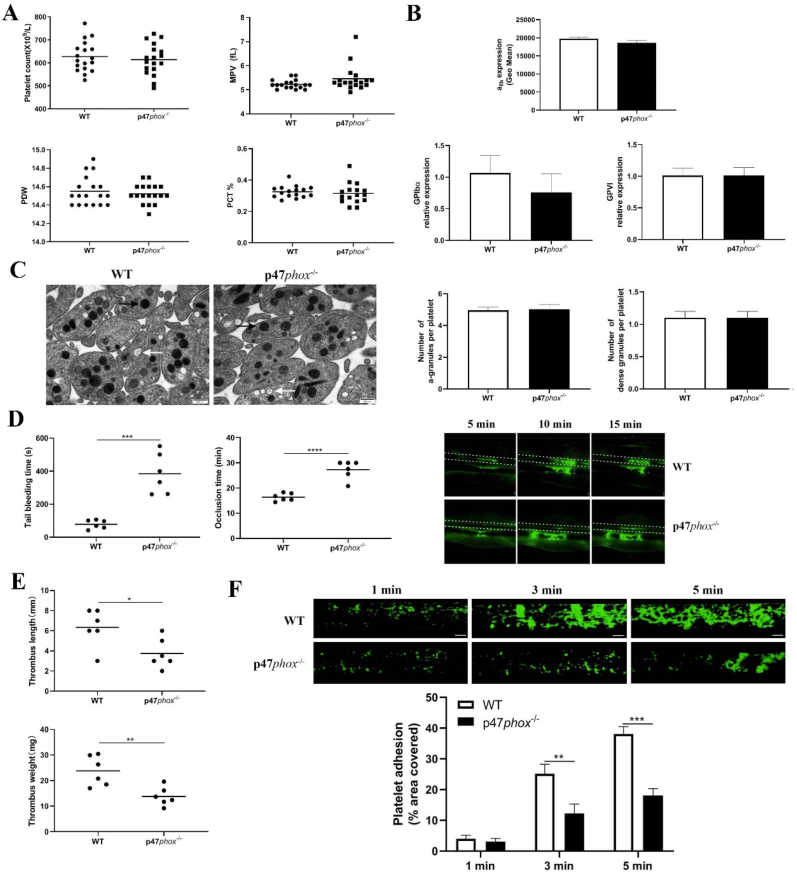
Using p47phox-/- mice, we first evaluated whether p47phox deficiency affects platelet production or turn over through measuring platelet count, mean platelet volume (MPV), platelet distribution width (PDW) and plateletcrit (PCT) and found no significant differences of these parameters between wild-type (WT) and p47phox-/- mice (p > 0.05) (Fig. 1A). As platelet surface receptors αIIbβ3, GPIbα and GPVI plays a critical role in platelet function after ligand engagement, we also measured the expression of these receptors by flow cytometry and qRT-PCR and showed equivalent protein or mRNA levels in WT and p47phox-/- mice (p > 0.05) (Fig. 1B). Furthermore, electron microscopy analysis demonstrated that p47phox deficiency did not affect the organization of platelet ultrastructure, or the number and size of α- and dense granules (Fig. 1C).
Fig. 1.
Platelet parameters, surface receptors expression, ultrastructure, tail bleeding, thrombus formation in wild-type or p47phox-/-mice. (A) Platelet count, mean platelet volume (MPV), platelet distribution width (PDW), plateletcrit (PCT) measured by an automatic blood analyzer (mean). (B) αIIbβ3 expression by flow cytometry using FITC-conjugated anti-mouse αIIb antibody; GPIbα and GPVI expression by quantitative real-time PCR (represented as a ratio relative to an internal control, β-actin) (mean ± SE, n = 3–4) (Student t-test). (C) Platelet ultrastructure analysis (α-granules and dense granules) by electron microscopy. Scale bar: 0.5 μm (x 30, 000 magnification). Black arrow: α-granule; white arrow: dense granule. The number of α-granules and dense granules was counted in 60 WT and 60 p47phox-/- platelets (mean ± SD) (Student t-test). (D) Tail bleeding time and FeCl3-induced arterial thrombus formation in wild-type (WT) and p47phox-/- mice (mean, n = 6). Representative image of arterial thrombus formation at different time point was shown. (E) Analysis of venous thrombosis. Mice underwent ligation of inferior vena cava (IVC) to initiate venous thrombus formation. After 24 h, the IVC samples were collected for measuring the thrombus length and weight (mean, n = 6). (F) Platelet adhesion on collagen under flow conditions. Whole blood from WT or p47phox-/- mice was labelled with mepacrine and perfused through fibrillar collagen-coated bioflux plates at a shear rate of 40 dyn/cm2 for 5 min. Platelet adhesion (covered area) was quantified at different time points (mean ± SE, n = 6) (one-way ANOVA). *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
In order to assess whether p47phox plays a role in in vivo hemostasis and arterial thrombus formation, we performed tail bleeding assay and FeCl3-induced mesenteric arteriole thrombus formation. As seen in Fig. 1D, a significantly prolonged bleeding time and delayed arterial thrombus formation was found in p47phox-/- mice compared to those in WT mice (p < 0.001), indicating that p47phox deficiency significantly impairs in vivo hemostasis and arterial thrombus formation. Beyond arterial thrombosis, platelets are also shown to be involved in venous thrombus formation [29]. We then assessed whether p47phox deficiency affects venous thrombosis using deep vein thrombosis (DVT) model (stenosis of the inferior vena cava) and reported that venous thrombus formation was significantly inhibited in p47phox-/- mice compared to that in WT mice as demonstrated by significantly shorter venous thrombus length (p < 0.05) and lighter venous thrombus weight (p < 0.01) (Fig. 1E). Moreover, we also evaluated the role of p47phox in vitro thrombus formation under arterial flow conditions using a microfluidic whole-blood perfusion system with collagen-coated BioFlux plates. As seen in Fig. 1F, thrombi formation was significantly inhibited in p47phox-/- platelets compared to that in WT platelets. Taken together, these data indicate that p47phox deficiency significantly impairs platelet hemostatic function as well as arterial and venous thrombus formation.
3.2. p47phox deficiency reduces platelet aggregation and granule secretion and αIIbβ3 activation
As platelet aggregation is critical for platelet hemostatic function, we next investigated the role of p47phox in platelet aggregation and activation and found that p47phox-/- platelets presented a significantly reduced aggregation in response to CRP (0.25 μg/ml) (Fig. 1A) or thrombin (0.01 U/ml) (Fig. 1B) stimulation compared with WT platelets (p < 0.05). Agonist-induced platelet granule secretion plays critical roles in the amplification of platelet signaling and subsequent activation and aggregation [30], we also detected platelet dense-granule (ATP release) and alpha-granule (P-selectin expression) secretion after agonist stimulation and revealed that ATP release (Fig. 2A and 2B) and P-selectin expression (Fig. 2C) was both significantly decreased in p47phox-/- platelets after either CRP or thrombin stimulation (p < 0.05). At the same time, p47phox-/- platelets also presented lower integrin αIIbβ3 activation after CRP (p < 0.01) or thrombin (p < 0.05) stimulation than WT platelets as shown by significantly reduced JON/A binding (Fig. 2D).
Fig. 2.
Platelet aggregation, granule recreation and αIIbβ3 activation. Platelets were isolated from WT or p47phox-/- mice and treated with CRP (0.25 μg/ml) (A) or thrombin (0.01 U/ml) (B) followed by analysis of platelet aggregation and ATP release (dense granule secretion) in a Lumi-Aggregometer Model 700. (C) P-selectin expression (α-granule secretion) and (D) αIIbβ3 activation (presented by JON/A binding) was measured by flow cytometry. Data were shown as mean ± SE (n = 4–6) (Student t-test). *p < 0.05; **p < 0.01. P-selectin expression was presented as the %-positive staining of anti-CD62P antibody. THR: Thrombin.
3.3. p47phox-/- platelets show decreased platelet spreading and clot retraction
We next investigated whether p47phox deficiency affects platelet spreading and clot retraction, two processes which are regulated by early- and late-αIIbβ3 outside-in signaling, respectively [31]. As seen in Fig. 3A, the spreading of p47phox-/- platelets on collagen or fibrinogen was significantly inhibited compared to WT platelets (p < 0.01). Consistent with platelet spreading profiles, thrombin-mediated clot retraction was also significantly decreased in p47phox-/- platelets compared to WT platelets (Fig. 3B). Since activation of αIIbβ3 outside-in signaling causes the phosphorylation of c-Src, Syk, and PLCγ2, which mediates clot retraction [5,6], we next measured the phosphorylation of Syk and PLCγ2 under the conditions of clot retraction and found significantly lower phosphorylation level of Syk and PLCγ2 in p47phox-/- platelets than WT platelets (p < 0.01) (Fig. 3C and 3D). Taken together, p47phox deficiency affects integrin αIIbβ3 outside-in signaling transduction.
Fig. 3.
Platelet spreading and clot retraction. (A) Platelets were placed on glass coverslips pre-coated with collagen or fibrinogen at 37 °C for 90 min followed by staining with Alexa Fluor-546-labelled phalloidin. Covered area was quantified by Image J software and analyzed by student t-test for comparison. Images (X100) are representative of three independent experiments (mean ± SD, n = 3). (B) Clot retraction was initiated after addition of thrombin (1 U/ml). Representative images at 30, 60, and 90 min from three independent experiments were shown. Data were quantified as the clot volume (%) and expressed as mean (n = 3) (Two-way ANOVA) (C) Under the condition of clot retraction, the phosphorylation level of Syk and PLCγ2 was detected by western blot and (D) quantified as a ratio relative to the total level (mean ± SD, n = 3) (Two-way ANOVA). *p < 0.05; **p < 0.01; ***p < 0.001.
3.4. Decreased ROS generation and impaired ERK1/2 and p38 MAPK signaling in p47phox-/- platelets
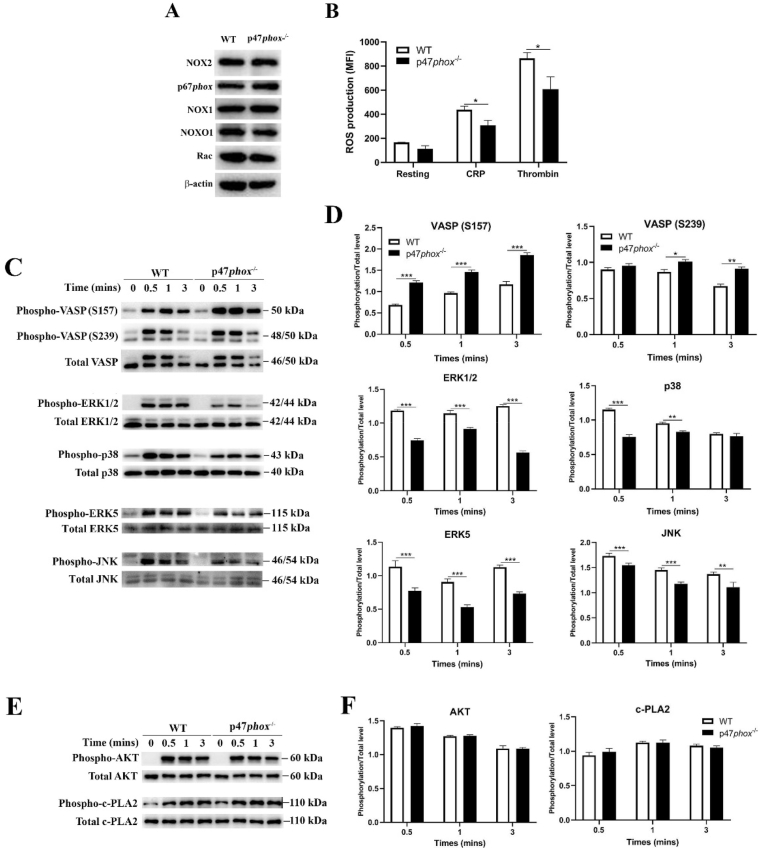
As p47phox is believed as the regulatory subunit for NOX2 complex [32], we then evaluated whether p47phox deficiency affects the expression of other subunits of NOX2 or NOX1. As seen in Fig. 4A, knockout of p47phox did not influence the expression of NOX2, p67phox, NOX1, NOXO1 and Rac. However, p47phox deficiency significantly decreased intracellular ROS generation in platelets after either CRP or thrombin stimulation (Fig. 4B). Meanwhile, the phosphorylation level of vasodilator-stimulated phosphoprotein (VASP) (S157/239), a downstream target of NO/cGMP signaling, was significantly elevated in p47phox-/- platelets after CRP stimulation (Fig. 4C and 4D), indicating that NO/cGMP signaling is enhanced in platelets after p47phox deficiency, consistent with the inhibitory role of NO/cGMP in platelet activation and function [33]. As mitogen-activated protein kinases (MAPKs), consisting of extracellular signal-related kinases 1/2 (ERK1/2), c-jun NH2-terminal kinases (JNK) and p38 MAPK, are sensitive to oxidative stress [34], we next measured the phosphorylation of ERK1/2 (Thr202/Tyr204), p38 MAPK (Thr180/Tyr182), ERK5 (Thr218/Tyr220) and JNK (Thr183/Tyr185) and found a significantly reduced phosphorylation level in CRP-stimulated p47phox-/- platelets (Fig. 4C and 4D). However, p47phox deficiency did not affect the phosphorylation level of AKT (Thr308) and cytosolic phospholipase A2 (c-PLA2) (Ser505) in CRP-stimulated platelets (Fig. 4E and 4F).
Fig. 4.
ROS generation and phosphorylation of VASP, ERK1/2, p38 MAPK, ERK5, JNK, AKT and c-PLA2. (A) Western blot analysis of the expression of NOX2, p67phox, NOX1, NOXO1 and Rac in WT and p47phox-/- platelets. (B) ROS generation in platelets after stimulation with CRP (2 μg/ml) or thrombin (0.5 U/ml) was expressed as mean fluorescent intensity (MFI) (mean ± SE, n = 6) (Student t-test). (C) The phosphorylation level of VASP, ERK1/2, p38, ERK5 and JNK in CRP-stimulated platelets was detected by western blot and (D) quantified as a ratio relative to the total level (mean ± SD, n = 3) (Two-way ANOVA). (E) The phosphorylation level of AKT and c-PLA2 was also detected and (F) quantified (mean ± SD, n = 3) (Two-way ANOVA). *p < 0.05; **p < 0.01; ***p < 0.001.
3.5. p47phox translocates to membrane to interact with both NOX1 and NOX2 after platelet activation
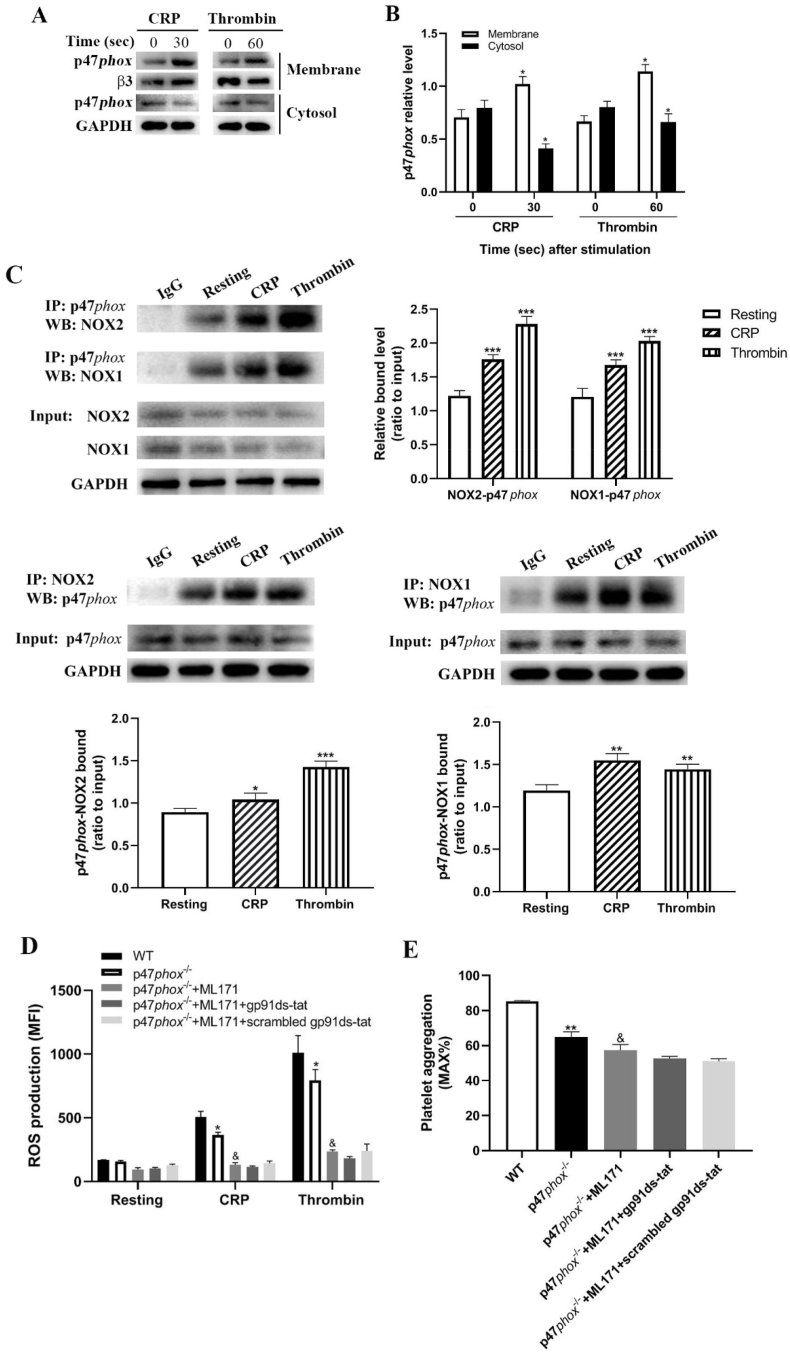
As p47phox involves in NADPH-derived ROS generation through translocation to membrane, we next isolated platelet membrane and cytosol to measure the expression of p47phox and found a significantly increased level of p47phox in the membrane and reduced level in the cytosol in CRP- or thrombin-stimulated platelets compared to unstimulated platelets (Fig. 5A and 5B), indicating that p47phox translocates to membrane after platelet activation. However, how p47phox regulates platelet ROS generation remains unclear. To investigate this issue, we used immunoprecipitation to study which subunit p47phox binds after translocation to the membrane. As seen in Fig. 5C, p47phox binds to both NOX2 and NOX1 in CRP- or thrombin-stimulated platelets as demonstrated by significantly increased binding after stimulation compared to unstimulated platelets (under resting condition). To further assess the role of p47phox in platelet ROS generation relative to NOX1 and NOX2, p47phox-/- platelets were treated with ML171 (NOX1 inhibitor) and/or gp91-ds-tat (NOX2 inhibitor) and we found that CRP- or thrombin-treated p47phox-/- platelets presented significantly decreased ROS production which was further reduced after inhibition of NOX1 (Fig. 5D), indicating that other regulatory subunit might also interact with NOX1 to regulate ROS generation except p47phox. Interestingly, inhibition of NOX2 did not cause further significant decrease of ROS generation on the basis of NOX1 inhibition and p47phox deficiency (Fig. 5D), suggesting that p47phox is the sole regulatory subunit of NOX2. Moreover, consistent with the profile of ROS generation, inhibition of NOX1 further significantly reduced the aggregation of p47phox-deficient platelet after CRP stimulation. However, addition of NOX2 inhibitor did not further decrease the aggregation of p47phox-deficient platelet after inhibition of NOX1 (Fig. 5E).
Fig. 5.
p47phox translocation and interaction with NOX1 and NOX2. (A) Platelets were treated with CRP (5 μg/ml) for 30s or thrombin (1 U/ml) for 60s followed by isolation of the membrane and cytosol to measure the expression of p47phox and (B) p47phox expression was quantified as a ratio relative to the internal control β3 or GAPDH. Data were presented as mean ± SD (n = 3). Compared to corresponding 0, *p < 0.05. (C) Immunoprecipitation analysis of the interaction of p47phox with NOX2 or NOX1 in platelets after stimulation with CRP (5 μg/ml) or thrombin (1 U/ml). Compared to resting, *p < 0.05; **p < 0.01; ***p < 0.001. (D) ROS generation in p47phox-/- platelets in the presence of ML171 (5 μM) (NOX1 inhibitor) and/or gp91ds-at (50 μM) (NOX2 inhibitor) after CRP (2 μg/ml) or thrombin (0.5 U/ml) stimulation. (E) Platelet aggregation in p47phox-/- platelets in the presence of ML171 (5 μM) (NOX1 inhibitor) and/or gp91ds-at (50 μM) (NOX2 inhibitor) after CRP (0.25 μg/ml) stimulation. Data were presented as mean ± SE (n = 6–8) (One-way ANOVA). Compared to WT, *p < 0.05; **p < 0.01. Compared with p47phox-/-, &p < 0.01.
3.6. Inhibition of p47phox activity impairs human platelet ROS generation and platelet function
To investigate whether p47phox plays similar roles in human platelets, we used PR-39, which inhibits p47phox activity through binding to its Src homology 3 domains [35], to treat human platelets. As indicated in Fig. 6A, PR-39 treatment significantly decreased ROS generation in platelets after thrombin stimulation compared to control. In addition, significantly reduced platelet aggregation and ATP release (Fig. 6B) were also found in PR-39-treatment platelets compared to control-treated platelets. Furthermore, thrombin-induced clot retraction was also impaired after RP-39 treatment as demonstrated by significantly higher clot volume than control at different time points (Fig. 6C). Taken together, these data indicate that p47phox is also involved in the regulation of ROS generation and platelet function in human platelets, consistent with its role in mouse platelets.
Fig. 6.
Effect of inhibition of p47phox on human platelet function. Human platelets were incubated with PR-39 (6 μM) (p47phox activity inhibitor) for 2 h followed by analysis of (A) ROS generation after thrombin (1 U/ml) treatment (mean ± SE, n = 4) which was quantified as a fold change and assessed by student t-test; (B) platelet aggregation and ATP release in response to CRP (2 μg/ml) (mean ± SE, n = 4) (Student t-test) which was expressed relative to control; and (C) clot retraction induced by thrombin (1 U/ml) (mean, n = 3) (Two-way ANOVA). *p < 0.05; ***p < 0.001.
4. Discussion
NADPH oxidase is the major enzyme contributing to ROS generation in platelets after stimulation [2]. As a member of NADPH oxidase, NOX1 and NOX2 are widely expressed in platelets. However, conflicting results are reported regarding the role of NOX2 in platelet ROS generation [16,19,20]. Although p47phox is generally thought as the regulatory subunit for NOX2, it plays different roles in smooth muscle cells relative to NOX2 [21]. In the present study, using p47phox-/- mice, we characterized p47phox’s role in platelet function and ROS generation and found that p47phox regulates platelet function and intracellular ROS production.
NOX1 and NOX2 are found to be expressed in platelets and have been demonstrated to play a critical role in platelet activation and thrombus formation. However, conflicting results have been obtained in terms of the differential role of NOX1 and NOX2 in G protein-coupled receptor (GPCR)- or GPVI-dependent platelet aggregation and function [16,19,20,36]. A previous study showed that NOX1 plays a selective role in GPCR-dependent platelet aggregation and granule secretion, whereas NOX2 involves in both GPCR- and GPVI-dependent platelet function [16]. In contrast, NOX1 but not NOX2 participates in the regulation of GPVI-dependent platelet function [20,36]. In addition, a recent study found that NOX2 is dispensable for platelet ROS generation, platelet activation and aggregation as well as not essential in mediating experimental thrombosis [19]. In response to stimulation, NOX1 and NOX2 require cytoplasmic subunits for activation. In general, p47phox is thought to be the regulatory subunit of NOX2 and NOXO1 is the regulatory subunit of NOX1 [15]. Considering the controversial data on the role of NOX2 and NOX1 in platelet activation, whether p47phox regulates platelet function and thrombus formation remains poorly understood. In the present study, we demonstrated significantly reduced both GPVI- and GPCR-dependent platelet aggregation and granule release, platelet spreading and clot retraction along with impaired in vivo hemostasis and arterial and venous thrombus formation, indicating that p47phox positively regulates GPVI- and GPCR-dependent platelet activation and function. However, as p47phox knockout mice were used in the present study rather than p47phox platelet-specific conditional knockout mice, we cannot rule out the possible contribution of p47phox from other vascular cells such as neutrophils or monocytes to the impaired in vivo thrombus formation in p47phox knockout mice.
Reactive oxygen species (ROS) has been demonstrated to regulate platelet function and thrombus formation [2] as high levels of platelet ROS are associated with thrombotic diseases, hypertension, diabetes and metabolic syndromes [37]. Additionally, ROS scavengers, inhibitors of O2•- generation or pharmacologic antioxidants attenuated platelet activation and aggregation [38,39], whereas ROS donors such as 2,3-dimethoxy-1,4-naphthoquinone (DNMQ) enhanced platelet activation and thrombus formation [38]. NOX1 and NOX2 is a major source of ROS production in platelets. In our study, we found that p47phox deficiency did not affect the expression of other subunits of NOX1 or NOX2, such as NOX2, p67phox, NOX1, NOXO1 and Rac, but significantly reduced GPVI- or GPCR-dependent ROS generation. In addition, a significantly increased VASP phosphorylation (S157/239) was found in CRP-stimulated platelets, indicating that NO/cGMP signaling pathway is enhanced in p47phox-/- platelets, possibly due to reduced ROS generation as intracellular ROS scavenge platelet-derived NO in a fast reaction forming peroxynitrite (ONOO-) as an end product [40,41]. However, whether ROS affects platelet function through upregulation of NO/cGMP signaling pathway in p47phox knockout mice remains unclear as phosphotyrosine phosphatase might also be ROS targets [42]. In addition, ROS might play distinct roles in platelet function compared to NO/cGMP signaling as ROS has been shown to affect platelet integrin αIIbβ3 activation, aggregation and thrombus formation [43,44], whereas, NO/cGMP signaling participates in the inhibition of platelet adhesion, shape change, aggregation and secretion as well as intracellular calcium release [33]. In the present study, the increased NO/cGMP signaling activation might contribute to the attenuated platelet aggregation and activation, which is consistent with the inhibitory role of NO/cGMP signaling in platelet activation and function [33]. Moreover, we also found that the phosphorylation of ERK1/2 (Thr202/Tyr204), p38 MAPK (Thr180/Tyr182), ERK5 (Thr218/Tyr220) and JNK (Thr183/Tyr185) was significantly reduced in p47phox-/- platelets after stimulation, in accordance with several studies showing that MAPK signaling pathways are sensitive to oxidative stress caused by changes of ROS [34,45]. Interestingly, p47phox deficiency did not affect the phosphorylation of AKT and c-PLA2 in CRP-stimulated platelets.
As generally considered as the regulatory subunit of NOX2 and NOX1, p47phox and NOXO1 share a similar set of motifs and both of them have NH2-terminal phox homology domains that bind to the phospholipids in the membrane [15]. However, in some cases, NOX1 activity might require p47phox. A previous demonstrated that smooth muscle cells with p47phox deficiency had reduced superoxide production and a decreased proliferative response to growth factors compared with wild-type cells, whereas the response of gp91phox (NOX2)-defective smooth muscle cells was indistinguishable from that of wild-type cells [21], indicating that p47phox and NOX2 plays different role in smooth muscle cells. In our study, we found that p47phox translocates to membrane from cytosol after CRP or thrombin stimulation as demonstrated by increased level in the membrane and decreased expression in the cytosol. Following immunoprecipitation analysis revealed that p47phox interacts with both NOX2 and NOX1 in either CRP- or thrombin-stimulated platelets, indicating that NOX1 activity might require p47phox in platelets. Further analysis of the role of p47phox relative to NOX2 or NOX1 in ROS generation demonstrated that reduced ROS generation in p47phox-/- platelets was further decreased after inhibition of NOX1, implying that NOX1 activity might also require other regulatory subunits such as NOXO1 [46] apart from p47phox in platelets. However, inhibition of NOX2 did not induce the further decrease of ROS generation on the basis of NOX1 inhibition and p47phox deficiency, suggesting that p47phox is the sole regulatory subunit of NOX2 in platelet.
In conclusion, our study demonstrates that p47phox positively regulates platelet function, in vivo hemostasis and arterial and venous thrombosis as well as ROS generation through interaction with both NOX1 and NOX2, indicating that p47phox might be a novel therapeutic target for treating thrombotic or cardiovascular diseases. However, as p47phox is also expressed in other vascular cells except platelets, a generalized approach to target p47phox should be cautious for treating thrombotic or cardiovascular diseases. Moreover, a risk of bleeding should also be considered in case of targeting p47phox as loss of p47phox increases tail bleeding time in mice.
Declaration of competing interest
All authors have no conflict of interest to declare.
Acknowledgement
This work was supported by National Natural Science Foundation of China (grant no. 81970124, 81400082, 81641151 and 81700178), the Natural Science Foundation of Jiangsu Province (grant no. BK20140219 and BK20170259), the funding for the Distinguished Professorship Program of Jiangsu Province, the Shuangchuang Project of Jiangsu Province, the Six Talent Peaks Project in Jiangsu Province (WSN-133), the 333 projects of Jiangsu Province (BRA2017542), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (18KJA320010 and 17KJA320008), Jiangsu Province’s Key Provincial Talents Program (ZDRCA2016054), Jiangsu Province's Graduate Scientific Research Innovation Program (KYCX18-2186, KYCX19-2231 and KYCX19-2234) and Youth Science and Technology Innovation Team of Xuzhou Medical University.
Contributor Information
Lingyu Zeng, Email: zengly2000@163.com.
Kailin Xu, Email: lihmd@163.com.
Jianlin Qiao, Email: jianlin.qiao@gmail.com.
References
- 1.Qiao J.L., Shen Y., Gardiner E.E., Andrews R.K. Proteolysis of platelet receptors in humans and other species. Biol. Chem. 2010;391:893–900. doi: 10.1515/BC.2010.081. [DOI] [PubMed] [Google Scholar]
- 2.Qiao J., Arthur J.F., Gardiner E.E., Andrews R.K., Zeng L., Xu K. Regulation of platelet activation and thrombus formation by reactive oxygen species. Redox Biol. 2017;14:126–130. doi: 10.1016/j.redox.2017.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hynes R.O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 4.Li Z., Delaney M.K., O'Brien K.A., Du X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 2010;30:2341–2349. doi: 10.1161/ATVBAHA.110.207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark E.A., Shattil S.J., Ginsberg M.H., Bolen J., Brugge J.S. Regulation of the protein tyrosine kinase pp72syk by platelet agonists and the integrin alpha IIb beta 3. J. Biol. Chem. 1994;269:28859–28864. [PubMed] [Google Scholar]
- 6.Suzuki-Inoue K., Hughes C.E., Inoue O., Kaneko M., Cuyun-Lira O., Takafuta T., Watson S.P., Ozaki Y. Involvement of Src kinases and PLCgamma2 in clot retraction. Thromb. Res. 2007;120:251–258. doi: 10.1016/j.thromres.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wonerow P., Pearce A.C., Vaux D.J., Watson S.P. A critical role for phospholipase Cgamma2 in alphaIIbbeta3-mediated platelet spreading. J. Biol. Chem. 2003;278:37520–37529. doi: 10.1074/jbc.M305077200. [DOI] [PubMed] [Google Scholar]
- 8.Phillips D.R., Jennings L.K., Edwards H.H. Identification of membrane proteins mediating the interaction of human platelets. J. Cell Biol. 1980;86:77–86. doi: 10.1083/jcb.86.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shattil S.J., Kashiwagi H., Pampori N. Integrin signaling: the platelet paradigm. Blood. 1998;91:2645–2657. [PubMed] [Google Scholar]
- 10.Finkel T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown D.I., Griendling K.K. Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circ. Res. 2015;116:531–549. doi: 10.1161/CIRCRESAHA.116.303584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nunez-Cordoba J.M., Martinez-Gonzalez M.A. Antioxidant vitamins and cardiovascular disease. Curr. Top. Med. Chem. 2011;11:1861–1869. doi: 10.2174/156802611796235143. [DOI] [PubMed] [Google Scholar]
- 13.Goszcz K., Deakin S.J., Duthie G.G., Stewart D., Leslie S.J., Megson I.L. Antioxidants in cardiovascular therapy: panacea or false hope? Front. Cardiovasc. Med. 2015;2:29. doi: 10.3389/fcvm.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lassegue B., Griendling K.K. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler. Thromb. Vasc. Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 16.Delaney M.K., Kim K., Estevez B., Xu Z., Stojanovic-Terpo A., Shen B., Ushio-Fukai M., Cho J., Du X. Differential roles of the NADPH-oxidase 1 and 2 in platelet activation and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2016;36:846–854. doi: 10.1161/ATVBAHA.116.307308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rae J., Newburger P.E., Dinauer M.C., Noack D., Hopkins P.J., Kuruto R., Curnutte J.T. X-Linked chronic granulomatous disease: mutations in the CYBB gene encoding the gp91-phox component of respiratory-burst oxidase. Am. J. Hum. Genet. 1998;62:1320–1331. doi: 10.1086/301874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pignatelli P., Sanguigni V., Lenti L., Ferro D., Finocchi A., Rossi P., Violi F. gp91phox-dependent expression of platelet CD40 ligand. Circulation. 2004;110:1326–1329. doi: 10.1161/01.CIR.0000134963.77201.55. [DOI] [PubMed] [Google Scholar]
- 19.Sonkar V.K., Kumar R., Jensen M., Wagner B.A., Sharathkumar A.A., Miller F.J., Jr., Fasano M., Lentz S.R., Buettner G.R., Dayal S. Nox2 NADPH oxidase is dispensable for platelet activation or arterial thrombosis in mice. Blood Adv. 2019;3:1272–1284. doi: 10.1182/bloodadvances.2018025569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh T.G., Berndt M.C., Carrim N., Cowman J., Kenny D., Metharom P. The role of Nox1 and Nox2 in GPVI-dependent platelet activation and thrombus formation. Redox Biol. 2014;2:178–186. doi: 10.1016/j.redox.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barry-Lane P.A., Patterson C., van der Merwe M., Hu Z.Y., Holland S.M., Yeh E.T.H., Runge M.S. p47phox is required for atherosclerotic lesion progression in ApoE(-/-) mice. J. Clin. Invest. 2001;108:1513–1522. doi: 10.1172/JCI11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dharmarajah J., Arthur J.F., Sobey C.G., Drummond G.R. The anti-platelet effects of apocynin in mice are not mediated by inhibition of NADPH oxidase activity. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2010;382:377–384. doi: 10.1007/s00210-010-0552-3. [DOI] [PubMed] [Google Scholar]
- 23.Jackson S.H., Gallin J.I., Holland S.M. The p47phox mouse knock-out model of chronic granulomatous disease. J. Exp. Med. 1995;182:751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo Q., Wei G., Wu X., Tang K., Xu M., Wu Y., Liu Y., Li X., Sun Z., Ju W. Platycodin D inhibits platelet function and thrombus formation through inducing internalization of platelet glycoprotein receptors. J. Transl. Med. 2018;16:311. doi: 10.1186/s12967-018-1688-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiao J., Wu X., Luo Q., Wei G., Xu M., Wu Y., Liu Y., Li X., Zi J., Ju W. NLRP3 regulates platelet integrin alphaIIbbeta3 outside-in signaling, hemostasis and arterial thrombosis. Haematologica. 2018;103:1568–1576. doi: 10.3324/haematol.2018.191700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiao J.L., Wu Y.L., Liu Y., Li X.Q., Wu X.Q., Liu N., Zhu F., Qi K.M., Cheng H., Li D.P. Busulfan triggers intrinsic mitochondrial-dependent platelet apoptosis independent of platelet activation. Biol. Blood Marrow Transplant. 2016;22:1565–1572. doi: 10.1016/j.bbmt.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Wei G., Luo Q., Wang X., Wu X., Xu M., Ding N., Zhao Y., Zhong L., Wang J., Wu Y. Increased GPIbalpha shedding from platelets treated with immune thrombocytopenia plasma. Int. Immunopharm. 2019;66:91–98. doi: 10.1016/j.intimp.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Arthur J.F., Qiao J., Shen Y., Davis A.K., Dunne E., Berndt M.C., Gardiner E.E., Andrews R.K. ITAM receptor-mediated generation of reactive oxygen species in human platelets occurs via Syk-dependent and Syk-independent pathways. J. Thromb. Haemostasis. 2012;10:1133–1141. doi: 10.1111/j.1538-7836.2012.04734.x. [DOI] [PubMed] [Google Scholar]
- 29.Montoro-Garcia S., Schindewolf M., Stanford S., Larsen O.H., Thiele T. The role of platelets in venous thromboembolism. Semin. Thromb. Hemost. 2016;42:242–251. doi: 10.1055/s-0035-1570079. [DOI] [PubMed] [Google Scholar]
- 30.Estevez B., Du X. New concepts and mechanisms of platelet activation signaling. Physiology. 2017;32:162–177. doi: 10.1152/physiol.00020.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flevaris P., Stojanovic A., Gong H., Chishti A., Welch E., Du X. A molecular switch that controls cell spreading and retraction. J. Cell Biol. 2007;179:553–565. doi: 10.1083/jcb.200703185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Benna J., Dang P.M., Gougerot-Pocidalo M.A., Marie J.C., Braut-Boucher F. p47phox, the phagocyte NADPH oxidase/NOX2 organizer: structure, phosphorylation and implication in diseases. Exp. Mol. Med. 2009;41:217–225. doi: 10.3858/emm.2009.41.4.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walter U., Gambaryan S. cGMP and cGMP-dependent protein kinase in platelets and blood cells. Handb. Exp. Pharmacol. 2009:533–548. doi: 10.1007/978-3-540-68964-5_23. [DOI] [PubMed] [Google Scholar]
- 34.Son Y., Cheong Y.K., Kim N.H., Chung H.T., Kang D.G., Pae H.O. Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? J. Signal Transduct. 2011;2011:792639. doi: 10.1155/2011/792639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi J., Ross C.R., Leto T.L., Blecha F. PR-39, a proline-rich antibacterial peptide that inhibits phagocyte NADPH oxidase activity by binding to Src homology 3 domains of p47 phox. Proc. Natl. Acad. Sci. U. S. A. 1996;93:6014–6018. doi: 10.1073/pnas.93.12.6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vara D., Campanella M., Pula G. The novel NOX inhibitor 2-acetylphenothiazine impairs collagen-dependent thrombus formation in a GPVI-dependent manner. Br. J. Pharmacol. 2013;168:212–224. doi: 10.1111/j.1476-5381.2012.02130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonomini F., Rodella L.F., Rezzani R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 2015;6:109–120. doi: 10.14336/AD.2014.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pignatelli P., Carnevale R., Di Santo S., Bartimoccia S., Sanguigni V., Lenti L., Finocchi A., Mendolicchio L., Soresina A.R., Plebani A. Inherited human gp91phox deficiency is associated with impaired isoprostane formation and platelet dysfunction. Arterioscler. Thromb. Vasc. Biol. 2011;31:423–434. doi: 10.1161/ATVBAHA.110.217885. [DOI] [PubMed] [Google Scholar]
- 39.Krotz F., Sohn H.Y., Pohl U. Reactive oxygen species: players in the platelet game. Arterioscler. Thromb. Vasc. Biol. 2004;24:1988–1996. doi: 10.1161/01.ATV.0000145574.90840.7d. [DOI] [PubMed] [Google Scholar]
- 40.Clutton P., Miermont A., Freedman J.E. Regulation of endogenous reactive oxygen species in platelets can reverse aggregation. Arterioscler. Thromb. Vasc. Biol. 2004;24:187–192. doi: 10.1161/01.ATV.0000105889.29687.CC. [DOI] [PubMed] [Google Scholar]
- 41.Chakrabarti S., Clutton P., Varghese S., Cox D., Mascelli M.A., Freedman J.E. Glycoprotein IIb/IIIa inhibition enhances platelet nitric oxide release. Thromb. Res. 2004;113:225–233. doi: 10.1016/j.thromres.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 42.Salmeen A., Barford D. Functions and mechanisms of redox regulation of cysteine-based phosphatases. Antioxidants Redox Signal. 2005;7:560–577. doi: 10.1089/ars.2005.7.560. [DOI] [PubMed] [Google Scholar]
- 43.Begonja A.J., Gambaryan S., Geiger J., Aktas B., Pozgajova M., Nieswandt B., Walter U. Platelet NAD(P)H-oxidase-generated ROS production regulates alphaIIbbeta3-integrin activation independent of the NO/cGMP pathway. Blood. 2005;106:2757–2760. doi: 10.1182/blood-2005-03-1047. [DOI] [PubMed] [Google Scholar]
- 44.Krotz F., Sohn H.Y., Gloe T., Zahler S., Riexinger T., Schiele T.M., Becker B.F., Theisen K., Klauss V., Pohl U. NAD(P)H oxidase-dependent platelet superoxide anion release increases platelet recruitment. Blood. 2002;100:917–924. doi: 10.1182/blood.v100.3.917. [DOI] [PubMed] [Google Scholar]
- 45.Gaitanaki C., Konstantina S., Chrysa S., Beis I. Oxidative stress stimulates multiple MAPK signalling pathways and phosphorylation of the small HSP27 in the perfused amphibian heart. J. Exp. Biol. 2003;206:2759–2769. doi: 10.1242/jeb.00483. [DOI] [PubMed] [Google Scholar]
- 46.Schroder K., Weissmann N., Brandes R.P. Organizers and activators: cytosolic Nox proteins impacting on vascular function. Free Radic. Biol. Med. 2017;109:22–32. doi: 10.1016/j.freeradbiomed.2017.03.017. [DOI] [PubMed] [Google Scholar]