Abstract
Purpose
Diabetic retinopathy (DR), a microvascular complication of diabetes, is the leading cause of visual disability and blindness in diabetic patients. Chronic hyperglycemia leads to increased oxidative stress and inflammation in the retina, resulting in microvascular damage. Our recent in vitro studies have demonstrated that inhibition of interleukin-6 (IL-6) trans-signaling significantly reduces oxidative stress in retinal endothelial cells. The purpose of this study was to further explore the relationship between IL-6 trans-signaling and oxidative stress using a streptozotocin (STZ) induced mouse model of early diabetic retinopathy.
Methods
Diabetes was induced in eight week-old male C57BL/6J mice using STZ injections. sgp130Fc (mouse sgp130Fc protein) treatment was used for inhibition of IL-6 trans-signaling. Studies were conducted to evaluate the effects of IL-6 trans-signaling on oxidative balance at the systemic and retinal level.
Results
Decreased antioxidant capacity and increased oxidative stress was observed in diabetic mice, which returned to near-normal levels with sgp130Fc treatment. Similarly, superoxide levels, lipid peroxidation, and markers of oxidative DNA damage were increased in the diabetic retina, and these effects were abrogated by sgp130Fc treatment. Inhibition of IL-6 trans-signaling also restored normal expression of catalase and endothelial nitric oxide synthase in mouse retinas.
Conclusions
Inhibition of IL-6 trans-signaling significantly reduces diabetes-induced oxidative damage at the systemic level and in the retina. These findings provide further evidence for the role of IL-6 trans-signaling in diabetes-mediated oxidative stress.
Keywords: Diabetic retinopathy, Oxidative stress, IL-6 trans-signaling
Graphical abstract
Highlights
-
•
Decreased antioxidant capacity and increased oxidative stress in mice with DR.
-
•
Inhibition of L-6 trans-signaling restores catalase and eNOS in the retina.
-
•
Inhibition of IL-6 trans-signaling reduces retinal oxidative damage.
1. Introduction
Diabetic retinopathy (DR) is characterized by abnormal neovascularization of the retina, endothelial dysfunction, and vascular inflammation. DR pathogenesis is linked to several cytokines including vascular endothelial growth factor (VEGF), tumor necrosis factor (TNF), and interleukin-6 (IL-6) [[1], [2], [3]]. Other changes that contribute to DR development include increased advanced glycation end-products (AGEs), overexpression of integrins and adhesion molecules, and increased oxidative stress in the retina [[3], [4], [5], [6], [7]].
IL-6 is a pleiotropic cytokine known to be elevated in the serum and vitreous of DR patients [[8], [9], [10], [11], [12]]. Recent studies suggest the pro-inflammatory effects of IL-6 in vasculature are mainly mediated by a soluble form of the IL-6 receptor (sIL-6R) via a mechanism known as IL-6 trans-signaling [[13], [14], [15]]. IL-6 trans-signaling is implicated in the pathogenesis of several inflammatory diseases, including arthritis, pulmonary fibrosis, and cancer [13,16,17]. Levels of sIL-6R are elevated in patients with DR, suggesting a role for IL-6 trans-signaling in DR pathology [[16], [17], [18], [19], [20], [21]]. The commercially available compound sgp130Fc (soluble gp130-IgG1 fused chimera) selectively binds the IL6-sIL6R complex and does not interfere with IL-6 alone, allowing for selective inhibition of IL-6 trans-signaling.
Reactive oxygen species (ROS) play an important role in the pathology of retinal damage in DR. Hyperglycemia induces over-activation of cellular glucose metabolism, leading to increased flux through the tricarboxylic acid (TCA) cycle and elevated levels of superoxide-generating TCA intermediates [22]. Elevated ROS production disrupts the oxidative balance maintained by cellular antioxidant defense systems, leading to oxidative damage to DNA, lipids, and proteins, thus disrupting normal cellular function [6,[23], [24], [25]]. In addition to direct oxidative damage, superoxide production also activates the polyol, hexosamine, and protein kinase C pathways, along with production of AGEs [25,26].
We recently published that exogenous stimulation of IL-6 trans-signaling contributes to inflammation, increased ROS generation, and barrier dysfunction in retinal endothelial cells [27]. Selective inhibition of IL-6 trans-signaling using sgp130Fc could mitigate these effects [27]. In the present study, we evaluated the effects of IL-6 trans-signaling inhibition on both systemic and retinal oxidative stress in a streptozotocin (STZ)-induced mouse model of early diabetic retinopathy.
2. Materials and methods
2.1. Animal studies
Eight week-old male C57BL/6J mice were randomly assigned to four groups: controls, diabetic, diabetic with sgp130Fc treatment, and non-diabetic controls with sgp130Fc treatment (n = 8 per group). Diabetes was induced in two groups using streptozotocin (STZ) injections (65 mg/kg STZ in sodium citrate buffer 0.05 M, pH 4.5) daily for five days. After eight weeks of hyperglycemia, appropriate treatment groups received 5 mg/kg sgp130Fc (mouse sgp130Fc protein, R&D Systems, Minneapolis, MN) [28,29], by intraperitoneal injection twice weekly for 2 weeks. After 10 weeks, mice were euthanized and blood, tissues, and vitreous fluid were collected for molecular studies.
2.2. Measurement of oxidative stress markers in serum, plasma, and vitreous fluid
Total antioxidant capacity in serum was measured using colorimetric assay kit (Cayman Chemical, Ann Arbor, MI). Malondialdehyde (MDA) levels in plasma were measured by ELISA (Cat #K739-100, BioVision, Milpitas, CA). Protein carbonyl content in vitreous fluid was measured using Protein Carbonyl Colorimetric Assay kit (Cat # 10005020, Cayman Chemical, Ann Arbor, MI).
2.3. Immunohistochemistry
Retinal sections were evaluated for 8-hydroxy-2′-deoxyguanosine (8-OHdG), a marker of oxidative DNA damage [30], by immunohistochemistry, using anti-8-OHdG monoclonal antibody (1:200 dilution; #SC-66036, Santa Cruz Biotechnology, Dallas TX) and 3,3′-diaminobenzidine tetrahydrochloride (DAB) peroxidase substrate kit (Vector Laboratories, CA, USA). The fluorescent dye dihydroethidium (DHE) was used to measure superoxide levels in fresh frozen retinal tissue as previously published [31]. For malondialdehyde (MDA) staining, cryosections were incubated overnight with MDA antibody (Abcam #ab6463, Cambridge, MA; 1:200 dilution), and with AlexaFluor 488 secondary antibody (Invitrogen, Carlsbad, CA; 1:200 for 1hr). Images were captured using a Keyence BZ-X710 fluorescent microscope (Itasca, IL).
2.4. Western blotting
Mouse retinal homogenates were prepared in RIPA buffer by sonication. Proteins were separated by 4–15% SDS-PAGE gel and probed using primary antibodies for eNOS (ab199956, Abcam, Cambridge, UK) and Catalase (sc-271803). Intensities were normalized to β-actin levels (sc-47778).
2.5. Statistical analysis
Two-way ANOVA followed by Tukey multiple comparisons test or student's t-test were used to determine differences between experimental groups. Statistical analyses were performed using GraphPad Prism software (GraphPad Software Inc. V6.0, San Diego, CA, USA). Data in bar-plots are expressed as means ± SEM.
3. Results
3.1. Effects of sgp130Fc treatment on body weight and glucose levels
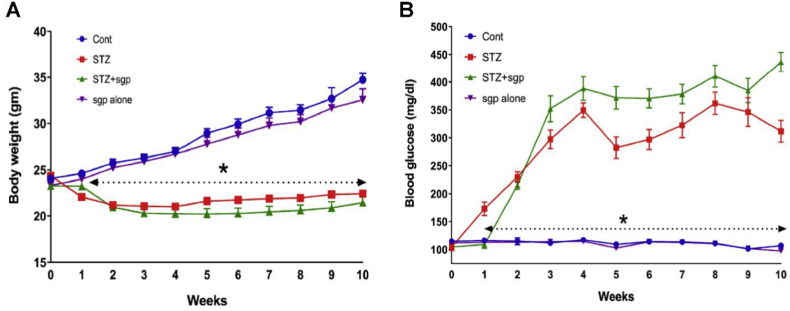
Blood glucose levels and body weight were monitored weekly for the entire duration of the study (10 weeks) (Fig. 1). The two groups with diabetes showed significantly decreased weight gain compared to non-diabetic groups (Fig. 1A). Treatment with sgp130Fc was initiated at week 8, and no adverse effects on body weight were observed (Fig. 1A). Blood glucose levels in non-diabetic animals were in the normal range of 100–120 mg/dL while levels in diabetic mice were consistently in the range of 300–400 mg/dL, with and without sgp130Fc treatment (Fig. 1B).
Fig. 1.
Effect of IL-6 trans-signaling inhibition on body weight and blood glucose levels in C57BL/6J diabetic mice. Body weight (A) and blood glucose levels (B) of all 4 groups of mice were monitored for the duration of the study. IL-6 trans-signaling inhibition using sgp130Fc treatment does not have a significant effect on the body weight and the blood glucose levels in both diabetic and non-diabetic mice. Data represents mean ± SEM, n = 8/group, *p < 0.05 vs control.
3.2. Protective effects of sgp130Fc in maintaining oxidative balance in diabetic mice
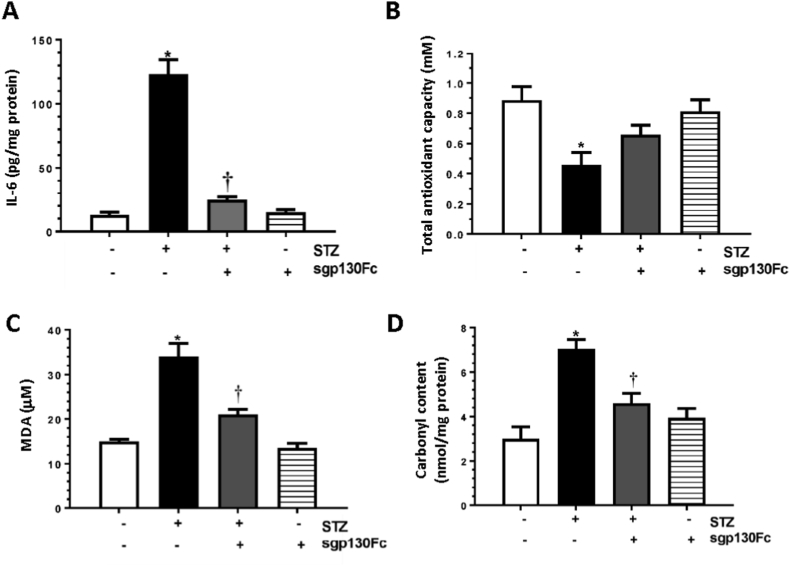
IL-6 levels in diabetic mice were significantly higher (~10-fold) than controls and significantly decreased following sgp130Fc treatment (Fig. 2A). In diabetic conditions, endogenous production of ROS overwhelms the antioxidant capacity leading to oxidative stress. Significantly lower antioxidant capacity was observed in diabetic mice serum (~0.5 fold) compared to control mice (0.45 vs 0.87 mM) (Fig. 2B). This decrease in antioxidant capacity was mitigated by treatment with sgp130Fc in diabetic mice. The antioxidant capacity in the serum of control mice treated with sgp130Fc was similar to untreated controls (0.81 vs 0.87 mM).
Fig. 2.
sgp130Fc treatment reduces oxidative stress in diabetic mice at systemic level and in the vitreous fluid. (A) Serum IL-6 levels, (B) total antioxidant capacity in serum, (C) MDA levels in plasma, and (D) carbonyl content in vitreous fluid were measured in control and diabetic mice with and without sgp130Fc treatment. Results are expressed as mean ± SEM. *p < 0.05 vs control. †p < 0.05 vs STZ.
Oxygen free radicals cause peroxidation of phospholipids, leading to accumulation of malondialdehyde (MDA); MDA levels in the blood are an excellent biomarker of lipid peroxidation. In diabetic animals, plasma MDA levels were ~2-fold higher than in non-diabetic controls (34.0 vs 14.5 μM) (Fig. 2C) and were significantly reduced by sgp130Fc treatment. Levels were unchanged in mice treated with sgp130Fc only compared to controls (14.0 vs 14.5 μM).
Oxidative damage to proteins results in carbonylation of amino acid side chains, which contribute to diabetes-mediated cellular dysfunction. Previous studies have shown that protein carbonylation is significantly increased in the vitreous of diabetic patients compared to control subjects [32], indicating the presence of oxidative stress. Protein carbonyl content was significantly increased in the vitreous fluid of diabetic mice compared to control mice (7.0 vs 2.9 nmol/mg protein) (Fig. 2D), and this effect was attenuated by sgp130Fc treatment. No significant change was observed in the protein carbonyl content in control mice treated with sgp130Fc (3.8 vs 2.9 nmol/mg).
3.3. Inhibition of IL-6 trans-signaling mitigates diabetes-induced superoxide generation and oxidative damage in the retina
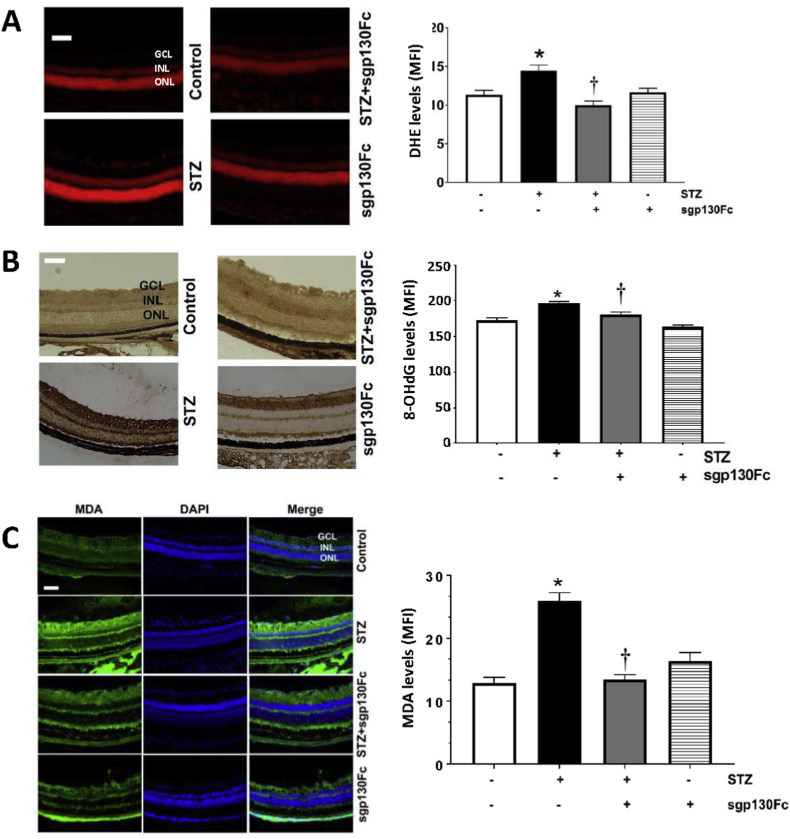
Retinal tissues were incubated with DHE, and superoxide generation was measured as total DHE fluorescence. Superoxide levels were increased (~1.5-fold) in the retinas of diabetic animals compared to controls (Fig. 3A). In diabetic animals treated with sgp130Fc, superoxide was reduced to levels seen in control animals, and there was no significant difference between sgp130Fc treated and untreated non-diabetic control animals.
Fig. 3.
sgp130Fc treatment reduces oxidative damage in diabetic mice retinas. Retinal sections were stained with (A) dihydroxyethidium (DHE) to measure ROS generation, (B) 8-OHdG, an oxidative DNA damage marker, and (C) MDA. Staining intensities were quantified, and results are expressed as mean ± SEM. *p < 0.05 vs control. †p < 0.05 vs STZ. MFI: Mean fluorescence intensity; GCL: Ganglion cell layer; INL: Inner nuclear layer; ONL: Outer nuclear layer. Scale bar = 100 μm.
A common marker of oxidative DNA damage is 8-oxo-deoxyguanosine (8-OHdG), which is produced during the oxidation of DNA by reactive oxygen species [33,34]. Studies have shown that 8-OHdG levels are increased in the serum and urine of diabetic patients [35]. We measured 8-OHdG levels in mice retinas by immunohistochemistry, and 8-OHdG immunoreactivity was weakly detected in non-diabetic mice, both with and without sgp130Fc treatment (Fig. 3B). 8-OHdG was strongly detected in diabetic mice, whereas levels in diabetic mice treated with sgp130Fc were significantly reduced, nearly to the levels of controls (Fig. 3B).
Different layers of retina were evaluated for lipid peroxidation using MDA staining. MDA levels were significantly increased in the ganglion cell layer (GCL), inner nuclear layer (INL), and outer nuclear layer (ONL) of diabetic mice retinas relative to non-diabetic controls (Fig. 3C), and this elevation was alleviated by treatment with sgp130Fc. Non-diabetic mice treated with sgp130Fc showed levels of MDA staining similar to untreated controls.
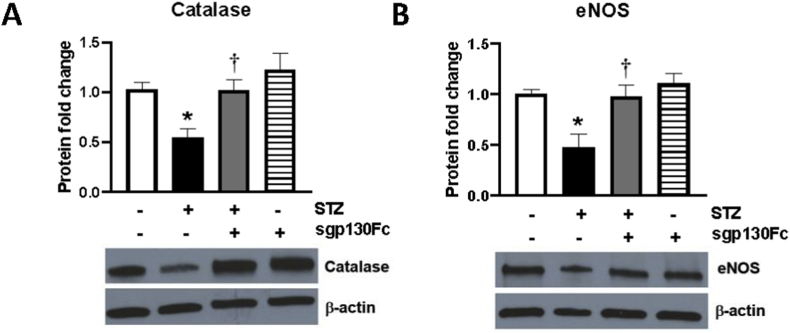
3.4. Inhibition of IL-6 trans-signaling restores normal expression of catalase and eNOS in the diabetic mouse retina
Earlier studies exploring the role of oxidative stress in the pathogenesis of diabetic retinopathy have shown that diabetes decreases expression of catalase [36,37] and endothelial nitric oxide synthase (eNOS) [38,39]. Mouse retinal homogenates were evaluated for catalase and eNOS protein levels (Fig. 4), and both enzymes were significantly reduced in diabetic animals (0.54-fold and 0.47-fold, respectively) as compared to non-diabetic controls. Inhibition of IL-6 trans-signaling using sgp130Fc restored the levels of both enzymes back to control levels. No significant change was observed in the catalase and eNOS protein levels in control mice treated with sgp130Fc.
Fig. 4.
Inhibition of IL-6 trans-signaling restores normal catalase and eNOS expression in mouse retinal tissue. (A) Catalase and (B) eNOS protein levels were measured by western blot in mouse retinal tissue. n = 4–8/group. Results are expressed as mean ± SEM. *p < 0.05 vs control, †p < 0.05 vs STZ.
4. Discussion
IL-6 signaling occurs via two mechanisms: classical signaling through a membrane-bound receptor (mIL-6R), or trans-signaling through a soluble receptor (sIL-6R). In trans-signaling, sIL-6R associates with IL-6 in circulation and the complex binds to gp130 on the cell membrane to initiate downstream signaling. Evidence suggests that IL-6 trans-signaling might be responsible for many of the pro-inflammatory effects of IL-6 [13]. Of clinical relevance, IL-6 trans-signaling can be selectively inhibited by the fusion protein sgp130Fc [40]. We recently published that stimulation of IL-6 trans-signaling increased oxidative stress, H2O2 levels, mitochondrial superoxide, and mitochondrial dysfunction in HRECs [27] and that these effects could be inhibited by treatment with sgp130Fc. The goal of this study was to determine the effects of selective inhibition of IL-6 trans-signaling on oxidative stress in an in-vivo model of diabetic retinopathy.
Diabetic retinopathy is a systemic disease, and the roles of vascular inflammation and oxidative stress in DR are well-known [3,7,41]. Chronic hyperglycemia is associated with retinal vascular inflammation, increased endothelial permeability, and barrier dysfunction [42,43]. These pro-inflammatory effects of hyperglycemia on retinal endothelial cells are mediated by inflammatory cytokines rather than increased cellular glucose uptake [44]. Additionally, oxidative stress is important in the development and progression of DR [23,24,45]. Compared to other tissues, the retina is particularly vulnerable to oxidative stress due to its high levels of glucose oxidation, oxygen consumption, and fatty acid content, which increase the risk of oxidative damage [46].
Recently, IL-6 has emerged as an important factor in diabetic retinopathy pathogenesis [[47], [48], [49]]. IL-6 is implicated in vascular inflammation and remodeling, and levels of IL-6 in vitreous fluid are correlated with disease severity [9,50,51]. While both IL-6 signaling and oxidative stress contribute to DR development, relatively little has been published about a potential relationship between these two processes and few mechanistic links have been proposed. IL-6 is reported to increase oxidative stress in vascular endothelial cells by inducing expression of the angiotensin II type 1 receptor [52]. In the retina specifically, IL-6−/− mice show significantly lower levels of superoxide generation in response to angiotensin-II stimulation than wildtype mice [51], suggesting a link between IL-6 and oxidative stress in retinal pathology. In the present study, we found that selective inhibition of IL-6 trans-signaling reduces systemic oxidative stress in diabetic mice and decreases diabetes-induced oxidative damage within the retina. To our knowledge, this is the first evidence specifically linking the inhibition of IL-6 trans-signaling with reduced retinal oxidative stress.
Catalase is one of the most crucial enzymes that mitigates oxidative stress and is responsible for the conversion of H2O2 to molecular oxygen and water. Low levels of catalase expression are correlated with higher H2O2 levels [53]. Endothelial nitric oxide synthase catalyzes the production of NO, a crucial mediator of vasodilation known to be reduced in oxidative stress [54]. In this study, we found reduced catalase and eNOS levels in diabetic mice retina. Several others have also reported reduced catalase and eNOS under diabetic conditions [[36], [37], [38], [39]]. To our knowledge, the relationship between IL-6 trans-signaling and catalase or eNOS expression has not yet been described. PI3K/Akt/mTOR signaling, a downstream effector of IL-6, has been shown to inhibit catalase in some cancer cells [55,56], but IL-6 classical signaling has also been linked with increased catalase activity [57]. IL-6 classical signaling has been reported to decrease eNOS activity [58,59], while elevated oxidative stress has been associated with increased eNOS activity [60]. Future work will aim to characterize the relationship between IL-6 trans-signaling and these enzymes, as well as the precise mechanism linking IL-6 trans-signaling to oxidative stress.
In conclusion, our study provides evidence supporting a novel link between IL-6 trans-signaling and the disruption of oxidative balance in the diabetic retina, thereby demonstrating the therapeutic potential of sgp130Fc in the treatment of DR.
Funding
This work was funded by an NIH/NEI grant (R01-EY026936) awarded to Shruti Sharma, PhD.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Caldwell R.B. Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Metab. Res. Rev. 2003;19(6):442–455. doi: 10.1002/dmrr.415. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson B.P., Schachat A.P. A review of clinical trials of anti-VEGF agents for diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2010;248(7):915–930. doi: 10.1007/s00417-010-1315-z. [DOI] [PubMed] [Google Scholar]
- 3.Tang J., Kern T.S. Inflammation in diabetic retinopathy. Prog. Retin. Eye Res. 2011;30(5):343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soedamah-Muthu S.S. Soluble vascular cell adhesion molecule-1 and soluble E-selectin are associated with micro- and macrovascular complications in Type 1 diabetic patients. J. Diabet. Complicat. 2006;20(3):188–195. doi: 10.1016/j.jdiacomp.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Gustavsson C. Vascular cellular adhesion molecule-1 (VCAM-1) expression in mice retinal vessels is affected by both hyperglycemia and hyperlipidemia. PloS One. 2010;5(9) doi: 10.1371/journal.pone.0012699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kowluru R.A., Mishra M. Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2015;1852(11):2474–2483. doi: 10.1016/j.bbadis.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Behl T., Kaur I., Kotwani A. Implication of oxidative stress in progression of diabetic retinopathy. Surv. Ophthalmol. 2016;61(2):187–196. doi: 10.1016/j.survophthal.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Koleva-Georgieva D.N., Sivkova N.P., Terzieva D. Serum inflammatory cytokines IL-1β, IL-6, TNF-α and VEGF have influence on the development of diabetic retinopathy. Folia Med. 2011;53(2):44–50. doi: 10.2478/v10153-010-0036-8. [DOI] [PubMed] [Google Scholar]
- 9.Funatsu H. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefe’s Arch. Clin. Exp. Ophthalmol. 2005;243(1):3–8. doi: 10.1007/s00417-004-0950-7. [DOI] [PubMed] [Google Scholar]
- 10.Hou T. Roles of IL-6-gp130 signaling in vascular inflammation. Curr. Cardiol. Rev. 2008;4(3):179. doi: 10.2174/157340308785160570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes T.C., Anderson M.E., Moots R.J. The many faces of interleukin-6: the role of IL-6 in inflammation, vasculopathy, and fibrosis in systemic sclerosis. Int. J. Rheumatol. 2011;2011 doi: 10.1155/2011/721608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauer J. Regulation of interleukin 6 receptor expression in human monocytes and monocyte-derived macrophages. Comparison with the expression in human hepatocytes. J. Exp. Med. 1989;170(5):1537–1549. doi: 10.1084/jem.170.5.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int. J. Biol. Sci. 2012;8(9):1237. doi: 10.7150/ijbs.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rose‐John S. Interleukin‐6 biology is coordinated by membrane‐bound and soluble receptors: role in inflammation and cancer. J. Leukoc. Biol. 2006;80(2):227–236. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- 15.Scheller J. The pro-and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta Mol. Cell Res. 2011;1813(5):878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 16.Becker C. TGF-β suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21(4):491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 17.Le T.-T.T. Blockade of IL-6 trans signaling attenuates pulmonary fibrosis. J. Immunol. 2014;193(7):3755–3768. doi: 10.4049/jimmunol.1302470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nowell M.A. Therapeutic targeting of IL-6 trans signaling counteracts STAT3 control of experimental inflammatory arthritis. J. Immunol. 2009;182(1):613–622. doi: 10.4049/jimmunol.182.1.613. [DOI] [PubMed] [Google Scholar]
- 19.Goumas F.A. Inhibition of IL‐6 signaling significantly reduces primary tumor growth and recurrencies in orthotopic xenograft models of pancreatic cancer. Int. J. Canc. 2015;137(5):1035–1046. doi: 10.1002/ijc.29445. [DOI] [PubMed] [Google Scholar]
- 20.Sharma S. Elevated serum levels of soluble TNF receptors and adhesion molecules are associated with diabetic retinopathy in patients with type-1 diabetes. Mediat. Inflamm. 2015:2015. doi: 10.1155/2015/279393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H. Increased levels of IL-6, sIL-6R, and sgp130 in the aqueous humor and serum of patients with diabetic retinopathy. Mol. Vis. 2016;22:1005. [PMC free article] [PubMed] [Google Scholar]
- 22.Nishikawa T. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404(6779):787. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 23.Hammes H.P. Diabetic retinopathy: hyperglycaemia, oxidative stress and beyond. Diabetologia. 2018;61(1):29–38. doi: 10.1007/s00125-017-4435-8. [DOI] [PubMed] [Google Scholar]
- 24.Calderon G.D. Oxidative stress and diabetic retinopathy: development and treatment. Eye. 2017;31(8):1122–1130. doi: 10.1038/eye.2017.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circ. Res. 2010;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du X.-L. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc. Natl. Acad. Sci. Unit. States Am. 2000;97(22):12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valle M.L. Inhibition of interleukin-6 trans-signaling prevents inflammation and endothelial barrier disruption in retinal endothelial cells. Exp. Eye Res. 2019;178:27–36. doi: 10.1016/j.exer.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLoughlin R.M. IL-6 trans-signaling via STAT3 directs T cell infiltration in acute inflammation. Proc. Natl. Acad. Sci. Unit. States Am. 2005;102(27):9589–9594. doi: 10.1073/pnas.0501794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho S.W. The soluble interleukin-6 receptor is a mediator of hematopoietic and skeletal actions of parathyroid hormone. J. Biol. Chem. 2013;288(10):6814–6825. doi: 10.1074/jbc.M112.393363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beckman K.B., Ames B.N. Oxidative decay of DNA. J. Biol. Chem. 1997;272(32):19633–19636. doi: 10.1074/jbc.272.32.19633. [DOI] [PubMed] [Google Scholar]
- 31.Pushpakumar S.B. Folic acid mitigates angiotensin-II-induced blood pressure and renal remodeling. PloS One. 2013;8(12) doi: 10.1371/journal.pone.0083813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Géhl Z. Diabetes-induced oxidative stress in the vitreous humor. Redox Biol. 2016;9:100–103. doi: 10.1016/j.redox.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Souza-Pinto N.C. Repair of 8-oxodeoxyguanosine lesions in mitochondrial dna depends on the oxoguanine dna glycosylase (OGG1) gene and 8-oxoguanine accumulates in the mitochondrial dna of OGG1-defective mice. Canc. Res. 2001;61(14):5378–5381. [PubMed] [Google Scholar]
- 34.Valavanidis A. Pulmonary oxidative stress, inflammation and cancer: respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int. J. Environ. Res. Publ. Health. 2013;10(9):3886–3907. doi: 10.3390/ijerph10093886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan H.-Z. The change of oxidative stress products in diabetes mellitus and diabetic retinopathy. Br. J. Ophthalmol. 2008;92(4):548–551. doi: 10.1136/bjo.2007.130542. [DOI] [PubMed] [Google Scholar]
- 36.Dave G.S., Kalia K. Hyperglycemia induced oxidative stress in type-1 and type-2 diabetic patients with and without nephropathy. Cell. Mol. Biol. (Noisy-Le-Grand) 2007;53(5):68–78. [PubMed] [Google Scholar]
- 37.Sindhu R.K. Dysregulation of hepatic superoxide dismutase, catalase and glutathione peroxidase in diabetes: response to insulin and antioxidant therapies. Clin. Exp. Hypertens. 2004;26(1):43–53. doi: 10.1081/ceh-120027330. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y. The Puerarin improves renal function in STZ-induced diabetic rats by attenuating eNOS expression. Ren. Fail. 2015;37(4):699–703. doi: 10.3109/0886022X.2015.1011500. [DOI] [PubMed] [Google Scholar]
- 39.Yu J.-W. Metformin improves the angiogenic functions of endothelial progenitor cells via activating AMPK/eNOS pathway in diabetic mice. Cardiovasc. Diabetol. 2016;15(1):88. doi: 10.1186/s12933-016-0408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jostock T. Soluble gp130 is the natural inhibitor of soluble interleukin‐6 receptor transsignaling responses. Eur. J. Biochem. 2001;268(1):160–167. doi: 10.1046/j.1432-1327.2001.01867.x. [DOI] [PubMed] [Google Scholar]
- 41.Kowluru R.A., Chan P.S. Oxidative stress and diabetic retinopathy. Exp. Diabetes Res. 2007;2007:43603. doi: 10.1155/2007/43603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joussen A.M. A central role for inflammation in the pathogenesis of diabetic retinopathy. Faseb. J. 2004;18(12):1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 43.Du Y., Sarthy V.P., Kern T.S. Interaction between NO and COX pathways in retinal cells exposed to elevated glucose and retina of diabetic rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287(4):R735–R741. doi: 10.1152/ajpregu.00080.2003. [DOI] [PubMed] [Google Scholar]
- 44.Busik J.V., Mohr S., Grant M.B. Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes. 2008;57(7):1952–1965. doi: 10.2337/db07-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kowluru R.A. Effect of reinstitution of good glycemic control on retinal oxidative stress and nitrative stress in diabetic rats. Diabetes. 2003;52(3):818–823. doi: 10.2337/diabetes.52.3.818. [DOI] [PubMed] [Google Scholar]
- 46.Anderson R.E., Rapp L.M., Wiegand R.D. Lipid peroxidation and retinal degeneration. Curr. Eye Res. 1984;3(1):223–227. doi: 10.3109/02713688408997203. [DOI] [PubMed] [Google Scholar]
- 47.El-Asrar A.M.A. Role of inflammation in the pathogenesis of diabetic retinopathy. Middle East Afr. J. Ophthalmol. 2012;19(1):70. doi: 10.4103/0974-9233.92118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mocan M.C., Kadayifcilar S., Eldem B. Elevated intravitreal interleukin-6 levels in patients with proliferative diabetic retinopathy. Can. J. Ophthalmol. 2006;41(6):747–752. doi: 10.3129/i06-070. [DOI] [PubMed] [Google Scholar]
- 49.Shimizu E. Plasma level of interleukin-6 is an indicator for predicting diabetic macular edema. Jpn. J. Ophthalmol. 2002;46(1):78–83. doi: 10.1016/s0021-5155(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 50.Kocak N. Comparison of vitreous and plasma levels of vascular endothelial growth factor, interleukin-6 and hepatocyte growth factor in diabetic and non-diabetic retinal detachment cases. Ann. Ophthalmol. 2010;42:10–14. Spec No. [PubMed] [Google Scholar]
- 51.Rojas M. Role of IL-6 in angiotensin II-induced retinal vascular inflammation. Invest. Ophthalmol. Vis. Sci. 2010;51(3):1709–1718. doi: 10.1167/iovs.09-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wassmann S. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ. Res. 2004;94(4):534–541. doi: 10.1161/01.RES.0000115557.25127.8D. [DOI] [PubMed] [Google Scholar]
- 53.Glorieux C. Regulation of catalase expression in healthy and cancerous cells. Free Radic. Biol. Med. 2015;87:84–97. doi: 10.1016/j.freeradbiomed.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 54.Pierini D., Bryan N.S. Advanced Protocols in Oxidative Stress III. Springer; 2015. Nitric oxide availability as a marker of oxidative stress; pp. 63–71. [DOI] [PubMed] [Google Scholar]
- 55.Glorieux C. Catalase expression in MCF-7 breast cancer cells is mainly controlled by PI3K/Akt/mTor signaling pathway. Biochem. Pharmacol. 2014;89(2):217–223. doi: 10.1016/j.bcp.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 56.Akca H. Tumour suppressor PTEN enhanced enzyme activity of GPx, SOD and catalase by suppression of PI3K/AKT pathway in non-small cell lung cancer cell lines. J. Enzym. Inhib. Med. Chem. 2013;28(3):539–544. doi: 10.3109/14756366.2011.654114. [DOI] [PubMed] [Google Scholar]
- 57.Yamada K. Possible involvement of catalase in the protective effect of interleukin-6 against 6-hydroxydopamine toxicity in PC12 cells. Brain Res. Bull. 1997;43(6):573–577. doi: 10.1016/s0361-9230(96)00336-x. [DOI] [PubMed] [Google Scholar]
- 58.Saura M. Stat3 mediates interelukin-6 inhibition of human endothelial nitric-oxide synthase expression. J. Biol. Chem. 2006;281(40):30057–30062. doi: 10.1074/jbc.M606279200. [DOI] [PubMed] [Google Scholar]
- 59.Hung M.-J. Interleukin-6 inhibits endothelial nitric oxide synthase activation and increases endothelial nitric oxide synthase binding to stabilized caveolin-1 in human vascular endothelial cells. J. Hypertens. 2010;28(5):940–951. doi: 10.1097/HJH.0b013e32833992ef. [DOI] [PubMed] [Google Scholar]
- 60.Ding H., Aljofan M., Triggle C.R. Oxidative stress and increased eNOS and NADPH oxidase expression in mouse microvessel endothelial cells. J. Cell. Physiol. 2007;212(3):682–689. doi: 10.1002/jcp.21063. [DOI] [PubMed] [Google Scholar]