Abstract
Background
Studies have shown that the primary causes of death in patients with acute coronary syndrome are arrhythmias and heart failure. The aim of this study is to evaluate the short-term prognosis of fragmented QRS (f-QRS) in patients with acute myocardial infarction (MI).
Methods
This study was a prospective and longitudinal analytic study performed on all patients with acute MI admitted to Rasht Heshmat Hospital Emergency during 2018–2019. Serial Electrocardiography (ECG) was performed in the emergency room after patient admission and was repeated 24 h after percutaneous coronary intervention and fibrinolytic therapy, as well as at the time of patient discharge. Short-term prognosis of f-QRS in patients was evaluated by a cardiologist within admission, 40 days after hospitalization and three months later again.
Results
In this study, 453 patients with MI were evaluated in two treatment methods of fibrinolytic and invasive with and without f-QRS. Based on the data of this study, the four study groups had no statistically significant difference in arrhythmia (p = 0.196). In addition, the effect of study groups on left ventricular ejection fraction index was not statistically significant (p = 0.597). The probability of adverse outcomes occurrence was not statistically significant among the four groups (p = 0.07).
Conclusion
The final results of this study showed that there was no significant difference between the four study groups and arrhythmia status. Therefore, f-QRS was not introduced as an independent predictor of arrhythmia in patients with acute MI.
Keywords: Fragmented QRS complexes, Non-ST elevated acute myocardial infarction, Acute coronary syndrome, Prognosis
1. Introduction
Acute coronary syndrome (ACS) comprises a range of clinical manifestations from ST-segment elevation myocardial infarction (STEMI) to non-ST-segment elevation myocardial infarction and unstable angina.1 Studies have shown that the primary causes of death in patients with ACS are arrhythmias and heart failure.2,3 The most effective treatment for arrhythmias during peri-infract period is rapid and complete revascularization. Percutaneous coronary intervention (PCI), if performed by a skilled person at an equipped center in a time period of fewer than 90 min from the time of patient arrival, can cause blood flow to the myocardial tissue in more than 90% of patients.4,5 However, fibrinolytic therapy can also be used if primary PCI is not available. If used within the first 2 h of symptom onset, fibrinolytic (thrombolytic) therapy is capable of restoring blood flow in approximately 75% of patients.6
Some thorough researches are ongoing to find a noninvasive way to classify life-threatening arrhythmias in patients with ACS to more accurate patterns. Fragmented QRS (f-QRS) is a noninvasive electrocardiographic parameter that is easy to evaluate.7 f-QRS complexes are unique electrocardiographic signals that indicate the altered ventricular conduction around areas with myocardial scar.8 f-QRS is defined as the presence of different RSR' patterns with or without Q-wave.7 The f-QRS complex is classified into various RSR’ patterns, including rSR’, rSr ', RSR’, notched S, and notched R.9 The fragmented narrow QRS is determined as the presence of an additional R wave (R′) or truncation in the S wave, or the presence of more than 1 R′ in 2 adjacent leads, associated with a region of the major coronary artery in the resting 12-lead electrocardiogram. The fragmented wide QRS (f-wQRS) consisting of different RSR patterns is defined by more than 2 R waves (R″) or more than 2 truncations in the R wave, or more than 2 truncations in the downstroke or upstroke of S wave.1
In recent years, numerous studies have shown that the presence of f-QRS in the electrocardiogram in the course of ACS can be a sign of life-threatening arrhythmia. On the other hand, some studies have shown that f-QRS in the superficial ECG is not a reliable predictor of myocardial scar, coronary disease in angiography, and long-term adverse outcomes.8 Up to the aforementioned explanation and as well as the lack of a comprehensive study considered these subjects in Iran, this article was focused on the evaluation of the short-term prognosis of f-QRS in patients with acute MI in two groups received invasive and fibrinolytic therapy.
2. Methods
2.1. The study population
This study was a prospective and longitudinal analytic study performed on all patients with acute MI admitted to Rasht Heshmat Hospital Emergency, from 2018 to 2019. Severe heart valve diseases, congenital heart disease, patients with pacemakers, diseases or abnormalities of organs such as cancer, liver or kidney failure, and neurological or mental illnesses were considered as the exclusion criteria. This study is based on the research proposal, approved by the research and technology directorate of Guilan University of Medical Sciences with Ethics Committee code of IR.GUMS.REC.1397.294.
2.2. Data collection
Demographic and clinical data including gender, age, Coronary Artery Disease (CAD) risk factors and types of MI were recorded. Serial ECG was performed in the emergency room after patient admission and was repeated 24 h after PCI and fibrinolytic therapy, as well as at the time of patient discharge. ECG was performed daily for each patient and electrocardiography was repeated until discharge or death of the patient, if any heart problems occurred. in addition, ECG was performed on all patients after invasive or fibrinolytic therapy. ECGs were compared with previous ECGs, if available, to confirm f-QRS.
In this study, the f-QRS is defined as:
Narrow f-QRS: f-QRS contains diverse RSR’ patterns with different morphologies of QRS complexes with the exception of bundle branch block, with or without Q-wave in 12-lead ECG observed at least in two adjacent leads associated with a myocardial range.
Fragmented wide QRS: In a wide QRS wave of bundle branch block (QRS> 120 ms), R wave or S wave with more than two notches is seen in 2 adjacent leads (F-LBBB QRS, F-RBBB QRS); In a wide QRS complex of premature ventricular contractions (PVCs), R waves have two notches and an interval of more than 40 ms between two notches of QRS waves in two or more adjacent leads are observed (f-PVCs).10
All patients underwent 24-h digital Holter monitoring, on average until the third day of admission. Considering the obtained data, patients were diagnosed with different abnormalities and arrhythmias (such as supraventricular extrasystole, ventricular extrasystole, bradycardia, pause, atrial fibrillation (AF), atrial flutter (AFL), and the presence of grade 2 or 3 atrioventricular (AV) block).
Echocardiography was also performed in all patients and patients with left ventricular ejection fraction (LVEF) less than 40% were considered as left ventricular systolic dysfunction (LVSD). All patients were recalled first on the 40th day and then three months after admission and underwent electrocardiography and echocardiography.
2.3. Prognosis of patients
In this study, based on the presence or absence of major adverse cardiovascular events (MACE) (consisting of congestive heart failure, cardiogenic shock, grade 2 and 3 heart block, ventricular tachycardia [VT] and fibrillation, repeated myocardial infarction [MI], revascularization, stroke, restenting, and cardiac surgery), short-term prognosis of f-QRS in patients was evaluated by a cardiologist during hospitalization, 40 days and three months after hospitalization by phone call and visit every three months in the outpatient clinics of Heshmat hospital.
2.4. Data analysis
The collected data were entered into SPSS version,21 software. The chi-squared test and odds ratio were used to compare the frequency of MACE in patients with acute MI in two groups received invasive and fibrinolytic therapy with and without f-QRS. Independent t-test and ANOVA test were used to compare, if the assumptions were established (if the parametric tests do not exit, nonparametric Mann–Whitney U test was applied). The logistic regression model, by controlling the effect of mediating and contextual variables, was used to investigate the relation of f-QRS in patients with acute MI in two groups receiving invasive and fibrinolytic therapy. Significance level of tests in this study was considered as p < 0.05.
3. Results
In this study, the 453 patients with acute MI admitted to Heshmat hospital were thoroughly investigated. The patients based on the received treatment method, were categorized in two approaches of fibrinolytic and invasive. They also were accurately monitored to diagnose having f-QRS. Because this study is a 40-day and 3-month follow-up, it reached to 109 patients with up to 10% volume loss in each group. Finally, 109 patients were placed in every four groups, consisting of the patients received fibrinolytic method with f-QRS, received fibrinolytic method without f-QRS, received invasive method with f-QRS, and received invasive method without f-QRS.
Based on the obtained results, the frequency distribution of sex (p = 0.322) and age group (p = 0.693), and history of hypertension (p = 0.196) and diabetes (p = 0.448), and as well as family history (p = 0.815) and smoking (p = 0.859) in the four study groups were distributed uniformly and did not have statistically significant differences. In addition, as shown in Table 1, low-density lipoprotein (LDL) (p = 0.001) and high-density lipoprotein (HDL) (p = 0.015) were statistically significant in the four groups.
Table 1.
Comparison of low-density lipoprotein and high-density lipoprotein amount in four study groups of patients.
| The Study groupa |
p | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Fib. -F |
Fib. +F |
Inv. -F |
Inv. +F |
|||||
| Mean ± SD | Min/Max | Mean ± SD | Min/Max | Mean ± SD | Min/Max | Mean ± SD | Min/Max | ||
| LDLa | 108.7 ± 37.1 | 41.0/224.0 | 113.5 ± 40.1 | 45.0/250.0 | 98.9 ± 32.5 | 31.0/211.0 | 93.4 ± 32.2 | 38.0/212.0 | .001 |
| HDLa | 43.7 ± 10.7 | 18.0/98.0 | 44.5 ± 10.0 | 26.0/93.0 | 41.4 ± 8.4 | 17.0/63.0 | 41.1 ± 8.3 | 23.0/67.0 | .015 |
Patients received fibrinolytic therapy with f-QRS (Fib. +F), Patients received fibrinolytic therapy without f-QRS (Fib. –F), Patients received invasive therapy with f-QRS (Inv. +F), Patients received invasive therapy without f-QRS (Inv. –F); f-QRS, fragmented QRS.
In accordance with the data in Table 2, f-QRS beginning time was not significantly different in two invasive and fibrinolytic groups (p = 0.089). In both treatment methods, f-QRS initiation was greater in the first 12 h than in the next 12 h.
Table 2.
Comparison of f-QRS beginning time in the two invasive and fibrinolytic groups.
| Variable | Duration | The Study group |
p | |||||
|---|---|---|---|---|---|---|---|---|
| Fib. +F |
Inv. +F |
Total |
||||||
| number | percent | number | percent | number | percent | |||
| f-QRS beginning time | First 12 h | 84 | 79.2% | 76 | 69.1% | 160 | 74.1% | .089 |
| After 12 h | 22 | 20.8% | 34 | 30.9% | 56 | 25.9% | ||
| Total | 106 | 100.0% | 110 | 100.0% | 216 | 100.0% | ||
(Fib. +F), patients received fibrinolytic therapy with f-QRS; (Inv. +F), patients received invasive therapy with f-QRS; f-QRS, fragmented QRS.
As can be observed in Table 3, the four study groups had no statistically significant difference in arrhythmia (p = 0.196). The data also demonstrated that based on the 24-h Holter monitoring, there was a significant difference in the type of arrhythmia in the four groups (p = 0.014).
Table 3.
Comparison of arrhythmia and 24-h Holter status in the 4 study groups.
| Variable | Status | The Study group |
p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fib. –F |
Fib. +F |
Inv. –F |
Inv. +F |
Total |
||||||||
| Number | % | Number | % | Number | % | Number | % | Number | % | |||
| arrhythmia | Yes | 98 | 81.7% | 95 | 89.6% | 103 | 88.0% | 99 | 90.0% | 395 | 87.2% | .196 |
| No | 22 | 18.3% | 11 | 10.4% | 14 | 12.0% | 11 | 10.0% | 58 | 12.8% | ||
| Total | 120 | 100.0% | 106 | 100.0% | 117 | 100.0% | 110 | 100.0% | 453 | 100.0% | ||
| 24 h Holter | VT | 5 | 22.7% | 4 | 36.4% | 6 | 42.9% | 7 | 63.6% | 22 | 37.9% | .014 |
| VF | 10 | 45.5% | 6 | 54.5% | 7 | 50.0% | 2 | 18.2% | 25 | 43.1% | ||
| Bradycardia | 7 | 31.8% | 1 | 9.1% | 0 | .0% | 0 | .0% | 8 | 13.8% | ||
| AF & AFL | 0 | .0% | 0 | .0% | 0 | .0% | 1 | 9.1% | 1 | 1.7% | ||
| Av block 2,3 | 0 | .0% | 0 | .0% | 1 | 7.1% | 1 | 9.1% | 2 | 3.4% | ||
| Total | 22 | 100.0% | 11 | 100.0% | 14 | 100.0% | 11 | 100.0% | 58 | 100.0% | ||
AF, atrial fibrillation; f-QRS, fragmented QRS; AFL, atrial flutter; VT, ventricular tachycardia; VF, ventricular fibrillation; (Fib. +F), patients received fibrinolytic therapy with f-QRS; (Fib. –F), patients received fibrinolytic therapy without f-QRS; (Inv. +F), patients received invasive therapy with f-QRS; (Inv. –F), patients received invasive therapy without f-QRS.
In accordance with the data in Table 4, the changes in the echo index from the first day to the 40th day in the four study groups were statistically significant (p = 0.001). In addition, the changes in LVEF parameter from the first day to the 90th day in each of the four study groups followed an upward trend.
Table 4.
Comparison of echo index (LVEF) on the first, 40th and 90th days in the four study groups.
| Variables | The Study Group |
p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fib. -F |
Fib. +F |
Inv. -F |
Inv. +F |
||||||
| Mean ± SD | Percentile 25/75 | Mean ± SD | Percentile 25/75 | Mean ± SD | Percentile 25/75 | Mean ± SD | Percentile 25/75 | ||
| LVEF of First Day | 35.4 ± 9.5 | 30.0/40.0 | 35.0 ± 8.8 | 30.0/40.0 | 35.2 ± 9.3 | 30.0/40.0 | 33.8 ± 9.7 | 25.0/40.0 | 0.567 |
| LVEF of 40th Day | 36.2 ± 9.2 | 30.0/40.0 | 35.8 ± 9.2 | 30.0/45.0 | 37.4 ± 11.3 | 30.0/50.0 | 35.5 ± 11.7 | 25.0/45.0 | 0.507 |
| LVEF of 90th Day | 36.1 ± 9.2 | 30.0/40.0 | 35.8 ± 9.2 | 30.0/45.0 | 37.8 ± 11.1 | 30.0/50.0 | 36.0 ± 11.7 | 25.0/45.0 | 0.336 |
| Difference of LVEF(1-40 day) | 0.79 ± 2.83 | 0.00/0.00 | 0.80 ± 3.59 | 0.0/5.00 | 2.14 ± 5.51 | 0.0/5.00 | 1.68 ± 5.34 | 0.0/5.00 | 0.001 |
| Difference of LVEF(40-90 day) | 0.04 ± 0.46 | 0.00/0.00 | 0.00 | 0.00 | 0.47 ± 1.85 | 0.00 | 0.59 ± 1.62 | 0.00/0.00 | 0.008 |
| Difference of LVEF(1-90 day) | 0.75 ± 2.87 | 0.00/0.00 | 0.80 ± 3.59 | 0.00/5.00 | 2.61 ± 5.63 | 0.00/5.00 | 2.27 ± 5.49 | 0.00/5.00 | 0.370 |
LVEF, left ventricular ejection fraction; (Fib. +F), patients received fibrinolytic therapy with f-QRS; (Fib. –F), patients received fibrinolytic therapy without f-QRS; (Inv. +F), patients received invasive therapy with f-QRS; (Inv. –F), patients received invasive therapy without f-QRS.
As shown in Table 4, in spite of the fact that the effect of study groups on LVEF index was not statistically significant (p = 0.597), but interaction of time and group effect was significant (p = 0.005).
As illustrated in Fig. 1, the trend of increasing LVEF index in the invasive group with f-QRS was higher than the other groups.
Fig. 1.
Curves of echo index (LVEF) for four study groups on the first, 40th, and 90th days. LVEF, left ventricular ejection fraction.
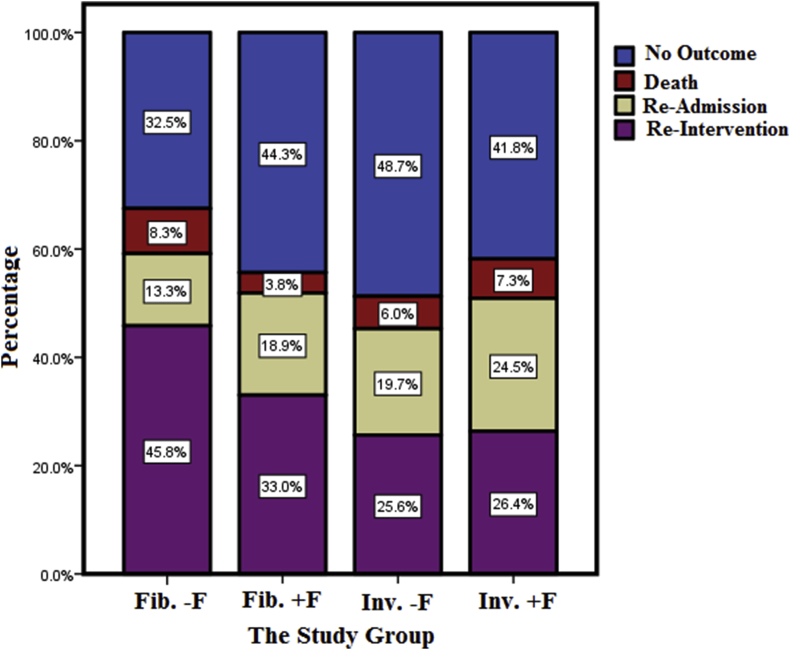
As tabulated in Table 5, the probability of adverse outcomes occurrence was not statistically significant among the four groups (p = 0.07), but the differences in the type of outcomes were statistically significant (p = 0.023).
Table 5.
The probability of adverse outcomes occurrence and the types of outcomes in the four study groups.
| Variables | Status | The Study group |
p | |||||
|---|---|---|---|---|---|---|---|---|
| Fib. –F | Fib. +F | Inv. –F | Inv. +F | Total | ||||
| Major cardiac outcomes | Without adverse outcomes | Number | 39 | 47 | 57 | 46 | 189 | .07 |
| % | 32.5% | 44.3% | 48.7% | 41.8% | 41.7% | |||
| With adverse outcomes | Number | 81 | 59 | 60 | 64 | 264 | ||
| % | 67.5% | 55.7% | 51.3% | 58.2% | 58.3% | |||
| Total | Number | 120 | 106 | 117 | 110 | 453 | ||
| % | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | |||
| Major cardiac outcomes | Without adverse outcomes | Number | 39 | 47 | 57 | 46 | 189 | .023 |
| % | 32.5% | 44.3% | 48.7% | 41.8% | 41.7% | |||
| Death | Number | 10 | 4 | 7 | 8 | 29 | ||
| % | 8.3% | 3.8% | 6.0% | 7.3% | 6.4% | |||
| Readmission | Number | 16 | 20 | 23 | 27 | 86 | ||
| % | 13.3% | 18.9% | 19.7% | 24.5% | 19.0% | |||
| Reintervention | Number | 55 | 35 | 30 | 29 | 149 | ||
| % | 45.8% | 33.0% | 25.6% | 26.4% | 32.9% | |||
| Total | Number | 120 | 106 | 117 | 110 | 453 | ||
| % | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | |||
(Fib. +F), patients received fibrinolytic therapy with f-QRS; (Fib. –F), patients received fibrinolytic therapy without f-QRS; (Inv. +F), patients received invasive therapy with f-QRS; (Inv. –F), patients received invasive therapy without f-QRS.
In this study, the highest mortality rate (8.3%) and the highest reinterventions rate (45.8%) were observed in fibrinolytic therapy without f-QRS (Fig. 2).
Fig. 2.
Comparison between different types of outcomes in the four study groups.
Multiple logistic regression model with backward LR method was used to evaluate the effect of study groups on adverse outcomes. Among the variables given in the original model, only two variables of age and study group remained in the final logistic model in which the chance of adverse outcome occurrence (death, rehospitalization and reintervention) became 1.02 times with age.
In addition, the effect of fibrinolytic group without f-QRS compared with the invasive group without f-QRS (reference group) was statistically significant so that the chance of adverse outcomes of this group increased 1.98 times.
4. Discussion
Acute MI is the result of the onset of stenosis or coronary artery obstruction, which can lead to severe ischemia and myocardial necrosis, as well as subsequently lead to an increased mortality rate and poor prognosis in patients. Therefore, estimating the risk of MI occurrence is an important issue in health cares. Better identification of electrocardiographic criteria is needed to identify MI more accurately. One of the important parameters that are still being investigated is the complexes of f-QRS. Based on many researches, f-QRS can be considered as a predictor of relapse of severe heart attacks, sudden cardiac death, increased mortality rate, and an indicator of early treatment intervention for patients. On the other hand, other studies have shown that f-QRS in superficial electrocardiogram is a reliable predictor of myocardial scar, coronary artery disease, or long-term adverse events such that autopsy of patients with MI, and left ventricular aneurysm have confirmed the significant myocardial necrosis along with islands of living myocardial tissue scattered in fibrotic tissue.7,12 However, some other researches have shown contradictory results in which f-QRS on superficial ECG is not a reliable predictor of myocardial scar, coronary angiography, and long-term adverse outcomes.8
Based on the results of this study, the comparison of the percentage of arrhythmia in the four study groups showed that there was no significant difference between the four groups and the arrhythmia status. Therefore, f-QRS was not introduced as an independent predictor of arrhythmia in patients with acute MI. Daszyk et al 12 showed that there were no statistically significant differences in conduction disorders and other arrhythmias in patients with f-QRS compared with patients without f-QRS at their discharge time. Sheng et al concluded that in patients with ACS who underwent successful revascularization (TIMI = 3), the presence of f-QRS was not associated with a higher incidence of arrhythmias compared to patients without f-QRS in the short-time follow-up.15
Investigation in Holter status showed that there was a significant relationship between this variable and the four study groups (p = 0.014). Of all patients, 31.8% of patients with VT were in the invasive group with f-QRS, which can be said that f-QRS is an effective parameter in the prognosis of these patients. About 40% of patients with ventricular fibrillation (VF) were in the fibrinolytic group without f-QRS. There is a little and sometimes conflicting information about VF during the acute phase of MI. In the hospital, VF often occurs with inferior MI treated with fibrinolysis. Outside the hospital, VF appears to be associated with anterior MI.13 Almost all the patients with bradycardia were in the fibrinolytic therapy group without f-QRS, and therefore f-QRS did not affect their prognosis. About two-thirds of patients with AF and AFL, as well as AV block 2.3 were in the invasive treatment group with f-QRS, indicating a poor prognosis for these variables.
Comparison of the echo index (LVEF) in the first, 40th and 90th days of LVEF showed that there was no significant difference between four study groups. There was also significant difference between the 40th day and 90th day. Up to Table 4 data, changes in echo index from the first day to the 40th day were statistically significant in the four study groups. The highest average of differences was related to the invasive group without f-QRS. Totally, the most changes happened in the invasive group. This index remained unchanged in the fibrinolytic group. In general, LVEF changes from the first day to 90th day in all four groups had an upward trend.
A comparison of the percentage of adverse outcomes in the four groups showed that about one-third of the adverse outcomes were in the fibrinolytic group without f-QRS. There was a statistically significant difference in the types of adverse outcomes. Almost one-thirds of total reported death were in the fibrinolytic group without f-QRS and almost the same number were in the invasive group with f-QRS and therefore, f-QRS did not affect the type of treatment group and death rate. Investigation of rehospitalization percentages showed that the highest rehospitalization rate happened in patients receiving invasive treatment with f-QRS. Comparison of adverse outcomes in two treatment methods of fibrinolytic and invasive showed that the percentage of adverse outcomes in fibrinolytic group were more than invasive one; however, this difference was not statistically significant. There was a statistically significant difference in the type of adverse outcomes between the two treatment groups. The mortality rate and rehospitalization in invasive treatment group were almost higher than fibrinolytic group, but reintervention rate in fibrinolytic treatment group was more than invasive treatment. The highest mortality rate and the highest rate of reintervention were observed in patients receiving fibrinolytic therapy without f-QRS.
Based on numerous researches, f-QRS can be considered as a predictor of recurrence of severe heart attacks, sudden cardiac death, increased mortality, and an indicator of early treatment intervention for patients. However, in other studies it was found that f-QRS in superficial electrocardiograms is a reliable predictor of myocardial scar, coronary artery disease, or long-term side effects; since autopsy of patients with MI and left ventricular aneurysm confirmed significant myocardial necrosis with living myocardial tissue islands scattered in fibrotic tissue.8,11,12,14 In Sheng et al15 study, the rate of malignant cardiac arrhythmia, LVSD, and mortality in patients with f-QRS were 13.6%, 29.2%, and 23.7%, respectively. Results showed that patients with acute MI with f-QRS, especially patients with STEMI, had higher values of malignant cardiac arrhythmia, LVSD, and mortality compared with the group without f-QRS. In addition, the presence of f-QRS was considered as an indication for early therapeutic intervention in patients. Das et al (2009) showed that f-wQRS was an independent predictor of mortality.7
5. Conclusion
The final results of this study showed that there was no significant difference between the four study groups and arrhythmia status. Therefore, f-QRS was not introduced as an independent predictor of arrhythmia in patients with acute MI. Investigation in Holter status also showed that there was a relationship between this variable and the four study groups.
In this study, there was no significant difference between the four study groups and the first day of LVEF. But, there was significant difference between 40th day and 90th day. In accordance with the obtained data, changes in echo index from the first day to 40th day were statistically significant in four study groups.
The results showed that about two-thirds of adverse outcomes were in the fibrinolytic study group without f-QRS. There was also a statistically significant difference between the four study groups and major cardiac outcomes. In other word, f-QRS had no effect on the type of treatment groups and the death rate.
Funding
This study was financially supported by the research and technology directorate of Guilan University of Medical Sciences, Rasht, Iran (Grant No.97072111).
Conflict of interest
All authors have none to declare.
Acknowledgment
The authors would like to appreciate the vice-chancellor for research and technology of the university, the head and staff members of Dr. Heshmat hospital, and all patients who voluntarily participated in this study.
References
- 1.Kumar A., Cannon C.P. Acute coronary syndromes: diagnosis and management, part I. Mayo Clin Proc. 2009;84(10):917–938. doi: 10.4065/84.10.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorenek B., Lundqvist C.B., Terradellas J.B. Cardiac arrhythmias in acute coronary syndromes: position paper from the joint EHRA, ACCA, and EAPCI task force. Eur Heart J Acute Cardiovasc Care. 2015;4(4):386. doi: 10.1177/2048872614550583. [DOI] [PubMed] [Google Scholar]
- 3.Hersi A., Alhabib K.F., Alsheikh-Ali A.A. Short-term and long-term mortality associated with ventricular arrhythmia in patients hospitalized with acute coronary syndrome: findings from the Gulf RACE registry-2. Coron Artery Dis. 2013;24(2):160–164. doi: 10.1097/MCA.0b013e32835c49ed. [DOI] [PubMed] [Google Scholar]
- 4.Ramana R.K., Lewis B.E. Percutaneous coronary intervention in patients with acute coronary syndrome: focus on bivalirudin. Vasc Health Risk Manag. 2008;4(3):493–505. doi: 10.2147/vhrm.s2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernando L., Canovas E., Freites A. Prevalence and prognosis of percutaneous coronary intervention-associated nephropathy in patients with acute coronary syndrome and normal kidney function. Rev Esp Cardiol. 2015;68(4):310–316. doi: 10.1016/j.rec.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 6.Ali M.R., Salim Hossain M., Islam M.A. Aspect of thrombolytic therapy: a review. Sci world J. 2014;2014:586510. doi: 10.1155/2014/586510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das M.K., Michael M.A., Suradi H. Usefulness of fragmented QRS on a 12-lead electrocardiogram in acute coronary syndrome for predicting mortality. Am J Cardiol. 2009;104(12):1631–1637. doi: 10.1016/j.amjcard.2009.07.046. [DOI] [PubMed] [Google Scholar]
- 8.Li M., Wang X., Mi S.-H. Short-term prognosis of fragmented QRS complex in patients with non-ST elevated acute myocardial infarction. Chinese Med J. 2016;129(5):518. doi: 10.4103/0366-6999.176989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonakdar H., Moladoust H., Kheirkhah J. Significance of a fragmented QRS complex in patients with chronic total occlusion of coronary artery without prior myocardial infarction. Anatol J Cardiol. 2016;16:106–112. doi: 10.5152/akd.2015.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pietrasik G., Zareba W. QRS fragmentation: diagnostic and prognostic significance. Cardiol J. 2012;19(2):114–121. doi: 10.5603/cj.2012.0022. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee S., Changawala N. Fragmented QRS complex: a novel marker of cardiovascular disease. Clin Cardiol. 2010;33(2):68–71. doi: 10.1002/clc.20709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daszyk A.M., Zygmund K., Mitręga K.A., Cebula S., Kalarus Z., Średniawa B. Fragmentation of the QRS complex in patients with acute coronary syndrome treated invasively. Kardiol Pol. 2016;74(7):644–649. doi: 10.5603/KP.a2015.0251. [DOI] [PubMed] [Google Scholar]
- 13.Braunwald E., Zipes D., Libby P. 6e éd. WB Saunders company; 2001. Heart Disease: A Textbook of Cardiovascular Medicine. [Google Scholar]
- 14.Das M.K., Zipes D.P. Fragmented QRS: a predictor of mortality and sudden cardiac death. Heart Rhythm. 2009;6(3 Suppl):S8–S14. doi: 10.1016/j.hrthm.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 15.Sheng Q-h, Hsu C.-C., Li J-p, Hong T., Huo Y. Correlation between fragmented QRS and the short-term prognosis of patients with acute myocardial infarction. J Zhejiang Univ – Sci B. 2014;15(1):67–74. doi: 10.1631/jzus.B1300091. [DOI] [PMC free article] [PubMed] [Google Scholar]