Abstract
The potential role of soluble neprilysin (sNEP) as a biomarker has been poorly documented. Hence, the present systematic review emphasizes to explore sNEP as an emerging biomarker for heart failure (HF), cardiovascular diseases, diabetic kidney diseases, and so on. A systematic review was performed using an online database search in PubMed, Science Direct, Scopus, and Cochrane Library. Articles reporting biomarker's performance to diagnose various diseases in human participants were included. The results of the search outcome were 4723 articles. Based on the inclusion criteria of the systematic review, finally, 12 articles fulfilled the selection criteria. In these studies, 8 cohort study, 2 cross-sectional study, 1 case–control, and 1 prospective cohort study were identified. All these studies clearly suggested sNEP as a potential biomarker for diagnosis of various diseases (HF, cardiovascular diseases, diabetic kidney diseases, metabolic syndrome). sNEP may be a potential biomarker for HF, cardiovascular diseases, diabetic kidney disease, and so on.
Keywords: Soluble neprilysin, Biomarker, Heart failure, Diabetic kidney disease, A systematic review
1. Introduction
Neprilysin (NEP) is a zinc-dependent membrane metallopeptidase with a molecular weight of 90 kDa and contains glycosylation sites.1 NEP is highly conserved in mammals, and there is only a 6-amino-acid variance in sequences between humans and rats.2 It is recognized by several names as enkephalinase, neutral endopeptidase, common acute lymphoblastic leukemia antigen, endopeptidase 24.11, and CD10.3,4 NEP is a familiar enzyme that was defined and fully characterized several decades ago. It is abundant and expressed by the highest concentrations in the proximal tubules and also expressed in the kidneys, lungs, endothelial cells, vascular smooth muscle cells, cardiac cells, fibroblasts, neutrophils, adipocytes, testes, and brain. NEP is also relatively indiscriminate in the cardiovascular system, and NEP cleaves numerous vasoactive peptides and others. Studies have documented an alternative processing form of NEP, soluble NEP (sNEP), which exists in the plasma and urine.5,6 sNEP has an enzymatic activity to degrade peptides equally to membrane-bound NEP.
The brush border of proximal tubular cell expression of NEP, the most susceptible elasticity of epithelial cells, was one of the main reasons to start testing its existence in urine.7 In addition to this protein, many other proteins were also in urinary exosomes.8 Furthermore, its contribution to blood pressure regulation and the development of inhibitors has fetched this protein to the prime of medical interest.9,10 Then, findings of the wide distribution of NEP and its unexpectedly extensive potentially important roles for the endopeptidase in cardiovascular, renal, pulmonary, gastrointestinal, and neurological functions.
Furthermore, an insufficient attempt was made to evaluate the sNEP as a biomarker for heart failure (HF), cardiovascular disease, kidney disease, and so on. The purpose of the present systematic review was to provide the cumulative research output about whether sNEP really plays a key role as a versatile biomarker for diagnosing various diseases.
2. Materials and methods
2.1. Literature search strategies for identification of relevant studies
The literature search was conducted in PubMed, Scopus, Science Direct, Google Scholar, and Cochrane Library using the following keywords: ‘Neprilysin’ plus ‘biomarker’ without language restriction. The searches were performed independently by 2 investigators. We have to screen all the abstracts available in English. Abstracts are manually screened based on the eligibility criteria and exclusions cross-checked by a member of the team. Any studies that fulfill the inclusion criteria were then to be reviewed in full. Full-text articles will be screened independently by two investigators for eligibility. Disagreement about any article's eligibility will be resolved by consent. All suggested articles will be screened for eligibility by using the same criteria as for the original articles. If necessary, we will revise the literature search to find articles similar to those missed in the original search.
2.2. Study inclusion and exclusion criteria
The systematic review considered the following inclusion criteria: (1) full research articles in English, (2) articles published after 2009, (3) human participants in both gender with sample size more than 20, and (4) assessment of sNEP as a biomarker are considered. Similarly, the exclusion criteria included the following: (1) abstracts and conference papers, (2) articles in other than the English language, (3) other than human studies such as animal and computational studies, (4) tissue expression studies about NEP, (5) human participants with sample size less than 20, and (6) articles published before 2009.
2.3. Data abstraction and data management
Each study will be extracted by one skilled scientist. The extraction will be reviewed and confirmed by at least one other methodologist. Any disagreements will be resolved by discussion among the team. Data will be extracted into standard forms.
2.4. Data synthesis
All included studies will be summarized in tables that tabulate the study populations, study design, biomarkers evaluated, and outcomes of the studies.
3. Results and discussion
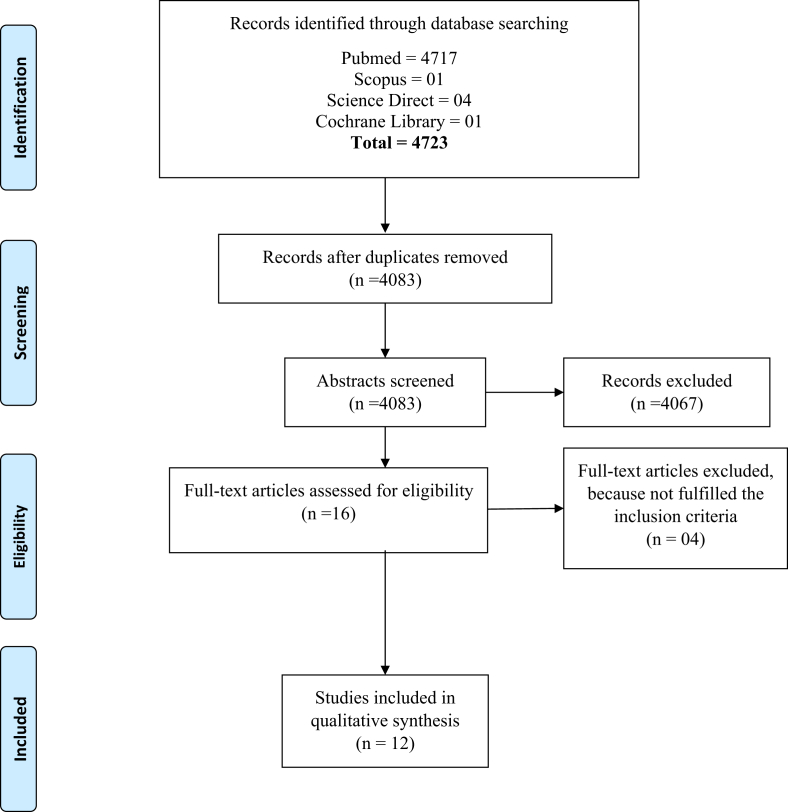
Two reviewers independently completed the selection and review of articles. Full-text articles found in the initial search were cross-checked with the reference. Studies that satisfied the full-text article selection criteria included the following: (1) primary research, (2) a sample size of ≥20 with any disease, (3) assessment of sNEP as a biomarker, (4) full-text article in English, (5) a general population (e.g., not a single gender), and (6) studies carried out after 2009. The systematic review search yielded about 4722 unique references from four databases (Fig. 1). All the articles based on inclusion criteria were reviewed and the duplication was removed. The studies published before the year 2009 were excluded from the review. After screening the abstracts, 12 articles met the selection criteria (Table 1).
Fig. 1.
PRIMA 2009 flow diagram.
Table 1.
Summarized studies included in the review.
| Reference | Study population | Biomarkers analyzed | Analytical method used for neprilysin estimation | Brief conclusion of the study | Type of study |
|---|---|---|---|---|---|
| A. Bayes-Genis et al, 2015 | 797 patients with HF | Plasma sNEP, NT-pro BNP, hscTnT, ST2 | Modified sandwich immunoassay | sNEP independently associated with HF-related death than NT-pro BNP | Cohort |
| Thomas A Zelniker et al, 2018 | 144 patients of cardiac arrest of nontraumatic origin out-of-hospital | Plasma sNEP, hsTnT, hsCRP, NT-pro BNP | Human neprilysin DuoSet ELISA research kit | sNEP independently associated with all-cause mortality in patients with out-of-hospital cardiac arrest of nontraumatic origin | Cohort |
| Pajenda et al, 2017 | 90 critically ill patients | Urine NEP | Human NEP ELISA | Elevated urinary NEP is indicative of proximal tubular cell stress or injury. | Cohort |
| Sridevi Gutta et al, 2018 | 60 patients with type 2 DM with a history of microalbuminuria and macroalbuminuria | ACE2 and NEP | Human ELISA kit | ACE2 and NEP as noninvasive biomarkers to assess kidney damage in patients with diabetes at an early stage. | Cross-sectional |
| Mattia Arrigo et al, 2018 | 50 patients (28 patients with acute HF and 22 acute noncardiac dyspnea) | sNEP | Modified sandwich immunoassay | Short-term clinical improvement after acute dyspnea or mid-term improvement after total artificial heart transplantation are associated with an increase rather than a decrease in sNEP | Cohort |
| Antoni Bayes-Genis et al, 2016 | 98 patients with chronic HF | sNEP | Fluorometric assay | Circulating sNEP as a biotarget in heart failure | Cohort |
| Antoni Bayés-Genís et al, 2015 | 1069 patients with HF | sNEP | Modified sandwich immunoassay | High levels of neprilysin are found in the circulation of patients with HF and that neprilysin concentrations are indicators of adverse outcomes for both cardiovascular mortality and morbidity. |
Cohort |
| Julio Nu ~ nez et al, J 2017 | 1021 consecutive ambulatory patients with heart failure | sNEP, NT-pro BNP | Modified sandwich immunoassay | Elevated sNEP levels predicted an increased risk of recurrent all-cause, cardiovascular, and AHF admissions in ambulatory patients with heart failure. | Cohort |
| Elena Guillén-Gómez et al, 2018 | 21 patients with type 2 diabetic [12 without DN (control patients) and 9 with incipient DN (DN basal)] | Urine NEP, VCAM-1 | Specific enzyme-linked immunosorbent assay | Neprilysin and VCAM-1 as potential new tools as DN progression biomarkers | Case control |
| Kristina F. Standeven et al, 2011 | 318 clinically healthy white men of European origin, characterized for the presence of the MetS | sNEP, tissue plasminogen activator, insulin, plasminogen activator inhibitor-1 |
Modified fluorescence | Obesity and the development of insulin resistance is associated with increased plasma NEP levels | Cross-sectional |
| Julio Núñez et al, 2016 | 210 patients consecutively admitted for AHF | NT-pro BNP, hscTnT, sNEP | Modified sandwich immunoassay | sNEP was associated with the risk of long-term recurrent all-cause and AHF rehospitalizations |
Prospective cohort |
| Reddy et al, 2019 | 1536 patients in the general population | sNEP, ANP, BNP, NT-pro ANP, NT-pro BNP | Sandwich ELISA | sNEP and natriuretic peptide did not correlate. | Large community-based cohort study |
HF = heart failure; sNEP = soluble neprilysin; NT-pro BNP = N-terminal brain natriuretic peptide; hscTnT = high sensitive cardiac troponin T; ST2 = suppression of tumorigenicity 2; NEP = neprilysin; ACE2 = angiotensin-converting enzyme 2; IL6 = interleukin; VCAM-1 = human vascular cell adhesion molecule-1; STEMI = ST-segment elevation myocardial infarction; DM = diabetes mellitus; DN = diabetic nephropathy; MetS = metabolic syndrome; AHF = acute heart failure; ANP = A-type natriuretic peptide; BNP = brain natriuretic peptide; NT-pro ANP = N-terminal pro-ANP; NT-pro BNP = N-terminal pro-BNP.
3.1. Association among soluble neprilysin, HF, and cardiovascular disease
The circulating sNEPhas recently emerged as a potential biomarker for the prognosis of cardiovascular death and hospital admission for patients with acute and chronic HF.
The factors which determine the readmission in the hospital for acute HF are not well described. However, the risk of recurrent hospitalization is significantly increased in these patients. To address this problem, Núñez et al11 conducted an experimental study to evaluate the association between sNEP and the risk of long-term repeated hospitalizations in a cohort of patients with AHF, because, NEP (NEP) is an enzyme with a pivotal role in the pathophysiology of HF. In their study, 210 patients with AHF were enrolled, and unplanned hospital admission was the primary end point. The decision on the clinical management of the primary end point was based on sNEP levels. Finally, sNEP was found to be significantly associated with the risk of any rehospitalization. Another interesting cohort study has emphasized the direct comparison of sNEP with existing markers for HF to establish the prognostic marker. Seven hundred ninety-seven consecutive ambulatory patients with HF participated and were followed up for 4.7 years. A primary end point has been cardiovascular death or HF hospitalization. A secondary end point explored cardiovascular death alone. sNEP remained independently associated with both the end points than other biomarkers and concluded that sNEP is a novel independent prognostic biomarker for HF.12
Elevated sNEP levels predicted as an increased risk of recurrent all-cause, cardiovascular, and AHF admissions in outpatients with HF have been revealed in the study of 1021 consecutive ambulatory patients with HF with a median follow-up period of 3.4 years. The conclusion points were the number of all-cause, cardiovascular, and AHF hospitalizations during follow-up.13
Arrigo et al14 measured sNEP concentration at admission and before discharge in 50 patients admitted for acute dyspnea. They confirmed that increased sNEP has been observed in immediate improvement after acute dyspnea or significant improvement after total artificial heart transplantation. Another study also observed from 98 patients with HF sNEP as a potential biomarker and also as a biotarget in HF.15
3.2. Association of neprilysin with type 2 diabetes mellitus and kidney function
Chronic kidney disease (CKD), which is considered by an advanced deterioration in the glomerular filtration rate over more than 3 months, is a devastating disease and is frequently accompanied by albuminuria. The fundamental mechanism of the development of diabetic kidney disease to end-stage renal disease is not fairly understood. In routine clinical practice, kidney diseases do not have any symptoms and biomarkers at its early stages.16 Hence, the evaluation of new biomarkers for the early detection and management of diabetic CKD is inevitable.
A cross-sectional study was performed on 60 patients with microalbuminuria and macroalbuminuria. Patients were categorized as nondiabetic (ND) and diabetic with normoalbuminuria, microalbuminuria, and macroalbuminuria. In their study, angiotensin 2 (ACE2) and NEP were evaluated in all the groups and concluded that ACE2 and NEP as noninvasive biomarkers to evaluate diabetic kidney disease progression at an early stage.17 Guillén-Gómez et al18 conducted a similar kind of study in 21 patients with diabetic nephropathy. However, they adopted the urinary proteome analysis to identify biomarkers of a clinical outcome, and tandem mass tag has been used for quantification of biomarkers. Finally, the observation revealed that NEP and VCAM-1 are promising biomarkers in the diagnosis and therapeutic management of DN.
Acute kidney injury (AKI) is one of the complications observed in critically ill patients who are in the intensive care units which causes predominantly sepsis. The new potential biomarker screening is extremely important to avoid complications due to AKI. Pajenda et al19 carried out a research study to address the aforementioned problem which included 90 patients who are admitted to the intensive care units. The urinary NEP has been estimated in all the patients and compared them with 55 healthy controls. They confirmed that raised urinary NEP is suggestive of proximal tubular cell stress or injury.
3.3. Miscellaneous
3.3.1. Cardiac arrest
Medical management of postcardiac arrest may be challenging; its mortality rate remains high20 and is characterized by myocardial dysfunction.21 Besides, early risk identification is an urgent need for patients with out-of-hospital cardiac arrest (OHCA). In this setting, biomarkers are the prognostic tool that assists to understand pathophysiological processes and support in decision-making.22 Zelniker et al23 sought to study the potential prognostic role of sNEP to understand the pathophysiology in patients with OHCA. One hundred forty-four patients with the successful return of natural circulation after OHCA of nontraumatic origin were enrolled, and sNEP was measured. They were followed up at least 30 days, and the primary end point was time to all-cause mortality. This study confirmed that sNEP was independently associated with all-cause mortality in patients with OHCA of nontraumatic origin and useful in the stratification of risk in these patients.
3.3.2. Obesity
NEP is produced by adipocytes,24 predicted that it might have a key role as an adipokine regulating characteristics of adipocyte function. Inhibition of NEP in obese insulin-resistant Zucker rats improved whole-body insulin-mediated glucose disposal and dual Angiotensin-converting enzyme (ACE)/NEP inhibition induced deep insulin sensitization and improved myocardial glucose uptake.25, 26, 27 It proved that NEP directly involved in the development of insulin resistance. Standeven et al28 conducted an interesting study to investigate the association of insulin resistance between NEP activity, obesity and components of the metabolic syndrome in 318 healthy individuals characterized for insulin resistance using the homeostasis model assessment and the presence of the metabolic syndrome (MetS) according to the International Diabetes Federation (IDF) definition. They concluded that increased plasma NEP activity associated significantly with obesity and the development of insulin resistance.
3.3.3. General population
Reddy et al29 have conducted a large community-based cohort study in the general population with 1536 participants. This study is unique because there are no such studies conducted previously. They measured plasma sNEPalong with natriuretic peptide and assessed the ventricular structure. The conclusion from this study is that there was no correction between NEP and natriuretic peptides. This result was contradicted in previous studies. However, this study has been conducted on the general population which should also be considered.
3.3.4. Pathophysiology of NEP
NEP is extensively scattered in several tissues, which include the kidney, lung, brain, heart, and vasculatures. Notably, the kidney is the richest source, which was identified with the use of a NEP monoclonal antibody in porcine renal tissues.30 A critical characteristic of NEP is that it cleaves and degrades a range of bioactive peptides. From this perception, NEP has high significance to cardiovascular and renal regulation, and accepting the modulations of these substrates by NEP is critical for understanding therapeutic and diagnostic associations.
NEP is responsible for the degradation of endogenous natriuretic peptides that have important adaptive cardiovascular effects, including direct effects to inhibit the renal sodium reabsorption, suppress the secretion of aldosterone from the adrenal gland,31, 32, 33 and inhibit cardiac inflammation and fibrosis.34, 35, 36 The amount of NEP is raised in chronic HF, increased further during acute decompensation, and is connected with a contrary outcome in patients with a reduced ejection fraction.6,13,37 NEP inhibition has positive effects on cardiac remodeling35,38,39 and decreases the morbidity and mortality of patients with HF with decreased systolic function.40
NEP is expressed on the surface of mature adipocytes24,41 which seemingly shed the enzyme into the plasma where its soluble form can be measured. Consumption of a high-fat diet raises circulating levels of NEP, and visceral fat contains high levels of the enzyme.41, 42, 43 Moreover, the improved renal sympathetic nerve activity that describes patients with obesity leads to stimulation of NEP within the kidney.34 People with obesity have increased levels of NEP in proportion to their body mass,42,43 and circulating levels of sNEP are predominantly elevated in patients with obesity with HF with a preserved ejection fraction.44
4. Conclusion
In conclusion, the present systematic review validates that measurement of sNEP, a versatile biomarker, seems to comparatively provide good insight into the diagnosis of HF, cardiovascular diseases, diabetic kidney disease, AKI, cardiac arrest, and obesity. Large cohort studies are needed to confirm our validation.
Ethics approval and consent to participate
Not applicable. This study was a systematic review.
Consent for publication
Not applicable.
Data statement
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Funding source
Nil.
Authors' contributions
K.R. and G.P. contributed to research idea and study design; data acquisition and data analysis; manuscript drafting. K.R. contributed in mentorship. All authors approve the final version of the manuscript.
Acknowledgments
Nil.
Contributor Information
Kumaresan Ramanathan, Email: kumaresanramanatha@gmail.com.
Giri Padmanabhan, Email: kpgiri@gmail.com.
References
- 1.Turner A.J., Matsas R., Kenny A.J. Endopeptidase-24.11 and neuropeptide metabolism. Biochem Soc Trans. 1985;13:39–42. doi: 10.1042/bst0130039. [DOI] [PubMed] [Google Scholar]
- 2.Oefner C., D'Arcy A., Hennig M., Winkler F.K., Dale G.E. Structure of human neutral endopeptidase (neprilysin) complexed with phosphoramidon. J Mol Biol. 2000;296:341–349. doi: 10.1006/jmbi.1999.3492. [DOI] [PubMed] [Google Scholar]
- 3.Stephenson S.L., Kenny A.J. The hydrolysis of alpha-human atrial natriuretic peptide by pig kidney microvillar membranes is initiated by endopeptidase-24.11. Biochem J. 1987;243:183–187. doi: 10.1042/bj2430183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vijayaraghavan J., Scicli A.G., Carretero O.A., Slaughter C., Moomaw C., Hersh L.B. The hydrolysis of endothelins by neutral endopeptidase 24.11(enkephalinase) J Biol Chem. 1990;265:14150–14155. [PubMed] [Google Scholar]
- 5.Aviv R., Gurbanov K., Hoffman A., Blumberg S., Winaver J. Urinary neutral endopeptidase 24.11 activity: modulation by chronic salt loading. Kidney Int. 1995;47:855–860. doi: 10.1038/ki.1995.128. [DOI] [PubMed] [Google Scholar]
- 6.Bayes-Genis A., Barallat J., Galan A. Soluble neprilysin is predictive of cardiovascular death and heart failure hospitalization in heart failure patients. J Am Coll Cardiol. 2015;65:657–665. doi: 10.1016/j.jacc.2014.11.048. [DOI] [PubMed] [Google Scholar]
- 7.Truong L.D., Shen S.S. Immunohistochemical diagnosis of renal neoplasms. Arch Pathol Lab Med. 2011;135(1):92–109. doi: 10.5858/2010-0478-RAR.1. [DOI] [PubMed] [Google Scholar]
- 8.Gonzales P.A., Pisitkun T., Hoffert J.D. Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol. 2009;20(2):363–379. doi: 10.1681/ASN.2008040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skidgel R.A., Erdos E.G. Angiotensin converting enzyme (ACE) and neprilysin hydrolyze neuropeptides: a brief history, the beginning and follow-ups to early studies. Peptides. 2004;25(3):521–525. doi: 10.1016/j.peptides.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Judge P., Haynes R., Landray M.J., Baigent C. Neprilysin inhibition in chronic kidney disease. Nephrol Dial Transplant. 2015;30(5):738–743. doi: 10.1093/ndt/gfu269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Núñez Julio, Núñez Eduardo, Miñana Gema. Serum neprilysin and recurrent hospitalizations after acute heart failure. Int J Cardiol. 2016;220:742–744. doi: 10.1016/j.ijcard.2016.06.271. [DOI] [PubMed] [Google Scholar]
- 12.Bayes-Genis Antoni, Barallat Jaume, Gala'n Amparo. Multimarker strategy for heart failure prognostication. Value of neurohormonal biomarkers: neprilysin vs NT-proBNP. Rev Esp Cardiol. 2015;68(12):1075–1084. doi: 10.1016/j.rec.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Nu∼ nez Julio, Nu∼ nez Eduardo, Barallat Jaume. Serum neprilysin and recurrent admissions in patients with heart failure. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.005712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arrigo Mattia, Nougué Héleńe, Launay Jean-Marie, Mebazaa Alexandre, Vodovar Nicolas. Plasma neprilysin concentration duringrecovery from acute illness. Eur Heart J. 2018:1–2. doi: 10.1093/eurheartj/ehy456. [DOI] [PubMed] [Google Scholar]
- 15.Bayes-Genis Antoni, Prickett Timothy C., Mark Richards A. Jaume Barallat, Josep Lupón. Soluble neprilysin retains catalytic activity in heart failure. J Heart Lung Transplant. 2016;35(5):684–685. doi: 10.1016/j.healun.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Collins A.J., Couser W.G., Dirks J.H. World Kidney Day: an idea whose time has come. Natl Med J India. 2006;19:55–57. [PubMed] [Google Scholar]
- 17.Gutta Sridevi, Grobe Nadja, Kumbaji Meenasri, Osman Hassan, Saklayen Mohammad, Gengxin Khalid M. Elased. Increased urinary angiotensin converting enzyme 2 and neprilysin in patients with type 2 diabetes. Am J Physiol Ren Physiol. 2018;315:F263–F274. doi: 10.1152/ajprenal.00565.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guillén-Gómez E., Bardají-de-Quixano B., Ferrer S. Urinary proteome analysis identified neprilysin and VCAM as proteins involved in diabetic nephropathy. J Diabetes Res. 2018;2018 doi: 10.1155/2018/6165303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pajenda Sahra, Mechtler Karl, Wagner Ludwig. Urinary neprilysin in the critically ill patient. BMC Nephrol. 2017;18:172. doi: 10.1186/s12882-017-0587-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nolan J.P., Soar J., Cariou A. European resuscitation council and European society of intensive care medicine guidelines for post-resuscitation care 2015: section 5 of the European resuscitation council guidelines for Resuscitation2015. Resuscitation. 2015;95:202–222. doi: 10.1016/j.resuscitation.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Perkins G.D., Olasveengen T.M., Maconochie I. European resuscitation council guidelines for resuscitation: 2017 update. Resuscitation. 2018;123:43–50. doi: 10.1016/j.resuscitation.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Vasan R.S. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113:2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 23.Zelniker Thomas A., Spaich Sebastian, Jan Stiepak, Steger Florian, Katus Hugo A., Preusch Michael R. Serum neprilysin and the risk of death in patients with out of-hospital cardiac arrest of non-traumatic origin. Eur Heart J: Acute Cardiovasc Care. 2018:1–6. doi: 10.1177/2048872618815062. [DOI] [PubMed] [Google Scholar]
- 24.Schling P., Schafer T. Human adipose tissue cells keep tight control on the angiotensin II levels in their vicinity. J Biol Chem. 2002;277(50):48066–48075. doi: 10.1074/jbc.M204058200. [DOI] [PubMed] [Google Scholar]
- 25.Arbin V., Claperon N., Fournie-Zaluski M.C., Roques B.P., Peyroux J. Acute effect of the dual angiotensin-converting enzyme and neutral endopeptidase 24-11 inhibitor mixanpril on insulin sensitivity in obese Zucker rat. Br J Pharmacol. 2001;133(4):495–502. doi: 10.1038/sj.bjp.0704098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arbin V., Claperon N., Fournie-Zaluski M.C., Roques B.P., Peyroux J. Effects of dual angiotensinconverting enzyme and neutral endopeptidase 24-11 chronic inhibition by mixanpril on insulin sensitivity in lean and obese Zucker rats. J Cardiovasc Pharmacol. 2003;41(2):254–264. doi: 10.1097/00005344-200302000-00015. [DOI] [PubMed] [Google Scholar]
- 27.Wang C.H., Leung N., Lapointe N. Vaso peptidase inhibitor omapatrilat induces profound insulin sensitization and increases myocardial glucose uptake in Zucker fatty rats: studies comparing a vaso peptidase inhibitor, angiotensin-converting enzyme inhibitor, and angiotensin II type I receptor blocker. Circulation. 2003;107(14):1923–1929. doi: 10.1161/01.CIR.0000062646.09566.CC. [DOI] [PubMed] [Google Scholar]
- 28.Standeven Kristina F., Hess Katharina, Carter Angela M. Obesity and the metabolic syndrome. Int J Obes (Lond) 2011;35(8):1031–1040. doi: 10.1038/ijo.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy Yogesh N.V., Iyer Seethalakshmi R., Scott Christopher G. Soluble neprilysin in the general population: clinical determinants and its relationship to cardiovascular disease. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kenny A.J., Bowes M.A., Gee N.S., Matsas R. Endopeptidase-24.11: a cell-surface enzyme for metabolizing regulatory peptides. Biochem Soc Trans. 1985;13:293–295. doi: 10.1042/bst0130293. [DOI] [PubMed] [Google Scholar]
- 31.Miura S., Nakayama A., Tomita S., Matsuo Y., Suematsu Y., Saku K. Comparison of aldosterone synthesis in adrenal cells, effect of various AT1 receptor blockers with or without atrial natriuretic peptide. Clin Exp Hypertens. 2015;37:353–357. doi: 10.3109/10641963.2014.987391. [DOI] [PubMed] [Google Scholar]
- 32.Miura S.I., Suematsu Y., Matsuo Y. The angiotensin II type 1 receptor-neprilysin inhibitor LCZ696 blocked aldosterone synthesis in a human adrenocortical cell line. Hypertens Res. 2016;39:758–763. doi: 10.1038/hr.2016.72. [DOI] [PubMed] [Google Scholar]
- 33.Martin F.L., Stevens T.L., Cataliotti A. Natriuretic and antialdosterone actions of chronic oral NEP inhibition during progressive congestive heart failure. Kidney Int. 2005;67:1723–1730. doi: 10.1111/j.1523-1755.2005.00269.x. [DOI] [PubMed] [Google Scholar]
- 34.Kirchgessner T.G., Uysal K.T., Wiesbrock S.M., Marino M.W., Hotamisligil G.S. Tumor necrosis factor-alpha contributes to obesity-related hyperleptinemia by regulating leptin release from adipocytes. J Clin Invest. 1997;100:2777–2782. doi: 10.1172/JCI119824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Lueder T.G., Wang B.H., Kompa A.R. Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ Heart Fail. 2015;8:71–78. doi: 10.1161/CIRCHEARTFAILURE.114.001785. [DOI] [PubMed] [Google Scholar]
- 36.Pu Q., Amiri F., Gannon P., Schiffrin E.L. Dual angiotensin-converting enzyme/neutral endopeptidase inhibition on cardiac and renal fibrosis and inflammation in DOCA-salt hypertensive rats. J Hypertens. 2005;23:401–409. doi: 10.1097/00004872-200502000-00023. [DOI] [PubMed] [Google Scholar]
- 37.Bayés-Genís A., Barallat J., Pascual-Figal D. Prognostic value and kinetics of soluble neprilysin in acute heart failure: a pilot study. JACC Heart Fail. 2015;3:641–644. doi: 10.1016/j.jchf.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Maki T., Nasa Y., Tanonaka K., Takahashi M., Takeo S. Direct inhibition of neutral endopeptidase in vasopeptidase inhibitor-mediated amelioration of cardiac remodeling in rats with chronic heart failure. Mol Cell Biochem. 2003;254:265–273. doi: 10.1023/a:1027337601863. [DOI] [PubMed] [Google Scholar]
- 39.Bäcklund T., Palojoki E., Saraste A. Ef fect of vasopeptidase inhibitor omapatrilat on cardiomyocyte apoptosis and ventricular remodeling in rat myocardial infarction. Cardiovasc Res. 2003;57:727–737. doi: 10.1016/s0008-6363(02)00721-6. [DOI] [PubMed] [Google Scholar]
- 40.McMurray J.J., Packer M., Desai A.S. PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 41.Ong W.K., Tan C.S., Chan K.L. Identification of specific cell-surface markers of adipose-derived stem cells from subcutaneous and visceral fat depots. Stem Cell Reports. 2014;2:171–179. doi: 10.1016/j.stemcr.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Standeven K.F., Hess K., Carter A.M. Neprilysin, obesity and the metabolic syndrome. Int J Obes (Lond). 2011;35:1031–1040. doi: 10.1038/ijo.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rice G.I., Jones A.L., Grant P.J., Carter A.M., Turner A.J., Hooper N.M. Circulating activities of angiotensin-converting enzyme, its homolog, angiotensin-converting enzyme 2, and neprilysin in a family study. Hypertension. 2006;48:914–920. doi: 10.1161/01.HYP.0000244543.91937.79. [DOI] [PubMed] [Google Scholar]
- 44.Goliasch G., Pavo N., Zotter-Tufaro C. Soluble neprilysin does not correlate with outcome in heart failure with preserved ejection fraction. Eur J Heart Fail. 2016;18:89–93. doi: 10.1002/ejhf.435. [DOI] [PubMed] [Google Scholar]