Abstract
OBJECTIVE: To improve conventional chemotherapeutic efficacy, it is significant to identify novel molecular markers for chemosensitivity as well as possible molecules accelerating cell-killing mechanisms. In this study, we attempted to elucidate how MK2206, an allosteric Akt inhibitor, enhances the cisplatin (CDDP)-induced cytotoxicity and apoptosis in testicular cancer. MATERIALS AND METHODS: We checked three testicular cancer cell lines for the expression of phospho(p)-Akt and its downstream molecules targets by Western blot. The potential antitumor effects were analyzed by MTT assay in vitro and by subcutaneous xenograft models in vivo. The cell invasion was analyzed by transwell invasion assay, and the activities of Akt signaling pathway and expression of apoptosis-related proteins were measured by Western blot. RESULTS: Our results indicated that there was overactivation of p-Akt and its downstream molecules in testicular cancer cell lines compared with normal testis epithelium cells. MK2206 (600 nM) inhibited cell invasion in TCAM-2 and P19 cell lines and significantly increased the susceptibility of testicular cancer to CDDP. Combined with CDDP, MK2206 potentiated CDDP-induced cytotoxicity and apoptosis, with repressed expression of p-Akt and its downstream targets. The subcutaneous xenograft models also showed that a combined CDDP/MK2206 therapy completely suppressed tumor growth without any side effects. CONCLUSION: These results suggested that the concomitant use of MK2206 could enhance the CDDP-induced cytotoxicity and apoptosis in testicular cancer with the suppressed expression of Akt pathway.
Introduction
Testicular cancer is the most common solid cancer in men between 15 and 35 years of age [1]. Cisplatin (CDDP)-based treatment is the first-line chemotherapy used in testicular cancer patients, either alone or combined with surgery, although the use of chemotherapy is associated with significant toxicity. However, a proportion of patients relapsed or acquired resistance to chemotherapy. To improve chemotherapeutic efficacy and explore the molecular mechanisms, it is important to detect new molecular markers for chemosensitivity and identify possible target-associated cell-killing mechanisms in testicular cancer.
Akt is a serine-threonine kinase activated by growth factors or survival factors to regulate cell growth, survival, proliferation, and metabolism [2]. Recent evidence indicates that Akt is frequently constitutively activated in many types of human cancer, and activated PI3K/Akt signaling is the central effector of many downstream signaling pathways that regulate various cellular responses of tumors, including cell growth, tumorigenesis, progression, survival, and chemosensitivity [3,4]. Dominant-negative mutants of Akt enhance the cytotoxicity of chemotherapeutic agents, suggesting an important role of Akt in drug resistance [5]. Some studies have shown that Akt activation seems to be related with CDDP resistance of ovarian cancer cells and bladder cancer cells [6,7]. Thus, chemotherapeutic agents that could be sensitized by Akt inhibition are highly desirable to guide the clinical application of Akt inhibitors.
MK2206 is a highly potent and selective allosteric Akt inhibitor which is under development for the treatment of solid tumors. Previous reports have demonstrated that MK2206 has antitumor activity alone and enhanced anti-tumor efficacy in combination use with chemotherapeutic agents in solid tumors [8,9], but there were no reports about the anti-tumor effects of MK2206 combined with chemotherapeutic agents in testicular cancer. In the present study, we investigated the effectiveness of MK2206 to enhance CDDP-induced cytotoxicity and apoptosis through the suppression of phospho(p)-Akt expression and its downstream molecules. This combined treatment may provide a potential new therapeutic option by targeting the Akt signaling pathway to increase CDDP sensitivity in testicular cancer.
Materials and Methods
Antibodies and Reagents
Antibodies were obtained as follows: anti-Akt, p-Akt (ser473), p-4EBP1, p-mTOR, p-70S6K, p-GSK3β, Ki-67, survivin (71G4B7), bax, cleaved caspase-3, Snail, Slug and ZEB1 from Cell Signaling Technology (Beverly, MA); anticaspase-8 and anticaspase-9 from BD Pharmingen (San Diego, CA); MK2206 from Active Biochem (Maplewood, NJ); and CDDP from WAKO (Osaka, Japan).
Cell Culture
Three testicular cancer cells lines (TCAM-2, NCCIT and P19) and one testicular epithelial cell (15P-1) line were obtained from ATCC. Cells were maintained in DMEM medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2.
MTT Assay
Cell viability was assessed by MTT assay. Briefly, 1×104 cancer cells with 100 μl suspension were grown in each well of 96-well plates. After 24 hours of incubation, cells were treated with or without different concentrations of drugs for another 24 hours. Then, 20 μl MTT working solution (5 mg/ml; Sigma) was added to each culture well and incubated for 4 hours. The absorbance (A) of each well was measured by a microculture plate reader at 540 nm. The percentage of cell viability = (A of experimental wells / A of control wells) × 100. These experiments were performed at least three times independently.
Drug Treatment and Preparation of Cellular Extracts
For the dose-response and the time-course studies, cells were grown to 70-80% confluency in 100-mm dishes for 48 hours. Then cells were treated with CDDP at the corresponding concentrations of IC30. For analysis of Akt pathway, cells were treated with CDDP at the concentration corresponding to IC30 with or without treatment of 600 nM MK2206. After treatment, cells were washed with phosphate-buffered saline (PBS) and lysed in an appropriate volume of ice-cold cell lysis buffer (20 mM Tris, PH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin) supplemented with 1 mM phenylmethylsulfonyl fluoride. Cellular lysates were clarified by centrifugation at 15000 × g for 20 minutes, and the protein concentrations of the lysates were determined by a detergent-compatible protein assay kit (Bio-Rad, Hercules, CA). Portions of the same cellular extracts were used for Western blot analysis.
Western Blot Analysis
Thirty to fifty micrograms of protein lysates were separated by SDS-PAGE on a 10% to 15% Tris-HCL minigel, and transferred onto a PVDF membrane following standard methods. After blocking, membranes were probed with appropriate dilutions of primary antibodies overnight, followed by incubation with horseradish peroxidase-conjugated secondary antibodies 1 hours. Proteins were visualized by the chemiluminescent detection system.
Transwell Invasion Assay
For transwell invasion assays, cells were suspended in serum-free DMEM. The cell suspensions TCAM-2 and P19 (5×104) were then added to the 8.0 μm pore polyethylene terephthalate filter insert of a 24-well transwell cell culture chamber (BD Falcon, Franklin Lakes, NJ, USA) and were incubated for 24 hours with medium containing 10% FBS in the bottom of the chamber. Residual cells on the upper side of chambers were removed by scraping with cotton swabs and the cells that attached to the lower side of the membrane were fixed with 70% ethanol and stained with hematoxylin. The number of invaded cells was counted in 3 randomly selected fields (×200) under microscope. Each experiment was triplicated and performed three times independently.
Mouse Xenograft Model
To produce a subcutaneous xenograft model, 5×106 P19 cells in 100 μl phosphate buffered saline were inoculated subcutaneously into each flank of pathogen-free 5-week-old female BALB/cAJcl-nu/nu mice (Nihon Clea, Japan). After growing for 7 days, mice with tumor xenograft were divided into four groups (5 mice each group) and treated for 2 weeks as follows: (a) vehicle alone (DMSO); (b) CDDP at 6 mg/kg weekly; (c) MK2206 at 50 mg/kg weekly; (d) combination of (b) and (c). We administered CDDP or MK2206 by intraperitoneal injection. The doses and injection regimens for these drugs were based on reports published previously [10,11]. Mice body weight and tumor size were measured every other day. Tumor volume was calculated using the equation, volume=width2×length/2. All mice were sacrificed on day 15 after initial treatment. Tumors were excised and formalin fixed for Ki-67 immunostaining. Approval for these studies was obtained from the Ethics Committee of Shandong Provincial Hospital Affiliated to Shandong First Medical University.
Statistical Analysis
Statistical data was analyzed using Statistical Package for Social Sciences (SPSS, version 17.0) software. All data used were representative of at least three independent experiments. Quantitative data were expressed as mean ± S.D. Comparisons were carried out by the Mann-Whitney U test and Kruskal-Wallis test. P values <.05 were considered statistically significant.
Results
Expression status of the Akt signaling pathway in testicular cancer cell lines
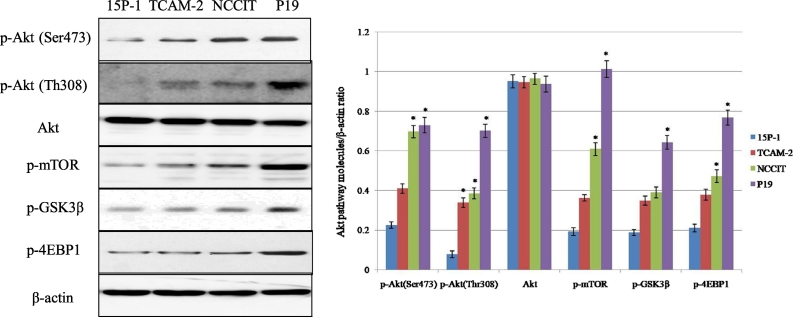
In the present study, the change of p-Akt expression and its downstream molecules were analyzed using 15P-1 (normal testicular epithelial cell) and three testicular cancer cell lines (TCAM-2, NCCIT and P19). Three testicular cancer cell lines had overexpression of p-Akt (Ser473), p-Akt (Thr308), and downstream targets, including p-4EBP1, p-GSK3β, p-mTOR, and the cell line P19 had the strongest expression of those molecules (Figure 1). There were no or weak expressions of Akt signaling in normal testicular epithelial cell 15P-1.
Figure 1.
The expression status of the Akt signaling pathway in testicular cancer cell lines and one testicular epithelial cell line. Western blot shows the expression of Akt and its downstream molecular targets. Bars represent SD. *P < .05 vs. 15P-1.
The Effect of MK2206 on p-Akt Expression, Cell Proliferation, and Cell Invasion in Testicular Cancer Cell Lines
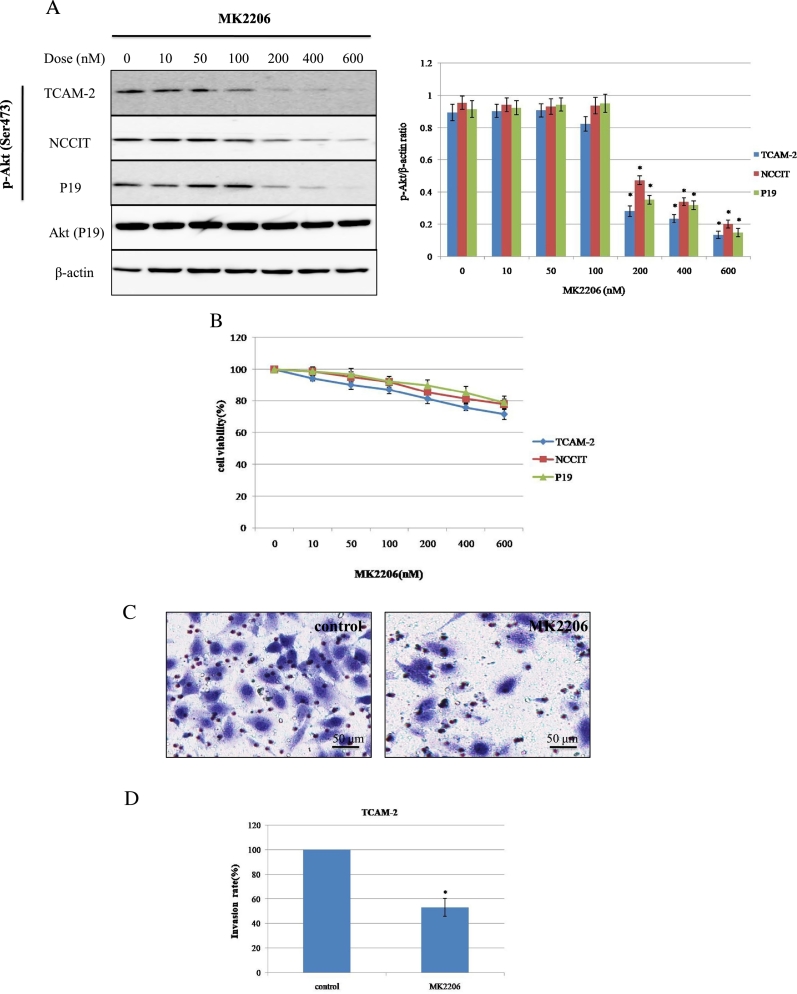
To investigate the effects of MK2206 on p-Akt expression, the three cell lines were treated with increasing doses of MK2206 for 24 hours. Dose-dependent decrease in the p-Akt (Ser473) expression were shown in these cell lines (Figure 2A). The cell viability rates after exposure to 600 nM of MK2206 were 74.67±2.54, 77.74±2.90, 78.93±3.03% in TCAM-2, NCCIT and P19, respectively (Figure 2B). In this study, we used the concentration (600 nM) at which MK2206 alone successfully suppressed p-Akt expression, but repressed cell viability only slightly and did not induce significant apoptosis (Figure 2, A and B, and 4A).
Figure 2.
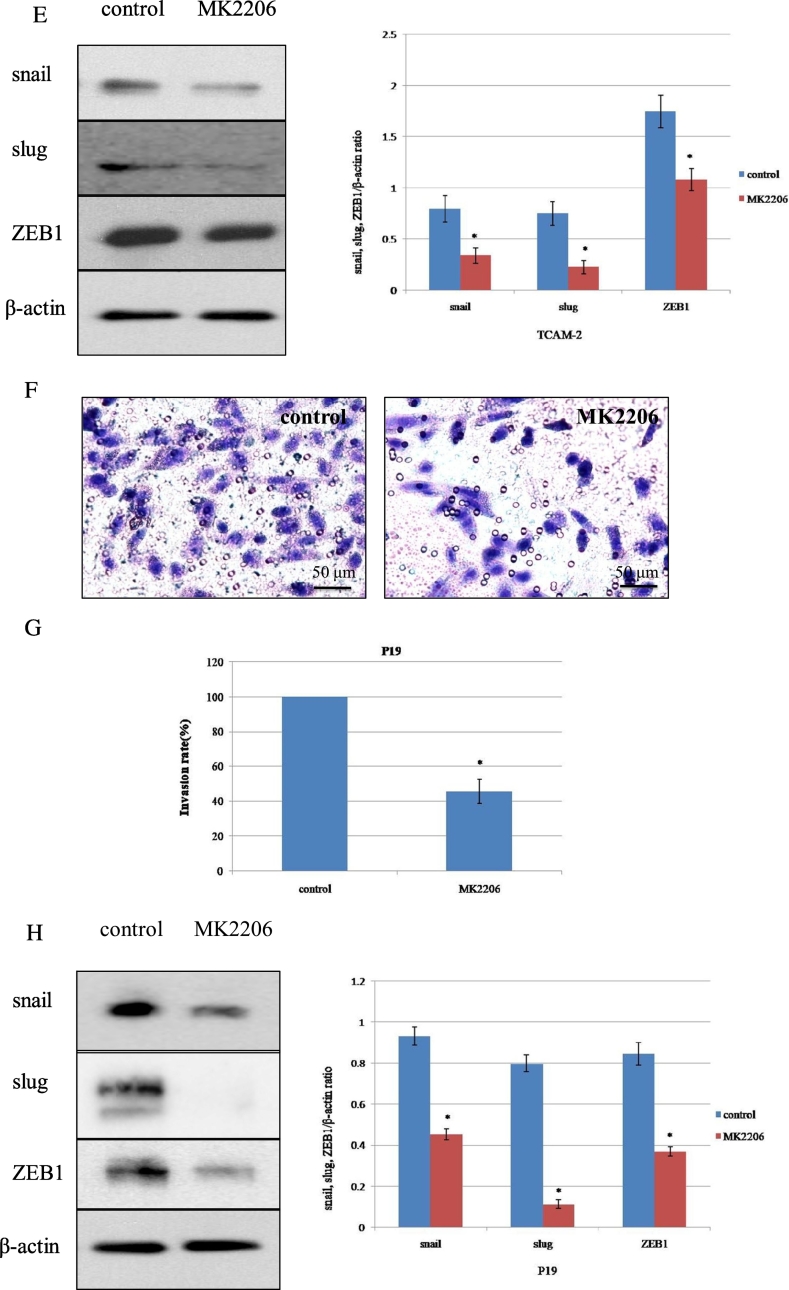
The effect of MK2206 treatment on p-Akt expression, cell viability, and cell invasion in testicular cancer cell lines. (A) Cell lines were exposed to MK2206 at the indicated dose for 24 hours, and the expression level of p-Akt was examined using Western blot. The Akt expression of P19 cell line was shown. *P < .05 vs. 100 nM. (B) Cell viability curves after treatment with MK2206 as in (A). Mean values from at least 3 independent experiments are shown; bars represent SD. (C-D and F-G) The invasive capability of TCAM-2 and P19 were evaluated by transwell invasion assay. The cell suspension (5×104 cells) and reagents (DMSO or MK2206) were added to the insert of a transwell cell culture chamber and incubated for 24 hours with medium containing 10% FBS in the bottom of the chamber. The cells that attached to the lower side of the membrane were fixed with 70% ethanol and stained with hematoxylin. Each experiment was triplicated, and the average number of cells in 5 microscopic fields (×200) was defined as the number of invasive cells. Representative microscopic image (C and F) and quantitated cell numbers (mean ± SD) of 3 independent experiments (D and G) were shown. (E and H) TCAM-2 and P19 cells were treated with control (DMSO) or MK2206 (600 nM) for 24 hours, and the abundance of indicated proteins related with invasion was examined using Western blot. SD = standard deviation. *P < .05 vs. control.
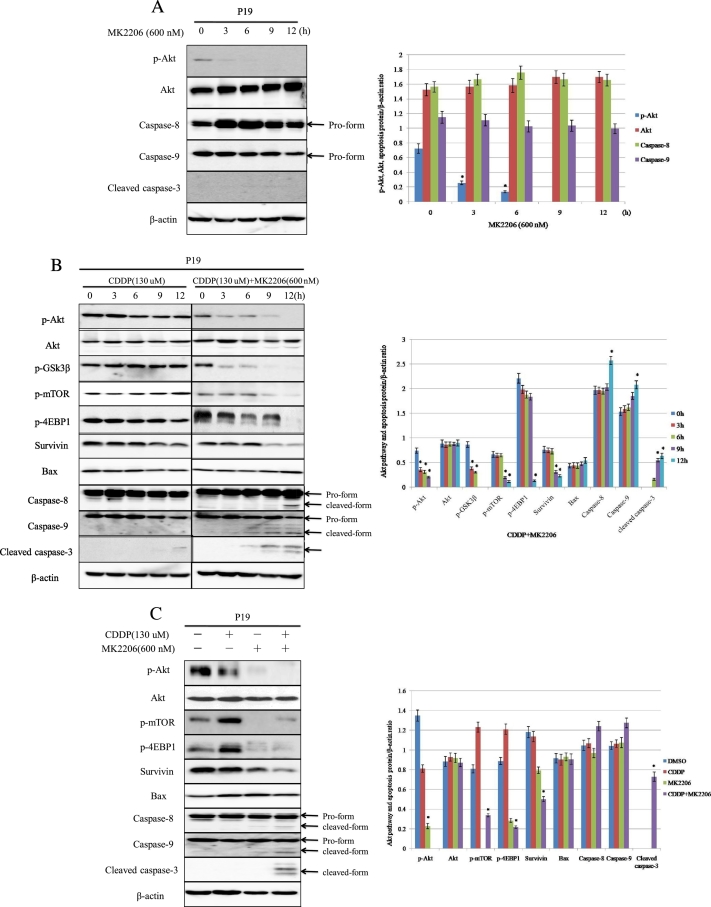
Figure 4.
MK2206 enhances CDDP-induced apoptosis through the suppression of Akt pathway activity in testicular cancer cell lines. (A) Cells were exposed to MK2206 (600 nM) alone for the indicated times. “0 hours” refers to the time for addition of reagents. *P < .05 vs. 0 hours. (B) Cells were treated with CDDP at a concentration corresponding to IC30 (130 μM), with or without addition of 600 nM of MK2206 for the indicated times, and the abundance of indicated protein was examined using Western blot. *P < 0.05 vs. 0 hours. (C) Cells were harvested after treatment with or without CDDP at indicated doses corresponding to IC30 for each cell line in the presence or absence of 600 nM of MK2206 for 12 hours. Bars represent SD. *P < 0.05 vs. DMSO or CDDP.
The effects of MK2206 on cell proliferation were elucidated by Matrigel invasion chamber assay (MTT), corresponding to the same concentrations as those of WB. TCAM-2 and P19 were treated with control (DMSO) or MK2206 (600 nM) and incubated for 24 hours before the invasion assay was taken. Invasion capability of TCAM-2 and P19 treated with MK2206 were decreased significantly compared with the control group (Figure 2, C and D, and 2, F and G). The invasion assay result was influenced by decreased cell proliferation because cell proliferation was repressed approximately to 20% by MK2206 at 600 nM, which could inhibit cell invasion rate approximately to 50% in TCAM-2 cells, and the invasion assay result of P19 was similar with TCAM-2 (Figure 2, D and G). If normalized, the decrease in cell invasion rate would be roughly 30%. To clarify the functional relevance of molecules that were associated with tumor invasion, the expression of Snail, Slug, and ZEB1 was checked using WB after treatment with MK2206. The WB results demonstrated that pretreatment with MK2206 for 24 hours inhibited the expression of Snail, Slug, and ZEB1 (Figure 2E). Meanwhile, Akt phosphorylation expression was also decreased when exposed to MK2206 (600 nM) for 24 hours in TCAM-2 (Figure 2A). We also tried invasion assay in P19, and the observation that MK2206 had an inhibited effect on invasion in P19 was similar with TCAM-2 (Figure 2H). These data may indicate that the decreased expression of Akt phosphorylation was associated with tumor invasion inhibition through suppression of Snail, Slug, and ZEB1.
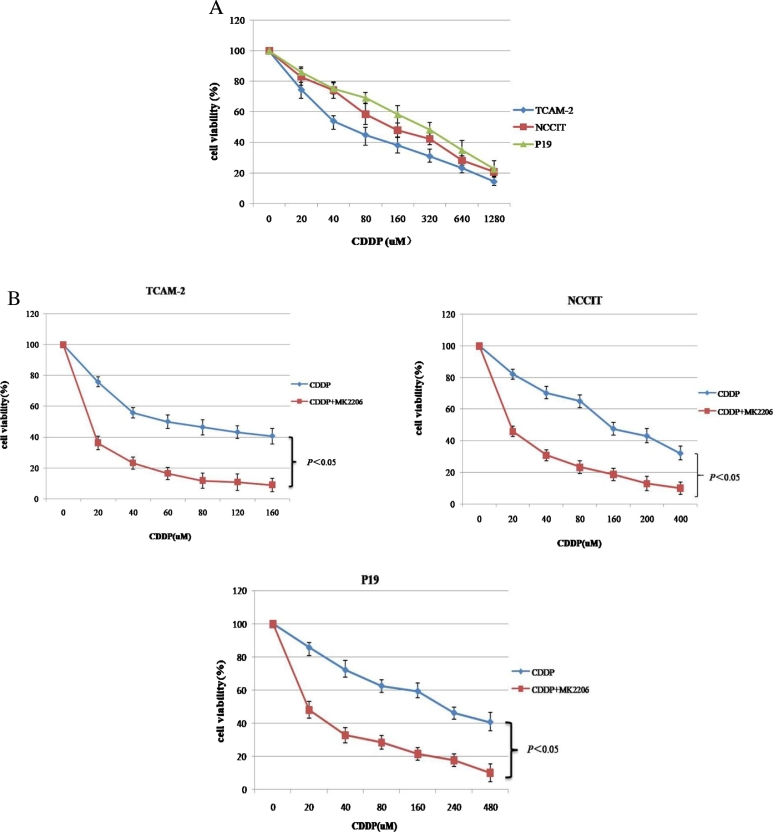
Sensitivity to CDDP in Testicular Cancer Cell Lines and Enhancement of CDDP-Induced Cytotoxicity by MK2206
The sensitivity to CDDP differed among cells: IC50 monitored by MTT assay was 76.0±4.6, 165.3±8.2, 221.5±11.4 μM in TCAM-2, NCCIT, and P19, respectively (Figure 3A). Combination with MK2206 (600 nM) significantly enhanced sensitivity to CDDP in a dose-dependent manner. Significant differences in cell viability rates were observed, statistical differences between CDDP and CDDP+MK2206 were P < .05, P < .05, and P < .05 in TCAM-2, NCCIT and P19, respectively (Figure 3B).
Figure 3.
The effect of combinatorial treatment with MK2206 and CDDP in testicular cancer cell lines. (A) Cell viability was evaluated 24 hours after treatment with CDDP at indicated concentrations using MTT assay among testicular cancer cell lines. (B) MTT assay showing effect of CDDP treatment with or without MK2206 (600 nM) in indicated cell lines. Average values from at least 3 independent experiments are shown; bars represent SD.
MK2206-Mediated Enhancement of CDDP-Induced Apoptosis Involved with Inhibition of the Akt Pathway
To explore the molecular mechanisms underlying the effects of MK2206 in CDDP-induced apoptosis, p-Akt, p-GSK3β, p-4EBP1, p-mTOR, survivin, Bax, and caspases were examined after treatment with CDDP, MK2206, and CDDP+MK2206 in a time-dependent manner in P19 (Figure 4, A and B). Western blot analysis demonstrated that MK2206 (600 nM) alone could inhibit the p-Akt expression, but it could not induce cleaved forms of apoptosis targets (caspases-8/caspase-9/caspase-3) in the time course (Figure 4A). Concomitant use of CDDP (130 μM) and MK2206 (600 nM) did not change the expression of Akt, but it suppressed p-Akt, p-GSK3β from 3 hours, p-mTOR from 9 hours, and p-4EBP1 from 12 hours. The activation of caspase-8/caspase-9/caspase-3, as shown by a significant increase in their cleaved products, occurred from 9 hours after exposure to CDDP+MK2206, but CDDP itself caused slight cleaved forms of caspase-3 only in 12 hours, with no significant change in phosphorylation of Akt, 4EBP1, and mTOR. The downregulation of survivin, as an antiapoptotic protein, was observed in 9 hours after treatment with CDDP combined with MK2206, but there was no obvious change in bax, an apoptotic protein, when treated with CDDP+MK2206 in time course (Figure 4B).
To further investigate the effects of the Akt pathway on the CDDP-induced apoptosis, WB was performed using P19 cells, which were incubated for 12 hours with the indicated reagents (Figure 4C). Single treatment with CDDP (130 μM) did not induced cleaved forms of caspase-8/caspase-9/caspase-3 and did not cause the activation of bax and remarkable inhibition of survivin. Interestingly, single use of CDDP increased the expression of p-4EBP1 and p-mTOR. CDDP combined with MK2206 induced significant suppression of p-Akt, p-4EBP1, p-mTOR, survivin, and activated caspases in the cell line, contrary to MK2206 or CDDP single treatment. These findings suggested that inhibition of the Akt pathway played an important role in the enhancement of CDDP-induced apoptosis.
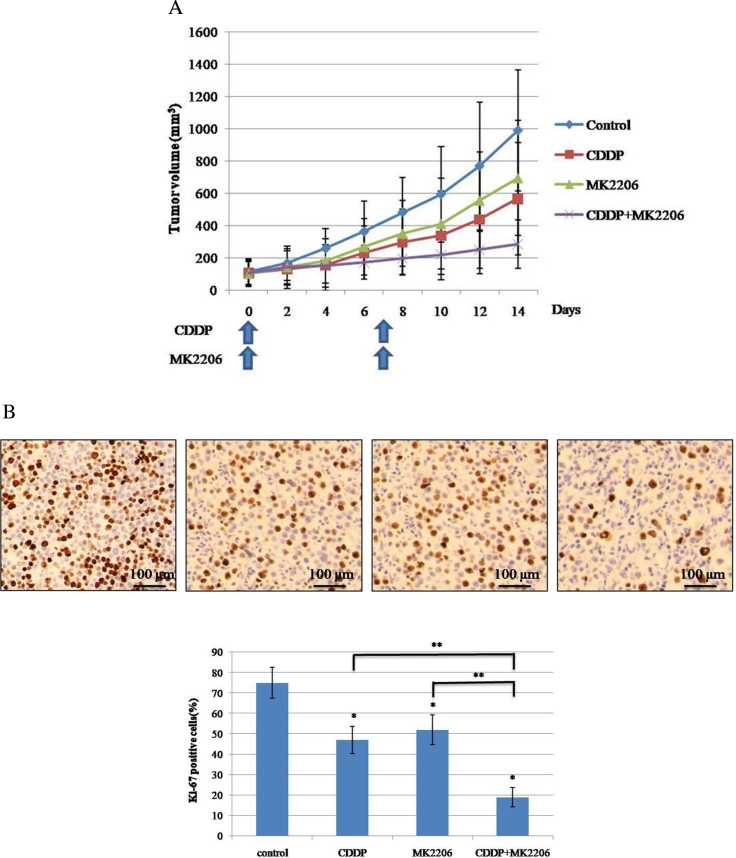
MK2206 Effectively Enhanced CDDP-Induced Cytotoxicity in a Mouse InVivo Model
For the clinical use of MK2206 for testicular cancer, we examined the effects of MK2206 alone or in combination with CDDP to the in vivo growth of P19 cells, using a subcutaneous xenograft model. CDDP and MK2206 were given weekly. To investigate the safety of this combination, body weight was assessed. During the treatment duration, combination treatment of MK2206 and CDDP did not have an adverse effect on body weight in comparison with MK2206 or CDDP alone. In our conditions, MK2206 or CDDP only partially delayed tumor cell growth and the tumor grew steadily, but the CDDP/MK2206 combination therapy suppressed tumor growth remarkably (Figure 5A). The tendency of tumor growth inhibition was observed among groups. Ki-67 immunostaining of tumors demonstrated that CDDP/MK2206 combination therapy of could reduce tumor cells proliferation significantly when compared with CDDP treatment alone (Figure 5B). These results indicated that MK2206 could enhance CDDP-induced cytotoxicity in vivo as well as in vitro.
Figure 5.
The effect of combinatorial treatment with CDDP and MK2206 on the growth of a P19 subcutaneous xenograft tumor in vivo. (A) The change of subcutaneous P19 tumor volume measured using the Vernier caliper. After tumors reached the indicated volume, tumor-bearing mice were treated with indicated drugs as shown in the figure and described in section “Materials and methods”, and the effect of the indicated drugs on tumor growth was measured using the Vernier caliper. (B) Histological examination of the effect of CDDP and MK2206 on a P19 tumor in vivo. At day 15, the mice were sacrificed and the tumors were dissected. The expression of Ki-67 was assessed by immunohistochemistry. The percentage of Ki-67-positive cells in each treatment group was compared (*P < 0.05 vs. control; **P < 0.05, CDDP+MK2206 vs. CDDP; CDDP+ MK2206 vs. MK2206).
Discussion
This study has shown that MK2206, an Akt inhibitor, specifically enhanced the CDDP-induced cytotoxicity and apoptosis with suppression of the Akt pathway in testicular cancer cell lines. We demonstrated that MK2206 sensitized those cancer cells to CDDP via the inhibition of phosphorylated Akt (p-Akt) and its downstream molecules in the Akt signaling pathway. These results indicated that the Akt pathway is a novel candidate and plays a critical role to influence chemosensitivity to CDDP in testicular cancer.
Akt, as one of the important target in PI3K/Akt signaling pathway, has 3 isoforms: Akt1, Akt2, and Akt3 [12]. Overactivation of Akt can influence many downstream effectors and mediate multiple pathways that favor tumorigenesis, and correlate with tumor progression and reduced survival in human cancer [13,14]. Meanwhile, the finding of PI3K/Akt mutations is notable, as prior studies in several tumor types have identified mutational activation of the PI3K-Akt pathway as a potential mechanism of resistance to cytotoxic chemotherapy, including cisplatin [15,16]. For example, the increased activation of p-Akt in human lung tumor is inversely correlated with CDDP sensitivity in their primary derived culture counterpart [17]. Zhang has demonstrated that Cx32 expression may induce CDDP resistance by activating the EGFR‑Akt signaling pathway in ovarian cancer cells [18]. The results of Yang indicated that TCF21 inhibited gastric cancer growth and chemoresistance through the AKT signaling pathway [19]. One important mechanism involved in CDDP resistance is the down-regulation of PTEN by induction of microRNAs, such as miR-93 in ovarian cells [20] and miR-221 in osteosarcoma cells [21]. PTEN, the phosphatase and tensin homolog, as a tumor suppressor, negatively regulates the Akt pathway by hydrolyzing phosphatidylinositol triphosphate to phosphatidylinositol biphosphate. Loss of PTEN strongly associates with the activation of Akt in tumor cell lines, and recent studies have shown that reduced PTEN expression is common in testicular tumors [22].
MK2206, a highly selective allosteric inhibitor of Akt, is being tested in both preclinical settings and clinical trials as an anticancer agent. MK2206 by itself causes cell proliferation inhibition of cell lines, including breast cancer lines, hepatocellular cancer lines, nasopharyngeal carcinoma cell lines, and so on [23,24]. Meanwhile, MK2206 could synergistically enhance antitumor efficacy with some chemotherapeutic agents, and suppress cell invasion coincided with decreased Akt phosphorylation [25,26]. In the current study, MK2206 (600 nM) alone inhibited Akt phosphorylation effectively using the concentration that could inhibit cell proliferation slightly, but cell invasion rate was suppressed significantly by MK2206 (600 nM) with the inhibition of Snail, Slug and ZEB1, which were transcription factors related with epithelial to mesenchymal transition (EMT). In addition, it has been reported that the expression of EMT pathways including Snail, Slug and ZEB1 were responsible for tumor invasion, metastasis, and decreased chemosensitivity, and inhibition of those targets increased the chemosensitivity [[27], [28], [29]]. Our results may show that decreased Akt phosphorylation played a critical role in the inhibition of cell invasion and repression of Snail, Slug and ZEB1.
To investigate the role of Akt in the CDDP-induced cytotoxicity, the effect of cell viability was checked in testicular cancer cell lines. CDDP combined with MK2206 clearly inhibited cell viability in comparison with single use of CDDP. Previous data have suggested that chemosensitivity to CDDP could be regulated by Akt pathway in some human cancer, such as non-small-cell lung cancer and gastric cancer[30,31]. Therefore, Akt inhibition is suspected to have a mechanism to promote CDDP susceptibility. We found the contribution of Akt pathway inhibition to apoptosis using MK2206 by showing the enhancement of CDDP-induced apoptosis in CDDP+MK2206. Our results suggested that 4EBP1, GSK3β and mTOR seem to act as the downstream targets of the Akt pathway to promote apoptosis. Generally, p-4EBP1 and p-mTOR have a close relation to protein synthesis, and p-GSK3β is related with cell cycle and metabolism. The activation of p-4EBP1, p-mTOR and p-GSK3β has been reported to increase in the regulation of tumor growth, angiogenesis and drug-resistance [32]. Previous reports have suggested that piperlongumine induces caspase-dependent apoptosis in human breast cancer cells via the inhibition of p-Akt, p-70S6K, and p-4EBP1 [33]. SGK1 inhibition exhibits significant antitumor effects against prostate cancer by inducing autophagy-dependent apoptosis via the mTOR-Foxo3a pathway [34]. NSCLC cells treated with cryptotanshinone reduced cell growth, and induced G0/G1 cell cycle arrest and the activation of apoptosis through PI3K/Akt/GSK3β pathway inhibition [35]. From our data, considerable apoptosis and increased chemosensitivity to CDDP were detected in combination use of CDDP and MK2206, depending on the suppression of the Akt signaling pathway, including reduction of p-4EBP1, p-mTOR and p-GSK3β. Our results demonstrated that the expression level of survivin protein decreased from 9 hours, corresponding to downregulation of Akt pathway molecules and activating caspase pathway. These data indicated that the change of survivin protein might mediate apoptosis through blockade of the Akt pathway. In addition, our findings suggested that MK2206 might inhibit the molecular targets of Akt pathway, leading to acceleration of CDDP-induced apoptosis and enhancement of chemosensitivity. Here, we showed that the combination therapy may be effective to overcome the preexisting resistance to a chemotherapeutic drug of testicular cancer, that is, promotion of chemosensitivity. But another important subject of chemotherapy against testicular cancer is acquired chemoresistance during the chemotherapy, that is, treatment refractory. To explore the effectiveness of this combination therapy against chemoresistance, we need further experiments using CDDP-resistant testicular cancer cell lines in the future. If this treatment were also effective against a chemoresistant situation, indication of this combination therapy would be further expanded.
To investigate the clinical usefulness and safety, we used a subcutaneous xenograft tumor model. In this model, MK2206 was administered intraperitoneally at 50mg/kg once per week. This dose was used referring to the previous document [7]. Furthermore, this concentration could still enhance cancer-specific CDDP-induced cytotoxicity, and significant tendency of tumor growth inhibition was found in the combination therapy group. When we consider the clinical application of MK2206 at a low concentration with CDDP, which is one of the most frequently used agents for testicular cancer, is a very attractive choice.
Conclusions
In summary, we identified that MK2206 could promote CDDP-induced cytotoxicity and apoptosis in testicular cancer cells by the repression of Akt activation and its downstream molecular targets. Akt target therapy using MK2206 will develop as a promising strategy applied to enhance chemosensitivity to CDDP in testicular cancer.
Declarations of interest
None
Acknowledgments
This study was supported by the grants the Natural Science Foundation of Shandong Province (No. ZR2017BH036), Key Research and Development Program of Shandong Province (No. 2018GSF118142) and Medical Science and Technology Development Plan Project of Shandong Province (No. 2017WS289).
Contributor Information
Qiang Fu, Email: qiangfu68@163.com.
Keqin Zhang, Email: 270638805@qq.com.
References
- 1.Chia V.M., Li Y., Quraishi S.M. Effect modification of endocrine disruptors and testicular germ cell tumor risk by hormone-metabolizing genes. Int J Androl. 2010;33:588–596. doi: 10.1111/j.1365-2605.2009.00975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Datta S.R., Brunet A., Greenberg M.E. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 3.Sun Y., Zhao S., Tian H. Depletion of PI3K p85alpha induces cell cycle arrest and apoptosis in colorectal cancer cells. Oncol Rep. 2009;22:1435–1441. [PubMed] [Google Scholar]
- 4.Liu N., Rowley B.R., Bull C.O. BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110α and p110δ activities in tumor cell lines and xenograft models. Mol Cancer Ther. 2013;12:2319–2330. doi: 10.1158/1535-7163.MCT-12-0993-T. [DOI] [PubMed] [Google Scholar]
- 5.Guerrero-Zotano A., Mayer I.A., Arteaga C.L. PI3K/AKT/mTOR: role in breast cancer progression, drug resistance, and treatment. Cancer Metastasis Rev. 2016;35:515–552. doi: 10.1007/s10555-016-9637-x. [DOI] [PubMed] [Google Scholar]
- 6.Yang X., Fraser M., Moll U.M. Akt-mediated cisplatin resistance in ovarian cancer: modulation of p53 action on caspase-dependent mitochondrial death pathway. Cancer Res. 2006;66:3126–3136. doi: 10.1158/0008-5472.CAN-05-0425. [DOI] [PubMed] [Google Scholar]
- 7.Sun D, Sawada A, Nakashima M, et al. MK2206 potentiates cisplatin-induced cytotoxicity and apoptosis through an interaction of inactivated Akt signaling pathway. Urol Oncol. 2015; 33:111.e17-26. [DOI] [PubMed]
- 8.Winder A., Unno K., Yu Y. The allosteric AKT inhibitor, MK2206, decreases tumor growth and invasion in patient derived xenografts of endometrial cancer. Cancer Biol Ther. 2017;18:958–964. doi: 10.1080/15384047.2017.1281496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sangai T., Akcakanat A., Chen H. Biomarkers of response to Akt inhibitor MK2206 in breast cancer. Clin Cancer Res. 2012;18:5816–5828. doi: 10.1158/1078-0432.CCR-12-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ou D.L., Lee B.S., Lin L.I. Vertical blockade of the IGFR-PI3K/Akt/mTOR pathway for the treatment of hepatocellular carcinoma: the role of survivin. Mol Cancer. 2014;13:2. doi: 10.1186/1476-4598-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsui Y., Watanabe J., Ikegawa M. Cancer-specific enhancement of cisplatin-induced cytotoxicity with triptolide through an interaction of inactivated glycogen synthase kinase-3beta with p53. Oncogene. 2008;27:4603–4614. doi: 10.1038/onc.2008.89. [DOI] [PubMed] [Google Scholar]
- 12.Bellacosa A., Testa J.R., Staal S.P. A retroviral oncogene, Akt, encoding a serine-threonine kinase containing an SH2-likeregion. Science. 1991;254:274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 13.Hers I., Vincent E.E., Tavaré J.M. Akt signalling in health and disease. Cell Signal. 2011;23:1515–1527. doi: 10.1016/j.cellsig.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Franks S.E., Briah R., Jones R.A., Moorehead R.A. Unique roles of Akt1 and Akt2 in IGF-IR mediates lung tumorigenesis. Oncotarget. 2016;7:3297–3316. doi: 10.18632/oncotarget.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang G., Li M., Zhu X. Knockdown of Akt sensitizes osteosarcoma cells to apoptosis induced by cisplatin treatment. Int J Mol Sci. 2011;12:2994–3005. doi: 10.3390/ijms12052994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhuang X.M., Zhou B. CXCR4 enhances cisplatin resistance of human tongue squamous cell carcinoma. J Oral Pathol Med. 2019;48:122–128. doi: 10.1111/jop.12813. [DOI] [PubMed] [Google Scholar]
- 17.Liu L.Z., Zhou X.D., Qian G., Shi X., Fang J., Jiang B.H. AKT1 amplification regulates cisplatin resistance in human lung cancer cells through the mammalian target of rapamycin/p70S6K1 pathway. Cancer Res. 2007;67:6325–6332. doi: 10.1158/0008-5472.CAN-06-4261. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y., Tao L., Fan L.X. Cx32 mediates cisplatin resistance in human ovarian cancer cells by affecting drug efflux transporter expression and activating the EGFR Akt pathway. Mol Med Rep. 2019;19:2287–2296. doi: 10.3892/mmr.2019.9876. [DOI] [PubMed] [Google Scholar]
- 19.Yang Z., Jiang X., Li D. TCF21 inhibits proliferation and chemoresistance through the AKT pathway in human gastric cancer. Gene. 2019;682:42–44. doi: 10.1016/j.gene.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Fu X., Tian J., Zhang L. Involvement of microRNA-93, a new regulator of PTEN/Akt signaling pathway, in regulation of chemotherapeutic drug cisplatin chemosensitivity in ovarian cancer cells. FEBS Lett. 2012;586:1279–1286. doi: 10.1016/j.febslet.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Zhao G., Cai C., Yang T. MicroRNA-221 induces cell survival and cisplatin resistance through PI3K/Akt pathway in human osteosarcoma. PLoS One. 2013;8 doi: 10.1371/journal.pone.0053906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ichimura K., Fukushima S., Totoki Y. Recurrent neomorphic mutations of MTOR in central nervous system and testicular germ cell tumors may be targeted for therapy. Acta Neuropathol. 2016;131:889–901. doi: 10.1007/s00401-016-1557-x. [DOI] [PubMed] [Google Scholar]
- 23.Wilson J.M., Kunnimalaiyaan S., Gamblin T.C. MK2206 inhibits hepatocellular carcinoma cellular proliferation via induction of apoptosis and cell cycle arrest. J Surg Res. 2014;191:280–285. doi: 10.1016/j.jss.2014.05.083. [DOI] [PubMed] [Google Scholar]
- 24.Ma B.B., Liu V.W., Hui C.W. Preclinical evaluation of the Akt inhibitor MK2206 in nasopharyngeal carcinoma cell lines. Invest New Drugs. 2013;31:567–575. doi: 10.1007/s10637-012-9896-5. [DOI] [PubMed] [Google Scholar]
- 25.Jin R., Nakada M., Teng L. Combination therapy using Notch and Akt inhibitors is effective for suppressing invasion but not proliferation in glioma cells. Neurosci Lett. 2013;534:316–321. doi: 10.1016/j.neulet.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Chen X., Cui D., Bi Y. AKT inhibitor MK-2206 sensitizes breast cancer cells to MLN4924, a first-in-class NEDD8-activating enzyme (NAE) inhibitor. Cell Cycle. 2018;17:2069–2079. doi: 10.1080/15384101.2018.1515550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao L., Wan Q., Li F. MiR-363 inhibits cisplatin chemoresistance of epithelial ovarian cancer by regulating snail-induced epithelial-mesenchymal transition. BMB Rep. 2018;51:456–461. doi: 10.5483/BMBRep.2018.51.9.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura R., Ishii H., Endo K. Reciprocal expression of slug and snail in human oral cancer cells. PLoS One. 2018;13 doi: 10.1371/journal.pone.0199442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren J., Chen Y., Song H. Inhibition of ZEB1 reverses EMT and chemoresistance in docetaxel-resistant human lung adenocarcinoma cell line. J Cell Biochem. 2013;114:1395–1403. doi: 10.1002/jcb.24481. [DOI] [PubMed] [Google Scholar]
- 30.Chen W., Liu X., Yuan S. HSPA12B overexpression induces cisplatin resistance in non-small-cell lung cancer by regulating the PI3K/Akt/NF-κB signaling pathway. Oncol Lett. 2018;15:3883–3889. doi: 10.3892/ol.2018.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou D., Liu W., Liang S. Apoptin‐derived peptide reverses cisplatin resistance in gastric cancer through the PI3K-AKT signaling pathway. Cancer Med. 2018;7:1369–1383. doi: 10.1002/cam4.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engelman J.A., Luo J., Cantley L.C. The evolution of phosphatidyli-nositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 33.Shrivastava S. Kulkarni P, Thummuri D, et al. Piperlongumine, an alkaloid causes inhibition of PI3K/Akt/mTOR signaling axis to induce caspase-dependent apoptosis in human triple-negative breast cancer cells. Apoptosis. 2014;19:1148–1164. doi: 10.1007/s10495-014-0991-2. [DOI] [PubMed] [Google Scholar]
- 34.Liu W., Wang X., Liu Z. SGK1 inhibition induces autophagy-dependent apoptosis via the mTOR-Foxo3a pathway. Br J Cancer. 2017;117:1139–1153. doi: 10.1038/bjc.2017.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim S.A., Kang O.H., Kwon D.Y. Cryptotanshinone induces cell cycle arrest and apoptosis of NSCLC cells through the PI3K/Akt/GSK-3β pathway. Int J Mol Sci. 2018;19:2739. doi: 10.3390/ijms19092739. [DOI] [PMC free article] [PubMed] [Google Scholar]