Abstract
Oncolytic viruses have demonstrated efficacy in numerous tumor models including non-small cell lung cancer (NSCLC). One limitation of viral therapy for metastatic lung cancer is that systemic administration can be hindered by complement and antiviral immunity. Thus, we investigated whether ex vivo-infected blood outgrowth endothelial cells (BOECs) could be used to deliver VSV-IFNβ in preclinical models of NSCLC. BOECs were obtained from human donors or C57/Bl6 mice. VSV was engineered to produce GFP or IFNβ. Human and murine BOECs could be infected by VSV-GFP and VSV-IFNβ. Infected BOECs resulted in killing of NSCLC cells in vitro and shielded VSV-IFNβ from antibody neutralization. Mouse BOECs localized to lungs of mice bearing syngeneic LM2 lung tumors, and infected murine BOECs reduced tumor burden in this model. In an immune-deficient A549 xenograft model, mice treated with VSV-IFNβ-infected human BOECs exhibited superior antitumor activity and survival of mice (n = 10, P < .05 compared to VSV-IFNβ alone). We conclude that BOECs can be used as a carrier for delivery of oncolytic VSV-IFNβ. This may be an effective strategy for clinical translation of oncolytic virotherapy for patients with metastatic NSCLC.
Introduction
Oncolytic virotherapy has gained traction in recent years with the recent FDA approval of talimogene laherpaprevec (T-vec) for melanoma [1]. This oncolytic virus is given to patients with intradermal metastatic melanoma and shows clinical activity with abscopal immune responses after intratumoral injection. Intratumoral injections, however, are not practical for diseases that are predominantly visceral, such as non-small cell lung cancer (NSCLC). Intravenous delivery of oncolytic virus would permit targeting of visceral tumors, but antiviral antibodies and complement reduce viral burden and neutralize infection of tumor cells [2]. Another disadvantage might be the potential for increased systemic toxicity.
Approaches to mitigate this in the past include utilizing immune suppression to quell the neutralizing antibody response or modifications to the virus to avoid recognition of the virus either by removal of common antigens or by nanoparticle shielding [[3], [4], [5], [6]]. As the antitumor immune response may be a major determinant of viral efficacy, immune suppression not only has the potential to adversely impact efficacy but also increases the potential for toxicity.
Finally, cellular carriers have been proposed as a vehicle for delivering oncolytic virotherapy to tumors [7,8]. Several types of cells have been used including T cells, mesenchymal stem cells, cancer cells, and blood outgrowth endothelial cells (BOECs) [[9], [10], [11], [12], [13]]. An optimal cellular carrier requires three features. First, it must be easily infected by virus. Second, it must carry the virus specifically to the tumor bed while hiding it from immunologic recognition. Finally, it must release progeny virus to act upon distant tumor sites. BOECs have been used to effectively deliver measles virus intratumorally to glioma [13]. Here, we describe experiments that demonstrate the potential of BOECs carrying vesicular stomatitis virus (VSV) expressing IFNβ as a systemic delivery system in mouse models of metastatic non-small cell lung cancer (NSCLC). Our findings support the potential for clinical translation of BOECs as a novel carrier for VSV-IFNβ.
Materials and Methods
Cell Lines and Cell Culture
Lung cancer cell lines, H2009 and H2030, were cultured in RPMI 1640 media (Gibco, Life Technologies, Gaithersburg, MD) containing 10% calf serum (Sigma-Aldrich, St. Louis, MO) (R10 medium). African green monkey kidney Vero cells (CCL-81) were cultured in DMEM (Gibco, Life Technologies) supplemented with 5% calf serum. The above cell lines were obtained from ATCC (American Tissue Culture Collection). A urethane-induced murine lung cancer cell line, LM2, was generously provided by the laboratory of Dr. Alvin M. Malkinson (Department of Pharmaceutical Sciences, University of Colorado, Denver, Colorado) and was maintained in MEM Alpha medium (Minimal Essential Medium Alpha, Gibco, Life Technologies) supplemented with L-glutamine and 10% calf serum. A549 human lung adenocarcinoma cells transfected with firefly luciferase (Luc-A549) were kindly provided by Dr. Panyam (University of Minnesota, Minneapolis, MN) and cultured in R10 medium. Dr. Panyam's laboratory obtained the cells from Caliper Life Sciences (Hopkinton, MA). Human and mouse blood outgrowth endothelial cells (hBOECs and mBOECs) were obtained from Dr. Robert Hebbel as cryopreserved aliquots saved from prior studies [14]. These cells were thawed, re-established in culture and further expanded to the desired number of cells. BOECS were cultured in EGM-2 Bullit Kit medium (Lonza, Walkersville, MD) supplemented with 10% fetal bovine serum (Sigma-Aldrich). Mouse BOEC medium was further supplemented with 0.05 mM N6,2’-O-dibutyryladenosine 3′ 5′ cyclic monophosphate sodium salt (Sigma-Aldrich, cat. no. D0627). To induce cellular attachment for tissue culture, the plates used for BOEC growth were coated with type 1 collagen (Corning Incorporated, Tewksbury, MA). Of note, while collagen was used to grow the BOECs, when used in co-culture experiments, collagen was not used.
Viruses
VSV (vesicular stomatitis virus, Indiana strain) engineered to produce green fluorescent protein (GFP) (cat. no. OV2018), human interferon-β (hIFNβ) (cat. no. OV2010), or mouse interferon-β (mIFNβ) (cat. no. OV2014) were obtained from Imanis Life Sciences (Rochester, MN). All viral stocks were grown in Vero cells. Titer values were determined by using serial dilution assays on Vero cells employing the Spearman and Karber equation as previously described [15] to obtain the tissue infective dose of 50% (TCID50).
VSV Infection of BOECs
mBOECs or hBOECs were seeded and incubated in 6-well plates at 100,000 cells for 96 h, 150,000 cells for 72 h, 200,000 cells for 48 h, and 250,000 cells for 24 h in EBM2 culture media. After incubation, cells were rinsed with Opti-MEM (Gibco, Life Technologies) and then incubated for 2 h with either Opti-MEM or Opti-MEM containing either VSV-mIFNβ (for mBOECs), VSV-hIFNβ (for hBOECs), or VSV-GFP at either a multiplicity of infection (MOI) of 1 or 10 as indicated. Cell viability was determined by counting viable cells after exposure to trypan blue and was normalized to untreated cells. Each experiment was performed in triplicate. Medium samples were removed at the indicated times and stored at −80 °C for VSV titer determination.
NSCLC Cells Co-Cultured with VSV-Infected BOECs
H2009 cells were seeded in 6-well plates at 150,000 cells per well in R10 medium. Separately, hBOECs or mBOECs were seeded into 6-well plates and grown in supplemented EGM-2 medium. After overnight incubation, BOECS were infected with VSV-GFP, VSV-IFNβ, or PBS control at MOI of 1 or 10 as described above. After 6 h of infection, BOECS were harvested by trypsinization, counted by trypan blue exclusion, centrifuged, washed, and suspended in R10 medium. BOECs infected with VSV or control were transferred to wells containing H2009 cells and co-cultured for 72 h. Cell number was measured by counting viable cells after exposure to trypan blue. Cell survival was normalized to H2009 cells treated with non-infected BOECs. BOECs do not adhere to tissue culture plates unless coated with type I collagen, so BOECs do not interfere with cell counting of H2009 cells.
Anti-VSV Plasma Preparation
Five C57/bl6 mice were immunized with three monthly subcutaneous injections (0.1 mL) of 1 × 108 TCID50/dose of VSV-GFP. One month after the final immunization, blood was collected weekly by facial vein bleeding and captured in BD Microtainer (Becton, Dickinson and Company) tubes with K2EDTA and then placed on ice. Blood was then centrifuged at 2000×g for 5 minutes, and the clear layer stored at -80 °C. The anti-VSV plasma was then pooled, aliquoted, and stored at -80 °C.
Plasma-Neutralizing Antibody Assay
Serial dilutions of plasma from VSV immunized mice were incubated with 2.6 × 106 TCID50 of VSV-mIFNβ for 1 h at 37 °C. These mixtures were then added to Vero cells contained in wells of a 96-well plate and incubated for 48 h. Wells were examined for cytopathic effects. Neutralizing titer was determined to be the dilution value of plasma that prevented the presence of cytopathic effects.
VSV Protection by BOECs In Vitro
BOECs infected with VSV-GFP or naked VSV-GFP virus were incubated with serial dilutions of anti-VSV neutralizing antibodies for 1 hour at 37 °C and added to cultures of NSCLC cells in 96-well plates. NSCLC cells were incubated with infected BOECs at 1:10 ratio or with VSV-GFP at MOI of 1.6. After 24 h incubation, the spread of VSV-GFP was monitored by fluorescence microscopy. Medium samples were removed from NSCLC-treated cells and stored at -80 °C for VSV titer determination.
Viral Titer Assessment
Viral titers were measured by infection of Vero cells (7000 cells/well) in 96-well plates with 1:5 serial dilutions of medium from the treated cells. The TCID50 was assessed using the Spearman and Karber method [15]. For some samples the titer was measured using a viral plaque assay to determine plaque-forming units/mL. Vero cells (6 × 105/well) were seeded onto 6-well plates and inoculated with serial dilutions in triplicate of medium samples; cells were then overlayed with (0.5%) agarose-DMEM medium mixture. After 24 h of incubation, cells were fixed with a 3:1 ratio of a methanol-acetic acid mixture, the agarose overlay removed, and cells stained with Coomassie brilliant blue (Sigma-Aldrich). Plaque numbers were counted, normalized to volume, and expressed as the mean +/− SD.
Cell Transfection
mBOECs were transfected with a previously described vector [16] encoding firefly luciferase (FLuc), green fluorescent protein (GFP), and blastocidin resistance gene using Lipofectamine 2000 (Invitrogen, Life Technologies, Waltham, MA) following the manufacturer's instruction. Blastocidin resistance was used to select for mBOECs that produce firefly luciferase (mBOEC-FLuc).
mBOEC Trafficking in A/J Mouse Model
2 × 105 LM2 cells in 0.1 mL 1X PBS were tail vein injected into five 8-week old A/J mice (cat. no. 000646, Jackson laboratory, Bar Harbor, ME) using a 27-gauge needle. Thirty-four days after tumor cell injection, mice received an intravenous (IV) injection of 1 × 106 mBOEC-FLuc cells contained in 0.1 mL of 1× PBS. One control animal had only LM2 cells injected by IV. Mice were given i.p. injections of 3 mg D-Luciferin sodium salt (cat. no. LUCNA-1G, Gold Biotechnology, Inc., St. Louis, MO) 10 minutes prior to imaging for FLuc activity. Luminescent imaging was performed with IVIS Spectrum (PerkinElmer, Inc., Waltham, MA) according to the manufacturer's instructions. Mice were euthanized after injection of D-Luciferin, and lungs and livers were resected and imaged at indicated time points.
Syngeneic Murine Model of NSCLC
2 × 105 LM2 cells in 0.1 mL 1X PBS were tail vein injected into 8-week old A/J mice using a 27-gauge needle. 20, 22, 24, 41, 43, and 45 days after tumor cell injection, mice received either an IV injection of 1 × 106 mBOECs (n = 5), 1 × 108 TCID50 of VSV-mIFNβ (n = 5), or 1 × 106 VSV-mIFNβ infected mBOECs (n = 5) contained in 0.1 mL 1xPBS. VSV-mIFNβ-infected mBOECs were prepared as above. Five age-matched mice without induction of lung tumors and untreated were used to determine normal lung weight. On day 48 mice were sacrificed and their lungs removed and weighed. Lung weights were normalized to the lung weight of untreated normal mice.
Quantitative RT-PCR for VSV Nucleocapsid (N) mRNA in Treated Lungs
Quantitative RT-PCR (20 μL) reaction contained 200 nM forward N specific primer (5′ TGATAGTACCGGAGGATTGACGAC 3′) and 200 nM reverse N specific primer (5′ CCTTGCAGTGACATGACTGCTCTT 3′); 1× Express superscript mix for one-step SYBR green ER and 1× Express SYBR green ER universal qPCR supermix; 1× ROX reference dye; nuclease-free water; and RNA template. One cycle of reverse transcription reaction (5 min at 50 °C) was used, followed by a denaturation step (2 min at 95 °C) and 40 cycles of amplification (15 sec at 95 °C and 1 min at 60 °C). Fluorescence was detected employing an Applied Biosystems 7300 (Waltham, MA). Samples were quantitated by comparison with a standard curve generated by amplification (PCR Supermix, Invitrogen) (Forward 5′ TGACAGCTCTTCTGCTCAGATCCA 3′) (Reverse 5′ TTCTGACTTAGCATACTTGCCAAT 3′) of 409-bp in vitro transcribed RNA (Improm-II reverse transcription system) encoding a 298 nucleotide portion of the VSV nucleocapsid gene (bases 971–1380) cloned into pGEM-T Easy vector (Promega). All samples and standards were run as three independent reactions.
Total RNA Preparation from Mouse Lung Tissue
Lungs were dissected from treated tumor bearing mice and flash frozen in liquid N2; 100 mg pieces were transferred into gentleMACS M tube containing 1 mL Trizol reagent (Life Technologies, Waltham, MA) and the RNA_01 program employed on the gentleMACS dissociator. The homogenate was centrifuged for 10 minutes at 5000×g (4 °C) and the liquid phase transferred to a fresh tube. Following this step, the rest of the RNA isolation follows the Trizol reagent instructions.
Human lung cancer xenograft experiment
1 × 106 Luc-A549 cells in 0.2 mL 1X PBS were tail vein injected into 8-week old, female Fox Chase SCID Beige (cat. no. 250, Charles River, Wilmington, MA) mice using a 27-gauge needle. Fourteen, 16, and 29 days after tumor cell injection, mice received either an IV injection of 1X PBS (n = 10), 1 × 106 mBOECs (n = 10), 1 × 108 TCID50 of VSV-mIFNβ (n = 10), or 1 × 106 VSV-mIFNβ-infected mBOECs (n = 10) contained in 0.2 mL 1xPBS. VSV-mIFNβ-infected mBOECs were prepared as above. Luminescent imaging was performed as above using an IVIS Spectrum. Bioluminescence reflecting tumor burden was quantitated using Living Image software (v. 4.3.1) according to the manufacturer's protocol. Mice were sacrificed if they lost more than 20% body weight or if they were moribund. Kaplan–Meier survival curves were generated in GraphPad Prism software (v. 6.0). All animal procedures were performed according to guidelines of the Institutional Animal Care and Use Committee at the University of Minnesota (Protocol # 1501-32207A).
Statistical Analysis
In vitro experiments were performed in triplicate. Results are expressed as a mean and standard deviation. Statistical analysis of in vitro and in vivo data were done using 2-sided paired t-tests with p value <.05 considered significant. Animal survival was estimated using Kaplan–Meier methodology. GraphPad Prism software (v. 6.0) was used to generate Kaplan–Meier curves.
Results
BOECs are Readily Infected by VSV-GFP and VSV-IFNβ
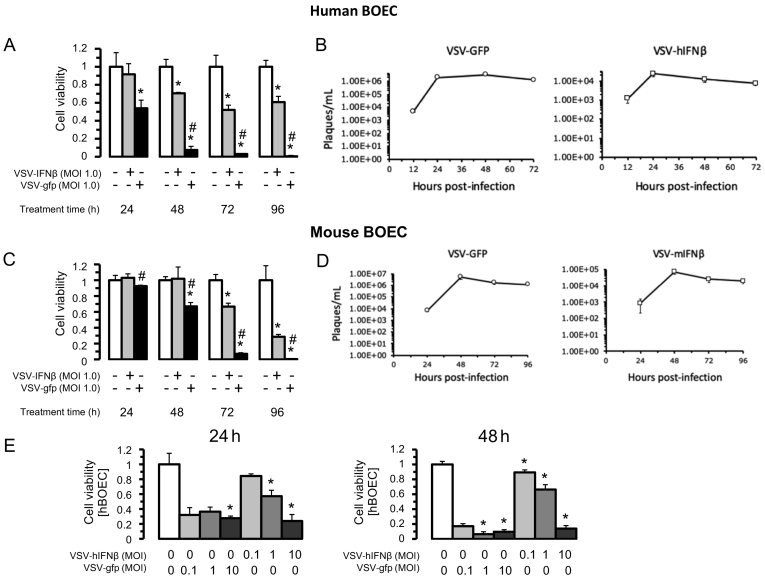
We first evaluated in vitro whether VSV engineered to express GFP (VSV-GFP) or VSV-IFNβ could infect and lyse BOECs. Human BOECs (hBOECs) derived from healthy donors and murine BOECs (mBOECs) derived from C57/Bl6 mice were cultured in vitro and infected at an MOI of 1.0 (Figure 1, A–D). Cells were evaluated for viability daily for 96 hours. VSV-GFP completely lysed hBOECS by 96 hours, whereas VSV-hIFNβ killed 50% of hBOECS by 96 hours (Figure 1A). Similarly, VSV-GFP completely lysed mBOECs by 96 hours, whereas VSV-hIFNβ killed approximately 70% of mBOECs by 96 hours (Figure 1C). Viral titer peaked between 24 and 48 hours under all conditions, but titers were >100-fold less for VSV-IFNβ vis a vis VSV-GFP (Figure 1, B and D). Increasing the viral dose of VSV-hIFNβ to an MOI of 10 improved oncolysis with nearly 90% of hBOECs killed by 48 hours (Figure 1E). These data show that VSV-GFP and VSV-IFNβ readily infect BOECs and that cells survive between 24–48 hours post-infection; this 24–48 hour window of time is sufficient to allow BOECs to deliver virus in vivo. Additionally, these results suggest that viral spread is more limited with VSV-IFNβ and thus BOECs need to be infected at a higher MOI.
Figure 1.
Susceptibility of BOECs to VSV-IFNβ infection. Human BOEC (hBOECs) were infected with VSV-GFP or VSV-IFNβ at MOI of 1. A) Cell viability was assayed by trypan blue exclusion from time of infection to 96 hours post-infection. B) Viral titers were determined from media employing viral plaque assay after hBOEC infection daily up to 72 hours post-infection. C-D) Murine BOECs (mBOECs) derived from C57/Bl6 mice were infected with VSV-GFP and VSV-IFNβ as in A-B. *denotes P < .02 compared to PBS control. # denotes P < .05 between VSV-GFP and VSV-IFNβ. E) hBOECs were infected at the indicated MOI with both VSV-GFP and VSV-IFNβ and assayed for cell viability at indicated time points.
Infected BOECs Lyse NSCLC Cells In Vitro
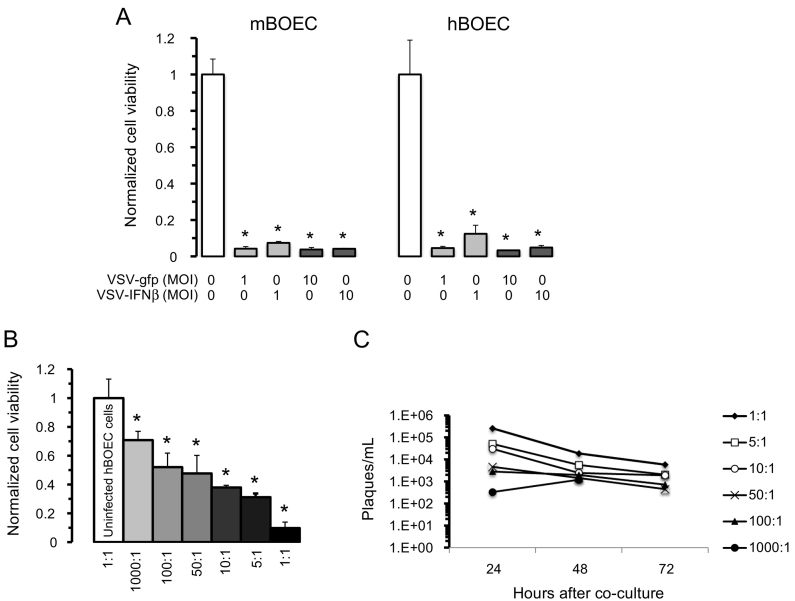
Having established that BOECs can be infected, we next evaluated in vitro whether infected BOECs can transfer virus to NSCLC cells and kill them. A previous in vitro study showed that H2009 cells are highly sensitive to viral oncolysis by either VSV-GFP or VSV-IFNβ at low MOI [15]. H2009 cells were co-cultured with either human or murine BOECs in a 1:1 ratio carrying VSV-GFP or VSV-hIFNβ and evaluated for cell viability. Nearly complete oncolysis was observed at an MOI of 1 or 10 (Figure 2A). To better represent a clinical scenario in which tumor cells would outnumber BOECs, we repeated this experiment using an increasing ratio of tumor cells to BOECs. A dose–response was observed, with a higher ratio of infected BOECs resulting in more effective killing by 72 hours (Figure 2B). Maximum killing was seen at a 1:1 ratio. Not surprisingly, viral titers at 24 hours mirrored the cell viability data, with the highest titer observed for the 1:1 ratio (Figure 2C).
Figure 2.
VSV can infect BOECs and transfer infection to NSCLC cells in vitro. A) An equal number of VSV-infected BOECs were co-cultured with H2009 cells and cell viability measured after 72 hours by trypan blue exclusion (Note: BOECs are not adherent to wells in absence of collagen-coating and are washed away prior to counting). Cell viability is normalized to control (PBS-treated BOECs co-cultured with H2009 cells). *indicates statistical significance (P < .002) compared to control. B) Co-culture of H2009 cells to VSV-hIFNβ-infected hBOECs (MOI =10) was done at varying ratios of H2009:hBOECs as indicated. Cell viability was normalized to H2009 cells treated with uninfected hBOEC cells. *indicates statistical significance (P < .03) compared to control. C) Viral titer was determined from medium harvested from a ratio of H2009 cells to VSV-hIFNβ infected hBOECs. Viral titers were determined using viral plaque assay.
Infected BOECs Lyse NSCLC Cells in the Presence of Antiviral Neutralizing Antibodies
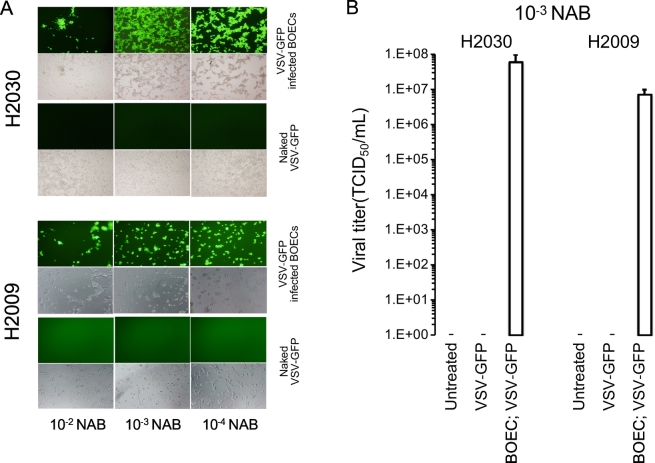
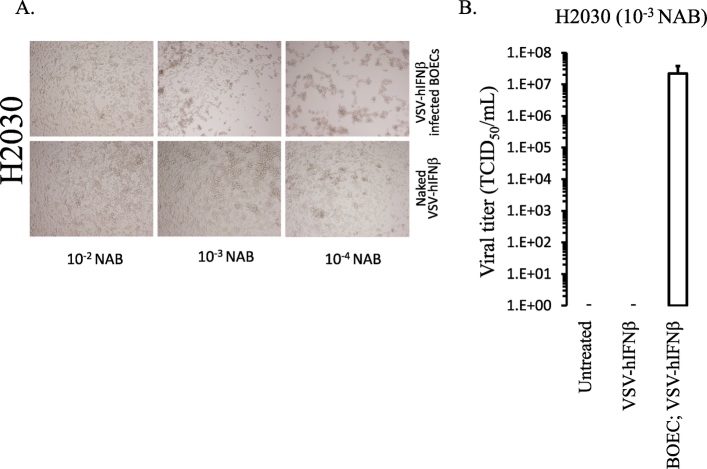
To determine whether BOECs shield VSV from neutralizing antibodies, we co-cultured NSCLC cells with VSV-GFP-infected BOECs or naked VSV-GFP in the presence or absence of anti-VSV neutralizing antibodies at increasing titers. In the presence of anti-VSV neutralizing antibodies, naked VSV-GFP was completely neutralized as seen by the absence of GFP expression (Figure 3A) or titratable virus (Figure 3B) in NSCLC lines. However, even in the presence of a high titer of anti-VSV neutralizing antibodies, infected BOECs transferred virus to both H2009 and H2030 cell lines (Figure 3, A and B). Similar results were observed for H2030 cells co-cultured with human BOECs infected with VSV-hIFNβ (Supplemental Figure 1). These data suggest that BOECs effectively prevent antibody-mediated neutralization and could overcome the effect of virus neutralization.
Figure 3.
Virus from infected BOECs were protected from neutralizing antibodies. A) H2009 and H2030 cells were treated with VSV-GFP or VSV-GFP infected BOECS in the presence of decreasing concentration of anti-VSV neutralizing antibodies (NAB) and assayed using fluorescence microscopy 24 hours after infection. B) VSV titer from cell medium of co-cultured VSV infected BOECs or naked VSV-GFP with H2009 cells. TCID50 is tissue culture infective dose 50%.
Supplemental Figure 1.
VSV-hIFNβ from infected BOECS is shielded from neutralizing antibodies. H2030 was treated in vitro with VSV-hIFNβ or BOECS infected with VSV-hIFNβ in the presence of decreasing concentration of anti-VSV neutralizing antibodies (NAB). A) Photomicrographs were taken 24 hours after infection to assess cytopathic effect. B) VSV-hIFNβ titer from cell medium of co-cultured VSV-hIFNβ infected BOECs or naked VSV-hIFNβ with H2030 cells.
BOECs Target Tumor Tissue In Vivo
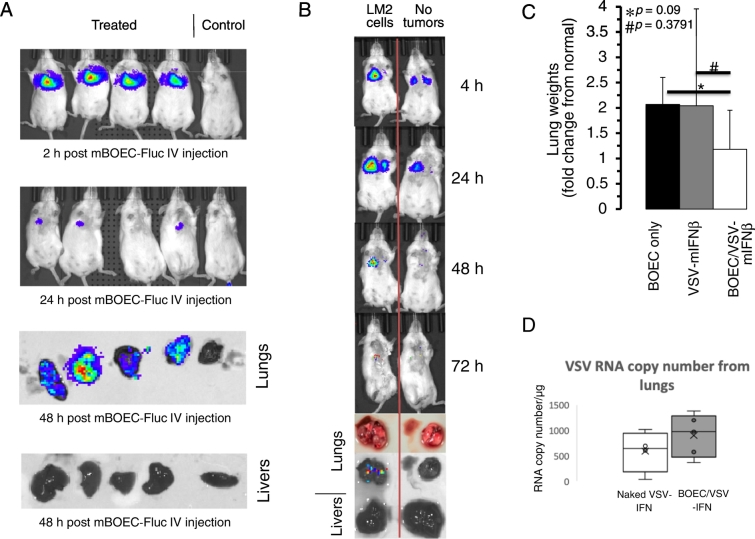
Though others have shown that BOECs can target tumor tissue in vivo using a syngeneic source of BOECs [13,17,18], it would be advantageous to be able to use an allogeneic cell source as an “off-the-shelf” product. Thus, mBOECs expressing firefly luciferase (mBOEC-fLuc) derived from C57/Bl6 mice were used in A/J mice bearing LM2 lung adenocarcinoma tumors. Mice were injected with LM2 cells by tail vein to establish tumors in the lung. 34 days later, 1 × 106 intravenous mBOECs-Fluc were injected by tail vein. Immediately after tail vein injection, in vivo luminescence imaging detected a bright signal in the lungs as expected (Figure 4A). mBOECs persisted in lung tissue at 24 hours, but by 48 hours, luminescence was not detectable in vivo. Upon sacrifice, lung tissue continued to show luciferase expression; however, other than the lungs, no luminescence was detected in the mouse including the liver (Figure 4A). As compared to mice without lung tumors, luminescence in the lung was brighter and persisted longer in mice with lung tumors (Figure 4B). This finding confirms that allogeneic mBOECs are able to persist in vivo and are retained in lung tissue in mice bearing tumors in the lungs.
Figure 4.
Trafficking of mBOEC cells to lung tumor growth in mice and preliminary efficacy in immune competent mice. A) A/J mice were IV injected with lung adenocarcinoma cells, and 34 days later were IV injected with mBOEC-fLuc cells. Luminescent imaging of mice along with resected lungs and livers are shown at the indicated times. The control mouse did not receive mBOEC-fLuc cells as a negative control. B) A single A/J mouse was IV injected with LM2 cells, and 34 d later was IV injected with mBOEC-fLuc cells. A second mouse without tumors was IV injected with mBOEC-fLuc cells at the same time as the first mouse. Luminescent imaging of mice along with resected lungs and livers are shown at the indicated times. C) 2 × 105 LM2 cells were IV injected into A/J mice and were treated with mBOECs (n = 5), VSV-mIFNβ (n = 5), or VSV-mIFNβ infected mBOECs (n = 5) by tail vein injection on days 20, 22, 24, 41, 43, and 45. On day 48 mice were sacrificed and their lungs removed and weighed. Lung weights were normalized to the lung weight of untreated normal mice (n = 5). Relative tumor burden, normalized to untreated normal lung weight, at necropsy is shown for each experimental group. Results are expressed as the mean +/− standard deviation. # P = .3791 for mBOEC cells alone compared to VSV-mIFNβ alone and * P = .09 for mBOEC cells alone compared to VSV-mIFNβ infected mBOEC cells. D) RNA from lungs of treated mice was extracted on day 27 and subjected to RT-PCR for VSV-N RNA to estimate the copy number in lung tissue compared between naked VSV-IFNβ and BOEC-infected with VSV-IFNβ (n = 4 for naked VSV-IFNβ and 5 for BOEC-VSV-IFNβ).
VSV-IFNβ-Infected BOECs May Reduce Tumor Burden In Vivo
We next sought to determine whether allogeneic BOECs infected with oncolytic virus can reduce tumor burden in vivo (Figure 4C). Mice were injected with LM2 by tail vein and then treated with mBOEC-VSV-IFNβ, VSV-IFNβ alone, or mBOEC alone IV every other day for 3 treatments. Treatment was repeated 3 weeks later, and then mice were sacrificed. Lung weight (normalized to age-matched normal mouse lungs) was used as a surrogate measure of lung tumor burden. There was a trend toward decreased lung tumor burden in the BOEC-VSV-IFNβ-treated mice as compared to control (P < .09), while naked virus was no different from control. We measured VSV-N RNA from lung homogenates of mice on day 27 and observed that there was greater VSV-N RNA detectable in lungs treated with BOECs infected with VSV-IFNβ compared to naked VSV (Figure 4D), though this was not statistically significant (P = .3). As the results are not statistically significant, these results require further investigation in order to conclude that BOEC mediated delivery will be effective in immune competent mice.
Infected BOECs Lead to Antitumor Activity In Vivo
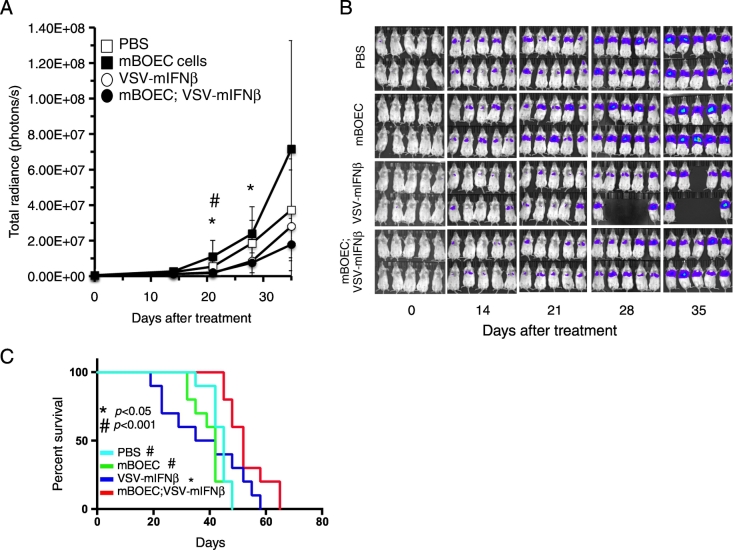
To test the efficacy of hBOECs in targeting human NSCLC, we used A549 cells expressing fLuc (Luc-A549) to establish metastatic tumors in lungs of SCID mice. Mice were treated every other day for 3 doses of either VSV-IFNβ (1x108 TCID50), 1x106 VSV-IFNβ-infected BOECs (MOI = 10), BOEC alone, or PBS injections (n = 10/group). Mice were imaged for luminescence as a measure of tumor burden (Figure 5, A and B). As compared to controls, VSV-IFNβ-infected BOECs controlled tumor burden more effectively than controls. VSV-IFNβ alone also demonstrated some efficacy as compared to controls as might be expected in this immune-deficient model; however, there was also increased toxicity of VSV-IFNβ in these mice, resulting in early death in the naked VSV-IFNβ group. These mice receiving naked VSV-IFNβ were losing weight and were not very active. They did not exhibit limb paralysis and therefore it is not clear that it was neurotoxicity. The BOEC-treated mice succumbed to disease burden at later time points. Survival of mice was also improved in the VSV-IFNβ-infected BOEC group, which was significantly prolonged compared to both naked VSV-IFNβ, BOEC alone, and PBS treated mice (Figure 5C). These mice also ultimately succumbed to tumor growth in the lungs.
Figure 5.
Systemic delivery of VSV infection by infected mBOECs to orthotopically implanted lung tumors. A) Luc-A549 tumor bearing SCID Beige mice were given either PBS, VSV-mIFNβ, mBOECs, or VSV-mIFNβ infected mBOECs. Tumor burden was estimated using levels of luciferase activity measured in radiance. *P < .02 for mBOEC infected with VSV-mIFNβ compared to mBOEC cells alone. # P < .03 for mBOEC infected with VSV-mIFNβ compared to PBS control. B) Bioluminescent imaging to detect the Luc-A549 cell signal in mice was performed at the indicated times. C) Survival of mice was determined using Kaplan–Meier method. Systemic delivery of VSV-mIFNβ infected mBOECs significantly prolonged the life of mice with lung cancer compared to PBS, mBOECs, or VSV-mIFNβ treatments. # P < .001 for PBS or mBOEC cells alone compared to VSV-mIFNβ infected mBOEC cells and * P < .05 for VSV-mIFNβ alone compared to VSV-mIFNβ infected mBOEC cells.
Discussion
The current study demonstrates that BOECs can be used as a carrier cell to deliver oncolytic VSV-IFNβ to metastatic lung tumors in murine models of NSCLC. BOECs are easily obtained from a peripheral blood draw, are rapidly grown in cell culture to large numbers, and reliably cryopreserved without altering their genome or phenotype, which facilitates the potential for clinical translation [19]. BOECs have been previously shown to carry gene therapy to tumors after systemic administration in models of melanoma and lung cancer [17,18,20]. Similarly, BOECS have been used to facilitate gene therapy of hemophilia in a canine model demonstrating the scalability of BOECS for clinical use [21]. BOECs have also been previously used to carry oncolytic measles virus in laboratory models of glioblastoma after intratumoral injections [13].
The current work adds to this literature by demonstrating that BOECs can successfully carry VSV by intravenous route, but also demonstrating that it might not be required to use an autologous source of BOECs. While we have not formally compared an autologous source of BOECs to allogeneic source, the above experiments in the LM2 model show that C57/Bl6 derived BOECs are able to persist in the lungs of A/J mice for at least 48 hours. This is significant as the requirement of an autologous cell source makes clinical translation much more expensive and time-consuming. For oncolytic virotherapy, since BOECs are meant only as delivery vehicles, their persistence in the circulation is not needed. Therefore, the use of an allogeneic cell source might mean less potential for long-term toxicity as these cells are not likely to be persistent for the long-term, though this needs to be empirically tested. Moreover, if allogeneic sources are adequate, it also means that donor derived BOECs could be cryopreserved as an “off-the-shelf” therapeutic and delivered without the delay and expense that would be required if using an autologous source. While xenogeneic cancer cells have been used as oncolytic viral carriers, we are not aware of other literature using allogeneic cell sources [11].
Several limitations of this study are worthy of mention. We showed an improvement in survival utilizing BOECs in an immune-deficient model of NSCLC. Our prior data with intratumoral injection of VSV-IFNβ in immune-competent mice showed that the immune response plays a large role in efficacy. We previously showed that VSV-IFNβ results in a robust T cell infiltrate and immunologic memory [15]. Additionally, we have observed that T cell depletion results in complete abrogation of antitumor activity in the LM2 model (unpublished data). Therefore, the result from the immune-deficient model does not take into account the potential role the immune response might have on outcomes. Our data in the LM2 model suggest that BOECs might be effective in an immune-competent model; however, we do not have long-term survival data as LM2 cells do not sustainably express luciferase marker to monitor disease noninvasively. Additionally, our metastatic model does not include other visceral sites of metastasis, such as the liver. Finally, the data as presented are not statistically significant and require further investigation to make more definitive conclusions. Future experiments utilizing an autochthonous model of lung cancer may be more informative.
It is difficult to know from the above results how BOECs might compare to other cell carrier sources in terms of efficacy. In a peritoneal ovarian cancer model, mesenchymal stem cells (MSCs) were successfully used to deliver measles virus via an intraperitoneal route [22]. One concern with MSCs is the inability to traffic out of the lungs to other sites of tumor [23]. It is possible that BOECs in these experiments are being trapped in the lungs, which happen to be the site where tumors are in this lung cancer model. Certainly, as the lungs are the first organ reached after intravenous injection, the delivery of virus to lung tissue may be much easier than delivery to other sites of systemic disease, such as other viscera or bone. The model used does not account for this, and thus the current results may not necessarily translate to other metastatic disease. However, in experiments done previously, BOECs have been shown to traffic out of the lung within 24 hours of tail vein injection and distribute systemically in normal mice [24]. Thus, our data showing the retention of BOECs in the lungs in mice bearing lung tumors suggests that BOECs traffic to the tumor microenvironment preferentially. Transgenic T cell carriers have been used to deliver VSV in acute myeloid leukemia models, and likewise showed an ability to traffic to tumors as well as shield VSV from antibody neutralization [9]. Whether this trafficking would translate to solid tumors is not clear as immune cell exclusion is a common feature of many solid tumors. Future experiments should be aimed at comparison of different cell carriers to determine the ideal method to employ and include orthotopic visceral metastases in other organs. Furthermore, future experiments should aim to utilize immune competent model systems whenever possible.
In conclusion, our results suggest that BOECs might be a viable method of delivering oncolytic VSV in future clinical trials. Further work using BOECs as carriers in immune competent models would be critical prior to clinical translation. If effective, future gene-modified BOECs could be used to enhance tumor targeting or immune therapy in combination with oncolytic virotherapy. VSV is currently being tested in several early phase clinical trials in patients with solid tumors using both intratumoral and intravenous injection (NCT03647163, NCT02923466, NCT03017820). The pharmacokinetic studies, particularly with intravenous injection, will be informative on how to move forward with cell carrier research with VSV.
The following are the supplementary data related to this article.
Declaration of Competing Interests
M.R.P is site principal investigator for a clinical trial using VSV-IFNβ-NIS in patients with advanced solid tumors. He receives research support from Vyriad, the manufacturer, for his conduct of the clinical trial. This funding source has no role in the current manuscript.
Funding
This work was funded by grants from A Breath of Hope Lung Foundation and Minnesota Masonic Charities. The funding sources have had no role in the conduct of the research or in any aspect of the development of this manuscript.
Acknowledgments
Acknowledgements
The authors would like to thank Brenda Koniar, CVT, University of Minnesota Academic Health Center, Center for Translational Medicine, for her assistance with tail vein injections performed for this project. The authors would also like to acknowledge the University of Minnesota, University Imaging Centers (http://uic.umn.edu) for their assistance.
Author Contributions
Conceptualization: M.R.P and R.A.K. Methodology: B.A.J, M.R.P, and R.A.K. Investigation: B.A.J, M.R.P, and Y.J. Writing – Original Draft: M.R.P. and B.A.J Writing – Review and Editing: M.R.P, R.A.K, and B.A.J Funding Acquisition: M.R.P. and R.A.K. Resources: R.A.K, M.R.P, and R.P.H.
References
- 1.Andtbacka R.H., Kaufman H.L., Collichio F., Amatruda T., Senzer N., Chesney J., Delman K.A., Spitler L.E., Puzanov I., Agarwala S.S. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J Clin Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 2.Tesfay M.Z., Kirk A.C., Hadac E.M., Griesmann G.E., Federspiel M.J., Barber G.N., Henry S.M., Peng K.W., Russell S.J. PEGylation of vesicular stomatitis virus extends virus persistence in blood circulation of passively immunized mice. J Virol. 2013;87:3752–3759. doi: 10.1128/JVI.02832-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peng K.W., Myers R., Greenslade A., Mader E., Greiner S., Federspiel M.J., Dispenzieri A., Russell S.J. Using clinically approved cyclophosphamide regimens to control the humoral immune response to oncolytic viruses. Gene Ther. 2013;20:255–261. doi: 10.1038/gt.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tresilwised N., Pithayanukul P., Holm P.S., Schillinger U., Plank C., Mykhaylyk O. Effects of nanoparticle coatings on the activity of oncolytic adenovirus-magnetic nanoparticle complexes. Biomaterials. 2012;33:256–269. doi: 10.1016/j.biomaterials.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 5.Mendez N., Herrera V., Zhang L., Hedjran F., Feuer R., Blair S.L., Trogler W.C., Reid T.R., Kummel A.C. Encapsulation of adenovirus serotype 5 in anionic lecithin liposomes using a bead-based immunoprecipitation technique enhances transfection efficiency. Biomaterials. 2014;35:9554–9561. doi: 10.1016/j.biomaterials.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almstatter I., Mykhaylyk O., Settles M., Altomonte J., Aichler M., Walch A., Rummeny E.J., Ebert O., Plank C., Braren R. Characterization of magnetic viral complexes for targeted delivery in oncology. Theranostics. 2015;5:667–685. doi: 10.7150/thno.10438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willmon C., Harrington K., Kottke T., Prestwich R., Melcher A., Vile R. Cell carriers for oncolytic viruses: Fed Ex for cancer therapy. Mol Ther. 2009;17:1667–1676. doi: 10.1038/mt.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell S.J., Peng K.W. The utility of cells as vehicles for oncolytic virus therapies. Curr Opin Mol Ther. 2008;10:380–386. [PubMed] [Google Scholar]
- 9.Melzer M.K., Zeitlinger L., Mall S., Steiger K., Schmid R.M., Ebert O., Krackhardt A., Altomonte J. Enhanced Safety and Efficacy of Oncolytic VSV Therapy by Combination with T Cell Receptor Transgenic T Cells as Carriers. Mol Ther Oncolytics. 2019;12:26–40. doi: 10.1016/j.omto.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramirez M., Garcia-Castro J., Melen G.J., Gonzalez-Murillo A., Franco-Luzon L. Patient-derived mesenchymal stem cells as delivery vehicles for oncolytic virotherapy: novel state-of-the-art technology. Oncolytic Virother. 2015;4:149–155. doi: 10.2147/OV.S66010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Power A.T., Wang J., Falls T.J., Paterson J.M., Parato K.A., Lichty B.D., Stojdl D.F., Forsyth P.A., Atkins H., Bell J.C. Carrier cell-based delivery of an oncolytic virus circumvents antiviral immunity. Mol Ther. 2007;15:123–130. doi: 10.1038/sj.mt.6300039. [DOI] [PubMed] [Google Scholar]
- 12.Na Y., Nam J.P., Hong J., Oh E., Shin H.C., Kim H.S., Kim S.W., Yun C.O. Systemic administration of human mesenchymal stromal cells infected with polymer-coated oncolytic adenovirus induces efficient pancreatic tumor homing and infiltration. J Control Release. 2019;305:75–88. doi: 10.1016/j.jconrel.2019.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei J., Wahl J., Nakamura T., Stiller D., Mertens T., Debatin K.M., Beltinger C. Targeted release of oncolytic measles virus by blood outgrowth endothelial cells in situ inhibits orthotopic gliomas. Gene Ther. 2007;14:1573–1586. doi: 10.1038/sj.gt.3303027. [DOI] [PubMed] [Google Scholar]
- 14.Hebbel R.P. Blood endothelial cells: utility from ambiguity. J Clin Invest. 2017;127:1613–1615. doi: 10.1172/JCI93649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel M.R., Jacobson B.A., Ji Y., Drees J., Tang S., Xiong K., Wang H., Prigge J.E., Dash A.S., Kratzke A.K. Vesicular stomatitis virus expressing interferon-beta is oncolytic and promotes antitumor immune responses in a syngeneic murine model of non-small cell lung cancer. Oncotarget. 2015;6:33165–33177. doi: 10.18632/oncotarget.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stish B.J., Oh S., Chen H., Dudek A.Z., Kratzke R.A., Vallera D.A. Design and modification of EGF4KDEL 7Mut, a novel bispecific ligand-directed toxin, with decreased immunogenicity and potent anti-mesothelioma activity. Br J Cancer. 2009;101:1114–1123. doi: 10.1038/sj.bjc.6605297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dudek A.Z., Bodempudi V., Welsh B.W., Jasinski P., Griffin R.J., Milbauer L., Hebbel R.P. Systemic inhibition of tumour angiogenesis by endothelial cell-based gene therapy. Br J Cancer. 2007;97:513–522. doi: 10.1038/sj.bjc.6603883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bodempudi V., Ohlfest J.R., Terai K., Zamora E.A., Vogel R.I., Gupta K., Hebbel R.P., Dudek A.Z. Blood outgrowth endothelial cell-based systemic delivery of antiangiogenic gene therapy for solid tumors. Cancer Gene Ther. 2010;17:855–863. doi: 10.1038/cgt.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin Y., Weisdorf D.J., Solovey A., Hebbel R.P. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jevremovic D., Gulati R., Hennig I., Diaz R.M., Cole C., Kleppe L., Cosset F.L., Simari R.D., Vile R.G. Use of blood outgrowth endothelial cells as virus-producing vectors for gene delivery to tumors. Am J Physiol Heart Circ Physiol. 2004;287:H494–H500. doi: 10.1152/ajpheart.00064.2004. [DOI] [PubMed] [Google Scholar]
- 21.Matsui H., Shibata M., Brown B., Labelle A., Hegadorn C., Andrews C., Hebbel R.P., Galipeau J., Hough C., Lillicrap D. Ex vivo gene therapy for hemophilia A that enhances safe delivery and sustained in vivo factor VIII expression from lentivirally engineered endothelial progenitors. Stem Cells. 2007;25:2660–2669. doi: 10.1634/stemcells.2006-0699. [DOI] [PubMed] [Google Scholar]
- 22.Mader E.K., Maeyama Y., Lin Y., Butler G.W., Russell H.M., Galanis E., Russell S.J., Dietz A.B., Peng K.W. Mesenchymal stem cell carriers protect oncolytic measles viruses from antibody neutralization in an orthotopic ovarian cancer therapy model. Clin Cancer Res. 2009;15:7246–7255. doi: 10.1158/1078-0432.CCR-09-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hakkarainen T., Sarkioja M., Lehenkari P., Miettinen S., Ylikomi T., Suuronen R., Desmond R.A., Kanerva A., Hemminki A. Human mesenchymal stem cells lack tumor tropism but enhance the antitumor activity of oncolytic adenoviruses in orthotopic lung and breast tumors. Hum Gene Ther. 2007;18:627–641. doi: 10.1089/hum.2007.034. [DOI] [PubMed] [Google Scholar]
- 24.Milbauer L.C., Enenstein J.A., Roney M., Solovey A., Bodempudi V., Nichols T.C., Hebbel R.P. Blood outgrowth endothelial cell migration and trapping in vivo: a window into gene therapy. Transl Res. 2009;153:179–189. doi: 10.1016/j.trsl.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]