ABSTRACT
Background
Anaplastic thyroid carcinoma (ATC) is an aggressive type of thyroid cancer, and its metastasis requires cell motility. Ceramide is involved in a variety of biological processes, including inflammation, cell signaling, cell motility, and induction of apoptosis, however has not previously been reported to inhibit the motility of ATC cells. We evaluated the effect of short chain C6-ceramide on motility of ATC cells.
Methods
Cell motility of 8305C thyroid carcinoma cell line treated with C6-ceramide was assessed using a transwell migration assay and a pseudopodia formation assay.
Results
Treatment with 10 µM C6-ceramide resulted in significantly fewer migratory cells than control treatment in a transwell migration assay (P < 0.002). In condition medium, 82.6% of C6-ceramide–treated cells formed lamellipodia. Importantly, treatment with 10 µM C6-ceramide drastically decreased the number of cells forming lamellipodia by 17.6% (P < 0.01).
Conclusion
Our results suggest that treatment with a low concentration of ceramide may prevent metastasis and recurrence of ATC by inhibiting cell motility. Further studies are necessary to investigate the mechanism of inhibition of cell motility by ceramide. Ceramide shows promise as a therapeutic treatment for ATC.
Keywords: anaplastic thyroid carcinoma, ceramide, glucosylceramide, migration, motility
Anaplastic thyroid carcinoma (ATC) is a rare and aggressive type of thyroid cancer that accounts for 2% of all thyroid carcinoma cases.1 It is associated with a median overall survival time of 3–10 months, with a median 1-year survival rate of 20%, and a median 10-year overall survival rate of less than 2%, despite aggressive multimodal management.2, 3 Only 10% of patients present with local disease; 40% present with locoregional disease and 50% present with distant metastasis.4 ATC arises from follicular thyroid cells, and is the most dedifferentiated subtype of thyroid cancer; ATC cells do not retain any of the biological features of follicular cells. ATC usually disseminates and metastasizes widely, leading to increased mortality.
It was recently reported that the accumulation of multiple genetic alterations that can activate the PI3K/Akt and MAPK pathways promotes thyroid carcinoma aggressiveness and progression to poorly differentiated and undifferentiated (anaplastic) thyroid carcinoma.5 Establishment of cancer dissemination and metastasis requires cell motility.6, 7 Localized actin polymerization at the leading edge of cells pushes the membrane forward in finger-like structures known as filopodia and sheet-like structures known as lamellipodia.8, 9 Cancer cell motility governed by formation of pseudopodia is a strong candidate as a therapeutic target for cancer metastasis. Molecular mechanisms of regulation of ATC migration and invasion remain unclear.
Sphingolipids are a class of lipids that have a long-chain sphingoid base backbone. They exert a variety of biological functions such as mediating cell growth, differentiation, and apoptosis.10,11,12 Ceramide, an important sphingolipid that forms in response to treatment with chemotherapeutic reagents and cell-death ligands such as Fas ligand and tumor necrosis factor alpha, has been implicated in mediating apoptosis in cancer cells.13,14,15 Treatment with exogenous ceramides has been shown to induce apoptosis in human cells both in vitro and in vivo.14, 16 We reported that dietary glucosylceramides exhibit anti-tumor activity by inducing apoptosis of head and neck squamous cell carcinoma in vivo.17
Ceramide liposomes were shown to inhibit peritoneal metastasis in a murine xenograft model of human ovarian cancer, and knockdown of phosphatidylinositol 3-kinase C2β abrogates this effect.18 This suggests that ceramide may have potential as a therapy for metastatic disease, but its role in metastasis of other cancers remains unclear. We investigated the effect of ceramide in thyroid cancer by evaluating the motility of anaplastic thyroid cancer cells after treatment with C6-ceramide.
MATERIALS AND METHODS
Antibodies and reagents
Isothiocyanate-conjugated phalloidin and anti–β-actin antibody (A5441) were purchased from Sigma (St. Louis, MO). C6-ceramide was obtained from Matreya (Pleasant Gap, PA).
Cell culture
Undifferentiated thyroid carcinoma cell line 8305C was obtained from RIKEN (Saitama, Japan) and grown in RPMI 1640 medium supplemented with 10% fetal bovine serum. Cells were maintained at < 80% confluence under standard incubator conditions (humidified atmosphere, 95% air, 5% CO2, 37°C). No mycoplasma contamination was observed in any cell line.
Cell migration assay
8305C cells (1 × 105 / well) were plated onto transwell chambers and treated with 10 µM C6-ceramide for 3 hours or PBS as a negative control. Migratory cells that passed through the membrane and attached to the lower surface were stained with 4’,6-diamidino-2-phenylindole (DAPI) and imaged using fluorescence microscopy. Cell migration was evaluated by counting the number of cells in 10 randomized visual fields.
Analysis of pseudopodium formation
8305C cells (2.5 × 105 / well) were incubated on BD Falcon culture slides and treated with 10 µM C6-ceramide for 3 hours or PBS as a negative control, according to the method described by Kitatani et al. After incubation, cells were washed twice with PBS and fixed with 4% formaldehyde for 10 minutes. Fixed cells were treated with 0.1% Triton X-100 for 10 minutes, followed by staining with tetramethylrhodamine isothiocyanate (TRITC)-conjugated phalloidin and DAPI for 5 minutes. Cells were imaged using confocal microscopy. Pseudopodia include filopodia and lamellipodia. Lamellipodia are thin and veil- like extensions at the edge of cells that contain a dynamic array of actin filaments. Cells were considered to have formed lamellipodia if F-actin enrichment was observed at the edge of cells. The rate of lamellipodium formation per cell was assessed by blinded quantification of fluorescence microscopy. The lamellipodium formation was evaluated by counting the rate of cells with lamellipodium in 10 randomized visual fields.
Statistical analysis
Values are shown as the mean ± SE. Statistical comparisons between control and C6-ceramide–treated cells were performed using the Mann–Whitney U test.
RESULTS
C6-ceramide inhibits cell migration
We evaluated the effect of C6-ceramide on 8305C cell motility using a transwell migration assay. Treatment with C6-ceramide led to significantly fewer migratory cells adhered to the lower surface (19.7 ± 0.8 vs. 133.7 ± 2.3 for the negative control; P = 0.002) (Fig. 1).
Fig. 1.
Cell migration assay.
Undifferentiated thyroid cancer cells were permitted to migrate in a transwell chamber after control treatment (a) or treatment with C6-ceramide (C6-Cer) (b). Migratory cells that adhered to the lower membrane surface were stained with DAPI and subsequently counted. Treatment with C6-ceramide (C6-Cer) resulted in significantly fewer migratory cells (19.7 ± 0.8) than the negative control (133.7 ± 2.3) (c); * indicates P = 0.002. Values are mean ± SE of an independent experiment.
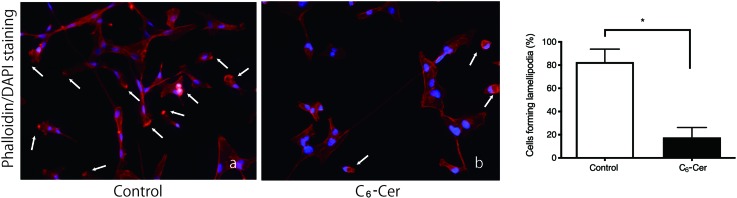
C6-ceramide inhibits lamellipodium formation
The effect of C6-ceramide on cell motility and migration was assessed by examining the formation of pseudopodia. Pseudopodia, such as lamellipodia and filopodia, are enriched for F-actin; thus, we stained 8305C cells with TRITC-conjugated phalloidin to visualize F-actin and DAPI to visualize nuclei in order to count the number of cells with pseudopodia. Treatment with C6-ceramide significantly inhibited lamellipodium formation; 17.6% of cells treated with C6-ceramide formed lamellipodia, whereas 82.6% of control cells formed lamellipodia (Fig. 2).
Fig. 2.
C6-ceramide inhibits lamellipodium formation.
Undifferentiated thyroid cancer cells were treated with PBS (a) or C6-ceramide (C6-Cer) (b) and stained with phalloidin (red) and DAPI (blue). Lamellipodia are indicated with white arrows. Lamellipodia formed in 17.6% of cells treated with C6-ceramide (C6-Cer) and in 82.6% of control cells. * indicates a significant difference, P = 0.01. Values are mean ± SE of an independent experiment.
DISCUSSION
ATC typically disseminates and metastasizes widely, which significantly increases mortality. The development of novel therapeutic drugs to prevent recurrence and metastasis would be very helpful for treating patients with a poor prognosis. Establishment of cancer recurrence and metastasis requires cell motility6, 7; therefore, inhibition of cell migration might prevent recurrence and metastasis. Mechanisms of cell migration in ATC are not well understood.
Ceramide is known as an intracellular mediator of apoptosis and can also exert a variety of other biological functions, including mediating inflammatory reactions, cell signaling, and cell motility. Kitatani et al. reported that ceramide inhibited cell motility in ovarian cancer.18 We evaluated the effect of ceramide on motility of ATC cells and observed that ceramide treatment significantly reduced the number of cells that could move across a membrane.
Cancer cells move within tissues during invasion and metastasis. In the course of metastasis, epithelial-derived cancer cells undergo the epithelial–mesenchymal transition, in which they obtain motility and lose epithelial characteristics. Intracellular adhesion is reduced and the actin cytoskeleton is remodeled, resulting in migration through the stroma. Ceramide is known to be involved in cell motility during the epithelial–mesenchymal transition, and a decrease in C18 ceramide has been shown to increase lymph node metastasis.19
The PI3K–Akt pathway regulates cell proliferation and is also actively involved in cell motility.20 Ceramide induces inactivation and dephosphorylation of Akt.21 The PI3K–Akt pathway is reported to be involved in anaplastic transformation of papillary thyroid cancer.5 C6-ceramide may inhibit the epithelial–mesenchymal transition in ATC.
Cancer cells migrate when their actin cytoskeleton is remodeled for promotion of cell motility. Actin polymerization at the leading edge induces the formation of membrane protrusions known as pseudopodia. These protruded membranes contact the substrate and form novel integrin-dependent adhesions between the cell and the substrate, and the nucleus and cell body then translocate by actomyosin contractile forces.7 We found that treatment with a low concentration of C6-ceramide inhibited lamellipodium formation of ATC.
Cell migration involves multiple processes that are regulated by various signaling molecules. Recent studies in Dictyostelium cells, leukocytes, and fibroblasts indicate that phosphatidylinositol 3 kinase (PI3K) plays a central role in the amplification of internal signaling asymmetry and thus helps to establish cell polarity and define the leading edge of the cell. Concomitant treatment with C2-ceramide and sorafenib inhibits cell growth and proliferation via a mechanism involving the PI3K/AKT/mTOR and Erk signaling pathways, and was also shown to inhibit cell migration and the epithelial–mesenchymal transition.22 In particular, the EGF protein family, which includes EGF, TGF-β, and a number of mitogens with similar amino acid sequences, can also modulate a number of integrin-dependent functions, including cell adhesion, migration, and cytoskeletal organization. Ceramide was reported to impair actin polymerization through increased RhoA/Rho kinase signaling and regulate foam cell formation.23 We hypothesize that ceramide inhibits cytoskeletal remodeling and regulates cell migration in ATC.
In our study, we found that 10 µM C6-ceramide inhibits cell motility of ATC. However, inhibitory effect of cell motility on normal cell have not been evaluated in this study. Influence of ceramide on cell growth has not been evaluated in this study. Furthermore, C6-ceramide might inhibit cell motility for another cancers. Thus, further research is expected.
In conclusion, this study demonstrates the potential of low-concentration ceramide to prevent metastasis and recurrence of ATC. We found that ceramide inhibits formation of lamellipodia and inhibits motility of ATC cells. The relationship between ceramide and cell motility should be further investigated.
Acknowledgments
Acknowledgments: This study was funded by a Grant-in-Aid for Scientific Research C of the Japan Society for the Promotion of Science (16K11233).
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1Neff RL,Farrar WB,Kloos RT,Burman KD Anaplastic thyroid cancer. Endocrinol Metab Clin North Am. 2008;37:525-38, xi. . 10.1016/j.ecl.2008.02.003 [DOI] [PubMed]
- 2.Smallridge RC,Copland JA. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin Oncol. 2010;22:486-97. 10.1016/j.clon.2010.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wendler J,Kroiss M,Gast K,Kreissl MC,Allelein S,Lichtenauer U,et al. Clinical presentation, treatment and outcome of anaplastic thyroid carcinoma: results of a multicenter study in Germany. Eur J Endocrinol. 2016;175:521-9. 10.1530/EJE-16-0574 [DOI] [PubMed] [Google Scholar]
- 4.Ranganath R,Shah MA,Shah AR. Anaplastic thyroid cancer. Curr Opin Endocrinol Diabetes Obes. 2015;22:387-91. 10.1097/MED.0000000000000189 [DOI] [PubMed] [Google Scholar]
- 5.Xing M. Genetic alterations in the phosphatidylinositol-3 kinase/Akt pathway in thyroid cancer. Thyroid. 2010;20:697-706. 10.1089/thy.2010.1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells A,Grahovac J,Wheeler S,Ma B,Lauffenburger D. Targeting tumor cell motility as a strategy against invasion and metastasis. Trends Pharmacol Sci. 2013;34:283-9. 10.1016/j.tips.2013.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamazaki D,Kurisu S,Takenawa T. Regulation of cancer cell motility through actin reorganization. Cancer Sci. 2005;96:379-86. 10.1111/j.1349-7006.2005.00062.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lauffenburger DA,Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359-69. 10.1016/S0092-8674(00)81280-5 [DOI] [PubMed] [Google Scholar]
- 9.Nürnberg A,Kitzing T,Grosse R. Nucleating actin for invasion. Nat Rev Cancer. 2011;11:177-87. 10.1038/nrc3003 [DOI] [PubMed] [Google Scholar]
- 10.Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274:1855-9. 10.1126/science.274.5294.1855 [DOI] [PubMed] [Google Scholar]
- 11.Okazaki T,Bell RM,Hannun YA. Sphingomyelin turnover induced by vitamin D3 in HL-60 cells. Role in cell differentiation. J Biol Chem. 1989;264:19076-80. [PubMed] [Google Scholar]
- 12.Vesper H,Schmelz EM,Nikolova-Karakashian MN,Dillehay DL,Lynch DV,Merrill AH Jr. Sphingolipids in food and the emerging importance of sphingolipids to nutrition. J Nutr. 1999;129:1239-50. 10.1093/jn/129.7.1239 [DOI] [PubMed] [Google Scholar]
- 13.Hannun YA,Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139-50. 10.1038/nrm2329 [DOI] [PubMed] [Google Scholar]
- 14.Obeid L,Linardic C,Karolak L,Hannun Y. Programmed cell death induced by ceramide. Science. 1993;259:1769-71. 10.1126/science.8456305 [DOI] [PubMed] [Google Scholar]
- 15.Kolesnick RN,Krönke M. Regulation of ceramide production and apoptosis. Annu Rev Physiol. 1998;60:643-65. 10.1146/annurev.physiol.60.1.643 [DOI] [PubMed] [Google Scholar]
- 16.Inamine M,Suzui M,Morioka T,Kinjo T,Kaneshiro T,Sugishita T,et al. Inhibitory effect of dietary monoglucosylceramide 1-O-beta-glucosyl-N-2′-hydroxyarachidoyl-4,8-sphingadienine on two different categories of colon preneoplastic lesions induced by 1,2-dimethylhydrazine in F344 rats. Cancer Sci. 2005;96:876-81. 10.1111/j.1349-7006.2005.00127.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujiwara K,Kitatani K,Fukushima K,Yazama H,Umehara H,Kikuchi M,et al. Inhibitory effects of dietary glucosylceramides on squamous cell carcinoma of the head and neck in NOD/SCID mice. Int J Clin Oncol. 2011;16:133-40. 10.1007/s10147-010-0141-y [DOI] [PubMed] [Google Scholar]
- 18.Kitatani K,Usui T,Sriraman SK,Toyoshima M,Ishibashi M,Shigeta S,et al. Ceramide limits phosphatidylinositol-3-kinase C2β-controlled cell motility in ovarian cancer: potential of ceramide as a metastasis-suppressor lipid. Oncogene. 2016;35:2801-12. 10.1038/onc.2015.330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karahatay S,Thomas K,Koybasi S,Senkal CE,ElOjeimy S,Liu X,et al. Clinical relevance of ceramide metabolism in the pathogenesis of human head and neck squamous cell carcinoma (HNSCC): attenuation of C18-ceramide in HNSCC tumors correlates with lymphovascular invasion and nodal metastasis. Cancer Lett. 2007;256:101-11. 10.1016/j.canlet.2007.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue G,Hemmings BA. PKB/Akt-dependent regulation of cell motility. JNCI Journal of the National Cancer Institute. 2013;105:393-404. 10.1093/jnci/djs648 [DOI] [PubMed] [Google Scholar]
- 21.Lin C,Chen C,Lin Y. Ceramide in apoptotic signaling and anticancer therapy. Curr Med Chem. 2006;13:1609-16. 10.2174/092986706777441986 [DOI] [PubMed] [Google Scholar]
- 22.Jiang S,Wang Q,Feng M,Li J,Guan Z,An D,et al. C2-ceramide enhances sorafenib-induced caspase-dependent apoptosis via PI3K/AKT/mTOR and Erk signaling pathways in HCC cells. Appl Microbiol Biotechnol. 2017;101:1535-46. 10.1007/s00253-016-7930-9 [DOI] [PubMed] [Google Scholar]
- 23.Singh RK,Haka AS,Brumfield A,Grosheva I,Bhardwaj P,Chin HF,et al. Ceramide activation of RhoA/Rho kinase impairs actin polymerization during aggregated LDL catabolism. J Lipid Res. 2017;58:1977-87. 10.1194/jlr.M076398 [DOI] [PMC free article] [PubMed] [Google Scholar]