Abstract
Objective:
To investigate the association between hypertension and outcome in patients with Coronavirus Disease 2019 (COVID-19) pneumonia.
Methods:
We performed a systematic literature search from several databases on studies that assess hypertension and outcome in COVID-19. Composite of poor outcome, comprising of mortality, severe COVID-19, acute respiratory distress syndrome (ARDS), need for intensive care unit (ICU) care and disease progression were the outcomes of interest.
Results:
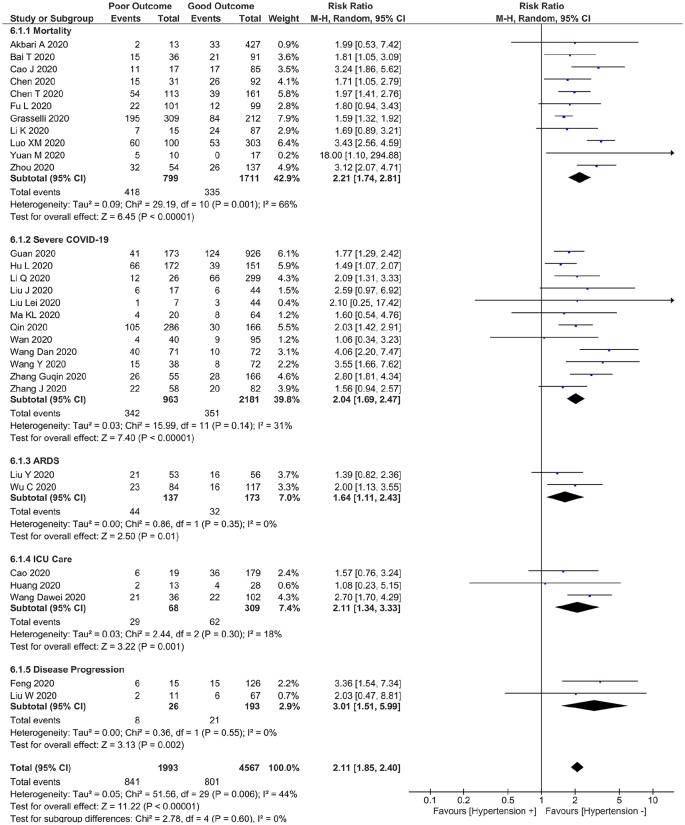
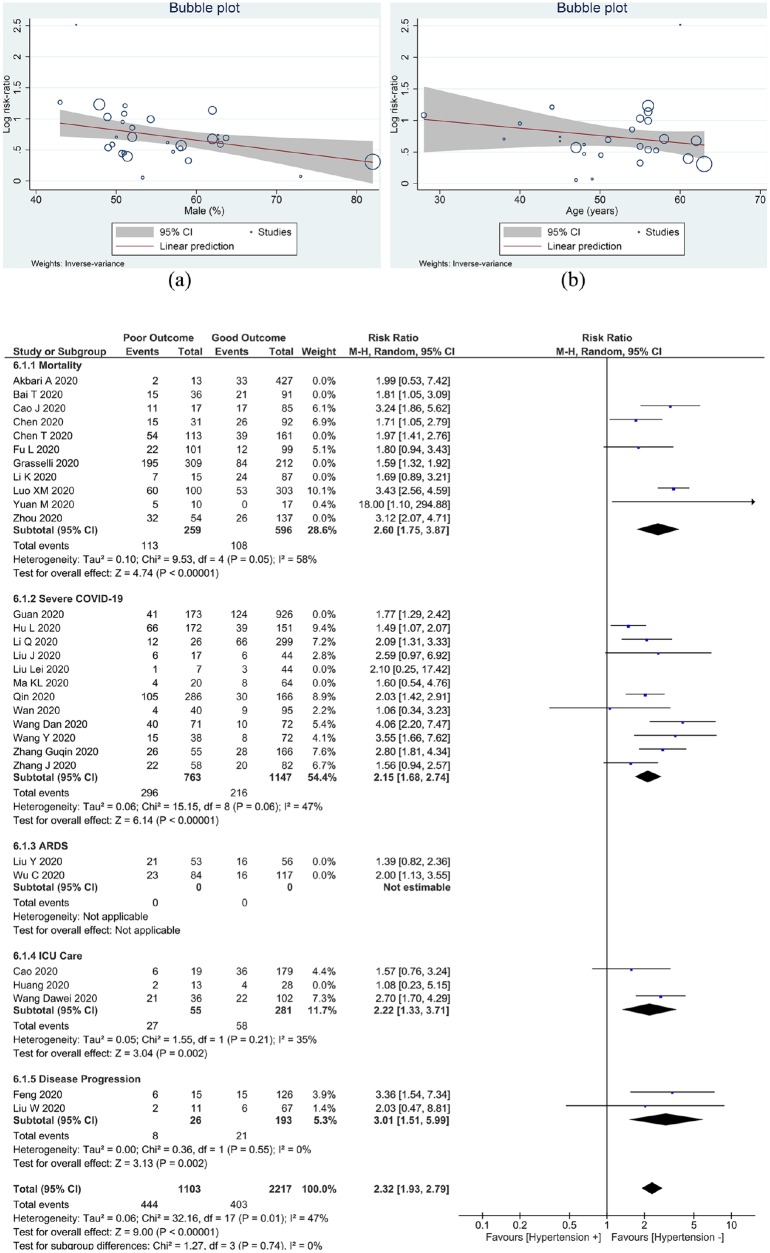
A total of 6560 patients were pooled from 30 studies. Hypertension was associated with increased composite poor outcome (risk ratio (RR) 2.11 (95% confidence interval (CI) 1.85, 2.40), p < 0.001; I2, 44%) and its sub-group, including mortality (RR 2.21 (1.74, 2.81), p < 0.001; I2, 66%), severe COVID-19 (RR 2.04 (1.69, 2.47), p < 0.001; I2 31%), ARDS (RR 1.64 (1.11, 2.43), p = 0.01; I2,0%, p = 0.35), ICU care (RR 2.11 (1.34, 3.33), p = 0.001; I2 18%, p = 0.30), and disease progression (RR 3.01 (1.51, 5.99), p = 0.002; I2 0%, p = 0.55). Meta-regression analysis showed that gender (p = 0.013) was a covariate that affects the association. The association was stronger in studies with a percentage of males < 55% compared to ⩾ 55% (RR 2.32 v. RR 1.79).
Conclusion:
Hypertension was associated with increased composite poor outcome, including mortality, severe COVID-19, ARDS, need for ICU care and disease progression in patients with COVID-19.
Keywords: Hypertension, coronavirus, COVID-19, severity, mortality
Introduction
Coronavirus disease 2019 (COVID-19) has spread across the globe, and has been officially confirmed by the World Health Organization (WHO) as a public health emergency.1 COVID-19 has infected more than 1.4 million people and killed more than 80,000 people globally, with the total number of cases and deaths increasing every day. Most COVID-19 patients present with mild or no symptoms, but a few portions of patients may develop severe pneumonia with progression to lethal complications, including acute respiratory distress syndrome (ARDS), multi-organ failure and death. Determining risk factors to anticipate the severity of COVID-19 are essential for efficient resource allocation2, especially during this pandemic season.
Hypertension is one of the most prevalent chronic diseases, affecting approximately 31.1% of adults (1.39 billion) worldwide in the year 2010.3 It is postulated that hypertensive patients have increased angiotensin-converting enzyme 2 (ACE2) expression due to associated genetic polymorphism and use of angiotensin-converting enzyme inhibitor (ACE-I) or angiotensin-receptor blocker (ARB), which even though still controversial, may increase susceptibility and severity of COVID-19.4 This study was aimed at investigating the association between hypertension and poor outcome in patients with COVID-19 pneumonia by performing a systematic review and meta-analysis.
Materials and methods
Eligibility criteria
We included all research studies enrolling adult COVID-19 patients with information on hypertension and the specific outcome of interests – mortality, severe COVID-19, ARDS, intensive care unit (ICU) care and disease progression. We excluded: review articles, non-research letters, communications, and commentaries; studies with samples < 20; case reports and small case series; non-English language articles; research in paediatric populations (17 years of age and younger).
Search strategy and study selection
A comprehensive systematic literature search was performed using PubMed, SCOPUS, EuropePMC, and Google Scholar electronic databases using the search terms: (a) ‘COVID-19’ OR ‘SARS-CoV-2’ AND ‘hypertension’; (b) ‘COVID-19’ OR ‘SARS-CoV-2’ AND ‘Characteristics’. Records curated from the initial search were then screened for duplicates which were then removed. Two independent authors (IH and MAL) screened the title and abstracts for potential articles. The full-texts of remaining articles were evaluated by applying the inclusion and exclusion criteria. The systematic literature search was finalized on 7 April 2020. The study was carried out in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline.
Data extraction
A standardized forms containing first author, year, study design, age, gender, cardiovascular diseases, hypertension, diabetes, chronic obstructive pulmonary disease (COPD), need for ICU care and severe COVID-19 were used for data extraction. Two authors (MAL and RP) performed the data extraction independently.
The outcome of interest for this meta-analysis was the composite poor outcome which comprised of mortality, severe COVID-19, ARDS, need for ICU care and disease progression. Severe COVID-19 was defined according to the report of the WHO–China Joint Mission on COVID-19,5 while ARDS was defined as per WHO interim guidance on severe acute respiratory infection (SARI) of COVID-19.6
Statistical analysis
To perform meta-analysis, we used Review Manager 5.3 (Cochrane Collaboration) and Stata version 16 softwares. To calculate dichotomous variables, we used Mantel–Haenszel formula to generate pooled effect estimate in form of risk ratios (RRs) along with its 95% confidence intervals (CIs). Random-effects model was used regardless of heterogeneity, to account for interstudy variability. All p-values in this study were two-tailed, and statistical significance was set at ⩽ 0.05. To assess the influence of covariates, restricted-maximum likelihood random effects meta-regression was performed for age, gender, cardiovascular disease, diabetes and COPD. Each individual components of composite poor outcome was then sub-analysed. Sensitivity analysis by leave-one-out method was used to single out cause of heterogeneity and test for statistical robustness. To evaluate the small-study effects, a regression-based Harbord’s test for binary outcome was performed. Funnel-plot analysis was performed to assess the risk of publication bias qualitatively.
Results
Study selection and characteristics
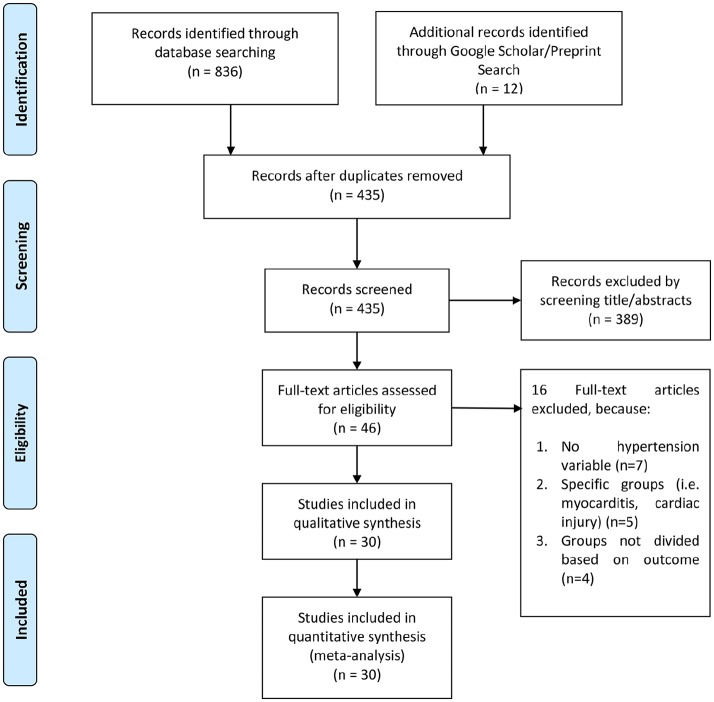
There were 848 records after initial database searching, of which 435 records remained after the removal of duplicates. After screening the title/abstract, 389 records were excluded. The 46 residual articles were then evaluated for eligibility by assessing its full-text article. 16 full-text articles were excluded because of: (a) no hypertension variable (n = 7); (b) specific groups (i.e. myocarditis, cardiac injury) (n = 5); (c) groups not divided based on outcome (n =4). Thereby, 30 studies with a total of 6560 patients were included in the final qualitative and quantitative synthesis (Figure 1, Table 1).7–35
Figure 1.
PRISMA flowchart.
Table 1.
Characteristics of the included studies.
| Authors | Study design | Samples | Male (%) | Mean overall age (median) (years) | Hypertension (%) |
Cardiovascular Comorbidity (%) | Diabetes (%) |
Respiratory Comorbidity (%) |
Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Akbari A 202036 | Observational Retrospective | 440 (13 vs 427) | 56.4 (61.5 vs 56.2) | 48 | 7.9 (15.3 vs 7.7) | 5.7 (15.3 vs 5.4) [CAD] | 7.5 (30.8 vs 6.8) | N/A | Mortality |
| Bai T 202015 | Observational Retrospective | 127 (36 vs 91) | 63 (77.8 vs 57.1 | 55 (67 vs 50) | 28.3 (41.7 vs 23.1) | 2.4 (5.6 vs 1.1) [CVD] | 11.8 (13.9 vs 11.0) | N/A | Mortality |
| Cao J 202027 | Observational Retrospective | 102 (17 vs 85) | 52 (76.5 vs 47.1) | 54 (72 vs 53) | 27.5 (64.7 vs 20) | 4.9 (17.6 vs 2.4) [CAD] | 10.8 (35.3 vs 5.9) | 9.8 (23.5 vs 7.1) [COPD] | Mortality |
| Chen 202031 | Observational Retrospective | 123 (31 vs 92) | 49 (71 vs 42 | 56 (72 vs 53) | 33.3 (48.4 vs 38.3) | 12.2 (25.8 vs 7.6) [CAD] | 11.4 (19.4 vs 8.7) | 4.9 (9.7 vs 3.3) [COPD] | Mortality |
| Chen T 202018 | Observational Retrospective | 274 (113 vs 161) | 62 (73 vs 55) | 62 (68.0 vs 51.0) | 34 (48 vs 24) | 8 (14 vs 4) [CVD] | 17 (21 vs 14) | 7 (10 vs 4) [CLD] | Mortality |
| Fu L 202030 | Observational Retrospective | 200 (34 vs 166) | 49.5 (16.2 vs 67.7) | <49 (5.9 vs 28.3), 50-59 (23.5 vs 27.1), 60-69 (20.6 vs 31.3), >70 (5 vs 13.2) | 50.5 (21.8 vs 12.1) | N/A | N/A | 4 (50.0 vs 15.6) [CLD] | Mortality |
| Grasselli 202026 | Observational Retrospective | 1591 | 82 | 63 | 49 | 21 | 17 | 4 [COPD] | Mortality |
| Li K 202014 | Observational Retrospective | 102 (15 vs 87) | 58 (73 vs 55) | 57 (69 vs 55) | 30 (47 vs 28) | 4 (13 vs 2) [CAD] | 15 (13 vs 15) | 2 (7 vs 1) [COPD] | Mortality |
| Luo X 202011 | Observational Retrospective | 403 (100 vs 303) | 47.9 (57 vs 44.9) | 56 (71 vs 49) | 28 (60 vs 17.5) | 8.9 (16 vs 6.6) [CAD] | 14.1 (25 vs 10.6) | 6.9 (17 vs 3.6) [COPD] | Mortality |
| Yuan M 20207 | Observational Retrospective | 27 (10 vs 17) | 45 (47 vs 40) | 60 (68 vs 55) | 19 (50 vs 0) | 11 (30 vs 0) [CAD] | 22 (60 vs 0) | N/ A | Mortality |
| Zhou 202031 | Observational Retrospective | 191 (54 vs 137) | 62 (70 vs 59) | 56 (69.0 vs 52.0) | 30.4 (48 vs 23) | 8 (24 vs 1) [CAD] | 19 (31 vs 14) | 3 (7 vs 1) [COPD] | Mortality |
| Guan 202033 | Observational Retrospective | 1099 (173 vs 926) | 58.1 (57.8 vs 38.2) | 47 (52.0 vs 45.0) | 15.0 (23.7 vs 13.4) | 2.5 (5.8 vs 1.8) [CAD] | 7.4 (16.2 vs 5.7) | 1.1 (3.5 vs 0.6) [COPD] | Severe COVID-19 |
| Hu L 202012 | Observational Retrospective | 323 (172 vs 151) | 51.4 (52.9 vs 49.7) | 61 (65 vs 56) | 32.5 (38.3 vs 25.8) | 12.7 (19.2 vs 5.3) [CVD] | 14.6 (19.2 vs 9.3) | 1.9 (3.5 vs 0) [COPD] | Severe COVID-19 |
| Li Q 202037 | Observational Retrospective | 325 (26 vs 299) | 51.4 (76.9 vs 49.2) | 51 (65 vs 49) | 24 (46.2 vs 22.1) | 5.5 (19.2 vs 4.3) [CAD] | 9.2 (19.2 vs 8.4) | 1.2 (7.7 vs 0.6) [COPD] | Severe COVID-19 |
| Liu J 202024 | Prospective Cohort | 61 (17 vs 44) | 50.8 (58.8 vs 47.7) | 40 (56 vs 41) | 19.7 (35.3 vs 13.6) | 1.6 (5.9 vs 0) [CVD] | 8.2 (1.6 vs 4.5) | 8.2 (1.6 vs 4.5) [COPD] | Severe COVID-19 |
| Liu L 202029 | Observational Retrospective | 51 (7 vs 44) | 62.7 (57.1 vs 63.7) | 45 (52 vs 44) | 7.8 (14.3 vs 6.8) | N/A | 7.8 (57.1 vs 0) | N/A | Severe COVID-19 |
| Ma KL 202022 | Observational Retrospective | 84 (20 vs 64) | 57.1 (60 vs 56.3) | 48 (58 vs 46.5) | 14.3 (20.0 vs 12.5) | 6 (10 vs 4.7) [CAD] | 11.9 (35 vs 4.7) | 6.0 (10.0 vs 4.7) [CLD] | Severe COVID-19 |
| Qin 202017 | Observational Retrospective | 452 (286 vs 166) | 52.0 (54.2 vs 48.2) | 58 (61 vs 53) | 29.5 (36.7 vs 18.1) | 5.9 (8.4 vs 1.8) [CVD] | 16.4 (18.5 vs 13.3) | 2.6 (3.1 vs 1.8) [COPD] | Severe COVID-19 |
| Wan 202016 | Observational Retrospective | 135 (40 vs 135) | 53.3 (52.5 vs 54.7) | 47 (56 vs 44) | 9.6 (10 vs 9.4) | 5.2 (15 vs 1) [CVD] | 8.9 (22.5 vs 3.1) | 0.7 (2.5 vs 0) [CLD] | Severe COVID-19 |
| Wang D 202038 | Observational Retrospective | 143 (71 vs 72) | 51 (62 vs 40.3) | 58 (65 vs 44) | 25.2 (43.7 vs 6.9) | 11.2 (16.9 vs 5.6) [CAD] | 9.1 (12.7 vs 5.6) | 7.0 (9.9 vs 4.2) [COPD] | Severe COVID-19 |
| Wang Y 202039 | Observational Retrospective | 110 (38 vs 72) | 43 (63.2 vs 33.3) | ⩽40 (53%), 41-60 (21%), >60 (36%) ⩽40 (7.9 vs 69.4), 41-60 (21.0 vs 18.1), >60 (71.0 vs 12.5) |
20.9 (39.5 vs 11.1) | N/A | 13.7 (21.0 vs 9.7) | 5.4 (10.5 vs 2.8) [COPD] | Severe COVID-19 |
| Zhang G 202032 | Observational Retrospective | 221 (55 vs 166) | 48.9 (63.6 vs 44.0) | 55 (62 vs 51) | 24.4 (47.3 vs 16.9) | 10 (23.6 vs 5.4) [CAD] | 10 (12.7 vs 9.0) | 2.7 (7.3 vs 1.2) [COPD] | Severe COVID-19 |
| Zhang J 202020 | Observational Retrospective | 140 (58 vs 82) | 50.7 (56.9 vs 46.3) | <30 (1.7 vs 4.9), 30-49 (15.5 vs 34.1), 50-69 (48.3 vs 50), ⩾70 (34.5 vs 11.0) | 30 (37.9 vs 24.4) | 5 (6.9 vs 3.7) [CAD] | 12.1 (13.8 vs 11.0) | 1.4 (3.4 vs 0) [COPD] | Severe COVID-19 |
| Liu Y 202048 | Observational Retrospective | 109 (53 vs 56) | 59 (52.8 vs 55.4) | 55 (61 vs 49) | 37 (21 vs 26) | 6.4 (5.7 vs 7.1) [CAD] | 11 (20.8 vs 1.8) | 3.7 (3.8 vs 3.6) [COPD] | ARDS |
| Wu C 202025 | Observational Retrospective | 201(84 vs 117) | 63.7 (71.4 vs 58.1) | 51 (58.5 vs 48) | 19.4 (27.4 vs 13.7) | 4 (6 vs 2.6) [CAD] | 10.9 (19 vs 5.1) | 2.5 [CLD] | ARDS |
| Cao 20209 | Observational Retrospective | 198 (19 vs 176) | 51 (89.5 vs 46.9) | 50.1 (63.7 vs 48.6) | 21.2 (31.6 vs 20.1) | 6.0 (26.3 vs 3.9) [CVD] | 7.6 (10.5 vs 7.3) | N/A | ICU Care |
| Huang 202021 | Observational Retrospective | 41 (13 vs 28) | 73 (85 vs 68) | 49.0 (49.0 vs 49.0) | 14.6 (15 vs 14) | 14.6 (23 vs 11) [CVD] | 19.5 (8 vs 25) | 2.4 (8 vs 0) [COPD] | ICU Care |
| Wang D 202019 | Observational Retrospective | 138 (36 vs 102) | 54.3 (61.1 vs 52.0) | 56 (66 vs 51) | 31.2 (58.3 vs 21.6) | 14.5 (25 vs 10.8) [CAD] | 10.1 (22.2 vs 5.9) | 2.9 (8.3 vs 1.0) [COPD] | ICU Care |
| Feng 202013 | Observational Retrospective | 141 (15 vs 126) | 51.1 (46.7 vs 51.6) | 44 (58 vs 41) | 14.9 (40.0 vs 11.9) | 2.1 (6.7 vs 1.6) [CVD] | 5.7 (13.3 vs 4.8) | 2.8 (13.3 vs 1.6) [COPD] | Disease Progression |
| Liu W 202023 | Observational Retrospective | 78 (11 vs 67) | 50 (63.6 vs 47.8) | 38 (55 vs 37) | 40 (18.2 vs 9.0) | N/A | 25 (18.2 vs 4.5) | 10 (9.1 vs 1.5) [COPD] | Disease Progression |
CAD, coronary artery disease; COVID-19, coronavirus disease 2019; CLD, chronic lung/pulmonary disease; CVD, cardiovascular disease; ICU, intensive care unit.
vs = Group A vs Group B.
Hypertension and outcome
This meta-analysis showed that hypertension was associated with increased composite poor outcome (RR 2.11 (1.85, 2.40), p <0.001; I2 44%, p = 0.006) (Figure 2). Sub-group analysis showed that hypertension was associated with increased mortality (RR 2.21 (1.74, 2.81), p < 0.001; I2 66%, p = 0.001), severe COVID-19 (RR 2.04 (1.69, 2.47), p < 0.001; I2 31%, p = 0.14), ARDS (RR 1.64 (1.11, 2.43), p = 0.01; I2 0%, p = 0.35), ICU care (RR 2.11 (1.34, 3.33), p = 0.001; I2: 18%, p = 0.30) and disease progression (RR 3.01 (1.51, 5.99), p = 0.002; I2 0%, p = 0.55).
Figure 2.
Hypertension and poor outcome. Forest plot shows that hypertension was associated with increased composite poor outcome and its sub-group which comprises of mortality, severe COVID-19, acute respiratory distress syndrome (ARDS), need for intensive care unit (ICU) care and disease progression in patients with COVID-19.
Sensitivity analysis indicate robustness of the effect estimate, removal of Luo XM et al. study reduces heterogeneity while maintaining the association with increased composite poor outcome (RR 2.02 (1.80, 2.27), p < 0.001; I2: 27%, p = 0.10) and mortality (RR 2.03 (1.65, 2.49), p < 0.001; I2 42%, p = 0.08).
Meta-regression
Meta-regression analysis showed that the association between hypertension and increased composite poor outcome was influenced by gender (p = 0.013) (Figure 3(a)), but not by age (p = 0.233) (Figure 3(b)), cardiovascular diseases (p = 0.464), diabetes (p = 0.882) and COPD (p=0.094).
Figure 3.
Meta-regression analysis showed that the association between hypertension and increased composite poor outcome was influenced by (a) gender, but not (b) age. (c) Sub-group analysis based on meta-regression results showed that studies of the association between hypertension and composite poor outcome was stronger in studies with a percentage of male <55%. ARDS: acute respiratory distress syndrome; ICU, intensive care unit.
Sub-group analysis
Sub-group analysis for studies with a percentage of males ⩾ 55% (RR 1.79 (1.58, 2.02), p < 0.001; I2 0%, p = 0.49) has lower RR for composite poor outcome compared to < 55% (RR 2.32 (1.93, 2.79), p < 0.001; I2 47%, p = 0.01) (Figure 3(c)).
Sub-group analysis for studies with median age ⩾ 55 years old (RR 2.17 (1.78, 2.64), p < 0.001; I2 68%, p < 0.001) has only a slightly higher RR for composite poor outcome compared to < 55 years old (RR 2.02 (1.68, 2.43), p < 0.001; I2 0%, p = 0.77).
Publication bias
Funnel-plot analysis showed a qualitatively symmetrical funnel plot for the association between hypertension and increased composite poor outcome (Figure 4(a)). Regression-based Harbord’s test showed no indication of small-study effects for hypertension and increased composite poor outcome (p = 0.219) (Figure 4(b)).
Figure 4.
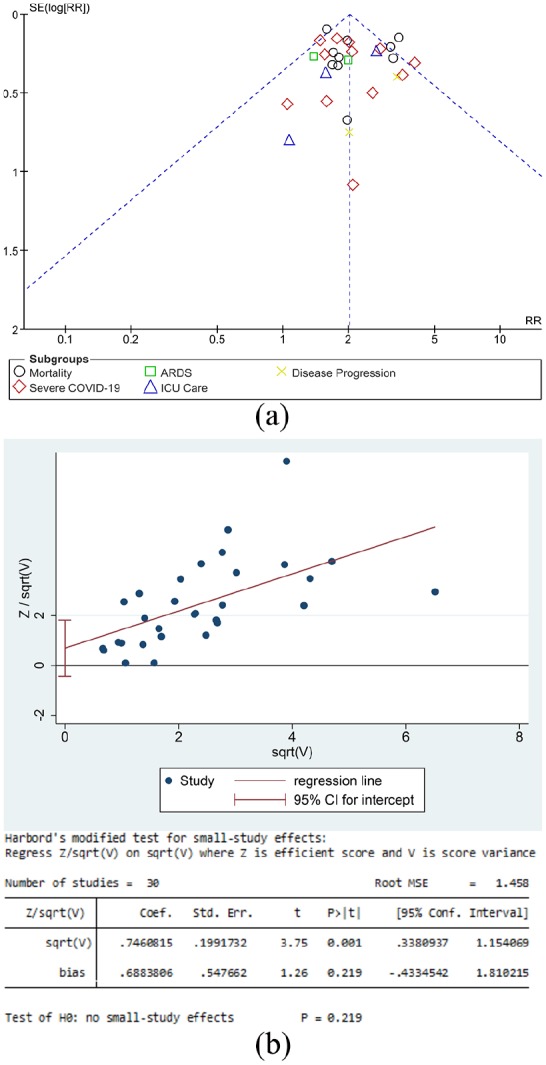
Publication bias analysis. (a) The funnel-plot analysis showed a qualitatively symmetrical funnel plot for the association between hypertension and increased composite poor outcome. (b) Regression-based Harbord’s test showed no indication of small-study effects for hypertension and increased composite poor outcome.
Discussion
Based on our meta-analysis, hypertension was shown to be associated with increased composite poor outcome that consists of mortality, severe COVID-19, ARDS, need for ICU care and disease progression in patients with COVID-19. This association was influenced by gender, but not age, cardiovascular disease, diabetes and COPD. The association between hypertension and increased poor outcome was stronger in studies with lower percentage of male patients.
It is still unclear whether hypertensive people are more likely to contract COVID-19 infection. However, individuals with hypertension tend to more severely affected by COVID-19, with ACE2 becomes a likely explanation.40 ACE2, a type 1 integral membrane glycoprotein which is found in the epithelial cells of cardiac, kidney, lung and intestinal tissue, converts angiotensin II to angiotensin 1–7.40,41 Thus, the presence of ACE2 neutralizes the inflammatory effects of angiotensin II, reduces the levels of proinflammatory cytokine interleukin-6, enhances the anti-inflammatory and antioxidant roles of angiotensin 1–7, increases the concentration of alveolar surfactant protein D and triggers vasodilation.42,43 It is likely that novel coronavirus responsible for COVID-19 worked through a similar pathway as the coronavirus that caused SARS-CoV, where they use ACE2 on the surfaces of epithelial cells as a receptor to attach and enter the host pneumocytes.40–42 Viral surface spike (S) protein of COVID-19 binds to ACE2 receptor following the spike protein activation by transmembrane protease serine 2 (TMPRSS2).44,45
Activation of the renin-angiotensin system (RAS) in various tissues is associated with conditions such as hypertension. Regular use of medications, including ARB and ACEI upregulates ACE2 expression, therefore facilitating the entry of SARS-CoV-2 into pneumocytes which ultimately increases the severity and fatality of infection.40,42 Overexpression of ACE2, which is supposed to provide protection for the lungs, is downregulated after viral uptake as the enzyme gets diminished, resulting in reduced angiotensin-II degradation, increased aldosterone secretion and consequently loss of potassium via urine.42
The interplay between hypertension, gender and RAS may be the explanation of our result regarding the association of hypertension and outcome in a lower percentage of male patients. In hypertensive females, there is greater activity of Angiotensin II type 2 receptor (AT2R) than angiotensin II type I (AT1R) which translates into attenuation of harmful response of AT1R activation.46 More expression and activation of AT1R are seen in hypertensive males and hypothesized in causing vasoconstriction, pro-inflammatory response, increasing oxidative stress, leading to ARDS in severe COVID-19.47,48 This condition provides an explanation for the higher incidence of severe COVID-19 in males compared to females. Oestrogen is postulated in bringing the predisposition towards ‘good’ RAS in females.49 Although we did not have data concerning the menopausal status of the women in the included studies, the majority of subjects included in the studies were in older age groups. This could explain why the association between hypertension and outcome was stronger in studies with a lower percentage of male patients. Nevertheless, this result has to be interpreted cautiously due to the known limitations of meta-regression, and is only a hypothesis-generating finding. An individual patient-level sub-group analysis (preferably with adjustment to various confounders) needs to be performed to confirm or deny this preliminary finding.
Currently, there is no scientific evidence showing that the termination of ACEI and ARB in patients with COVID-19 infection would be beneficial and the authorities in the field still recommend those patients to continue their regular anti-hypertensives. Theoretically, inhibiting the breakdown of angiotensin II (using ACEI) or blocking angiotensin AT1R (using ARB) could decrease inflammation systematically and specifically in the lung, heart and kidney, which ultimately reduces the potential complication of COVID-19, including ARDS, myocarditis and acute kidney injury.50
Discontinuation of ACEI or ARB in COVID-19 patients with hypertension might potentially be harmful as they continue to offer cardiovascular and renal protection. In fact, the use of ARB has been studied for the treatment of COVID-19 and its complications. Studies have shown that COVID-19 downregulates ACE2 expression and hinders it’s organoprotective effect.51 It is hypothesized that unregulated angiotensin-II activity leads to multiple organ injury.52,53 Furthermore, ACE2 has been shown to protect lungs from ARDS.54 Hence, drugs that may increase ACE2 may offer protection rather than harm.
Although ACE2 is singled out as the port of entry for COVID-19, it plays a substantial anti-inflammatory role in renin–angiotensin–aldosterone system (RAS) signalling. The elderly with hypertension have lower ACE2 levels and higher RAS signalling, which develops to extremely low ACE2 levels and markedly elevated RAS signalling after COVID-19 infection, resulting in a likely reduced incidence of disease, but greater severity.55 On the contrary, younger individuals without hypertension have higher ACE2 levels and lower RAS signalling, which turns to modestly low ACE2 levels and modestly enhanced RAS signalling after they are infected with COVID-19, resulting in a potentially increased incidence of disease, but lesser severity. This supports the suggestion of continuing treatment with RAS antagonists such as ACEI/ARB in order to prevent further lung injury due to COVID-19, including sepsis-associated lung injury.55 The effect estimate for subgroup analysis for age ⩾ 55 and < 55 years old differs slightly, in which ⩾ 55 years old was associated with slightly higher risk. However, because meta-regression did not demonstrate significant differences across age groups, the significance remains uncertain, and direct comparison between the older and younger patients is needed.
Regardless of potential benefit or harm associated with ACEI/ARB, controlling blood pressure is important to prevent cardiovascular complications. Data on hypertensive medications were lacking in the included studies, hence, we are unable to provide meta-analysis or meta-regression for ACEI/ARB.
Implications for clinical practice
Hypertension was associated with increased poor outcome in patients with COVID-19 and the association was not affected by age, gender, presence of cardiovascular disease, diabetes or COPD. This designates that hypertension may act as one of the potential prognostic factors for COVID-19 severity that should be sought in the triage.
Limitations
The label hypertension is not universal; the included studies may have different definition of hypertension. The stage of hypertension and whether they are controlled or poorly controlled were also unknown. Data on chronic hypertensive medications were not adequately reported by the included studies, and so cannot be analysed. Such data may provide insight on the ACEI/ARB impact on prognosis. Additionally, the included studies were subject to potential confounders that may weaken or strengthen the effect estimate. The result of the meta-regression has to be interpreted cautiously due to the known limitations of such analysis. Most of the articles included in the study were published at preprints servers, and as such are not yet peer reviewed. Majority of the studies originated from China, the patients perhaps overlapping across the reports. Most of the studies included were retrospective in design.
Conclusion
Hypertension was associated with increased mortality, severe COVID-19, ARDS, need for ICU care and disease progression in patients with COVID-19. The association was stronger in studies with a lower percentage of male patients.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Raymond Pranata  https://orcid.org/0000-0003-3998-6551
https://orcid.org/0000-0003-3998-6551
Michael Anthonius Lim  https://orcid.org/0000-0001-7631-6835
https://orcid.org/0000-0001-7631-6835
References
- 1. World Health Organization. Coronavirus disease (COVID-19) outbreak, https://www.who.int/westernpacific/emergencies/covid-19 (accessed 7 April 2020).
- 2. Pranata R, Huang I, Lukito AA, et al. Elevated N-terminal pro-brain natriuretic peptide is associated with increased mortality in patients with COVID-19 – systematic review and meta-analysis. Postgr Med J 2020. Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol 2020; 16: 223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med 2020; 8: e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization. Report of the WHO–China Joint Mission on Coronavirus Disease 2019 ( COVID-19 ), https://www.who.int/publications-detail/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19) (accessed 7 April 2020).
- 6. World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected, https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected (accessed 7 April 2020).
- 7. Yuan M, Yin W, Tao Z, et al. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PLoS One 2020; 15: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maddaloni E, Buzzetti R. Covid-19 and diabetes mellitus: unveiling the interaction of two pandemics. Diabetes Metab Res Rev 2020; e33213321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cao M, Zhang D, Wang Y, et al. Clinical features of patients infected with the 2019 novel coronavirus (COVID-19) in Shanghai, China. medRxiv. Epub ahead of print 4 March 2020. DOI: 10.1101/2020.03.04.20030395 [DOI]
- 10. Yanli L, Wenwu S, Jia L, et al. Clinical features and progression of acute respiratory distress syndrome in coronavirus disease 2019. medRxiv 2020; 1–9. [Google Scholar]
- 11. Luo X, Xia H, Yang W, et al. Characteristics of patients with COVID-19 during epidemic ongoing outbreak in Wuhan, China. medRxiv. Epub ahead of print 19 March 2020. DOI: 10.1101/2020.03.19.20033175. [DOI] [Google Scholar]
- 12. Hu L, Ph D, Chen S, et al. Risk factors associated with clinical outcomes in 323 COVID-19 patients in Wuhan, China. medRxiv. Epub ahead of print 25 March 2020. DOI: 10.1101/2020.03.25.20037721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feng Z, Yu Q, Yao S, et al. Early prediction of disease progression in 2019 novel coronavirus pneumonia patients outside Wuhan with CT and clinical characteristics. medRxiv. Epub ahead of print 23 February 2020. DOI: 10.1101/2020.02.19.20025296 . [DOI]
- 14. Li K, Chen D, Chen S, et al. Radiographic findings and other predictors in adults with Covid-19. medRxiv; 2 Epub ahead of print 27 March 2020. DOI: 10.1101/2020.03.23.20041673. [DOI] [Google Scholar]
- 15. Bai T, Tu S, Wei Y, et al. Clinical and laboratory factors predicting the prognosis of patients with COVID-19: an analysis of 127 patients in Wuhan, China. SSRN Electron J; 6 Epub ahead of print 5 March 2020. DOI: 10.2139/ssrn.3546118. [DOI] [Google Scholar]
- 16. Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID-19 patients in Northeast Chongqing. J Med Virol. Epub ahead of print 21 March 2020. DOI: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis 2020; 53: 1689–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020; 368: m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang J jin, Dong X, Cao Y yuan, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy Eur J Allergy Clin Immunol 2020; 1–12. [DOI] [PubMed] [Google Scholar]
- 21. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ma KL, Liu Z-H, Cao F-C, et al. COVID-19 myocarditis and severity factors: an adult cohort study. medRxiv. Epub ahead of print 23 March 2020. DOI: 10.1101/2020.03.19.20034124. [DOI] [Google Scholar]
- 23. Liu W, Tao Z-W, Lei W, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 2020; 133: 1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu J, Liu Y, Xiang P, et al. Neutrophil-to-lymphocyte ratio predicts severe illness patients with 2019 novel coronavirus in the early stage. medRxiv. Epub ahead of print 12 February 2020. DOI: 10.1101/2020.02.10.20021584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu C, Hu X, Song J, et al. Heart injury signs are associated with higher and earlier mortality in coronavirus disease 2019 (COVID-19). medRxiv. Epub ahead of print 29 February 2020. DOI: 10.1101/2020.02.26.20028589. [DOI] [Google Scholar]
- 26. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. Epub ahead of print 6 April 2020. DOI: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cao J, Tu W-J, Cheng W, et al. Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China. Clin Infect Dis. Epub ahead of print 2 April 2020. DOI: 10.1093/cid/ciaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ali A, Emami A, Javanmardi F, et al. Early epidemiological analysis of CoVID-19: first report from South of Iran. Res Sq. Epub ahead of print 2 April 2020. DOI: 10.21203/rs.3.rs-19915/v1. [DOI] [Google Scholar]
- 29. Lei L, Jian-ya G, Hu W, et al. Clinical characteristics of 51 patients discharged from hospital with COVID-19 in Chongqing, China. medRxiv. Epub ahead of print 23 February 2020. DOI: 10.1101/2020.02.20.20025536 [DOI] [Google Scholar]
- 30. Fu L, Fei J, Xiang H-X, et al. Influence factors of death risk among COVID-19 patients in Wuhan, China: a hospital-based case-cohort study. medRxiv 2020; 86: 2020.03.13.20035329. [Google Scholar]
- 31. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 6736: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang G, Hu C, Luo L, et al. Clinical features and outcomes of 221 patients with COVID-19 in Wuhan, China. medRxiv. Epub ahead of print 16 March 2020. DOI: 10.1101/2020.03.13.20035329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen M, Fan Y, Wu X, et al. Clinical characteristics and risk factors for fatal outcome in patients with 2019-coronavirus infected disease (COVID-19) in Wuhan, China. SSRN Electron J. Epub ahead of print 3 March 2020. DOI: 10.2139/ssrn.3546069. [DOI] [Google Scholar]
- 34. Wang J, Luo Q, Chen R, et al. Susceptibility analysis of COVID-19 in smokers based on ACE2. Preprints. Epub ahead of print 5 March 2020. DOI: 10.20944/preprints202003.0078.v1. [DOI] [Google Scholar]
- 35. Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Akbari A, Emami A, Javanmardi F, et al. Early Epidemiological analysis of CoVID-19: first report from South of Iran. Res Sq. Epub ahead of print 2020. [Google Scholar]
- 37. Li Q, Ling Y, Zhang J, et al. Clinical characteristics of SARS-CoV-2 infections involving 325 hospitalized patients outside Wuhan. Res Sq. Epub ahead of print 2020. [Google Scholar]
- 38. Wang D, Li R, Wang J, et al. Correlation analysis between disease severity and clinical and biochemical characteristics of 143 cases of COVID-19 in Wuhan, China: a descriptive study. Res Sq. Epub ahead of print 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang Y, Zhou Y, Yang Z, et al. Clinical characteristics of patients with severe pneumonia caused by the 2019 novel coronavirus in Wuhan, China. MedRxiv. Epub ahead of print 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ma RCW, Holt RIG. COVID-19 and diabetes. Diabet Med. Epub ahead of print 3 April 2020. DOI: 10.1111/dme.14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schiffrin EL, Flack J, Ito S, et al. Hypertension and COVID-19. Am J Hypertens. Epub ahead of print 6 April 2020. DOI: 10.1093/ajh/hpaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pal R, Bhansali A. COVID-19, diabetes mellitus and ACE2: the conundrum. Diabetes Res Clin Pract 2020; 108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rossi GP, Sanga V, Barton M. Potential harmful effects of discontinuing ACE-inhibitors and ARBs in COVID-19 patients. Elife; 9 Epub ahead of print 6 April 2020. DOI: 10.7554/eLife.57278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181: 271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr Clin Res Rev 220; 14: 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ramirez LA, Sullivan JC. Sex differences in hypertension: where we have been and where we are going. Am J Hypertens 2018; 31: 1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rabelo LA, Alenina N, Bader M. ACE2-angiotensin-(1-7)-Mas axis and oxidative stress in cardiovascular disease. Hypertens Res 2011; 34: 154–160. [DOI] [PubMed] [Google Scholar]
- 48. Guo J, Huang Z, Lin L, et al. Coronavirus Disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infec. J Am Heart Assoc 2020; 9: e016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cohall DH, Scantlebury-Manning T, James S, et al. Renin-angiotensin-aldosterone system gender differences in an Afro-Caribbean population. JRAAS 2015; 16: 539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zheng Y-Y, Ma Y-T, Zhang J-Y, et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol. Epub ahead of print 5 March 2020. DOI: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vaduganathan M, Vardeny O, Michel T, et al. Renin–Angiotensin–Aldosterone System inhibitors in patients with Covid-19. N Engl J Med 2020; 382: 1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res 2020; ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 2020; 63: 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005; 436: 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. AlGhatrif M, Cingolani O, Lakatta EG. The dilemma of coronavirus disease 2019, aging, and cardiovascular disease. JAMA Cardiol. Epub ahead of print 3 April 2020. DOI: 10.1001/jamacardio.2020.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]