Main Text
Cardiac alternans, referring to beat-to-beat alternations to the action potential duration (APD) of cardiomyocytes (Fig. 1), are associated with contractile dysfunction and arrhythmogenesis in multiple disease conditions. In tissue, these cellular phenomena can manifest as spatially concordant alternans (SCA), wherein all regions alternate with the same phase, or spatially discordant alternans (SDA), wherein different regions alternate with the opposite (period 2; Fig. 1) or otherwise offset (higher period) phase. Whereas SCA can directly affect cardiac output (mechanical force can alternate strong or weak associated with long or short APD), SDA also presents the possibility for transition to potentially fatal arrhythmias (1).
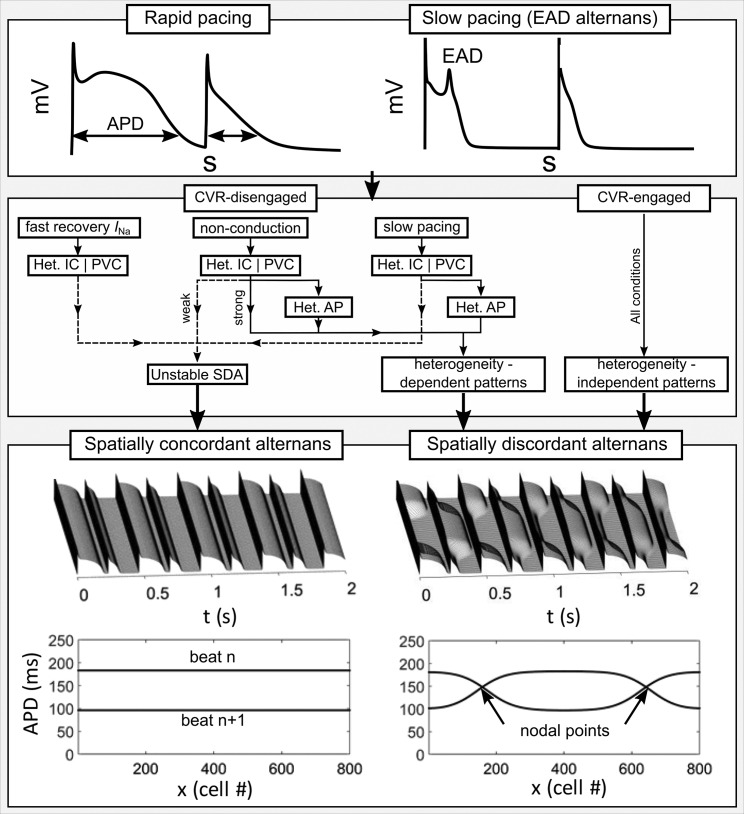
Figure 1.
Different mechanisms of SCA and SDA. The upper panel illustrates single-cell alternans emerging during rapid pacing or alternating EADs. The middle panel summarizes the different condition-dependent pathways to the final state of SCA or SDA. Het. IC refers to the conditions with heterogeneous initial conditions; PVC refers to the conditions that included an initial applied focal excitation; Het. AP refers to the conditions with underlying electrophysiological heterogeneity; and Unstable SDA refers to a transient period of SDA that eventually forms SCA. The lower panel, taken from Huang et al. in this issue, illustrates SCA and SDA in a one-dimensional strand of tissue.
Previous theoretical studies (1,2) have demonstrated the critical role of conduction velocity restitution (CVR) in the development of SDA (Fig. 1), mechanisms that are supported by experimental studies (3); this paradigm, which requires rapid pacing and/or slow recovery of the fast Na+ current (INa), is now well established and has received detailed nonlinear analysis (2,4). However, other experimental studies, such as those associated with heart failure, Brugada syndrome, and long QT syndromes, observe SDA emerging at normal- or slow-pacing rates and/or in the absence of slow INa recovery (5, 6, 7); these observations cannot be explained by mechanisms dependent on CVR. This is far from a trivial discrepancy; the efficacy of pharmacological treatment strategies is strongly linked to the governing arrhythmia mechanisms, and it is therefore vital to establish whether the dynamics of SDA in the absence of CVR are similar to or distinct from those in its presence.
To explore these pertinent gaps in the understanding of SDA mechanisms, Huang et al. in this issue of Biophysical Journal (8) built on two decades’ work from Zhilin Qu and colleagues to theoretically study the occurrence and dynamics of SDA emerging at slow-pacing rates or otherwise in the absence of CVR. These investigations reveal distinct dynamics compared to CVR-dependent SDA, highlighting the clinical importance of developing a greater understanding of SDA under all conditions.
In single cells, rapid pacing in combination with certain conditions (such as slow recovery of the calcium current or INa) can induce bifurcations of the APD restitution curve dependent on refractory properties—alternans. Incomplete recovery leads to a smaller current amplitude on the subsequent beat, which, for depolarizing currents, results in a reduced action potential (AP) upstroke velocity and/or plateau phase and ultimately shorter APD; this incomplete current activation permits full recovery for the next beat, and the cycle repeats. These refractory properties are also present in the intracellular calcium-handling system: rapid pacing can induce beat-to-beat alternations to the magnitude of intracellular calcium release and consequently the whole-cell calcium transient, which may induce APD alternans through interactions with calcium-sensitive currents such as that carried by the sodium-calcium exchanger (9,10).
At slow-pacing rates and in the absence of slow ion current recovery, however, other mechanisms are necessary to induce alternans, such as abnormal dynamics of the transient outward current (6) or alternating emergence of early afterdepolarizations (EADs; Fig. 1). These mechanisms may be the most relevant for clinically observed alternans in the absence of rapid pacing; yet, it is unclear how the manifestation in tissue may differ for alternans resulting from these disparate single-cell mechanisms.
The dynamics of alternans in tissue can be sensitive to heterogeneous initial conditions, which alone can be sufficient to initially induce transient SDA. Heterogeneous initial conditions can be imposed directly in simulation studies, but it is less clear how these conditions translate to the clinic. In this context, heterogeneous initial conditions may refer to repolarization heterogeneity, caused by either underlying AP heterogeneity or spatially complex previous excitations in electrically homogeneous tissue, or to the presence of abnormal focal excitations responsible for premature ventricular complexes (PVCs). Thus, AP heterogeneity, prior patterns of excitation, and prior PVCs all present the possibility of inducing perturbations that can impact the long-term dynamics of SDA. Huang et al. in this issue study the impact of all of these factors in combination with multiple conditions of CVR disengagement on the long-term dynamics and stability of nodal points (in one dimension) or lines (in two dimensions) that form at the boundary between different tissue regions in SDA (Fig. 1).
When CVR was engaged, multiple conditions (simple rapid pacing in fully homogeneous tissue, directly imposed heterogeneous initial conditions, PVCs, and AP heterogeneity) all led to stable SDA (Fig. 1). Moreover, the final state, referring to the number and location of nodal points or lines, was independent of the specific initial condition heterogeneity and largely independent of AP heterogeneity; nodal lines form perpendicular to AP propagation for multiple underlying heterogeneity structures. Thus, initial and underlying heterogeneities failed to influence the long-term dynamics emerging from rapid-pacing-induced alternans; CVR-induced SDA is the only stable solution.
The outcome is more variable in the absence of CVR, with long-term dynamics dependent on the method by which CVR was disengaged and the specific conditions of the simulation. In all cases without CVR, rapid pacing in isolation was insufficient for SDA; perturbations due to heterogeneous initial conditions or PVCs were necessary to induce a transient or sustained period of SDA. In conditions in which CVR was disengaged during rapid pacing by increasing the INa recovery rate, heterogeneous initial-condition- or PVC-induced SDA was unstable, with nodes disappearing to form SCA; in the presence of AP propagation, CVR is necessary for rapid-pacing-induced alternans to maintain stable SDA.
A second method to disengage CVR that the authors implemented was to apply rapid-pacing stimuli to the whole tissue simultaneously, i.e., global pacing; no conduction throughout the tissue occurs, and CVR cannot be engaged. Under these nonconduction conditions, weakly heterogeneous initial conditions behaved similarly to the rapid INa recovery condition, with SDA nodes disappearing and SCA forming (Fig. 1). However, in the presence of strongly heterogeneous initial conditions and/or underlying electrical heterogeneity, stable SDA could be obtained. The emergent SDA pattern was dependent on the specific initial condition heterogeneity and/or underlying tissue heterogeneity; SCA and heterogeneity-induced SDA are both stable solutions.
Finally, CVR was disengaged by slow pacing, with alternans induced via Ito or EAD-dependent mechanisms (Fig. 1). In combination with global pacing, similar features emerged to rapid global pacing, with both SCA and SDA being stable solutions in homogeneous and heterogeneous tissue. However, in the presence of conduction and at slow-pacing rates, SDA induced in homogeneous tissue was not stable and always resulted in SCA; underlying electrical heterogeneity was necessary to induce stable SDA. This final condition may be the most relevant for the clinical presentation of SDA observed at normal- or slow-pacing rates.
In summary, the stability of SDA and dependence on prior tissue state and perturbations are all affected by underlying CVR conditions and cellular alternan mechanisms. These observations may be critical for successful management of clinical conditions associated with T-wave alternans, and mechanistic analysis such as that presented here is essential to direct future research into novel treatment strategies.
Editor: Eric Sobie.
References
- 1.Qu Z., Garfinkel A., Weiss J.N. Mechanisms of discordant alternans and induction of reentry in simulated cardiac tissue. Circulation. 2000;102:1664–1670. doi: 10.1161/01.cir.102.14.1664. [DOI] [PubMed] [Google Scholar]
- 2.Echebarria B., Karma A. Instability and spatiotemporal dynamics of alternans in paced cardiac tissue. Phys. Rev. Lett. 2002;88:208101. doi: 10.1103/PhysRevLett.88.208101. [DOI] [PubMed] [Google Scholar]
- 3.Gizzi A., Cherry E.M., Fenton F.H. Effects of pacing site and stimulation history on alternans dynamics and the development of complex spatiotemporal patterns in cardiac tissue. Front. Physiol. 2013;4:71. doi: 10.3389/fphys.2013.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe M.A., Fenton F.H., Karma A. Mechanisms for discordant alternans. J. Cardiovasc. Electrophysiol. 2001;12:196–206. doi: 10.1046/j.1540-8167.2001.00196.x. [DOI] [PubMed] [Google Scholar]
- 5.Narayan S.M. T-wave alternans and the susceptibility to ventricular arrhythmias. J. Am. Coll. Cardiol. 2006;47:269–281. doi: 10.1016/j.jacc.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 6.Qu Z., Xie Y., Weiss J.N. T-wave alternans and arrhythmogenesis in cardiac diseases. Front. Physiol. 2010;1:154. doi: 10.3389/fphys.2010.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holley C.L., Cooper J.A. Macrovolt T-wave alternans and polymorphic ventricular tachycardia. Circulation. 2009;120:445–446. doi: 10.1161/CIRCULATIONAHA.109.861633. [DOI] [PubMed] [Google Scholar]
- 8.Huang C., Song Z., Qu Z. Spatially discordant repolarization alternans in the absence of conduction velocity restitution. Biophys. J. 2020;118:2574–2587. doi: 10.1016/j.bpj.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clusin W.T. Mechanisms of calcium transient and action potential alternans in cardiac cells and tissues. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H1–H10. doi: 10.1152/ajpheart.00802.2007. [DOI] [PubMed] [Google Scholar]
- 10.Restrepo J.G., Weiss J.N., Karma A. Calsequestrin-mediated mechanism for cellular calcium transient alternans. Biophys. J. 2008;95:3767–3789. doi: 10.1529/biophysj.108.130419. [DOI] [PMC free article] [PubMed] [Google Scholar]