Abstract
Objective
Allogeneic cartilage transplantation is used to treat severe osteochondral defects or cartilaginous injury. However, acute immune rejection has been a key problem interfering with graft healing.
Methods
Full-thickness osteochondral defects were performed in Sprague Dawley rats. The allograft implants were set into the defect region. Blood and spleen samples from Postoperative Day 3 onward were collected for inflammatory cell analysis, including analysis of monocytes, natural killer cells, CD4+CD25+Foxp3+ regulatory T cells, CD4+ T cells, and CD8+ T cells. Gross observation and histologic staining (hematoxylin and eosin, toluidine blue) were carried out at the same time point to assess the repair effect of the cartilage graft and the degree of immune rejection.
Results
Treatment with basic fibroblast growth factor, agarose gel, and allogeneic cartilage was similar to that of the autologous group. The percentage of monocytes in allografts was at a higher level in the spleen and blood; the frequency of CD4+ T cells in the allogeneic group was higher than in the autologous group and the other agarose groups at 6 weeks after transplantation. The number of regulatory T cells in the autograft was increased from Postoperative Week 1; similar results were observed in groups containing basic fibroblast growth factor beginning at Postoperative Week 3.
Conclusions
Allogeneic cartilage transplantation induces acute immune rejection, which compromises the validity of the implant. The combination of basic fibroblast growth factor and agarose gel facilitates the goal of immune privilege and promotes the success of the allograft tissues.
The translational potential of this article
This study investigated the combination of basic fibroblast growth factor (bFGF) and agarose gel facilitates promotes the success of the allograft tissues transplantation. This work may help clinicians find a new way to repair articular cartilage damage. This will affect the treatment of articular cartilage movement injuries and arthritis.
Keywords: Cartilage transplantation, Immune privilege, Inflammatory factors
Introduction
Nowadays, it is common to see articular cartilage defects caused by sports injury, which is difficult to manage for the limited healing capacity [1], [2]. Although cartilage defect less than 3 mm can be repaired by hyaline cartilage, Yamamoto et al. [4] argue that it is unable to repair cartilage defect that is larger than 4 mm [3], [4].
Numerous therapies have been attempted for repair of articular cartilage damage, including bone marrow stimulation [5], platelet-rich plasma, cells plus scaffold, and whole-tissue transplantation techniques [6], [7], [8]. Among these strategies, autologous osteochondral transplantation is an effective method for resurfacing osteochondral defects. The limitations, however, are equally obvious and hard to be avoided such as donor site morbidity and the availability of the graft. Thus, allogeneic cartilage could potentially be an alternative choice if the innate immune response could be modulated.
Host-versus-graft rejection is a frequent and serious complication of allogeneic tissue or organ transplantation and can lead to poor outcomes [9]. It is suggested long-term survivors of allogeneic hematopoietic stem cell transplantation cause a heavy burden of late side effects [10]. Luan and Iwata [11] proposed a promising method to treat rats with diabetes by implanting agarose gel incorporated with basic fibroblast growth factor (bFGF) subcutaneously; this protected the islet cells that replace agarose gel from attack by the host immune system. With this in mind, the goal of this study was to identify an ideal combination of the surgical site and implant source to reduce inflammation and prolong implant survival and integration of allogeneic cartilage transplantation.
Materials and methods
Animals and experimental groups
A total of 196 adult male Sprague Dawley rats (body weight: 200–300 g, age: 6–8 weeks) were obtained from the Animal Experimental Center of Dalian Medical University. The rats were divided into groups as shown in Figure 1. For all the groups, a full-thickness defect (3 mm in diameter) in the non–weight-bearing portion of the distal medial femur was used. There were seven transplant groups: (i) nontransplant control, (ii) autologous articular cartilage (AU), (iii) allogeneic (same species, different rat) articular cartilage (AL), (iv) allogeneic articular cartilage replacing agarose gel (ALA), (v) allogeneic articular cartilage replacing agarose gel with bFGF (ALAB), (vi) agarose gel (AG), and (vii) agarose gel with bFGF (AGB).
Figure 1.
Experimental design and study groups (28 rats per group; 7 rats per time point were used). bFGF = basic fibroblast growth factor.
Preparation of the bFGF device
A column-shaped agarose scaffold (3 mm in diameter and 3 mm in length) containing bFGF and heparin was produced based on the method used by Luan and Iwata [11]. In brief, 4.5% (w/v) agarose solution was prepared by mixing 450 mg agarose (Gene Company, Hong Kong, China) in 10 mL of double distilled water and autoclaving to dissolve and sterilise. The agarose solution was stored in a 50-mL tube and kept on ice to induce gelation. Then, the agarose gel was shaped into a 3 mm × 3 mm implant, frozen at −30 °C overnight, and freeze-dried for 24 hours. The bFGF solution (5 mL of 500 mg/mL solution; PeproTech Co., NJ, USA) was slowly dropped onto the agarose gel and allowed to absorb for 2 minutes. Finally, 15 mL of heparin solution (250 mg/mL; Changshan Biochemical, Hebei, China) was uniformly added, and the agarose gel with the mixed solution was then stored at 4 °C overnight before use.
Surgical procedures
All the experimental protocols and procedures were approved by the Institutional Animal Care and Use Committee of the Affiliated Zhongshan Hospital of Dalian University. The animals were housed at the Experimental Center of the Affiliated Zhongshan Hospital of Dalian University. After the Sprague Dawley rats were anaesthetised with 10% chloral hydrate (4 mL/kg), a medial peripatellar skin incision was made, and the joint was exposed via lateral dislocation of the patella; a tool was used to create a full-thickness articular cartilage defect (3.0 mm × 3.0 mm) in the trochlear groove of each femur. Some rats in different groups were implanted with the corresponding grafts except those in the nontransplant control group. Then, the grafts of agarose gel were implanted in those in ALA and AG groups; agarose gel with bFGF was implanted in those in ALAB and AGB groups. One week later, the implants in ALA and ALAB groups were replaced by the allogeneic articular cartilage in a second operation. After surgery, the rats were allowed to move freely and fed twice a day in their cages.
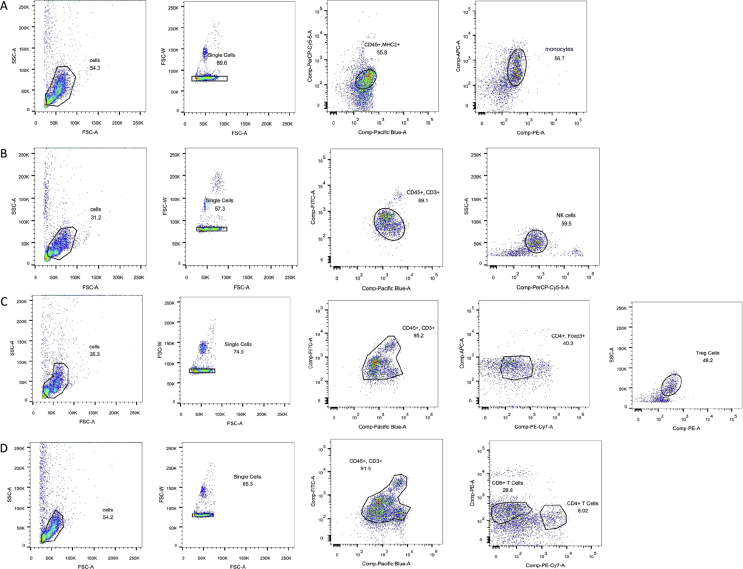
Flow cytometric analysis
All the fluorescently labelled cells were analysed using a FACScalibur. The following antibodies were used in flow cytometric analysis of the activation of inflammatory cells in blood and the spleen from the recipient rats: Rat CD45 HRZN V450, Rat Granulocytes RP-1, Rat CD8a PE MAB, Rat CD11b APC MAB (BD PMG, Franklin, NJ, USA), and Anti-Rat MHC Class II (eBioscience, San Diego, CA, USA) were used to monitor rat monocytes (Figure 2A). Anti-CD3 and anti-CD16 (BD PMG) were used to detect rat natural killer (NK) cells (Figure 2B). Anti-rat CD3, anti-CD4 (BD PMG), anti-CD25, and anti-Foxp3 (eBioscience, USA) were used to monitor rat CD4+CD25+Foxp3+ regulatory T cells (Tregs) (Figure 2C). Rat CD4+ T cells were analysed using Rat CD45 HRZN V450, Rat CD3 FITC MAB, Rat CD4 PE-CY7 MAB, (BD PMG, USA)and Anti-Rat CD25 (eBioscience, USA); CD8+ T cells were measured using Rat CD45 HRZN V450, Rat CD3 FITC MAB, Rat CD8a PE MAB, (BD PMG, USA) and Anti-Rat CD25 (eBioscience, USA) (Figure 2D).
Figure 2.
Analysis process of inflammatory cells. (A) CD45 + RP1 + CD8 + CD11b + MHC II → monocytes; (B) CD3 + CD16 → NK cells; (C) CD25 + Foxp3 + CD3 + CD4 → Tregs; (D) CD45 + CD3 + CD4 + CD25 → CD4+ T cells and CD8+ T cells. NK = natural killer; Tregs = regulatory T cells.
Histological study
Rats from all groups (n = 7, per time period, per group) were sacrificed at Days 3 and 7, at 3 and 6 weeks after transplantation. After the 6th week of the transplantation, the quality of the repaired tissue was evaluated according to the International Cartilage Repair Society Cartilage Injury Evaluation Package [12]. And then, the isolated knee joints were fixed in 10% formalin for 48 hours and were decalcified in 4% ethylene diamine tetraacetic acid (EDTA) (Invitrogen, USA) for approximately two months. Specimens were obtained from the distal part of the femur and embedded in paraffin; sagittal sections (3-μm) were cut and stained with hematoxylin and eosin (H&E) or toluidine blue (Sigma-Aldrich, St. Louis, MO, USA) according to standard protocols. Evaluation for the quality of the repaired tissue was performed by 2 of the authors (B.L. and J.Y.) who were blinded as to which treatment the rats received.
Statistical analysis
All data are reported as the mean and standard deviation. Gross examination of the tissue repair and the percentages of immune cells were compared using one-way analysis of variance.
Results
Macroscopic examination of the transplantation site
Gross examination of the cartilage allografts was performed 6 weeks after transplantation (Figure 3). Smooth integrated surfaces and good thickness were observed in the AU group. In the ALAB group, the graft was similar to that in the AU group. Groups AL and ALA showed only 50–75% filling of repaired cartilage and evident demarcation from the normal cartilage border. The other groups revealed poor outcomes without cartilage tissue implanted. Based on the International Cartilage Repair Society, the scores of the ALAB group were significantly higher than those of the AL and ALA groups (p < 0.01), and the differences between the AU group and the AL or ALA group were also significant (p < 0.001). However, there was no statistically significant difference between the ALAB and AU groups.
Figure 3.
Gross morphological observations of cartilage defects 6 weeks after transplantation. A comparison of the cartilage repair assessment score (International Cartilage Repair Society) between groups was conducted. Columns represent medians and interquartile with error bars. The cartilage repair assessment score was significantly higher in the AU group than that in the AL group (p < 0.001), so was the ALAB group compared with the ALA group (p < 0.01). Scale bar is 1 mm for all the images. ***, p < 0.001, ****, p < 0.0001. (i) non-transplant control, (ii) autologous articular cartilage (AU), (iii) allogeneic (same species, different rat) articular cartilage (AL), (iv) allogeneic articular cartilage replacing agarose gel (ALA), (v) allogeneic articular cartilage replacing agarose gel with bFGF (ALAB), (vi) agarose gel (AG) and (vii) agarose gel with bFGF (AGB).
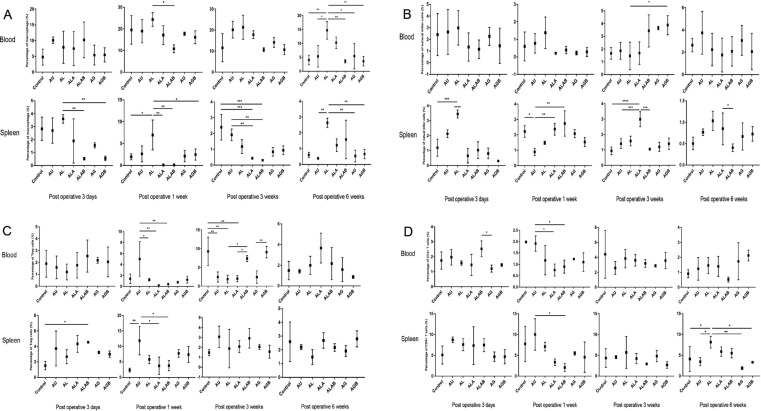
Changes of inflammatory cells
On the third day after operation, the number of monocytes in the spleen of those in Group AL was the highest and significantly higher than in those of the ALAB and AGB groups. In addition, from Week 1 to Week 6 after surgery, the blood monocytes in those of the AL group were significantly higher than those of the ALAB group (Figure 4A).
Figure 4.
Changes of the frequency of inflammatory cells in blood and spleen samples. (A) Blood samples: representative number of monocytes between the AL and ALAB groups at Postoperative Week 1 (*, P < 0.05). Statistical differences between the AL and the other five groups at Postoperative Week 6 (*, P < 0.05; **, P < 0.01). Spleen samples: change of monocytes among the AL and other groups at each time point (*, P < 0.05; **, P < 0.01; ***, P < 0.001). (B) Blood samples: representative frequency of NK cells in the AL and AGB group at Postoperative Week 3 (*, P < 0.05). Spleen samples: differences between the nontransplant control and AL (***, P < 0.001) and between the AL and ALA, ALAB, AG, and AGB groups at Postoperative Day 3 (****, P < 0.0001). Differences between the ALA group and the other groups after transplantation surgery (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001). (C) Blood samples: Tregs in the AU and AL, ALA, and ALAB groups at Postoperative Week 1 (*, P < 0.05; **, P < 0.01), percentage of Tregs in the AGB and AG, ALAB and ALA, and AL groups at Postoperative Week 3, respectively (*, P < 0.05; **, P < 0.01). Spleen samples: statistical differences between the ALAB and nontransplant control group at Postoperative Day 3 (*, P < 0.05). Change of Tregs between the AU and specific groups at Postoperative Week 1 (*, P < 0.05; **, P < 0.01). (D) Blood samples: percentage of CD4+ T cells in the AU and ALA and AU and ALAB groups at Postoperative Week 1, respectively (*, P < 0.05). Spleen samples: change of CD4+ T cells between the AU and ALAB groups at Postoperative Week 1 (*, P < 0.05). Statistical differences between the AL and the other four groups at Postoperative Week 6 (*, P < 0.05; **, P < 0.01). AG = agarose gel; AGB = agarose gel with bFGF; AL = allogeneic articular cartilage; ALA = allogeneic articular cartilage replacing agarose gel; ALAB = allogeneic articular cartilage replacing agarose gel with bFGF; AU = autologous articular cartilage; bFGF = basic fibroblast growth factor; NK = natural killer; Tregs = regulatory T cells.
The frequency of NK cells in the AL group was significantly higher than that in the other groups at day 3, and the difference was statistically significant. The number of NK cells in the group containing the allogeneic graft was significantly increased at different time points, whereas this remained low in the AU group always (Figure 4B).
The changes of CD4+ T cells were not remarkable in the two samples at the first 3 time points. By Week 6, the percentage of CD4+ T cells in the AL group was higher than that in the AU group and the other agarose groups (Figure 4D).
In blood samples, the number of Tregs in the AU group was the highest at Postoperative Week 1. Three weeks after operation, the Tregs in the control group, ALAB group, and AGB group increased compared with the other groups. In spleen tissues, the frequency of Tregs was increased in the AU group at Week 1 after operation, which was almost the same as that in blood samples (Figure 4C).
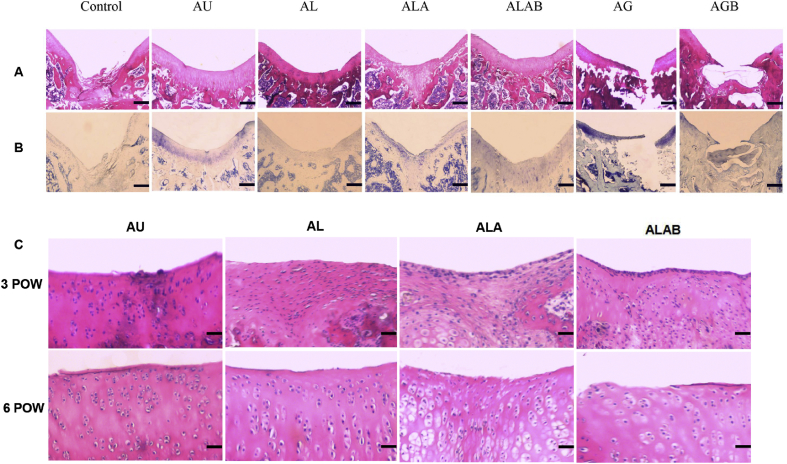
Histologic grading of the repair cartilages
The repaired tissues of the ALAB and AU groups were hyaline-like, with good integration, smooth surfaces, and adequate thickness. In contrast, the other groups did not appear to undergo ideal healing. On H&E staining, the ALA and AL groups showed that chondrocytes appear with small, condensed nuclei at Postoperative Weeks 3 and 6. In the ALAB and AU groups, chondrocytes were orderly and regularly arranged, and no evident of rejection was found (Figure 5C).
Figure 5.
Comparison of histological results after transplantation. (A and B) Comparison of all 6-week H&E and toluidine blue histology sections in 7 groups after implantation. Nontransplant control: amorphous reparative tissue filling the subchondral region. AU: intensively staining covering the defect. AL: the intensity of staining in the regenerated region was less than that of AU and ALAB. ALA: partly positive cartilage organisation in the area. ALAB: amounts of cartilage-like tissue restored in the full-thickness defect. AG and AGB showed the agarose gel occupied the space and hindered the reconstructive process. Scale bar is 1 mm for all the images. (C) Histological findings in knee cartilage in the transplantation site at Postoperative Week (POW) 3 and POW 6 (hematoxylin and eosin). At POWs 3 and 6, the ALAB and AU groups showed no obvious evidence of rejection. In the other two groups, chondrocytes with small, condensed nuclei were visible at each time point. Scale bar is 50 μm for all the images. AG = agarose gel; AGB = agarose gel with bFGF; AL = allogeneic articular cartilage; ALA = allogeneic articular cartilage replacing agarose gel; ALAB = allogeneic articular cartilage replacing agarose gel with bFGF; AU = autologous articular cartilage; bFGF = basic fibroblast growth factor; H&E = hematoxylin and eosin; NK = natural killer; Tregs = regulatory T cells.
The cartilage transplantation sections showed regenerating cartilaginous tissues in the ALAB and AU groups, which was more distinctive than those in the AL and ALA groups at 6 weeks after transplantation (Figure 5A and B).
Discussion
In previous studies, there were no published studies on investigating the effect of the combination of bFGF and agarose gel in promoting articular cartilage repair. However, bFGF plays a significant role in repair of cartilage because bFGF together with agarose gel could lower the host-versus-graft rejection grade and fasten hyaline cartilage restoration in lesions [13], [14], [15]. In this study, we assessed the ability and feasibility of this therapy to modulate acute inflammation and promote immune tolerance and healing of the defect in vivo.
In this study, monocytes and NK cells in the AL group first changed in spleen samples at time points of 3 days and were later higher than those in the other groups, especially for monocytes in blood samples. CD4+ T cells were increased at Postoperative Week 6 in the spleen. This finding suggested that immune cells play a critical role in the acute rejection stage and may influence the repair of the allogeneic cartilage graft. In the AU group, the percentage of CD4+CD25+Foxp3+ Tregs increased from Week 1; we observed a similar phenomenon in the ALAB group, which indicates agarose and bFGF may influence healing of allogeneic cartilage toward autologous articular cartilage because CD4+CD25+Foxp3+ Tregs are conducive to the establishment of immune tolerance [16].
Li et al [14], [18], Deng et al [17], and Yokoo et al. [19] have shown that bFGF was beneficial to cartilage formation, and exogenous bFGF could promote chondrocyte proliferation, cartilage maturation, and mesenchymal cell differentiation, which also stimulates cartilaginous matrix synthesis. In addition, cartilage differentiation is reduced if bFGF was absent [20]. However, some studies showed that the gene expression of Type II collagen was inhibited by bFGF [21]. Moreover, Sah et al. have proposed that different concentrations of bFGF led to opposite results on cartilage repair and therefore served a dual role in the regulation of cartilage metabolism [35]. The present investigation showed that bFGF inhibited the activation of inflammatory cells that would inhibit the release of cytokines that destroy the formation of graft tissue. Moreover, all groups containing bFGF, especially, both the ALAB and AGB groups, showed that the frequency of CD4+CD25+Foxp3+ Tregs remained at a high level in blood at Postoperative Week 3 compared with the other groups. Tregs can protect the implanted tissues from attack and may facilitate chondrocyte proliferation.
The term of immune privilege was first defined by Medawar [22] in the 1940s. They found that the allograft skin placed within the anterior chamber of the eye would consistently survive until leakage occurred at the blood-ocular barrier.
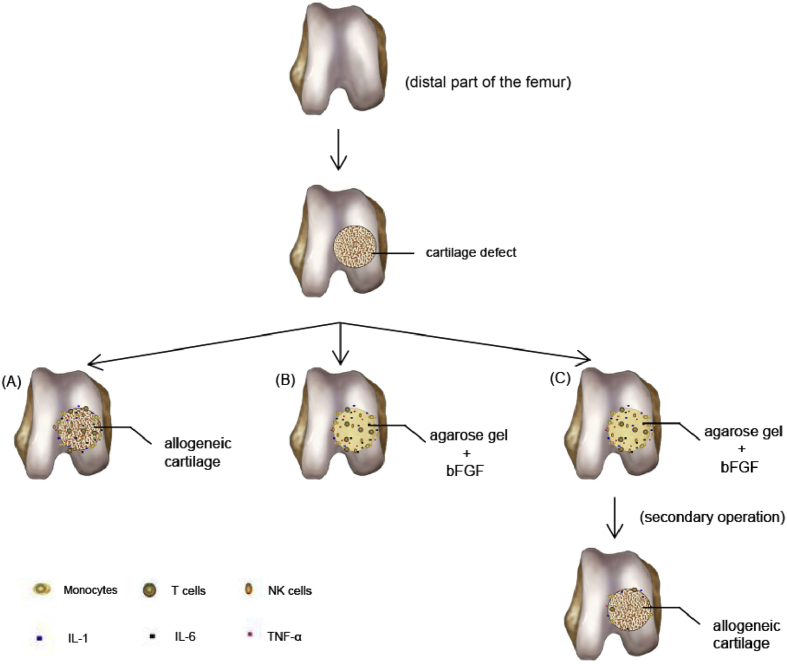
In this study, it is suggested that agarose gel and bFGF form a barrier around the region of transplantation and they act as an alloantigen that could not activate immune response and will not get to lymph nodes. It is shown that, as long as the compound space does not leak, the transplanted cartilage could survive and play a role in repairing the defect areas (Figure 6C).
Figure 6.
Different inflammation situations of allogeneic cartilage transplantation. (A) A column-shaped allogeneic cartilage transplanted into the defect, resulting in the activation of monocytes and other inflammatory cells, for instance, NK cells and T cells, which release interleukin-1 (IL-1), IL-6, and tumor necrosis factor alpha (TNF-α). (B) The same size of agarose gel containing bFGF was implanted into the defect; similar inflammatory rejection as allogeneic cartilage occurs around the graft. (C) One week later, since replacing the agarose gel and bFGF with the allograft, immune-privileged circumstance begins to form and the number of inflammatory cells and factors neighboring the graft reduces drastically. bFGF = basic fibroblast growth factor; NK = natural killer.
Previous murine studies demonstrated the effect of CD4+CD25+Foxp3+ Tregs for allograft survival and immune privilege through a variety of mechanisms, which are associated with CD62L expression and interleukin (IL)-17A–dependent mechanisms [23], [24], [25], [26].
Tregs that help to establish immune tolerance, especially after corneal allograft transplantation, are defined as expression of the Foxp3 transcription factor. In clinical transplantation, the Treg acts as a peripheral tolerance central regulator, raising the possibility to improve transplantation outcomes [27], [28]. Our findings showed that the action of Tregs was similar; there was high frequency of Tregs in both the AU group and ALAB group at different time points after transplantation. This concept is in accordance with the viewpoint of Taylor [29], who had proposed that understanding the molecular blocks of ocular immune privilege would then be applied to all allografts. Cartilage allograft transplantation was performed to achieve precise surface architecture without donor site morbidity [6], [30], [31], [32]. Lee et al. [33] showed that allogeneic chondrocytes dominated cartilaginous remodelling approximately 12–24 weeks after transplantation. Griffin et al. [34] concluded that collagen probably had a special mechanical role in cartilage repair at the early stage of maturation. Here, we found that the results of allograft transplantation were similar to those of autologous tissues. The damage from cytokines produced by monocytes and other inflammatory cells, including IL-1, IL-6, and tumor necrosis factor alpha (Figure 6, A and B), may stimulate chondrocytes to secrete proteinase A, which could destroy the cartilaginous matrix and inhibit chondrocyte proliferation. Furthermore, the size and function of chondrocytes can change in response to stimulation from inflammatory factors.
We did not follow the fate of the transplanted cartilage tissues further in this study. Chronic immune rejection and effect of cartilage repair are supposed to be observed in the long term indeed. Another limitation of this study is the use of the rat as a small animal model and a small group size; however, the overall number of rats was large enough, and statistical power was met. Correlative results should be obtained if this method is used for much larger cartilage defects or xenogeneic graft transplantation in future, and the experiment provides a good method for avoiding host-versus-graft response and promoting cartilage repair.
In conclusion, we demonstrated immune privilege was a relevant concept for cartilage repair. Osteochondral allograft transplantation can cause a severe acute immune rejection, which leads to loss of viability; it may involve both the innate and adaptive immune responses. The application of a combination of agarose gel and bFGF may establish its own microenvironment to locally suppress inflammation and to control the activities of immune cells and may serve as a level of protection against rejection as an immune-privileged tissue. To our knowledge, this is the first report of this strategy containing a growth factor, agarose gel, and full-thickness cartilage tissue to mitigate the acute immune responses for allogeneic cartilage repairing and facilitate graft survival. Clarifying the mechanism that is responsible for our findings may improve the success of osteochondral allotransplantation.
Funding
This study was not supported by any funding.
Conflict of Interest
The authors have no conflicts of interest to disclose in relation to this article.
Footnotes
This work was supported by China Pastdoctoral Science Foundation (NO. 174180), Doctoral Scientific Research Foundation of Liaoning Province (NO. 201601299) and the Scientific Technology Star Program of Dalian (NO. 2017RQ154).
Contributor Information
Baoyi Liu, Email: liubaoyi-513@163.com.
Dewei Zhao, Email: zhaodewei2016@163.com.
References
- 1.Caplan N., Kader D.F. Two-to 9 year outcome after autologous chondrocyte transplantation of the knee. In: Banaszkiewicz P.A., Kader D.F., editors. Classic papers in orthopaedics. Springer; London: 2013. pp. 165–168. [Google Scholar]
- 2.Grande D.A., Schwartz J.A., Brandel E., Chahine N.O., Sgaglione N. Articular cartilage repair. Nat Inst Health. 2013;4(4):281–285. doi: 10.1177/1947603513494402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charalambous C.P. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. In: Banaszkiewicz P.A., Kader D.F., editors. Classic papers in orthopaedics. Springer; London: 2013. pp. 377–379. [Google Scholar]
- 4.Yamamoto T., Wakitani S., Imoto K., Hattori T., Nakaya H., Saito M. Fibroblast growth factor-2 promotes the repair of partial thickness defects of articular cartilage in immature rabbits but not in mature rabbits. Osteoarthr Cartil. 2004;12(8):636–641. doi: 10.1016/j.joca.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Breinan H.A., Martin S.D., Hsu H.P., Spector M. Healing of canine articular cartilage defects treated with microfracture, a type-II collagen matrix, or cultured autologous chondrocytes. J Orthop Res. 2000;18(5):781–789. doi: 10.1002/jor.1100180516. [DOI] [PubMed] [Google Scholar]
- 6.Bedi A., Feeley B.T., Williams R.J. Management of articular cartilage defects of the knee. J Bone Jt Surg Am. 2010;92(4):994–1009. doi: 10.2106/JBJS.I.00895. [DOI] [PubMed] [Google Scholar]
- 7.Komarek J., Valis P., Repko M., Chaloupka R., Krbec M. Treatment of deep cartilage defects of the knee with autologous chondrocyte transplantation: long-term results. Acta Chir Orthop Traumatol Cech. 2010;77(4):291–295. [PubMed] [Google Scholar]
- 8.Bugbee W.D. Fresh osteochondral allografts. J Knee Surg. 2002;15(3):191–195. [PubMed] [Google Scholar]
- 9.Flowers M.E., Parker P.M., Johnston L.J., Matos A.V.B., Storer B., Bensinger W.I. Comparison of chronic graft-versus-host disease after transplantation of peripheral blood stem cells versus bone marrow in allogeneic recipients: long-term follow-up of a randomized trial. Blood. 2002;100(2):415–419. doi: 10.1182/blood-2002-01-0011. [DOI] [PubMed] [Google Scholar]
- 10.Shanis D., Merideth M., Pulanic T.K., Savani B.N., Battiwalla M., Stratton P. Female long-term survivors after allogeneic hematopoietic stem cell transplantation: evaluation and management. Semin Hematol. 2012;49(1):83–93. doi: 10.1053/j.seminhematol.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luan N.M., Iwata H. Long-term allogeneic islet graft survival in prevascularized subcutaneous sites without immunosuppressive treatment. Am J Transplant. 2014;14(7):1533–1542. doi: 10.1111/ajt.12739. [DOI] [PubMed] [Google Scholar]
- 12.Brittberg M., Winalski C.S. Evaluation of cartilage injuries and repair. J Bone Jt Surg Am. 2003;85-A(Suppl 2):58–69. doi: 10.2106/00004623-200300002-00008. [DOI] [PubMed] [Google Scholar]
- 13.Guo X., Zuo H., Cao C.X., Zhang Y., Geng D., Zhang Z.T. Abnormal expression of Col X, PTHrP, TGF-beta, bFGF, and VEGF in cartilage with Kashin-Beck disease. J Bone Miner Metab. 2006;24(4):319–328. doi: 10.1007/s00774-006-0690-3. [DOI] [PubMed] [Google Scholar]
- 14.Li X., Su G., Wang J., Zhou Z., Li L., Liu L. Exogenous bFGF promotes articular cartilage repair via up-regulation of multiple growth factors. Osteoarthr Cartil. 2013;21(10):1567–1575. doi: 10.1016/j.joca.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Chen B., Qin J., Wang H., Magdalou J., Chen L.B. Effects of denovirus-mediated bFGF, IL-1Ra and IGF-1 gene transfer on human osteoarthritic chondrocytes and osteoarthritis in rabbits. Exp Mol Med. 2010;42(10):684–695. doi: 10.3858/emm.2010.42.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roy S., Barners P.F., Garg A., Wu S., Cosman D., Vankayalapati R. NK cells lyse T regulatory cells that expand in response to an intracellular pathogen. J Immunol. 2008;180(3):1729–1736. doi: 10.4049/jimmunol.180.3.1729. [DOI] [PubMed] [Google Scholar]
- 17.Deng T., Huang S., Zhou S.X., He L.S., Jin Y. Cartilage regeneration using a novel gelatin-chondroitin-hyaluronan hybrid scaffold containing bFGF-impregnated microspheres. J Microencapsul. 2007;24(2):163–174. doi: 10.1080/02652040701233523. 2007. [DOI] [PubMed] [Google Scholar]
- 18.Li Q., Liu T.Y., Zhang L., Liu Y., Zhang W.J., Liu W. The role of bFGF in down-regulating alpha-SMA expression of chondrogenically induced BMSCs and preventing the shrinkage of BMSC engineered cartilage. Biomaterials. 2011;32(21):4773–4781. doi: 10.1016/j.biomaterials.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Yokoo N., Saito T., Uesugi M., Kobayashi N., Xin K.Q., Okuda K. Repair of articular cartilage defect by autologous transplantation of basic fibroblast growth factor gene-transduced chondrocytes with adeno-associated virus vector. Arthritis Rheum. 2005;52(1):164–170. doi: 10.1002/art.20739. [DOI] [PubMed] [Google Scholar]
- 20.Brandl A., Angele P., Roll C., Prantl L., Kujat R., Kinner B. Influence of the growth factors PDGF-BB, TGF-beta1 and bFGF on the replicative aging of human articular chondrocytes during in vitro expansion. J Orthop Res. 2010;28(3):354–360. doi: 10.1002/jor.21007. [DOI] [PubMed] [Google Scholar]
- 21.Kondo S., Cha S.H., Xie W.F., Sandell L.J. Cytokine regulation of cartilage-derived retinoic acid-sensitive protein (CD-RAP) in primary articular chondrocytes: suppression by IL-1, bfGF, TGFbeta and stimulation by IGF-1. J Orthop Res. 2001;19(4):712–719. doi: 10.1016/S0736-0266(00)00068-1. [DOI] [PubMed] [Google Scholar]
- 22.Medawar P.B. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29(1):58–69. [PMC free article] [PubMed] [Google Scholar]
- 23.Cunnusamy K., Chen P.W., Niederkorn J.Y. IL-17A-dependent CD4+CD25+ regulatory T cells promote immune privilege of corneal allografts. J Immunol. 2011;186(12):6737–6745. doi: 10.4049/jimmunol.1100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ermann J., Hoffmann P., Edinger M., Dutt S., Blankenberg F.G., Higgins J.P. Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood. 2005;105(5):2220–2226. doi: 10.1182/blood-2004-05-2044. [DOI] [PubMed] [Google Scholar]
- 25.Fu S., Yopp A.C., Mao X., Chen D., Zhang N., Chen D. CD4+ CD25+ CD62+ T-regulatory cell subset has optimal suppressive and proliferative potential. Am J Transplant. 2005;4(1):65–78. doi: 10.1046/j.1600-6143.2003.00293.x. [DOI] [PubMed] [Google Scholar]
- 26.Ochando J.C., Yopp A.C., Yang Y., Garlin A., Li Y., Boros P. Lymph node occupancy is required for the peripheral development of alloantigen-specific Foxp3+ regulatory T cells. J Immunol. 2005;174(11):6993–7005. doi: 10.4049/jimmunol.174.11.6993. [DOI] [PubMed] [Google Scholar]
- 27.Hori S., Nomura T., Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3.Science 2003.299:1057-1061. J Immunol. 2017;198(3):981–985. [PubMed] [Google Scholar]
- 28.Kang S.M., Tang Q., Bluestone J.A. CD4+CD25+ regulatory T cells in transplantation: progress, challenges and prospects. Am J Transplant. 2010;7(6):1457–1463. doi: 10.1111/j.1600-6143.2007.01829.x. [DOI] [PubMed] [Google Scholar]
- 29.Taylor A.W. Ocular immune privilege and transplantation. Front Immunol. 2016;7:37–40. doi: 10.3389/fimmu.2016.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCulloch P.C., Kang R.W., Sobhy M.H., Hayden J.K., Cole B.J. Prospective evaluation of prolonged fresh osteochondral allograft transplantation of the femoral condyle: minimum 2-year follow-up. Am J Sports Med. 2007;35(3):411–420. doi: 10.1177/0363546506295178. [DOI] [PubMed] [Google Scholar]
- 31.Gudas R., Stankevicius E., Monastyreckiene E., Pranys D., Kalesinskas R.J. Osteochondral autologous transplantation versus microfracture for the treatment of articular cartilage defects in the knee joint in athletes. Knee Surg Sport Traumatol Arthrosc. 2006;14(9):834–842. doi: 10.1007/s00167-006-0067-0. [DOI] [PubMed] [Google Scholar]
- 32.Gross A.E., Kim W., Las-Heras F., Backstein D., Safir O., Pritzker K.P. Fresh osteochondral allografts for post-traumatic knee defects:long-term followup. Clin Orthop Relat Res. 1997;466(8):1863–1870. doi: 10.1007/s11999-008-0282-8. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J.H., Prakash K.V., Pengatteeri Y.H., Park S.E., Koh H.S., Han C.W. Chondrocyte apoptosis in the regenerated articular cartilage after allogenic chondrocyte transplantation in the rabbit knee. J Bone Jt Surg Br. 2007;89(7):977–983. doi: 10.1302/0301-620X.89B7.18983. [DOI] [PubMed] [Google Scholar]
- 34.Griffin D.J., Ortved K.F., Nixon A.J., Bonassar L.J. Mechanical properties and structure-function relationships in articular cartilage repaired using IGF-I gene-enhanced chondrocytes. J Orthop Res. 2016;34(1):149–153. doi: 10.1002/jor.23038. [DOI] [PubMed] [Google Scholar]
- 35.Sah R.L., Chen A.C., Grodzinsky A.J., Trippel S.B. Differential effects of bFGF and IGF-I on matrix metabolism in calf and adult bovine cartilage explants. Arch Biochem Biophys. 1994;308(1):137–147. doi: 10.1006/abbi.1994.1020. [DOI] [PubMed] [Google Scholar]