Abstract
Objectives
The objective of this study was to investigate the effect of botulinum toxin type A (BTX-A)–induced quadriceps muscle atrophy on the cartilage and subchondral bone in an otherwise intact rat joint model.
Methods
The rat right quadriceps muscle atrophy was established by intramuscular injection of BTX-A. Twenty-four rats were divided randomly into 3 groups: The BTX-A–treated 4-week group; the BTX-A–treated 8-week group; and the control group injected with phosphate buffer saline were observed for 8 weeks. Muscle atrophy level was measured by weighing and histology examinations. Serum interleukin-1β level was tested by ELISA (enzyme linked immunosorbent assay); the subchondral bone was analysed by micro–computed tomography and the cartilage was measured by histology examinations (gross view, haematoxylin and eosin staining and Safranin-O/fast green staining) and immunohistochemistry test {collagen X [ColX]}.
Results
BTX-A intramuscular injection led to muscle atrophy. Characteristics of muscle atrophy appeared in two BTX-A–injected groups but not in the control group. Quadriceps atrophy did not affect interleukin-1β level in serum, but resulted in subchondral bone abnormal changes with reduced bone volume/total tissue volume and increased Structure Model Index. Furthermore, the more the severe cartilage damage, the higher the histologic damage scores, followed by the higher the percentage of collagen X–positive chondrocytes caused by muscle atrophy.
Conclusions
Quadriceps muscle atrophy triggered the subchondral bone abnormal change and cartilage degeneration, which would be a risk factor for development of osteoarthritis.
The translational potential of this article
Our results indicate that anti-quadriceps muscle atrophy can be a candidate therapeutic target in the prevention of knee osteoarthritis.
Keywords: Cartilage, Muscle atrophy, Osteoarthritis, Subchondral bone
Introduction
Osteoarthritis (OA) is one of the most common chronic joint diseases followed by pathological features including degenerative changes of articular cartilage, secondary bone hyperplasia, and narrowing of joint space on plain radiographs [[1], [2], [3]]. Pain, malformation, and dysfunction are the most significant clinical manifestations of OA [4]. People older than 50 years with heavily loaded and active joints often easily suffer OA. In addition, some studies demonstrated that evidence of radiographic knee osteoarthritis (KOA) increases up to 80% for people older than 65 years [5]. However, the causes and progression of OA are still not fully understood. Recently, a large number of researchers have considered OA as a whole joint issue and the result of a complex interaction among several factors [[6], [7], [8], [9]].
One of the earliest symptoms appeared in patients with OA is muscle weakness or atrophy [7,10]. In general, the atrophy of the thigh muscle in patients with KOA is often characterised as a decrease in mass, strength, function, and coordination of quadriceps. Previous clinical studies had shown that muscles and joints are functionally interdependent and that the muscles cause the joints to actively move, maintain joint stability, and absorb mechanical shocks [11,12]. In addition, the quadriceps muscle is an important component in maintaining the stress balance of the knee joint. The decrease in muscle mass and strength makes the joints prone to fatigue, thus affecting the nutrient supply of the cartilage [13]. Fisher [14] and Slemenda et al. [15] supposed that patients with OA always showed quadriceps weakness. Subsequently, Slemenda et al [15] also found that female patients with KOA have a 15% reduced knee extensor strength than those women who did not suffer from KOA. Recently, several clinical studies reported that the quality and strength of the lower limb muscles play an important role in the pathogenesis of OA [[16], [17], [18]]. Nevertheless, less evidence indicated that atrophy of muscle could result in subchondral bone aberrant remodelling in OA development.
Botulinum toxin type A (BTX-A), a macromolecular protein toxin produced by Clostridium botulinum can internalise toxins by receptor-mediated endocytosis after binding to the axon terminals of alpha motor neurons. Once it gets inside the cell, BTX-A will inhibit the release of acetylcholine (ACh) [19]. Owing to the low level of ACh, muscle fibres cannot be activated, thus causing paralysed muscle, attenuated muscle strength, thinned muscle fibre, and eventually atrophied muscle. Hence, in animal studies, BTX-A has been widely used for studies of the relationship between muscle atrophy and OA [[20], [21], [22], [23]]. The results of the studies found that quadriceps atrophy induced by intramuscular injection of BTX-A could promote cartilage degeneration and elevate the secondary inflammation, leading to significant OA in the rabbit [[20], [21], [22], [23]]. As subchondral bone abnormal change is of great importance in OA development, whether muscle atrophy incurs subchondral bone abnormal change is still unknown. Further studies are needed to evaluate subchondral bone in more detail.
The objective of this study was to use a rat quadriceps femoris atrophy model, to further explore the level of cartilage degeneration and subchondral bone abnormal remodelling after quadriceps femoris atrophy. We hypothesised that knee muscle atrophy is associated with not only knee cartilage degeneration but also subchondral bone abnormal changes, thereby providing evidence that muscle atrophy would be a risk factor for the onset and progression of OA.
Materials and methods
Animal model
All the related animal experiments were approved by the Animal Ethics Committee at Jinan University (Ethics Reference No.: 201812015-01), twenty-four male Sprague–Dawley rats aged 16 weeks (weight: 350 ± 20 g) were used in this study. All the animals raised were 4 to 6 rats per cage. We divided the animals into 3 groups, eight rats in BTX-A 4-w group received 6U/kg BTX-A injection in right quadriceps muscle of the hind limb, and they were sacrificed after 4 weeks. Eight rats in BTX-A 8-w group received 6U/kg BTX-A injection in right quadriceps muscle of the hind limb and were sacrificed after 8 weeks. The dose of BTX-A was referred to the previous studies in this area [[23], [24], [25]]. Rats in both of BTX-A groups received an intramuscular injection of phosphate buffer saline (PBS) in their left quadriceps muscle of the hind limb. Another eight rats in the control group received equal amounts of PBS in bilateral quadriceps muscle of hind limbs and were sacrificed after 8 weeks. All the rats were anaesthetised by intraperitoneal injection of 1% pentobarbital sodium. Each injection of BTX-A or PBS was divided equally among three lines of the thigh of the hind limb – medial, central, and lateral–corresponding to the vastus medialis, rectus femoris, and vastus lateralis muscle. At the end of the experimental period, samples were collected and recorded, including quadriceps muscle, blood, and knee joint. All the animals were acclimatised to local vivarium conditions at a temperature of 24–26 °C and a humidity of 70% with free access to water and a pelleted commercial diet in the mouse house under specific pathogen–free conditions and well taken care by staff of the animal house of the Jinan University.
Blood collection and serum analysis
We collected 5 ml blood sample by cardiac puncture before the heartbeat stopped. The blood sample was then centrifuged at 3000 g for 10 min. The serum were then stored at −20 °C until analysis. Interleukin-1β (IL-1β) was used as a marker for active inflammation and the levels were measured with the IL-1β ELISA kit in accordance with the manufacturer's instructions (IL-1β Elisa kit, lot: EK0392 Boster, Chian).
Muscle mass and muscle histology
The quadriceps muscle mass was measured with an electronic balance as soon as the quadriceps muscle was isolated from the rat thigh. After weighing, all the specimens were fixed in 4% paraformaldehyde, paraffin sections were made , and these slides were then stained with haematoxylin and eosin (H&E) stain. Finally, we used a microscope (Leica DMRB microscope, Germany) to assess changes in the muscle fibres.
Micro–computed tomography scanning
In this study, the structural change on the subchondral bone of rat knees was evaluated through micro–computed tomography (micro-CT) quantitatively. At the end of our experiment, the right knees of rats were extracted and dissected, and soft tissues surrounding the knee were moved out. Then, the specimens were fixed in 4% paraformaldehyde before analysis with high-resolution micro-CT (skyscan 1172; Bruker, Belgium) at custom isotropic resolution of 9 mm isometric voxel size with a voltage of 80 kV p and a current of 88 mA. Other scannerparameter setting including the exposure time was 54 min, and the rotation step was 0.4°, with the complete rotation over 180°. Select the subchondral bone of the tibia as region of interest, and used three dimensional data analysis software (CTAnalyzer, Skyscan, Bruker, Belgium) for further data analysis [26]. Three dimensional morphometric parameters were calculated to illustrate the subchondral bone structure: bone mineral density (BMD) representing the apparent density of porous trabeculae, bone volume/total tissue volume (BV/TV) describing the ratio of bone volume over tissue volume, and the Structure Model Index (SMI) assessing whether a bone structure is rod-like or plate-like [27,28].
Cartilage histology and immunohistochemistry
The right femoral condyle, tibial plateau of the rats was decalcified with 10% Ethylene Diamine Tetraacetic Acid (EDTA) decalcifying solution for 17 days and kept at 37 °C. After decalcification, the medial compartment of knees was embedded into paraffin. Serial section of the samples was cut at 5 μm thickness sagittal-oriented sections of the femoral condyle or tibial plateau. These slides were then stained with H&E and Safranin-O/fast green stains. Cartilage degradation was quantified according to Mankin grading system [29]. Two researchers scored the pathological sections independently under the same conditions and graded for OA by using a microscope (Leica DMRB). If there was a difference, it would be decided by a third researcher.
The standard Mankin criteria assess cartilage structure (0–6), the number of chondrocytes (0–3), matrix staining (0–4), and tidemark integrity (0–1), and the total score ranges between 0 (normal) and 14 (the most severe OA). For each joint, the samples show that the most severe OA changes in each of the knee cartilage region were graded. Each slide of the samples was observed at high magnification (enlarged 100 times), and the score was calculated based on the Mankin grading system.
Immunostaining was performed using a standard protocol as previously reported [[30], [31], [32]], the sections which were incubated firstly with primary antibodies to rabbit Collagen X (ColX) (Abcam, 1:50, ab58632) overnight at 4 °C and then with horseradish peroxidase–streptavidin–conjugated goat anti–rabbit secondary antibody (Cell signalling technology, 1:100, 7047) 1 h at room temperature. Then the sections were counterstained with horseradish peroxidase–streptavidin detection system (Cell signalling technology, DAB Substrate Kit, 8059S) and haematoxylin. Photographs of the selected areas were taken by a light microscope (Leica DMRB microscope, Germany). We counted the number of positively stained cells of the cartilage in the whole medial tibia per specimen and evaluated the difference statistically.
Statistical analysis
The statistical analysis was performed using the statistical software SPSS (version 13.0; SPSS Inc., Chicago, Illinois, USA). Comparisons within groups were analysed using the paired sample t test, whereas comparisons between groups were analysed using the one-way analysis of variance. Differences between groups were assessed by the Shapiro–Wilk test of normality and Levene's test for homogeneity of variance. The result of Levene's test was used to determine the post hoc testing strategy. If the results were not significant, least significant difference test (LSD-t) or the post hoc test was adopted. If Levene's test was significant, the analysis of variance was followed by Dunnett's T3 post hoc test for unequal variance. The level of significance was set at α = 0.05. Data were reported as mean ± stand error mean. The graphs were generated using GraphPad Prism 6 Software (GraphPad Software, San Diego, CA, USA).
Result
BTX-A injection led to muscle atrophy
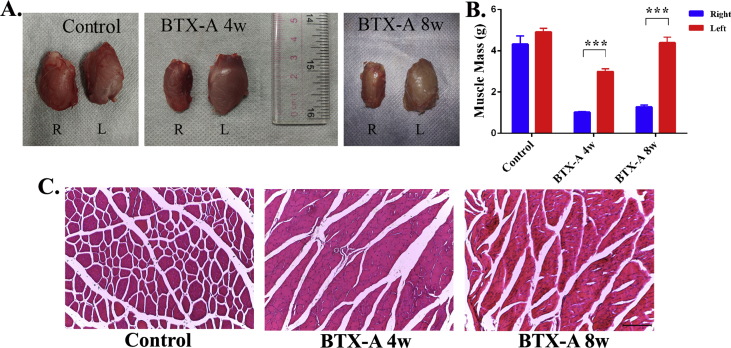
Compared with left quadriceps muscle, a significant decrease could be observed in the volume of the right quadriceps (Figure 1A). As shown in Figure 1B, the right quadriceps muscle mass was significantly lower than that of the left side in the BTX-A 4-w group (R:1.02 ± 0.03 g, L: 2.99 ± 0.14 g) and the BTX-A 8-w group (R: 1.28 ± 0.09 g, L: 4.39 ± 0.28 g) (p < 0.001), and there was no significant difference in the control group (R: 4.32 ± 0.40 g, L: 4.91 ± 0.18 g) (p = 0.196). In addition, the left quadriceps muscle mass between the BTX-A 8w group (4.39 ± 0.28 g) and control group (4.91 ± 0.18 g) showed no statistically significant difference (p = 0.152). Results of a cross-sectional histological slide of quadriceps musculature from 3 groups showed the significant quadriceps muscle atrophy in both BTX-A–injected groups, characterised by neovascularity and fat or inflammatory cells infiltration within muscle fibres of BTX-A groups, and it recovered partly in the BTX-A 8-w group compared with the BTX-A 4-w group (Figure 1C). While in the control group, the result demonstrated that the muscle fibres showed classic cylindrical dense structures (Figure 1C).
Figure 1.
(A) Examples of quadriceps muscles gross view in 3 groups, “R” means right side quadriceps muscles, “L” means left side quadriceps muscles, showing a significant atrophy after BTX-A injection. (B) Quantitatively analysed of the weight of bilateral quadriceps muscles mass of hind limbs in all 3 groups. (C) The cross-sectional histological slide of quadriceps musculature from 3 groups by H&E staining; Scale bar = 200 μm, ***p < 0.001. BTX-A = botulinum toxin type A; H&E staining = haemotoxylin and eosin staining.
Muscle atrophy did not affect the level of IL-1β in serum
The level of IL-1β in the serum was tested by an ELISA assay, and the results showed no significant difference between 3 groups (Control group: 40.67 ± 0.36 pg/ml; BTX-A 4-w group: 40.77 ± 0.36 pg/ml; and BTX-A 8-w group: 42.97 ± 1.76 pg/ml; P = 0.276) (Figure 2). This result indicated that quadriceps muscle atrophy did not affect the level of IL-1β in serum. But whether muscle atrophy would upregulate the inflammation level in synovial fluid of the knee joint still need further investigation.
Figure 2.
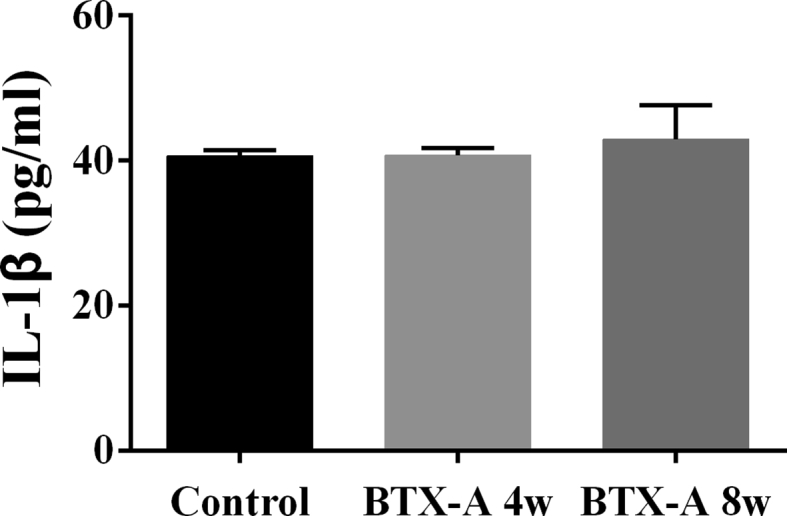
ELISA assay showing the IL-1β level in serum of 3 groups. IL-1β = interleukin-1β.
Quadriceps atrophy resulted in subchondral bone abnormal change
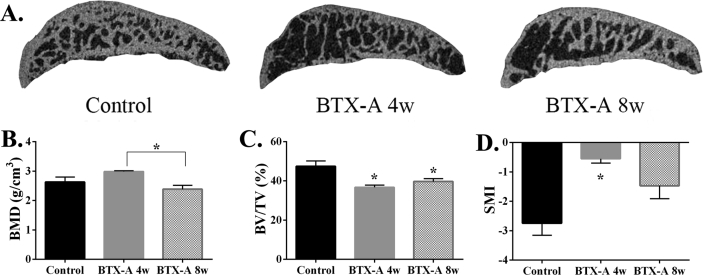
In contrast to right knees in control group rats, the result of micro-CT examination showed that the microarchitecture of the right knee subchondral bone had significantly changed in two BTX-A groups (Figure 3A). BMD showed no difference between the BTX-A groups and the control group (2.63 ± 0.17 g/cm3) statistically, but compared with BTX-A 8-w group (2.38 ± 0.13 g/cm3), the BMD of BTX-A 4-w group (2.98 ± 0.29 g/cm3) is significantly higher (*p < 0.05.) (Figure 3B). BV/TV in the BTX-A 4-w group (36.73 ± 1.13%) and BTX-A 8-w group (39.67 ± 1.48%) had significantly decreased than that of the control group (47.37 ± 2.79, *p < 0.05.), between two BTX-A groups, there was no difference (Figure 3C). SMI is the data to show the shape of the trabecular bone, an upregulated SMI indicated that trabecular bone transformed from rod-like to plate-like, and it is one of the characteristics of OA. Our result demonstrated that the SMI was significantly upregulated in the BTX-A 4-w group (−0.55 ± 0.15) than that of the BTX-A 8-w group (−1.47 ± 0.44, *p < 0.05.) and control group (−2.74 ± 0.41, *p < 0.05.), and there was no statistically significant difference between the BTX-A 8-w group and the control group (Figure 3D).
Figure 3.
(A) Micro-CT images of the tibia subchondral bone medial compartment (sagittal view) of rat in the 3 groups. (B–D) Quantitative analysis of microstructural parameters of the subchondral bone by micro-CT analysis. Micro-CT =micro–computed tomography.
Atrophy of quadriceps triggered cartilage degeneration
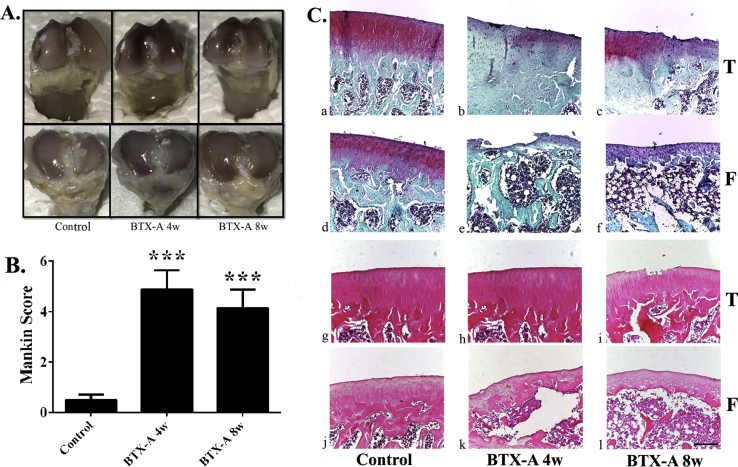
The result of gross view showed that the articular surface of the femoral condyles or tibial plateau of the rat right knee was relatively smooth and no obvious osteophyte formation in all 3 groups. But the colour of the cartilage was darker in the BTX-A 4-w group and BTX-A 8-w group than in the control group, this may suggest that the cartilage becomes thinner in BTX-A groups (Figure 4A). What is more, Safranin-O staining and H&E staining (Figure 4C) revealed mild OA lesions in the articular cartilage in the two BTX-A groups, the main pathological features including the declining of cartilage thickness, wearing of cartilage surface, and decreasing number of chondrocyte. In comparison with the control group (0.50 ± 0.22), the Mankin score was significantly higher in the BTX-A 4-w group (4.88 ± 0.77, p = 0.001) and BTX-A 8-w group (4.14 ± 0.74, p = 0.001), which reflected early stage OA changes of the right knee (Figure 4B).
Figure 4.
(A) Gross view of the knee joint in 3 groups. (B) Mankin score showed that cartilage degeneration of the right knee in 2 BTX-A groups. (C) Safranin-O/fast green staining (a–f) and H&E staining (g–l) of sagittal sections showing the subchondral tibia and femur medial compartment. Scale bar = 200 μm, ***p < 0.001. BTX-A = botulinum toxin type A; H&E staining = haemotoxylin and eosin staining.
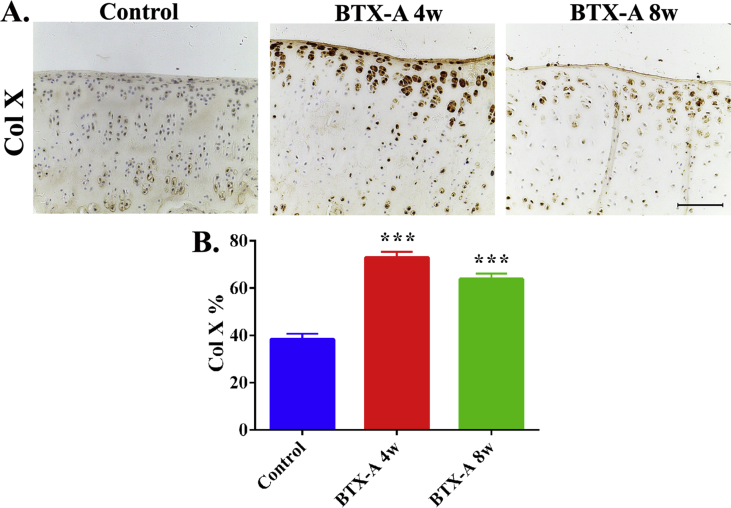
Besides, the result immunohistochemical staining showed that the percentage of ColX-positive cells (a marker of chondrocyte hypertrophy) in the BTX-A 4-w group (72.94% ± 2.48%, p < 0.001) and BTX-A 8-w group (63.85% ± 2.29%, p < 0.01) was significantly higher than that in the control group (38.37% ± 2.31%) (Figure 5). This result indicated that cartilage degeneration was more serious in the two BTX-A groups compared with the control group.
Figure 5.
(A) Immunostaining results showing the ColX-positive cells in the articular cartilage regions of the three groups. Scale bar, 100 μm. (B) Semiquantitative analyses percentage of ColX-positive chondrocytes. ***p < 0.001. ColX = collagen X.
Discussion
OA is characterised by articular cartilage degeneration, subchondral bone remodelling, and secondary inflammation, but the mechanisms that drive this disease are not completely understood. Recently, researchers have increasingly focused on muscle atrophy in OA development. In clinical studies, the results revealed that the quality and strength of the lower limb muscles play an important role in the pathogenesis and progress of OA [[16], [17], [18]]. In animal studies, researchers found that muscle weakness or atrophy would result in cartilage degradation and secondary inflammation of the joint in OA development [[20], [21], [22], [23]]. But whether muscle atrophy led to subchondral bone abnormal remodelling in OA is still largely unknown. In the present study, we demonstrated that BTX-A–induced muscle weakness or atrophy resulted in joint instability and that would not only subsequently lead to cartilage degradation, but subchondral bone abnormal remodelling, thereby accelerating progression of OA in the rat model.
In this study, our results demonstrated obvious quadriceps muscle atrophy in rats after intramuscular administration of BTX-A, which can be proved by reduction of quadriceps muscle weight and pathological changes of quadriceps muscle (Figure 1). And the atrophy is a transient and reversible progress [33], as shown here, the loss of muscle mass of right quadriceps muscle in rats of the BTX-A 4-w group is more serious than rats in the BTX-A 8-w group (Figure 1), which is consistent with the other study [22,23]. Therefore, BTX-A injection is a reliable method for inducing quadriceps muscle atrophy in rats. Besides, this study also provides evidence that quadriceps atrophy influences OA progression through promoting cartilage degeneration and subchondral bone remodelling (Figure 3, Figure 4, Figure 5). More interestingly, compared with the rats in BTX-A 4-w groups, the Mankin score of the right knee was significantly lower in BTX-A 8-w groups. Both the result of the quality of cartilage (Figure 4, Figure 5) and the BV/TV and SMI in the subchondral bone (Figure 3) indicated that the BTX-A 8-w groups show less severe OA characteristics, compared with the BTX-A 4-w groups. Therefore we could infer that these recoveries may result from the reversible effect of BTX-A in muscle and these results demonstrated that quality of muscle is an important factor to keep the joint healthy. However, previous publication demonstrated significant difference in bone thickness at 4 and 8 weeks after BTX-A injection [34]. This change might be similar to the disuse atrophy that occurs secondary to muscle atrophy [25]. If the bone does not receive continuous stimulation from muscles, the bone will become atrophic and osteoporotic [35]. In this study, although the rats remained mobile until sacrificed, we could not eliminate the effect of BTX-A on the bone. And we did not know if mobility is a contributing factor to observe recovery effects yet, which could also be a limitation in this study.
Secondary inflammation is a main factor in the pathogenesis of OA [32,[36], [37], [38]]. IL-1β is a well-known inflammatory cytokine in OA development [36,37]. Several previous studies reported that muscle atrophy by BTX-A injection would lead to secondary inflammation of the joint, including in the synovial fluid, synovium, or cartilage [22,23]. However, based on the result of the average Mankin score of the two BTX-A groups was below 5 (Figure 4) in this study, the level of IL-1β in serum showed no difference between three groups (Figure 2), indicating an early stage of OA or suggesting that muscle atrophy induced by BTX-A injection might not affect the level of IL-1β in serum. Besides, we did not collect serum samples at onset of our study, and IL-1β levels were taken at the point of muscle recovery in the BTX-A 8-w group and control group; these limitations could be factors of the negative result of IL-1β.
Subchondral bone abnormal remodelling is one of the main characteristic of OA [28,39]. Subchondral bone aberrant changes have been proved to be related to subchondral microfractures or microdamage [28]. In the early stage of OA, once the subchondral bone loss appears, anabolism of the osteoblast would increase, if the synthesis exceeds loss, the bone density of the subchondral bone tends to recover, which is the pathological change of the subchondral bone in the relatively early stages of OA [40]. Therefore, in this study the BMD in the BTX-A 4-w group and BTX-A 8-w group showed no difference compared with the control group (Figure 3). Alternatively, several studies suggested that bone resorption is one of the main pathological changes of subchondral bone in relatively early stage OA and osteoporosis only in the early stage of OA [41,42]. Therefore, the results showed that BV/TV in two BTX-A groups significantly downregulated and SMI in BTX-A 4-w group significantly upregulated in the subchondral bone compared with the control group, indicating abnormal bone resorption in the subchondral bone (Figure 3). Those were consistent with the characteristic of the early stage of OA [39,42]. In this present study, we found that quadriceps muscle atrophy resulted in joint instability and would subsequently trigger the subchondral bone abnormal change, which is a risk factor of OA development.
Numerous of studies of OA have focused largely on cartilage damage [30,32,43]. Previous publications have shown that the mechanical stress is great importance in OA development [30,44]. And it is well known that normal knee muscles would keep knee joint in good stability, especially the quadriceps muscle. When quadriceps atrophy occurs, the mechanical stress of knee joint will change, which may result in unstable joint and accelerate the degeneration of articular cartilage. Previous animal studies had reported that quadriceps muscle atrophy resulted in cartilage degeneration and osteophyte formation, which would contribute to OA development [[20], [21], [22], [23]]. In this study, the results showed that no large fissure and marginal osteophyte in the articular surface of the BTX-A groups (Figure 4A), but thinner thickness and darker colour of the cartilage could be seen after BTX-A injection (Figure 4A). The characteristic of cartilage damage in BTX-A 4-w and BTX-A 8-w groups can be found in the results of Safranin-O/fast green staining, H&E staining, and Mankin score (Figure 4). In addition, immunohistochemistry analysis result showed that percentage of ColX-positive cells upregulated significantly in two BTX-A injection groups, compared with the control group (Figure 5). Taken together, these results demonstrated that muscle atrophy would lead to the articular cartilage degenerative changes, thus playing a key role in OA development.
In conclusion, the results of this study showed that quadriceps muscle atrophy triggered the subchondral bone abnormal change and cartilage degeneration, and it would be a risk factor for development of OA.
Conflict of interest
The authors have no conflicts of interest to disclose in relation to this article.
Acknowledgements
The work was partially supported by grants from the National Natural Science Foundation of China (81672224 and 81871809), China Postdoctoral Science Foundation (2017M612850), Medical Scientific Research Foundation of Guangdong Province (B2019042) and Postdoctroal Fund of the First Affliliated Hospital, Jinan University (801311).
Contributor Information
Yuanfeng Chen, Email: chenyf2018@jnu.edu.cn.
Zhengang Zha, Email: zhzgg@vip.163.com.
References
- 1.Bijlsma J.W., Berenbaum F., Lafeber F.P. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377:2115–2126. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 2.Kinds M.B., Welsing P.M., Vignon E.P., Bijlsma J.W., Viergever M.A., Marijnissen A.C. A systematic review of the association between radiographic and clinical osteoarthritis of hip and knee. Osteoarthr Cartil. 2011;19:768–778. doi: 10.1016/j.joca.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Neogi T., Felson D., Niu J., Nevitt M., Lewis C.E., Aliabadi P. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ. 2009;339 doi: 10.1136/bmj.b2844. b2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arden N., Nevitt M.C. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol. 2006;20:3–25. doi: 10.1016/j.berh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Neustadt D.H. Intra-articular injections for osteoarthritis of the knee. Clevel Clin J Med. 2006;73:897–898. doi: 10.3949/ccjm.73.10.897. 901-894, 906-811. [DOI] [PubMed] [Google Scholar]
- 6.Felson D.T. Osteoarthritis as a disease of mechanics. Osteoarthr Cartil. 2013;21:10–15. doi: 10.1016/j.joca.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roos E.M., Herzog W., Block J.A., Bennell K.L. Muscle weakness, afferent sensory dysfunction and exercise in knee osteoarthritis. Nat Rev Rheumatol. 2011;7:57–63. doi: 10.1038/nrrheum.2010.195. [DOI] [PubMed] [Google Scholar]
- 8.Ayral X., Pickering E.H., Woodworth T.G., Mackillop N., Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis – results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthr Cartil. 2005;13:361–367. doi: 10.1016/j.joca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Egloff C., Hugle T., Valderrabano V. Biomechanics and pathomechanisms of osteoarthritis. Swiss Med Wkly. 2012;142 doi: 10.4414/smw.2012.13583. w13583. [DOI] [PubMed] [Google Scholar]
- 10.Palmieri-Smith R.M., Thomas A.C., Karvonen-Gutierrez C., Sowers M.F. Isometric quadriceps strength in women with mild, moderate, and severe knee osteoarthritis. Am J Phys Med Rehabil. 2010;89:541–548. doi: 10.1097/PHM.0b013e3181ddd5c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radin E.L., Yang K.H., Riegger C., Kish V.L., O'Connor J.J. Relationship between lower limb dynamics and knee joint pain. J Orthop Res. 1991;9:398–405. doi: 10.1002/jor.1100090312. [DOI] [PubMed] [Google Scholar]
- 12.Liikavainio T., Isolehto J., Helminen H.J., Perttunen J., Lepola V., Kiviranta I. Loading and gait symmetry during level and stair walking in asymptomatic subjects with knee osteoarthritis: importance of quadriceps femoris in reducing impact force during heel strike? The Knee. 2007;14:231–238. doi: 10.1016/j.knee.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 13.O'Hara B.P., Urban J.P., Maroudas A. Influence of cyclic loading on the nutrition of articular cartilage. Ann Rheum Dis. 1990;49:536–539. doi: 10.1136/ard.49.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher N.M., White S.C., Yack H.J., Smolinski R.J., Pendergast D.R. Muscle function and gait in patients with knee osteoarthritis before and after muscle rehabilitation. Disabil Rehabil. 1997;19:47–55. doi: 10.3109/09638289709166827. [DOI] [PubMed] [Google Scholar]
- 15.Slemenda C., Heilman D.K., Brandt K.D., Katz B.P., Mazzuca S.A., Braunstein E.M. Reduced quadriceps strength relative to body weight: a risk factor for knee osteoarthritis in women? Arthritis Rheum. 1998;41:1951–1959. doi: 10.1002/1529-0131(199811)41:11<1951::AID-ART9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Hortobagyi T., Garry J., Holbert D., Devita P. Aberrations in the control of quadriceps muscle force in patients with knee osteoarthritis. Arthritis Rheum. 2004;51:562–569. doi: 10.1002/art.20545. [DOI] [PubMed] [Google Scholar]
- 17.Segal N.A., Glass N.A., Felson D.T., Hurley M., Yang M., Nevitt M. Effect of quadriceps strength and proprioception on risk for knee osteoarthritis. Med Sci Sport Exerc. 2010;42:2081–2088. doi: 10.1249/MSS.0b013e3181dd902e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan J., Stehling C., Muller-Hocker C., Schwaiger B.J., Lynch J., McCulloch C.E. Vastus lateralis/vastus medialis cross-sectional area ratio impacts presence and degree of knee joint abnormalities and cartilage T2 determined with 3T MRI – an analysis from the incidence cohort of the Osteoarthritis Initiative. Osteoarthr Cartil. 2011;19:65–73. doi: 10.1016/j.joca.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brin M.F. Botulinum toxin: chemistry, pharmacology, toxicity, and immunology. Muscle Nerve Suppl. 1997;6:S146–S168. [PubMed] [Google Scholar]
- 20.Herzog W., Longino D. The role of muscles in joint degeneration and osteoarthritis. J Biomech. 2007;40:S54–S63. doi: 10.1016/j.jbiomech.2007.03.001. Suppl 1. [DOI] [PubMed] [Google Scholar]
- 21.Rehan Youssef A., Longino D., Seerattan R., Leonard T., Herzog W. Muscle weakness causes joint degeneration in rabbits. Osteoarthr Cartil. 2009;17:1228–1235. doi: 10.1016/j.joca.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Egloff C., Sawatsky A., Leonard T., Hart D.A., Valderrabano V., Herzog W. Effect of muscle weakness and joint inflammation on the onset and progression of osteoarthritis in the rabbit knee. Osteoarthr Cartil. 2014;22:1886–1893. doi: 10.1016/j.joca.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 23.Egloff C., Hart D.A., Hewitt C., Vavken P., Valderrabano V., Herzog W. Joint instability leads to long-term alterations to knee synovium and osteoarthritis in a rabbit model. Osteoarthr Cartil. 2016;24:1054–1060. doi: 10.1016/j.joca.2016.01.341. [DOI] [PubMed] [Google Scholar]
- 24.Gao Z.Y., Wu J.X., Liu W.B., Sun J.K. Reduction of adhesion formation after knee surgery in a rat model by botulinum toxin A. Biosci Rep. 2017;37 doi: 10.1042/BSR20160460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park Y.W., Kim S.G., Jo Y.Y. S100 and p65 expression are increased in the masseter muscle after botulinum toxin-A injection. Maxillofac Plast Reconstr Surg. 2016;38:33. doi: 10.1186/s40902-016-0079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gui T., Lin Y.K., Huan S.W., Li Y.H., Wang B.H., Yang J. Elevated expression of ICAM-1 in synovium is associated with early inflammatory response for cartilage degeneration in type 2 diabetes mellitus. J Cell Biochem. 2019;120:13177–13186. doi: 10.1002/jcb.28592. [DOI] [PubMed] [Google Scholar]
- 27.Hildebrand T., Ruegsegger P. Quantification of bone microarchitecture with the structure model index. Comput Methods Biomech Biomed Eng. 1997;1:15–23. doi: 10.1080/01495739708936692. [DOI] [PubMed] [Google Scholar]
- 28.Li G., Yin J., Gao J., Cheng T.S., Pavlos N.J., Zhang C. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther. 2013;15:223. doi: 10.1186/ar4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mankin H.J., Dorfman H., Lippiello L., Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53:523–537. [PubMed] [Google Scholar]
- 30.Chen Y., Sun Y., Pan X., Ho K., Li G. Joint distraction attenuates osteoarthritis by reducing secondary inflammation, cartilage degeneration and subchondral bone aberrant change. Osteoarthr Cartil. 2015;23:1728–1735. doi: 10.1016/j.joca.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y., Lin S., Sun Y., Guo J., Lu Y., Suen C.W. Attenuation of subchondral bone abnormal changes in osteoarthritis by inhibition of SDF-1 signalling. Osteoarthr Cartil. 2017;25:986–994. doi: 10.1016/j.joca.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y., Lin S., Sun Y., Pan X., Xiao L., Zou L. Translational potential of ginsenoside Rb1 in managing progression of osteoarthritis. J Orthop Translat. 2016;6:27–33. doi: 10.1016/j.jot.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Croes S.A., Baryshnikova L.M., Kaluskar S.S., von Bartheld C.S. Acute and long-term effects of botulinum neurotoxin on the function and structure of developing extraocular muscles. Neurobiol Dis. 2007;25:649–664. doi: 10.1016/j.nbd.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vegger J.B., Bruel A., Thomsen J.S. Vertical trabeculae are thinned more than horizontal trabeculae in skeletal-unloaded rats. Calcif Tissue Int. 2015;97:516–526. doi: 10.1007/s00223-015-0035-0. [DOI] [PubMed] [Google Scholar]
- 35.Kun-Darbois J.D., Libouban H., Chappard D. Botulinum toxin in masticatory muscles of the adult rat induces bone loss at the condyle and alveolar regions of the mandible associated with a bone proliferation at a muscle enthesis. Bone. 2015;77:75–82. doi: 10.1016/j.bone.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 36.Blom A.B., van der Kraan P.M., van den Berg W.B. Cytokine targeting in osteoarthritis. Curr Drug Targets. 2007;8:283–292. doi: 10.2174/138945007779940179. [DOI] [PubMed] [Google Scholar]
- 37.Jones A.R., Flannery C.R. Bioregulation of lubricin expression by growth factors and cytokines. Eur Cells Mater. 2007;13:40–45. doi: 10.22203/ecm.v013a04. discussion 45. [DOI] [PubMed] [Google Scholar]
- 38.Elsaid K.A., Jay G.D., Chichester C.O. Reduced expression and proteolytic susceptibility of lubricin/superficial zone protein may explain early elevation in the coefficient of friction in the joints of rats with antigen-induced arthritis. Arthritis Rheum. 2007;56:108–116. doi: 10.1002/art.22321. [DOI] [PubMed] [Google Scholar]
- 39.Zhen G., Wen C., Jia X., Li Y., Crane J.L., Mears S.C. Inhibition of TGF-beta signalling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19:704–712. doi: 10.1038/nm.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Botter S.M., van Osch G.J., Waarsing J.H., Day J.S., Verhaar J.A., Pols H.A. Quantification of subchondral bone changes in a murine osteoarthritis model using micro-CT. Biorheology. 2006;43:379–388. [PubMed] [Google Scholar]
- 41.Boyd S.K., Matyas J.R., Wohl G.R., Kantzas A., Zernicke R.F. Early regional adaptation of periarticular bone mineral density after anterior cruciate ligament injury. J Appl Physiol (1985) 2000;89:2359–2364. doi: 10.1152/jappl.2000.89.6.2359. [DOI] [PubMed] [Google Scholar]
- 42.Dedrick D.K., Goldstein S.A., Brandt K.D., O'Connor B.L., Goulet R.W., Albrecht M. A longitudinal study of subchondral plate and trabecular bone in cruciate-deficient dogs with osteoarthritis followed up for 54 months. Arthritis Rheum. 1993;36:1460–1467. doi: 10.1002/art.1780361019. [DOI] [PubMed] [Google Scholar]
- 43.Anderson D.D., Chubinskaya S., Guilak F., Martin J.A., Oegema T.R., Olson S.A. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res. 2011;29:802–809. doi: 10.1002/jor.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding L., Heying E., Nicholson N., Stroud N.J., Homandberg G.A., Buckwalter J.A. Mechanical impact induces cartilage degradation via mitogen activated protein kinases. Osteoarthr Cartil. 2010;18:1509–1517. doi: 10.1016/j.joca.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]