Abstract
Reconstruction of long-bone segmental defects (LBSDs) has been one of the biggest challenges in orthopaedics. Biomaterials for the reconstruction are required to be strong, osteoinductive, osteoconductive, and allowing for fast angiogenesis, without causing any immune rejection or disease transmission. There are four main types of biomaterials including autograft, allograft, artificial material, and tissue-engineered bone. Remarkable progress has been made in LBSD reconstruction biomaterials in the last ten years.
The translational potential of this article
Our aim is to summarize recent developments in the divided four biomaterials utilized in the LBSD reconstruction to provide the clinicians with new information and comprehension from the biomaterial point of view.
Keywords: Allograft, Artificial material, Autograft, Biomaterial, Long-bone segmental defect reconstruction, Tissue engineering
Abbreviations: LBSD, long-bone segmental defect; BMP-2 & 4, bone morphogenetic protein-2 & 4; VEGF, Vascular Endothelial Growth Factor; bFGF, basic Fibroblast Growth Factor; TGF-β, Transforming Growth Factor-β; PDGF, Platelet-Derived Growth Factor; FGF-2, Fibroblast Growth Factor-2; sRANKL, soluble RANKL; M-CSF, macrophage colony-stimulating factor; PET/CT, positron emission- and computed tomography; ALP, alkaline phosphatase; htMSCs, human tubal mesenchymal stem cells; rVEGF-A, recombinant vascular endothelial growth factor-A; rhBMP-2, recombinant human bone morphogenetic protein-2; PCL, polycaprolactone; TCP, tricalcium phosphate; rhBMP-7, recombinant human bone morphogenetic protein 7; PDLLA, poly(DL-lactide); β-TCP, β-tricalcium phosphate; PLA, poly(lactic acid); DBM, decalcified bone matrix; PPF, propylene fumarate; poly, (L-lactide-co-D,L-lactide); MSC, autologous mesenchymal stem cells; TEB, combining ceramic block with osteogenic-induced mesenchymal stem cells and platelet-rich plasma; MIC, fresh marrow-impregnated ceramic block; ALLO, partially demineralized allogeneic bone block; BMSC, bone marrow–derived mesenchymal stem cell; rADSC, rabbit adipose-derived mesenchymal stem cell; ADSC, allogenic adipose-derived stem cells; HDB, heterogeneous deproteinized bone; ASC, adipose-derived stem cell; SF, silk fibroin; CS, chitosan; nHA, nano-hydroxyapatite; BV, baculovirus
Background
Long-bone segmental defects (LBSDs) are defined as the bone loss in length longer than one and half of the long-bone diameter, or longer than one-fifth to one-fourth of the long-bone length. LBSDs have always been a great challenge in orthopaedics. Congenital bone disease, arthritis, osteomyelitis, bone nonunion, bone infection, bone exposure, trauma, and bone tumour excision can all be accompanied with an LBSD. An LBSD exists in any type of long-bone at any location, facing differing therapy complexity. In addition to the bone tissue, muscle, vessel, nerve, and even skin damage and loss can also be involved in an LBSD. That said, multiple surgical interventions are necessary. Because a long-bone has its critical role in movements and supporting, a segmental defect can totally lose and impair its function. Given that, a sped up recovery is always desirable. All these makes the LBSD reconstruction a very challenging surgical procedure with demanding postoperative care [[1], [2], [3], [4]]. Current clinical interventions and treatment options include autologous or allogenic bone grafting, distraction osteogenesis, bioactive pseudomembranes, and intramedullary nailing [2,5].
Requirements for biomaterials applied in LBSD reconstruction
In general, there are natural (bone, cartilage, corals, etc.) and synthetic (e.g., metallic, polymeric, ceramic, composite) biomaterials for clinical use. Ideally biomaterials for the LBSD reconstruction should be strong enough, biocompatible, and sometimes biodegradable. Immune rejection or disease transmission could be fatal. The mechanical strength and stiffness of the material and the adhesion area to the host bone need to be adequate to provide structural support and transmit the regeneration enhancing force. Blood supply needs to be guaranteed to support new bone growth and to shorten and optimize the rehabilitation process. Moreover, osteoinduction and osteoconduction are significant factors for recovery. The grafted/implanted materials should provide adequate porosity and interconnectivity for new bone to grow in, while its inner structure integrity should be kept during the bone growing and bone remodelling process.
Among all the aforementioned ones, osteoinduction is one of the most important requirements for an LBSD reconstruction biomaterial. Osteoinduction refers to the process that induces, stimulates, and regulates the production of calcified bone matrix, arousing osteogenesis. Osteoinduction involves recruitment of immature cells and stimulation of mesenchymal stem cells into the osteogenic lineage [6]. A material can be osteoinductive if possessing the following properties: (1) The material can mineralize in vivo, (2) the material is porous, (3) blood vessels can grow into the pores and cells can be transported into the core of the material through the pores, and (4) blood supply can limit the physiological Ca and/or P ion concentrations [7].
In addition, fast angiogenesis is critical for the LBSD reconstruction efficiency. It is noteworthy that LBSDs are frequently accompanied with vasculature disruption, leading to acute necrosis and hypoxia, forming a haematoma. Osteoprogenitor and mesenchymal cells are recruited at the defect by the activated factors, e.g., growth factors and cytokines. Mesenchymal cells migrate to the defect region, and capillaries grow into the region from bone marrow and endosteum. Then, the granulation tissue is replaced by fibrocartilage, and an external callus is formed by the periosteum, which is mineralized from the inside to woven bone. After that, bone remodelling and angiogenesis starts, with the necrotic bone removed and the fracture callus replaced by lamellar bone [[8], [9], [10]]. The foregoing process is believed to be associated with quite a few activating factors including bone morphogenetic protein-2 and 4 (BMP-2 & BMP-4), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF), and fibroblast growth factor-2 (FGF-2). Among these, the VEGF is expressed in angioblasts, chondroblasts, chondrocytes, osteoprogenitor cells, and osteoblasts. VEGF can act with soluble receptor activator for nuclear factor-κ B ligand (sRANKL) to promote osteoclastogenesis and act as a substitute for a macrophage colony-stimulating factor (M-CSF) in osteoclastogenesis [[11], [12], [13]]. Fibroblast growth factor-2 is able to stimulate angiogenesis and the proliferation and differentiation of osteoblasts [14,15].
Testing methods for the LBSD reconstruction biomaterials
In vivo tests and clinical reporting (and various reviews) are frequently carried out in the research of LBSD reconstruction biomaterials. In animal tests, LBSDs are created in rabbits, dogs, sheep, goats, and pigs. Each animal type shows both advantages and disadvantages, while sheep are most frequently used large animal models to study LBSD reconstruction. This is mainly because sheep have similar body weight and geometric proportions and load-bearing patterns in the tibias to humans; thus, human implants are suitable to be used in sheep [16]. After the LBSD reconstruction, bone union, bone/marrow formation, and intervening layer are observed histologically and radiographically by micro-computed tomography, X-ray, etc. Dual-energy X-ray absorptiometry (DEXA) can be performed for the bone mineral density and content measurement [17]. After the animal was sacrificed, mechanical properties of the healed defects are usually tested and assessed in the torsional load-to-failure tests, providing data of torsional strength, torsional stiffness, angular deformation to failure, and energy absorption to failure.
In clinical reports and reviews, comparable patient cases are collected and analyzed. Limb alignment and stability and bone healing can be observed radiographically and noninvasively. Given that, 18F-labelled sodium fluoride–based coregistration of positron emission tomography–computed tomography (PET/CT) can quantify the radiodensity and osteoblast activity. Moreover, full weight-bearing attainment, functional limb recovery, and patient satisfaction during the follow-up period are recorded.
Biomaterial types and recent developments
In the current narrative overview, we are concentrating on recent developments published from 2010 to 2018 in LBSD reconstruction biomaterials retrieved from the literature in PubMed and PubMed Central (summarized in Table 1).
Table 1.
Developments published from 2010 to 2019 in LBSD reconstruction biomaterials.
| Biomaterial typess | Developments published from 2010 to 2019 |
|---|---|
| Autograft |
|
| Allograft |
|
| Artificial materials |
|
| Tissue-engineered bone |
|
Autograft
An autograft is a natural biomaterial that has always been the gold standard for the LBSD reconstruction. Autogenous bones exhibit superior osteoinductivity, osteoconductivity, and osteogenesis compared with other types of materials. Fast healing and avoidance of immune rejection are also the advantages of autografts. Vascularized autografts have showed good results in reconstructing an LBSD, frequently with a higher fusion rate than the nonvascularized autografts [[18], [19], [20]]. Normally, autologous cortical bone grafts are adopted for segmental defects of 5–6 cm in length, while vascularized cortical autografts are applied for defects longer than 6 cm [21] or in case with severe loss of vascularized soft tissue [[22], [23], [24]].
However, disadvantages do exist including limited availability of graft material, risk of comorbidity, insufficient integration into the damaged bone, prolonged anaesthetic periods, donor site morbidity, and predisposition to failure. Common sources for autografts are ilia, fibulae, and ribs, which are always low in strength and limit the application of autograft mostly to the upper limb with relatively low load-bearing needs [25].
Recent reports on autografts in the LBSD reconstruction show the trend to incorporate bioactive factors into autogenous bones to promote osseointegration. For example, the combination of platelet-rich plasma with autologous cancellous bone graft could improve bone healing compared with the sole graft of autologous bone [26]. One report described a technique using a biomembrane forming around the previously placed antibiotic spacer and the biomembrane could enclose the later placed cancellous autograft in the LBSD reconstruction. Such a biomembrane could help to prevent resorption of the autograft and secrete growth factors. This biomembrane can also be applied in an LBSD reconstruction with other material types including allografts and artificial materials [27,28].
Based on the theory that mesenchymal stromal stem cells can produce fibroblastic cell lines with ossifying properties, the technique of percutaneous autologous bone marrow injection was newly applied to treat delayed and nonunion of long bones. This percutaneous injection technique is much less invasive and with much less morbidity than the traditional corticocancellous bone autograft. The 8-year clinical follow-up of 45 cases (26 tibiae, 16 femurs, and 3 humeri) of long-bone nonunion showed 69% healed tibiae and 63% healed femurs [29]. In another report, autologous bone marrow was injected in combination with an allograft decalcified bone matrix (DBM) in long-bone nonunions, and the union was attained after an average period of 8.1 months with a range of 2 months to 3 years [30]. In a similar report, 92% of patients showed union after a mean healing time of 15 ± 2.73 weeks (range: 12–22 weeks) [31]. This less-invasive percutaneous injection of osteogenic factors or medicine might be a developing direction for LBSD of a relatively small size.
Allograft
An allograft overcomes the limit of grafting resource accompanied with an autograft but brings concerns about rejection, disease transmission, delayed union, or nonunion. A nonunion situation is mostly due to the sterilization process before the allograft [32]. Common pretreatments on xenogenic bones before usage include freezing, freeze-drying, chemical sterilization, irradiation, and decalcification. It is worth to notice that none of the aforementioned treatments can fully prevent immune rejection, while the antiinfection at the reconstruction site can be weak and antibiotics need to be applied. Some research studies have tested the effects of supercritical CO to degrease and sterilize bone allografts as a single-step procedure. The biocompatibility test results fulfilled the ISO 10993 criteria, offering a promising pretreating method for allografting [33]. Treatments such as deproteinizing the allograft bones could help reduce immune rejection. A deproteinized bone might be utilized to avoid immune rejection, while maintaining the natural bone structure and mechanics. In a study by Jian et al. [34], although the deproteinized bone was only composed of collagen Type I and hydroxyapatite, it maintains the natural reticular porosity. It also exhibited good mechanical properties, cell adhesion rate, and histocompatibility.
Combining an autograft with an allograft in an LBSD reconstruction is believed to improve early-stage stability and weight-bearing capacity. It can also enhance union, especially in segmental defects of weight-bearing bones. This is probably due to the superior osteointegration of autogenous graft. In one study, six patient cases (18–49 years old, in average 33 years old) of posttraumatic long segment bone loss (10–20 cm in length, 15 cm in average) in the distal femur were reconstructed combining an allograft and a free vascularized fibular graft and followed up for 7–24 months. All the six cases exhibited union, and the average union time was 6 months, suggesting that this could be a promising single-stage technique for load-bearing LBSDs [35]. Moreover, an application of composite microsurgical free fibula inside a massive bony allograft was clinically tried in children after long-bone tumour resection, where the vascularized fibula could contribute to osteosynthesis [36]. In a study, a massive allograft was adopted together with a free fibula osteocutaneous flap to mechanically enhance the implantation and achieve biological incorporation. The 9 lower-limb reconstruction ambulated with partial weight-bearing at an average of 4.2 months and ambulated with full weight-bearing at an average of 8.2 months [37].
Bioactivating the allograft bone with some bioactive factors/cells and thereby enhancing union is another research direction in an LBSD allografts. In a study by Wang et al. [38], coating xenogenic bone with magnesium plasma could upregulate the alkaline phosphatase (ALP) gene activity and viability of human tubal mesenchymal stem cells (htMSCs), while it maintained the original mechanical properties of an allograft bone. This magnesium ion–activated xenogenic bone showed potential for faster union with an LBSD allograft [38]. Other bioactivating factors/cells include autologous concentrated bone marrow–derived cells [39], mesenchymal stem cells, osteogenic protein-1 [40], recombinant vascular endothelial growth factor-A (rVEGF-A) [41], and recombinant human bone morphogenetic protein-2 (rhBMP-2) [42], all showing positive healing results.
To sum up, recent developments in the LBSD allograft concentrate on overcoming some existing problems, especially delayed union or nonunion. In particular, combining the allograft with other more biocompatible materials such as an autograft or an osteocutaneous flap can promote biological incorporation. On the other hand, attempts such as incorporating bioactivating factors/cells to the xenogenic bones could enhance osteointegration to improve the therapeutic efficiency without destroying the original microstructure or compromising the mechanical properties of allografts.
Artificial materials
Recently, various artificial materials have become a hot research spot in LBSD treatments. Artificial materials can be designed, fine-tuned, and fabricated targeting at the intended clinical use, combining desired properties from each component. An artificial biomaterial could consist of bone (autogenous or allogenous), metal, ceramic (tricalcium phosphate, hydroxyapatite), polymer, hydrogel (alginate, chitosan), collagen, and silk fibroin. Problems that may accompany artificial materials in this LBSD reconstruction include insufficient bioactivity (such as insufficient osteoinductivity, osteoconductivity, and osteogenesis), incomplete or even no degradation, too fast degradation before new bone consolidation, immune rejection, and possible adverse effects to cells. Given these, recent studies have concentrated on inducing bioactivity to the artificial material, in addition to maintaining its satisfying mechanical strength and durability. The components capable of inducing bioactivity include autograft, allograft, DBM, etc.
A clinical research study used a cylindrical titanium mesh cage, combined with cancellous bone allograft and DBM putty, to restore the LBSD. The cage held the cancellous bone and the bone matrix in place during therapy and provided mechanical supporting to a certain degree. The one-year follow-up showed satisfying limb alignment, stability, and bone healing. Immediate full weight-bearing was initiated, and early limb functional recovery was achieved [43].
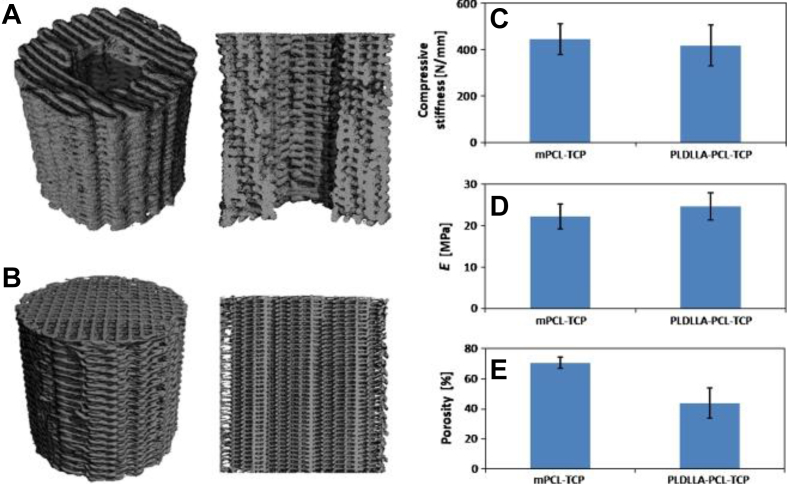
More recently, poly(dl-lactide)–tricalcium phosphate (TCP)–polycaprolactone (PCL) and mPCL-TCP composite biomaterials were reported. These composites mimicked the inner microstructure of cancellous bones and allowed for coagulating blood retention and new bone ingrowth (Fig. 1). They can withstand the physiological and mechanical stresses for up to 3 months. The compressive stiffness for poly(dl-lactide)–TCP–PCL and mPCL–TCP averaged 446 ± 66 N/mm and 418 ± 88 N/mm, respectively. It appears that these novel artificial materials have the potential to make up promising LBSD reconstructive biomaterials if combined with suitable osteogenic factors [44].
Figure 1.
Micro-CT 3D reconstructions of (A) a PDLLA–TCP–PCL and (B) mPCL–TCP composite (height 20 mm, diameter 18 mm). (C) Compressive stiffness values averaged 446 N/mm (SD = 66.3) for mPCL–TCP and 418 N/mm for PDLLA–TCP–PCL (SD = 88.1) scaffolds; (D) the elastic modulus 22.17 MPa (SD = 3.0) and 24.70 MPa (SD = 3.3). (E) Porosity was determined to be 70.55% for mPCL-TCP (SD = 3.78) composites and 43.76% for PDLLA–TCP-PCL (SD = 10.02) composites as determined by micro-CT analysis. Error bars represent standard deviations, n = 6 (Reichert et al., 2011) (No color used in print. Two-column fitting image.). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.) PCL = polycaprolactone; PDLLA = poly(dl-lactide); TCP = tricalcium phosphate;
There have been a large number of studies on artificial materials for the LBSD reconstruction since 2010. Such biomaterials include oxidized-irradiated alginate hydrogel [45], β-tricalcium phosphate (β-TCP) combined with cancellous autograft [46], DBM plus autogenous bone [47], hydroxyapatite–DBM (allograft) composite [48], β-TCP plus collagen composite [49], chitosan hydrogel [50], porous poly(lactic acid) (PLA)/DBM composite [51,52], and bone-like hydroxyapatite/polyamino acid composite [53]. All the aforementioned artificial materials were assessed for their capability in LBSD reconstruction, and the results were promising.
Attention should be paid to the fact that the material may interfere with cellular processes, e.g., affecting bone formation. For instance, a study described the artificial biomaterial of a porous propylene fumarate (PPF) sleeve surrounding a solid porous propylene fumarate intramedullary rod for mechanical support. The results indicated that this structure, especially the nonporous intramedullary rod, may decrease bone formation, possibly because of its hindering effects [54].
Tissue-engineered bone
Tissue engineering in the LBSD reconstruction requires a cell source to amplify and build the new bone tissue and a scaffold to hold and protect the cells. In addition, the reconstruction should allow nutrients and metabolic wastes to diffuse through and degrade in the end [55]. Osteoblasts, exogenous stem/progenitor cells, and mesenchymal stem cells, especially bone marrow–derived mesenchymal stem cells and adipose-derived mesenchymal stem cells, are most frequently seeded in biocompatible and biodegradable scaffolds to build the bone tissue engineering reconstruction. Bone marrow, periosteum, fat, muscle, cord blood, and embryonic or induced pluripotent stem cells can be a cell source for bone tissue engineering [[55], [56], [57], [58], [59]]. In addition, growth factors, such as hepatocyte growth factor and rhBMP-2, can be incorporated to stimulate cell differentiation and amplification. Hydroxyapatite, TCP, polyethylene, collagen, chondroitin sulphate, calcium silicate, medical-grade polycaprolactone, silk, hydroxyapatite, chitosan, poly(l-lactide-co-D,l-lactide), polyglycolide, autologous bone graft, alumina, baghdadite (Ca3ZrSi2O9), bioactive glass nanoparticles, and their combinations have been intensively studied as biocompatible scaffold materials [[60], [61], [62], [63], [64]]. Tissue engineering was demonstrated to be possible for the first time in 1930s, and since ca. 2010, growing amounts of research have been conducted on tissue engineering in LBSD reconstruction. A novel selective cell retention technology has been introduced in 2010: It allows for materials enriched with osteoprogenitors exerted from autologous bone marrow to substitute the traditional autografts [65]. This could be an attractive progress in the tissue engineering technique.
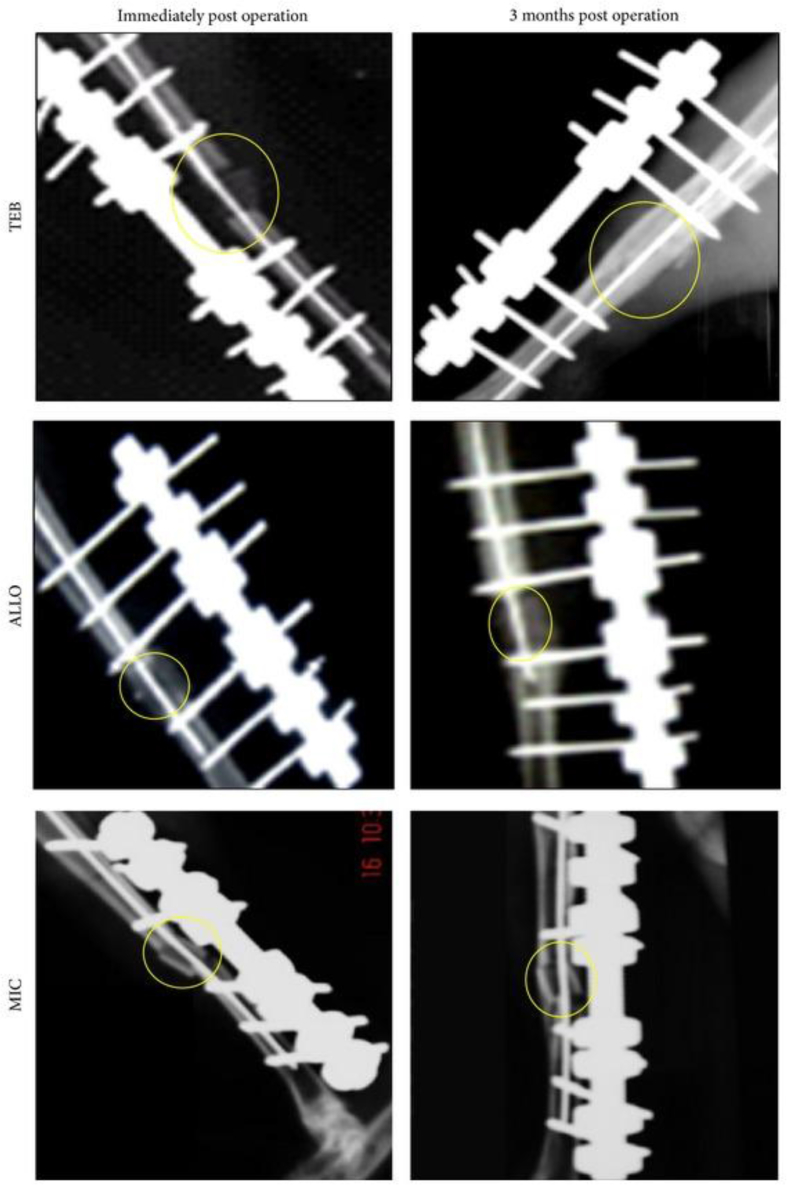
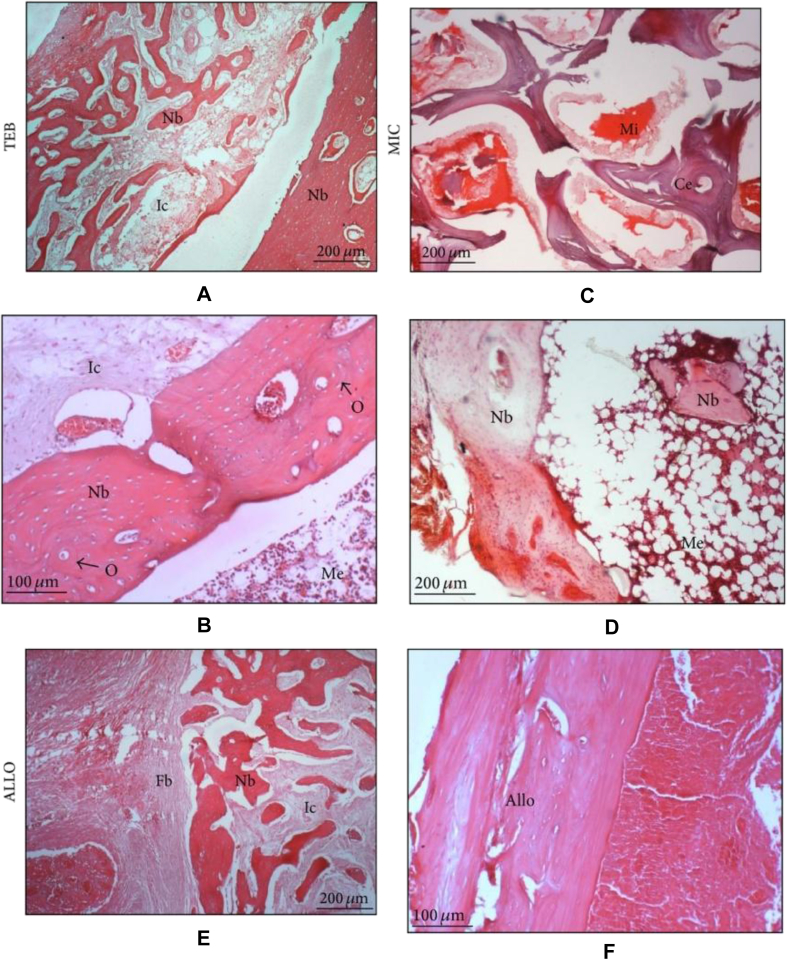
Ng et al [66] applied autologous mesenchymal stem cells (MSCs) and plasma-derived fibrin-impregnated ceramic block (combining ceramic block with osteogenic-induced mesenchymal stem cells and platelet-rich plasma, abbr. “TEB”) to restore segmental load-bearing bone defects. In the implanted composite, differentiated MSCs can express osteogenic genes and mineralize within the scaffold, while the plasma is a rich source of growth factors and the plasma-derived fibrin could promote osteogenic differentiation of MSCs in vitro and enhance osteogenesis in vivo. This in vivo study was conducted in rabbit tibias. Compared with the rabbits without implantation or implanted with fresh marrow–impregnated ceramic block and partially demineralized allogeneic bone block, the group implanted with TEB resumed normal gait pattern faster, achieved the union faster, scored higher new bone percentage, and showed higher compressive strength (Figure 2, Figure 3) [66].
Figure 2.
Radiological changes seen in the three test groups immediately: Day 21, Day 60, and Day 90 after operation (TEB: combining ceramic block with osteogenesis-induced mesenchymal stem cells and platelet-rich plasma; MIC: fresh marrow-impregnated ceramic block; ALLO: partially demineralized allogeneic bone block) TEB: defect bridged by uniform new bone, cut ends of cortex no longer distinguishable, graft no longer distinguishable. MIC: a slight increase in radiodensity surrounding and distinguishable from the graft (callus formation) with no bridging of cortex. ALLO: a slight increase in radiodensity surrounding and distinguishable from the graft bridging of one cortex with new bone formation (Ng et al., 2014, Open Access) (No color used in print. Two-column fitting image.). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Figure 3.
Histological sections from the middle segment of the implants three months after implantation (H&E) (TEB: combining ceramic block with osteogenic-induced mesenchymal stem cells and platelet-rich plasma; MIC: fresh marrow-impregnated ceramic block; ALLO: partially demineralized allogeneic bone block) (A) Abundant new bone was found in TEB. The section reveals new bones (Nb) forming a trabecular network amidst infiltrated cells (Ic) while new compact bone (Nb) was found at the right periphery (40x). (B) Here, the peripheral bone appeared more mature with lamellar and osteon features (O) adjacent to the well-formed intramedullary canal filled with marrow element (Me) (100x). (C) Residual ceramic (Ce) was noted in MIC. Mineral deposits (Mi) (stained red) were seen around the ceramic (40x). (D) The section reveals new bones (Nb) that are undergoing mineralization amidst infiltrated marrow element (Me) (40x). (E) Significant fibrous tissues (Fb) were noted in ALLO. The section reveals new bones (Nb) forming a trabecular network amidst infiltrated cells (Ic) (40x). (F) An intact allograft bone (Allo) (100x) (Ng et al., 2014, Open Access) (Color used in print. Single-column fitting image.). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In a study by Wang et al. [67], DBM scaffolds were seeded with expanded rabbit foetal bone marrow–derived mesenchymal stem cells (BMSCs) and cultivated in osteogenic media in vitro. The BMSC/DBM constructs were implanted in the prepared radial defects in rabbits and were compared with the groups implanted with DBM scaffolds alone. Significantly more new bone tissue was observed in the BMSC/DBM group.
In another study [68], β-TCP composite with rabbit adipose-derived mesenchymal stem cells (rADSCs) was constructed through tissue engineering. The cultivated composite biomaterial was then inserted in the 2-cm bone defect of radius in the middle and lower level in rabbits. Two control groups were treated with no implantation or implanted with solely β-TCP. After implantation, the rADSCs showed the potential to differentiate into osteoblast without provoking an immune rejection. The X-ray results on the 8th week showed superior healing situation in the rADSCs/β-TCP group compared with the control groups, with the bone connectivity and bone marrow cavity completely recovered. Six weeks after surgery, the rADSCs/β-TCP implant began to degrade and new bone growth could be observed in pores left after degradation. The degradation was significant 8 weeks after surgery, accompanied with recanalization of the bone marrow. In a similar study [69], allogenic adipose-derived stem cells (ADSCs) were combined with heterogeneous deproteinized bone (HDB) to repair segmental radial defects and obtained more desirable results compared with the heterogeneous deproteinized bone restored defects. In a clinical trial, the human autologous ADSCs were supplemented with the DBM and the autologous adipose-derived stem cell could fully differentiate into a 3D osteogenic-like implant [70].
One study compared the healing effects with a polycaprolactone–TCP composite material seeded with autologous BMSCs or rhBMP-7 and the gold standard autograft, all inserted in critical-sized tibial defects in sheep. Twelve months after surgery, the scaffold with rhBMP-7 showed superior healing results compared with the autograft gold standard. The MSC-seeded biomaterial construct, with significantly more bone formation, higher strength, and more even axial bone distribution at the interface. The results suggested that rhBMP-7 is more bioactive than the MSCs in osteogenesis and bone remodelling in in vivo circumstances [71].
Moreover, a 3D scaffold of silk fibroin/chitosan/nano-hydroxyapatite (SF/CS/nHA) seeded with BMSCs [72], autologous MSCs plus rhBMP-2 incorporated in deproteinated bone [73], and adipose-derived stem cells seeded in hybrid baculovirus (BV) have been introduced to restore LBSDs. The biomechanical properties were shown to be comparable with living bones and autologous bones [74].
Compared and contrasted to the foregoing three biomaterials involving autograft, allograft, and artificial material, tissue-engineered bone is a relatively new biomaterial applied in the LBSD reconstruction. Most research studies of tissue engineering for LBSD reconstructions are still experiments. However, much progress has been achieved and bone tissue engineering has consolidated its role as a promising technique and a research hot spot in the LBSD restoration, giving much hope to patients.
Conclusions
This narrative overview has provided an insight into the most developments in biomaterials applied in the LBSD reconstruction since 2010. The previously described four biomaterial types, namely autograft, allograft, artificial material, and tissue-engineered bone, are overlapping. For example, the scaffold for tissue engineering is frequently an artificial biomaterial seeded with MSCs, and an artificial biomaterial can consist of autograft or allograft. Developments in one type of the biomaterials can simultaneously bring novel techniques to other biomaterials. Currently, autografts are still the gold standard for the LBSD reconstruction, because of their satisfactory healing effects. The usage of allografts has become more and more reliable. That said, an allograft is also an invaluable assisting material combined with other materials for bone defect reconstructions. Future research in the LBSD reconstruction biomaterials may rely mainly on artificial biomaterials and bone tissue engineering, which possess flexibility in the material design and fabrication and may satisfy the requirements for the LBSD reconstruction to the maximum degree. In these said two fields, more work should definitely be carried out and more progress can be expected. Moreover, attaining a “perfect” LBSD reconstruction may not rely on just a single biomaterial type, but the union of two or more of them can be a wise choice.
Conflicts of interest statement
The author(s) have no conflicts of interest relevant to this article.
Acknowledgements
This work was supported by Shenzhen Science and Technology Innovation Commission (grant numbers JCYJ20170307173012031, JCYJ20170413162104773); National Natural Science Foundation of China (grant numbers 51802340, 81672227, 31870956); Science and Technology Planning Project of Guangdong Province (grant number 2017A030310318); and Chinese Academy of Sciences (grant number QYZDB-SSW-JSC030).
References
- 1.Griensven M.V., Biberthaler P., Balmayor E.R. Clinical approaches to the healing of long bone defects. In: Jan-Thorsten S., Dietmar W.H., editors. Advanced therapies in regenerative medicine. World Scientific Publishing; Singapore: 2015. pp. 217–232. [Google Scholar]
- 2.Lasanianos N.G. Current management of long bone large segmental defects. Orthop Traumatol. 2010;24:149–163. [Google Scholar]
- 3.Kadhim M., Holmes L., Gesheff M.G., Conway J.D. Treatment options for nonunion with segmental bone defects: systematic review and quantitative evidence synthesis. J Orthop Trauma. 2017;31:111. doi: 10.1097/BOT.0000000000000700. [DOI] [PubMed] [Google Scholar]
- 4.Roberts T.T., Rosenbaum A.J. Bone grafts, bone substitutes and orthobiologics. Organogenesis. 2012;8:114–124. doi: 10.4161/org.23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishida J., Shimamura T. Methods of reconstruction for bone defect after tumor excision: a review of alternatives. Med Sci Monitor Inter Med J Exper Clinic Res. 2008;14:RA107. [PubMed] [Google Scholar]
- 6.Capanna R., De Biase P. Osteoinduction: basic principles and developments. In: Kwok-Sui Leung K.S., Taglang G., Schnettler R., Alt V., Haarman H.J.T.M., Hartmut Seidel, editors. Practice of intramedullary locked nails. Springer; Berlin: 2006. pp. 23–42. [Google Scholar]
- 7.Marc Bohner R.J.M. A proposed mechanism for material-induced heterotopic ossification. Mater Today. 2019 doi: 10.1016/j.mattod.2018.10.036. [DOI] [Google Scholar]
- 8.Kanczler J.M., Oreffo R.O. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cells Mater. 2008;15:100–114. doi: 10.22203/ecm.v015a08. [DOI] [PubMed] [Google Scholar]
- 9.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 10.Plouët J. Angiogenesis. Annu Rev Med. 1999;47:300. [Google Scholar]
- 11.Pufe T., Petersen W., Tillmann B., Mentlein R. The splice variants VEGF121 and VEGF189 of the angiogenic peptide vascular endothelial growth factor are expressed in osteoarthritic cartilage. Arthritis Rheum. 2001;44:1082–1088. doi: 10.1002/1529-0131(200105)44:5<1082::AID-ANR188>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 12.Tatsuyama K., Maezawa Y., Baba H., Imamura Y., Fukuda M. Expression of various growth factors for cell proliferation and cytodifferentiation during fracture repair of bone. Eur J Histochem. 2009;44:269. [PubMed] [Google Scholar]
- 13.Uchida S., Maehara T., Hirai N., Kawai K., Shimizu H. Theta oscillation in the anterior cingulate and beta-1 oscillation in the medial temporal cortices: a human case report. J Clin Neurosci. 2003;10:371–374. doi: 10.1016/s0967-5868(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 14.Osdoby P., Anderson F., Maloney W., Collin-Osdoby P. Isolation and cultivation of osteoclasts and osteoclast-like cells. In: Koller M.R., Palsson B.O., Masters J.R.W., editors. Human cell culture. Springer; Dordrecht: 2002. pp. 147–169. [Google Scholar]
- 15.Walsh T.F., Toupence R.B., Young J.R., Song X.H., Ujjainwalla F., Devita R.J. Potent antagonists of gonadotropin releasing hormone receptors derived from quinolone-6-carboxamides. ChemInform. 2000;10:443–447. doi: 10.1016/s0960-894x(00)00024-x. [DOI] [PubMed] [Google Scholar]
- 16.Jewell E., Rytlewski J., Anglen J.O., Mckinley T.O., Shively K.D., Chu T.M.G. Surgical fixation hardware for regeneration of long bone segmental defects: translating large animal model and human experiences. Clin Rev Bone Miner Metabol. 2015;13:222–231. [Google Scholar]
- 17.Boer F.C.D., Patka P., Bakker F.C., Wippermann B.W., Lingen A.V., Vink G.Q.M. New segmental long bone defect model in sheep: quantitative analysis of healing with dual energy X-ray absorptiometry. J Orthop Res. 2010;17:654–660. doi: 10.1002/jor.1100170506. [DOI] [PubMed] [Google Scholar]
- 18.Estrella E.P., Wang E.H. A comparison of vascularized free fibular flaps and nonvascularized fibular grafts for reconstruction of long bone defects after tumor resection. J Reconstr Microsurg. 2017;33:194–205. doi: 10.1055/s-0036-1594299. [DOI] [PubMed] [Google Scholar]
- 19.Shimizu T., Yajima H., Y, Shigematsu K., Kawamura K., Takakura Y. Vascularized proximal fibular autograft for treatment of post-traumatic segmental bony defects in the distal radius. J Reconstr Microsurg. 2008;24:565–568. doi: 10.1055/s-0028-1090622. [DOI] [PubMed] [Google Scholar]
- 20.Laffosse J.M., Accadbled F., Abid A., Kany J., Darodes P., Sales D.G.J. Reconstruction of long bone defects with a vascularized fibular graft after tumor resection in children and adolescents: thirteen cases with 50-month follow-up. Revue De Chirurgie Orthopédique Et Réparatrice De L Appareil Moteur. 2007;93:555–563. doi: 10.1016/s0035-1040(07)92677-x. [DOI] [PubMed] [Google Scholar]
- 21.Hiroshi Y., Yasunori K., Koji S., Kenji K., Kenji K., Susumu T. Vascularized fibular grafting in the treatment of methicillin-resistant Staphylococcus aureus osteomyelitis and infected nonunion. J Reconstr Microsurg. 2004;21:13–20. doi: 10.1055/s-2004-818044. [DOI] [PubMed] [Google Scholar]
- 22.Rose P.S., Shin A.Y., Bishop A.T., Moran S.L., Sim F.H. Vascularized free fibula transfer for oncologic reconstruction of the humerus. Clin Orthop Relat Res. 2005;438:80. doi: 10.1097/01.blo.0000179586.34727.5b. [DOI] [PubMed] [Google Scholar]
- 23.Bae D.S., Waters PMSampson C.E. Use of free vascularized fibular graft for congenital ulnar pseudarthrosis: surgical decision making in the growing child. J Pediatr Orthop. 2005;25:755. doi: 10.1097/01.bpo.0000186241.29415.df. [DOI] [PubMed] [Google Scholar]
- 24.3Rd A.J., Berend K.R., Gunneson E.E., Urbaniak J.R. Free vascularized fibular grafting for the treatment of postcollapse osteonecrosis of the femoral head. Surgical technique. J Bone Joint Surg Am. 2003;85:87. doi: 10.2106/00004623-200403001-00012. [DOI] [PubMed] [Google Scholar]
- 25.Lauthe O., Soubeyrand M., Babinet A., Dumaine V., Anract P., Biau D.J. The indications and donor-site morbidity of tibial cortical strut autografts in the management of defects in long bones. Bone Joint Lett J. 2018;100-B(5):667–674. doi: 10.1302/0301-620X.100B5.BJJ-2017-0577.R2. [DOI] [PubMed] [Google Scholar]
- 26.Schneppendahl J., Jungbluth P., Lögters T.T., Sager M., Wild M., Hakimi M. Treatment of a diaphyseal long-bone defect with autologous bone grafts and platelet-rich plasma in a rabbit model. Vet Comp Orthop Traumatol. 2015;28:164–171. doi: 10.3415/VCOT-14-05-0079. [DOI] [PubMed] [Google Scholar]
- 27.Donegan D.J., Scolaro J., Matuszewski P.E., Mehta S. Staged bone grafting following placement of an antibiotic spacer block for the management of segmental long bone defects. Orthopedics. 2011;34 doi: 10.3928/01477447-20110922-16. [DOI] [PubMed] [Google Scholar]
- 28.Masquelet A.C., Begue T. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin N Am. 2010;41:27–37. doi: 10.1016/j.ocl.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Gross J.B., Diligent J., Bensoussan D., Galois L., Stoltz J.F., Mainard D. Percutaneous autologous bone marrow injection for treatment of delayed and non-union of long bone: a retrospective study of 45 cases. Bio Med Mater Eng. 2015;25:187–197. doi: 10.3233/BME-141235. [DOI] [PubMed] [Google Scholar]
- 30.Wilkins R.M., Chimenti B.T., Rifkin R.M. Percutaneous treatment of long bone nonunions: the use of autologous bone marrow and allograft bone matrix. Orthopedics. 2003;26:549–554. doi: 10.3928/0147-7447-20030502-04. [DOI] [PubMed] [Google Scholar]
- 31.Muhammad A., Muhammad I., Faheem Mubashir F., Ranjeet Kumar S., Muhammad Latif S., Ahmad Humayun S. Role of injecting bone marrow aspiration injection in treating delayed union and non-union. J Pak Med Assoc. 2014;64:S154. [PubMed] [Google Scholar]
- 32.Aponte-Tinao L.A., Ritacco L.E., Albergo J.I., Ayerza M.A., Muscolo D.L., Farfalli G.L. The principles and applications of fresh frozen allografts to bone and joint reconstruction. Orthop Clin N Am. 2014;45:257–269. doi: 10.1016/j.ocl.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Chang L., Chen Y.J., Chen Y.P., Chen C.T., Yu W.H. Biocompatibility of human bone allograft powder processed by supercritical CO. Formos J Musculoskelet Disord. 2011;2:55–61. [Google Scholar]
- 34.Jian Y.K., Tian X.B., Bo L.I., Bing Q., Zhou Z.J., Zheng Y. Properties of deproteinized bone for reparation of big segmental defect in long bone. Chin J Traumatol. 2008;11:152–156. doi: 10.1016/s1008-1275(08)60032-2. [DOI] [PubMed] [Google Scholar]
- 35.Venkatramani H., Sabapathy S.R., Dheenadayalan J., Devendra A., Rajasekaran S. Reconstruction of post-traumatic long segment bone defects of the lower end of the femur by free vascularized fibula combined with allograft (modified Capanna's technique) Eur J Trauma Emerg Surg. 2015;41:17. doi: 10.1007/s00068-014-0451-2. [DOI] [PubMed] [Google Scholar]
- 36.Weichman K.E., Dec W., Morris C.D., Mehrara B.J., Disa J.J. Lower extremity osseous oncologic reconstruction with composite microsurgical free fibula inside massive bony allograft. Plast Reconstr Surg. 2015;136:396–403. doi: 10.1097/PRS.0000000000001463. [DOI] [PubMed] [Google Scholar]
- 37.Halim A.S., Chai S.C., Wan I.W., Wan A.W., Mat Saad A.Z., Wan Z. Long-term outcome of free fibula osteocutaneous flap and massive allograft in the reconstruction of long bone defect. J Plast Reconstr Aesthet Surg. 2015;68:1755–1762. doi: 10.1016/j.bjps.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Wang W., Wong H., Chu P., Leung F., Cheung K., Yeung K., editors. Magnesium plasma modified bone allograft for large bone defect treatment. 2015 Shanghai thin film conference(2015上海薄膜国际会议) 2015. [Google Scholar]
- 39.Scaglione M., Fabbri L., Dell'Omo D., Gambini F., Guido G. Long bone nonunions treated with autologous concentrated bone marrow-derived cells combined with dried bone allograft. Musculoskelet Surg. 2014;98:101–106. doi: 10.1007/s12306-013-0271-2. [DOI] [PubMed] [Google Scholar]
- 40.Di B.C., Aldini N.N., Lucarelli E., Dozza B., Frisoni T., Martini L. Osteogenic protein-1 associated with mesenchymal stem cells promote bone allograft integration. Tissue Eng A. 2010;16:2967–2976. doi: 10.1089/ten.tea.2009.0637. [DOI] [PubMed] [Google Scholar]
- 41.Ruizibán M.A., Gonzalezlizán F., Diazheredia J., Elíasmartin M.E., Correa G.C. Effect of VEGF-A165 addition on the integration of a cortical allograft in a tibial segmental defect in rabbits. Knee Surg Sports Traumatol Arthrosc Official J Esska. 2013;23:1393–1400. doi: 10.1007/s00167-013-2785-4. [DOI] [PubMed] [Google Scholar]
- 42.Yasuda H., Yano K., Wakitani S., Matsumoto T., Nakamura H., Takaoka K. Repair of critical long bone defects using frozen bone allografts coated with an rhBMP-2-retaining paste. J Orthop Sci. 2012;17:299–307. doi: 10.1007/s00776-012-0196-x. [DOI] [PubMed] [Google Scholar]
- 43.Cobos J.A., Lindsey R.W., Gugala Z. The cylindrical titanium mesh cage for treatment of a long bone segmental defect: description of a new technique and report of two cases. J Orthop Trauma. 2000;14:54–59. doi: 10.1097/00005131-200001000-00011. [DOI] [PubMed] [Google Scholar]
- 44.Reichert J.C., Wullschleger M.E., Cipitria A., Lienau J., Cheng T.K., Schütz M.A. Custom-made composite scaffolds for segmental defect repair in long bones. Int Orthop. 2011;35:1229–1236. doi: 10.1007/s00264-010-1146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Priddy L.B., Chaudhuri O., Stevens H.Y., Krishnan L., Uhrig B.A., Willett N.J. Oxidized alginate hydrogels for bone morphogenetic protein-2 delivery in long bone defects. Acta Biomater. 2014;10:4390–4399. doi: 10.1016/j.actbio.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sasaki G., Watanabe Y., Miyamoto W., Yasui Y., Morimoto S., Kawano H. Induced membrane technique using beta-tricalcium phosphate for reconstruction of femoral and tibial segmental bone loss due to infection: technical tips and preliminary clinical results. Int Orthop. 2017;42:1–8. doi: 10.1007/s00264-017-3503-5. [DOI] [PubMed] [Google Scholar]
- 47.Ozdemir M.T., Kir M.Ç. Repair of long bone defects with demineralized bone matrix and autogenous bone composite. Indian J Orthop. 2011;45:226–230. doi: 10.4103/0019-5413.80040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hopp S.G., Dahners L.E., Gilbert J.A. A study of the mechanical strength of long bone defects treated with various bone autograft substitutes: an experimental investigation in the rabbit. J Orthop Res. 2010;7:579–584. doi: 10.1002/jor.1100070416. [DOI] [PubMed] [Google Scholar]
- 49.Goshima K., Nakase J., Xu Q., Matsumoto K., Tsuchiya H. Repair of segmental bone defects in rabbit tibia promoted by a complex of β-tricalcium phosphate and hepatocyte growth factor. J Orthop Sci. 2012;17:639–648. doi: 10.1007/s00776-012-0262-4. [DOI] [PubMed] [Google Scholar]
- 50.Luca L., Rougemont A.L., Walpoth B.H., Boure L., Tami A., Anderson J.M. Injectable rhBMP-2-loaded chitosan hydrogel composite: osteoinduction at ectopic site and in segmental long bone defect. J Biomed Mater Res A. 2015;96A:66–74. doi: 10.1002/jbm.a.32957. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y., Wang J., Wang J., Niu X., Liu J., Gao L. Preparation of porous PLA/DBM composite biomaterials and experimental research of repair rabbit radius segmental bone defect. Cell Tissue Bank. 2015;16:615–622. doi: 10.1007/s10561-015-9510-0. [DOI] [PubMed] [Google Scholar]
- 52.Chung R., Kalyon D.M., Valdevit A. Vol. 12. 2018. pp. 253–262. (Patient-specific and physiological load sustaining synthetic graft substitutes for fusion of critically sized segmental bone defects). [Google Scholar]
- 53.Yan L., Jiang D.M. Study of bone-like hydroxyapatite/polyamino acid composite materials for their biological properties and effects on the reconstruction of long bone defects. Drug Des Dev Ther. 2015;9:6497–6508. doi: 10.2147/DDDT.S96207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Henslee A.M., Spicer P.P., Yoon D.M., Nair M.B., Meretoja V.V., Witherel K.E. Biodegradable composite scaffolds incorporating an intramedullary rod and delivering bone morphogenetic protein-2 for stabilization and bone regeneration in segmental long bone defects. Acta Biomater. 2011;7:3627–3637. doi: 10.1016/j.actbio.2011.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bruder S., Fox B. Tissue engineering of bone. Cell based strategies. Clin Orthop Relat Res. 1999;367:68–83. doi: 10.1097/00003086-199910001-00008. [DOI] [PubMed] [Google Scholar]
- 56.Colnot C. Cell sources for bone tissue engineering: insights from basic science. Tissue Eng B Rev. 2011;17:449–457. doi: 10.1089/ten.teb.2011.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nguyen B.B., Moriarty R.A., Kamalitdinov T., Etheridge J.M., Fisher J.P. Collagen hydrogel scaffold promotes mesenchymal stem cell and endothelial cell coculture for bone tissue engineering. J Biomed Mater Res A. 2017;105:1123–1131. doi: 10.1002/jbm.a.36008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yousefi A.M., James P.F., Akbarzadeh R., Subramanian A., Flavin C., Oudadesse H. Prospect of stem cells in bone tissue engineering: a review. Stem Cells Int. 2016;2016 doi: 10.1155/2016/6180487. 13 pages. Article ID 6180487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Verboket R., Leiblein M., Seebach C., Nau C., Janko M., Bellen M. Autologous cell-based therapy for treatment of large bone defects: from bench to bedside. Eur J Trauma Emerg Surg. 2018;44(Suppl 1):1–17. doi: 10.1007/s00068-018-0906-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Candrian C., Kon E., Krishnakumar G.S., Filardo G., Roffi A., Gostynska N. The role of three-dimensional scaffolds in treating long bone defects: evidence from preclinical and clinical literature—a systematic review. BioMed Res Int. 2017;2:1–13. doi: 10.1155/2017/8074178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roseti L., Parisi V., Petretta M., Cavallo C., Desando G., Bartolotti I. Scaffolds for bone tissue engineering: state of the art and new perspectives. Mater Sci Eng C Mater Bio App. 2017;78:1246. doi: 10.1016/j.msec.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 62.Zhang X.Y., Fang G., Zhou J. Additively manufactured scaffolds for bone tissue engineering and the prediction of their mechanical behavior: a review. Materials. 2017;10:50. doi: 10.3390/ma10010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reichert J.C., Epari D.R., Wullschleger M.E., Berner A., Saifzadeh S., Nöth U. Bone tissue engineering. Reconstruction of critical sized segmental bone defects in the ovine tibia. Der Orthopäde. 2012;41:280–287. doi: 10.1007/s00132-011-1855-x. [DOI] [PubMed] [Google Scholar]
- 64.Zan Q., Wang C., Dong L., Cheng P., Tian J. Design and preparation of chitosan/HA composite scaffolds for tissue engineering with long-bone-like structure. Int J Mater Prod Technol. 2010;37:271. [Google Scholar]
- 65.Brodke D., Pedrozo H.A., Kapur T.A., Attawia M., Kraus K.H., Holy C.E. Bone grafts prepared with selective cell retention technology heal canine segmental defects as effectively as autograft. J Orthop Res. 2010;24:857–866. doi: 10.1002/jor.20094. [DOI] [PubMed] [Google Scholar]
- 66.Ng M.H., Duski S., Tan K.K., Yusof M.R., Low K.C., Rose I.M. Repair of segmental load-bearing bone defect by autologous mesenchymal stem cells and plasma-derived fibrin impregnated ceramic block results in early recovery of limb function. BioMed Res Int. 2015;2014:345910. doi: 10.1155/2014/345910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Z.X., Chen C., Zhou Q., Wang X.S., Zhou G., Liu W. The treatment efficacy of bone tissue engineering strategy for repairing segmental bone defects under osteoporotic conditions. Tissue Eng A. 2015;21:2346. doi: 10.1089/ten.TEA.2015.0071. [DOI] [PubMed] [Google Scholar]
- 68.Zi-Zheng W.U., Soo-Min L., Wang Z., Zhi L.I., Zhang J., Orthopedics D.O. Use of rADSCs/β-TCP bone tissue engineering complex for repairing of large segmental radial bone defect in rabbits. Fudan Univ J Med Sci. 2015;42:338–343. [Google Scholar]
- 69.Liu J., Zhou P., Long Y., Huang C., Chen D. Repair of bone defects in rat radii with a composite of allogeneic adipose-derived stem cells and heterogeneous deproteinized bone. Stem Cell Res Ther. 2018;9:79. doi: 10.1186/s13287-018-0817-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dufrane D., Docquier P.L., Delloye C., Poirel H.A., André W., Aouassar N. Scaffold-free three-dimensional graft from autologous adipose-derived stem cells for large bone defect reconstruction: clinical proof of concept. Medicine. 2015;94 doi: 10.1097/MD.0000000000002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reichert J.C. Queensland University of Technology; Queensland: 2010. Tissue engineering bone - reconstruction of critical sized segmental bone defects in a large animal model. PhD thesis. [Google Scholar]
- 72.Ruan S.Q., Deng J., Yan L., Huang W.L. Composite scaffolds loaded with bone mesenchymal stem cells promote the repair of radial bone defects in rabbit model. Biomed Pharmacother. 2017;97:600. doi: 10.1016/j.biopha.2017.10.110. [DOI] [PubMed] [Google Scholar]
- 73.Jian Y.K., Tian X.B., Qi-Hong L.I., Bo L.I., Zhi P., Zhao W.F. Biomechanical researches on tissue engineering bone constructed by deproteinated bone. Chin J Traumatol. 2010;13:32–36. [PubMed] [Google Scholar]
- 74.Lin C.Y., Chang Y.H., Sung L.Y., Chen C.L., Lin S.Y., Li K.C. Long-term tracking of segmental bone healing mediated by genetically engineered adipose-derived stem cells: focuses on bone remodeling and potential side effects. Tissue Eng A. 2014;20:1392. doi: 10.1089/ten.tea.2013.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]