Abstract
Background
Osteosarcoma, ranking as the second primary cause of cancer-related death, is the most common type of bone cancer. Doxorubicin (DOX) is used as a first-line treatment for osteosarcoma; however, the tumour recurrence rate remains high. Recent studies have suggested that DOX-induced migration and stemness in osteosarcoma cells might be the primary reason of recurrence and drug resistance. However, the underlying mechanisms remain unclear. Therefore, it is urgent to explore novel effective treatments to overcome DOX-induced drug resistance of osteosarcoma.
Methods
Osteosarcoma cells KHOS and U2OS were treated with DOX and apatinib (AP) alone or in combination. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and colony formation assays were performed to evaluate effects on proliferation. Flow cytometry analysis was carried out to assess cell apoptosis. Cell migration was determined by the transwell assay. Cancer stemness was detected by flow cytometry analysis of CD133+ cells and sphere-formation assay. Western blot assay was used to measure the expression of E-cadherin, N-cadherin, vimentin, matrix metalloproteinase 9 (MMP-9), signal transducer and activator of transcription 3 (STAT3)/p-STAT3, SRY-box2 (Sox2) and octamer-binding protein 4 (Oct4), and Nanog in treated osteosarcoma cells.
Results
Herein, we revealed that AP treatment significantly enhanced the sensitivity of osteosarcoma cells to DOX, reversed the DOX-induced stemness phenotype and metastasis capacity of osteosarcoma cells, and abolished the upregulation of p-STAT3, Sox2, Oct4, and Nanog. We further demonstrated that AP reversed DOX-induced stemness and migration of osteosarcoma cells through Sox2.
Conclusion
These results suggested that AP significantly abolished the DOX-induced stemness phenotype and metastasis capacity in osteosarcoma cells by inhibiting Sox2 via STAT3 signalling.
The translational potential of this article
Our study indicates that Doxorubicin-based chemotherapeutics may simulate cancer stem cells (CSCs) properties in osteosarcoma, leading to the resistance of osteosarcoma. Apatinib can reduce the Doxorubicin-induced chemoresistance through STAT3/Sox2 pathway inactivation. This study represents that Apatinib may act as an effective chemotherapy sensitiser for reducing chemoresistance correlated with CSCs in osteosarcoma.
Keywords: Apatinib, Cancer stemness, Doxorubicin, Osteosarcoma, SRY-box2, Signal transducer and activator of transcription 3
Introduction
Osteosarcoma, frequently occurring in children and adolescents, is one of the most common primary bone tumours in the world [1]. Owing to its high malignant and metastatic potential, the overall survival rates of osteosarcoma have remained low during the past several decades despite the tremendous advancements in medical technologies [1], [2]. The five-year survival rate of patients with osteosarcoma and a localised lesion is approximately 65–70%, whereas it could be as low as 19–30% in those with distant metastasis [3], [4]. Currently, the therapeutic strategies for newly diagnosed osteosarcoma mainly depend on chemotherapy and surgical resection [2], [5]. Doxorubicin (DOX) is a chemotherapy medication that is used to treat multiple human cancers, and currently, the combination chemotherapy of DOX and 3 to 4 cytotoxic agents (cisplatin, DOX, and ifosfamide) become the standard first-line treatment of osteosarcoma [6], [7]. However, the effects of DOX-based combination chemotherapy were recently reported to be significantly limited by drug resistance [8], [9]. Therefore, it is urgent to explore effective strategies to overcome drug resistance in the treatment of osteosarcoma caused by DOX.
Although the underlying mechanisms of DOX-induced drug resistance in osteosarcoma treatment remain unclear, increasing evidence suggested that cancer stem cells (CSCs) might be involved in it [9], [10]. CSCs are defined as a small proportion of tumour cells that possess differentiation and self-renewal characteristics [11], [12]. It was reported that CSCs were responsible for the initiation, progression, recurrence, and migration of multiple human tumours, such as breast tumour, lung tumour, and hepatocellular tumour [12]. Evidence revealed that heterogeneous osteosarcomas possessed a subtype of CSCs with elevated tumourigenicity and chemoresistance, and the existence of CSCs was considered to be one of the primary reasons for the failure of osteosarcoma treatment [9]. Multiple pathways, up to now, have been shown to be associated with CSCs, such as Notch, wingless (Wnt/β-catenin), and phosphoinositide 3 - kinase / protein kinase B (PI3K/Akt) signalling pathways [13], [14], [15]. Recently, DOX was reported to enhance the stemness of osteosarcoma by upregulating the expression of Kruppel - like factor 4 (KLF4) [10], suggesting that inhibition of stemness might be an important research direction for alleviating the resistance of osteosarcoma to DOX.
The process of tumour initiation and progression is determined to a considerable degree by the expression of genes that are responsible for controlling the nature and fate of target cells. Sox2, Oct4, and Nanog, acting as critical transcription factors in cells, were revealed to contribute to the induction of cancer stemness [16], [17], [18]. Overexpression of Sox2 was found in a variety of cancer cells, and Sox2 knockdown significantly attenuated tumourigenicity and inhibited the CSC phenotype of osteosarcoma [19], [20]. The activated STAT3 signalling pathway was frequently observed in multiple human cancers, indicating a critical role of STAT3 in cancer progression [21]. Recent research studies have revealed a pivotal role of the STAT3 pathway in cancer stemness induction [22]. Moreover, activation of STAT3 in breast cells was demonstrated to increase the expression of Sox2, resulting in the enhancement of cancer stemness [23]. However, the relationship between STAT3/Sox2 and DOX has not been reported.
Apatinib (AP), which specifically binds to the intracellular ATP-binding site of vascular endothelial growth factor receptor 2, is an important receptor tyrosine kinase inhibitor that is widely used in multiple cancer treatments [24]. Although AP was recently reported to regulate the progression of osteosarcoma by inhibiting STAT3 [25], whether AP involves in the osteosarcoma stemness still remains undetermined.
In the present study, we aimed to investigate the effects and underlying mechanisms of AP on DOX-induced migration and stemness of osteosarcoma cells in vitro, providing a novel therapeutic strategy for patients with osteosarcoma to overcome the DOX-induced chemoresistance.
Materials and methods
Cell culture
Two osteosarcoma cell lines KHOS and U2OS were purchased from the Cell Bank of the China Science Academy, Shanghai, and maintained at 37 °C in Rosewell Park Memorial Institute (RPMI-1640) medium (Gibco Laboratories, Grand Island, NY, USA) containing 10% foetal bovine serum (Gibco Laboratories) and 1% penicillin/streptomycin under 5% CO2 and 95% air.
Lentivirus production and infection
The lentiviral vector encoding the Complementary DNA (cDNA) of Sox2 (pCCL-Sox2) was designed and purchased from Vigenebio (Shanghai, China). For lentivirus production, 293T cells were transfected with 10 mg of pCCL-Sox2 plus 15 mg of packaging plasmids CMVDR8.91 using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) according to the protocols of the manufacturers. Forty-eight hours after transfection, viral supernatants were harvested and filtered with a 0.45-mm syringe filter (Millipore, Billerica, MA, USA). Then, viral supernatants were concentrated using an Amcions Ultra-15 Centrifugal Filter (Millipore) and stored at −80 °C. For infection, the KHOS and U2OS cells were cultured in 35-mm tissue culture plates in 1 ml of RPMI-1640 medium supplemented with 5 mg/ml polybrene (Sigma, Shanghai, China) and 5 ml of viral concentrates.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
The KHOS and U2OS cells were seeded into 96-well plates at the density of 2 × 105 cells/well. After incubation at 37 °C for 24 h, the cells were treated with AP (10 μM) or DOX (100 μM) for another 24 h. Then, the cells were collected and resuspended with the culture medium, and cell viability of the treated KHOS and U2OS cells was measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) uptake method. In brief, the treated KHOS and U2OS cells were seeded in 96-well plates at the concentration of 3000 cells/per well and cultured at 37 °C in an incubator with 5% CO2 and 95% air for 24, 48, or 72 h. MTT solution (6 mg/ml) was then added into each well and incubated at 37 °C for additional 4 h. The absorbance of the cell suspension was measured using an MRX Revelation 96-well multiscanner (Dynex Technologies, Chantilly, VA, USA) at 490 nm. The experiment was performed at least 3 times independently.
Colony formation assay
Cell proliferation of the treated KHOS and U2OS cells was detected by colony formation assay. In brief, the untreated KHOS and U2OS cells (1000 cells/per well) were plated into 35-mm Petri dishes and cultured overnight at 37 °C. Subsequently, different treatments were applied to the dishes for 48 h, after which the medium was replaced with the normal medium and further cultured for two weeks. Colonies were fixed using 4% paraformaldehyde and stained using 0.1% crystal violet solution. The number of colonies was calculated under a microscope at a magnification of 200×.
Apoptosis analysis
Cell apoptosis of the treated KHOS and U2OS cells was evaluated through flow cytometry analysis. In brief, after drug treatment for 48 h, the KHOS and U2OS cells were harvested by trypsinization and washed with precold phosphate-buffered saline (PBS) for three times. The cells were then stained with Annexin Fluorescein Isothiocyanate (V-FITC) (R&D Systems Inc, Minneapolis, USA) and propidium iodide (R&D Systems Inc) in the dark for 15 min. Finally, the apoptosis rate was detected using a Becton Dickinson Fluorescence Activating Cell Sorter (FACS) Calibur Flow Cytometer (BD Biosciences, CA, USA).
Transwell assay
Cell migration of the treated KHOS and U2OS cells was determined by the transwell assay using transwell chambers (8-μm pores; Corning Incorporated, MA, USA) coated with Matrigel matrix (BD Biosciences, CA, USA). In brief, the treated KHOS and U2OS cells were harvested and resuspended with RPMI-1640 medium at a concentration of 2 × 104 cells/ml. Then, 100 ml of the cell suspension was added into the upper chamber, and RPMI-1640 medium containing 10% foetal bovine serum was used as an attractant in the lower chamber. After culturing at 37 °C for 48 h, cells that migrated through the membrane were fixed and stained using 1% crystal violet.
Quantitative real-time polymerase chain reaction assay
Total RNAs of the treated KHOS and U2OS cells were prepared using the TRIzol reagent (Takara, Japan) and then transcribed into cDNA using a Takara RT kit (Takara) following protocols obtained from the manufacturers. The ABI Prism 7700 sequence detection system (PE Applied Biosystems, California, USA) was used to carry out the quantitative real-time polymerase chain reaction process with the following protocols: 95 °C, 5 mins; 35 cycles of 95 °C for 35 s, 60 °C for 45 s, and 72 °C for 90 s; and 72 °C, 10 mins. Sequences of primers used in the present study are the following: Sox2: forward 5′-CAC CTA CAG CAT GTC CTA CTC G-3′, reverse 5′-GGT TTT CTC CAT GCT GTT TCT T-3’; Oct4: forward 5′- GCT CGA GAA GGA TGT GGT CC-3′, reverse 5′- CGT TGT GCA TAG TCG CTG CT-3’; Nanog: forward 5′-GGA GTA GAG TGT AGA GGA GAA TGA GTT A-3′, reverse 5′-CTA ACT CTT TAA CTT CTT CCC AAA TC-3’; GAPDH: forward 5′- TGT TCG TCA TGG GTG TGA AC-3′, reverse 5′- ATG GCA TGG ACT GTG GTC AT-3’; GAPDH was used as the internal control, and the expression of each gene was calculated using the 2−ΔΔCT method.
Western blot assay
Total proteins of the treated KHOS and U2OS cells were extracted using the radio immunoprecipitation assay (RIPA) buffer (0.5 M Tris, 250 mM NaCl, 0.1% Nonidet P-40, 0.2 M Na3VO4, 0.2 M NaF) containing protease inhibitors (Roche, Mannheim, Germany). Protein concentration was determined using a Bicinchoninic Acid (BCA) kit (Pierce, Rockford, USA), according to the instructions provided by the manufacturers. A total of 50 μg of total proteins was loaded, and the proteins were isolated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The target proteins were transferred to polyvinylidene difluoride membranes (Millipore, Bedfordshire, UK) and then incubated with 5% skimmed milk for 2 h to block nonspecific binding sites. Subsequently, the membranes were subjected to incubation with primary antibodies against E-cadherin (1:500, ab15148; Abcam, UK), β-actin (1:10000, ab8226; Abcam, UK), N-cadherin (1:1000, ab18203; Abcam, UK), vimentin (1:2000, ab92547; Abcam, UK), MMP-9 (1:1000, ab194316; Abcam, UK), p-STAT3 (1:20000, ab76315; Abcam, UK), STAT3 (1:2000, ab119352; Abcam, UK), Sox2 (1:1000, ab97959; Abcam, UK), Oct4 (1:1000, ab181557; Abcam, UK), and Nanog (1:5000, ab109250; Abcam, UK) at 4 °C for 12 h. After washing three times with Tris-Buffered Saline Tween-20 (TBST), the membranes were incubated with horseradish peroxidase (HRP)–conjugated corresponding secondary antibodies (IgG-HRP, 1:2000; Abcam, UK) for 2 h, and signals were detected using the enhanced chemiluminescent reagents (ECL, Germany).
CD133+ analysis
After drug treatment, the KHOS and U2OS cells were harvested and resuspended with fresh culture medium at a concentration of 1 × 106 cells/ml. Before CD133+ analysis, the cells were placed on ice for at least 30 min, followed by the treatment with anti-CD133 antibody (catalogue #130-105-225; Miltenyi Biotec, Bergisch Gladbach, Germany) for 1 h. After washing three times with precold PBS, the cells were incubated with an Alexa 488-conjugated secondary antibody (Invitrogen) for 45 min and washed with precold PBS again before analysis by a BD FACS caliber flow cytometer (BD Biosciences).
Sphere-formation assay
After determining the number of osteosarcoma cells by trypan blue staining, the cell suspensions were serially diluted in the cultured medium until there was no multicellular adhesion in the solution and then seeded into ultralow attachment 6-well plates (Corning). On the next day, a phase-contrast microscope (Olympus, Hamburg, Germany) was used to examine each well, and the wells containing a single cell were marked. After culturing at 37 °C for 7 days, each well was photographed using the phase-contrast microscope (Olympus), and wells with a sphere were marked and counted.
Statistical analysis
Data in the present study were expressed as the mean ± standard deviation and analysed using the Student t test (two-group comparison) or one-way analysis of variance (multiple-group comparison), followed by the Tukey post hoc test. Differences between means were considered significant if P < 0.05.
Results
AP enhanced the sensitivity of osteosarcoma cells to DOX
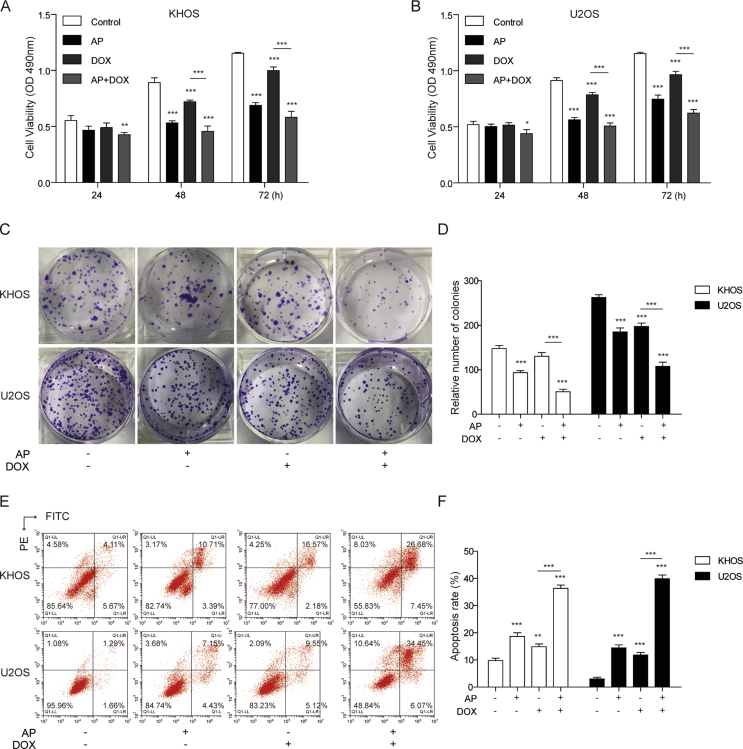
To determine whether AP involved in the effects of DOX on osteosarcoma cells, we first evaluated the cell viability of the KHOS and U2OS cells treated with AP alone, DOX alone, or both drugs in combination by MTT and colony formation assays. The results from the MTT assay showed that AP or DOX alone could reduce the cell viability of KHOS and U2OS cells, and the cell viability in the combination group of AP and DOX was significantly decreased than that in the DOX-alone group (Figure 1A and B). In the colony formation assay, we found that the colony number of the AP- or DOX-alone group was obviously less than that of the control group; moreover, the combination of AP and DOX significantly reduced the colony number compared with DOX alone (Figure 1C and D). In addition, we assessed the effects of AP, DOX alone, or both drugs in combination on cell apoptosis with flow cytometry analysis. Compared with the control group, the AP- or DOX-alone group showed a significant upregulation of the cell apoptosis rate of the KHOS and U2OS cells. Cell apoptosis of the KHOS and U2OS cells in the combination group of AP and DOX was remarkably higher than that in the DOX-alone group (Figure 1E and F). These findings suggested that AP could enhance the sensitivity of osteosarcoma to DOX.
Figure 1.
AP enhanced the sensitivity of osteosarcoma cells to DOX. (A and B) Cell viability of KHOS and U2OS cells treated with AP (2μM for both KHOS and U2OS), DOX (50 nM for KHOS, 25 nM for U2OS), or AP+DOX were evaluated by MTT assay at 24 h, 48 h, and 72 h. (C and D) Colony formation assay of KHOS and U2OS cells treated with AP, DOX, or both drugs in combination for 7 days. (E and F) After treating with DMSO, AP, DOX, or AP+DOX, KHOS and U2OS cells were subjected to cell apoptosis analysis using flow cytometry. Data are represented as the mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001. AP = apatinib; DOX = doxorubicin; MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; DMSO = dimethyl sulfoxide; PE = phycoerythrin; FITC = fluorescein isothiocyanate; SD = standard deviation.
AP inhibited DOX-induced migration of osteosarcoma cells
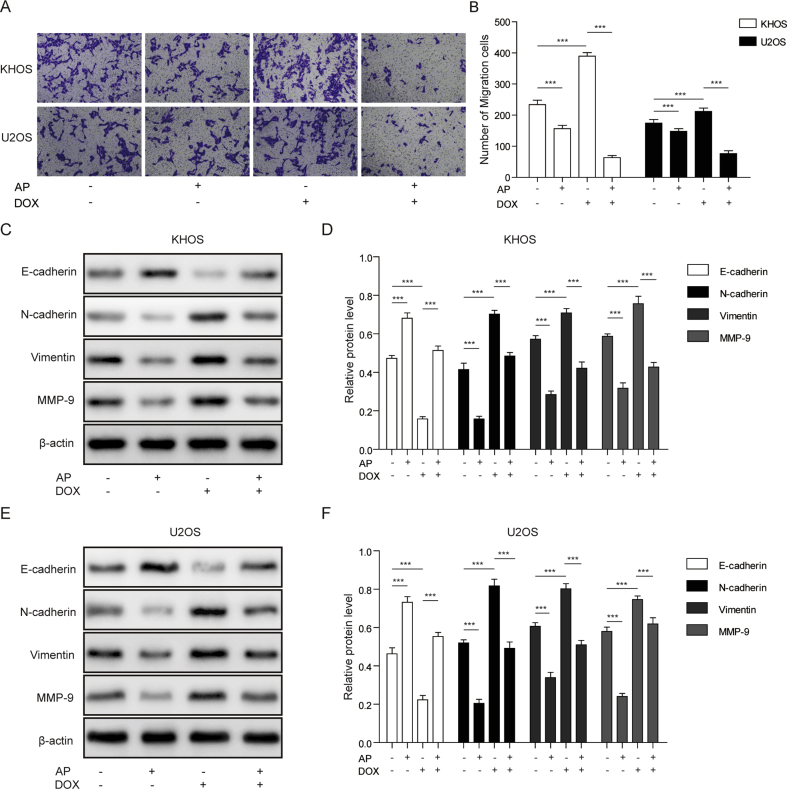
According to the transwell analysis, we found that AP treatment remarkably reduced the KHOS and U2OS migration cell number, whereas DOX treatment significantly increased the migration cell number compared with the control group. Compared with the DOX-alone group, the combination group of AP and DOX showed a lower migration cell number (Figure 2A and B). Moreover, to better characterise the effects of AP and DOX on migration, four migration-related proteins were evaluated by western blotting in KHOS and U2OS cells. Compared with the control group, AP treatment resulted in a significant upregulation of E-cadherin and a significant downregulation of N-cadherin, vimentin, and MMP-9 in KHOS and U2OS cells. However, DOX treatment significantly decreased the E-cadherin expression and increased N-cadherin, vimentin, and MMP-9 expression in KHOS and U2OS cells. Compared with the DOX-alone group, the combination treatment of AP and DOX caused a remarkable elevation of E-cadherin expression and a decrease of N-cadherin, vimentin, and MMP-9 expression in KHOS and U2OS cells (Figure 2C–F). These results indicated that AP treatment could inhibit DOX-induced migration of osteosarcoma cells.
Figure 2.
AP inhibited DOX-induced enhancement of migration of osteosarcoma. (A and B) Transwell assay was carried out to examine the effects of AP, DOX, or AP+DOX on migratory capacity of KHOS and U2OS cells. (C–F) Migration-related proteins (E-cadherin, N-cadherin, vimentin, and MMP-9) of AP-, DOX-, or AP+DOX–treated KHOS and U2OS cells were measured by western blot assay. Data are represented as the mean ± SD. ***P < 0.001. AP = apatinib; DOX = doxorubicin; MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; MMP = matrix metalloproteinase; SD = standard deviation.
AP reversed DOX-induced cancer stemness of osteosarcoma cells
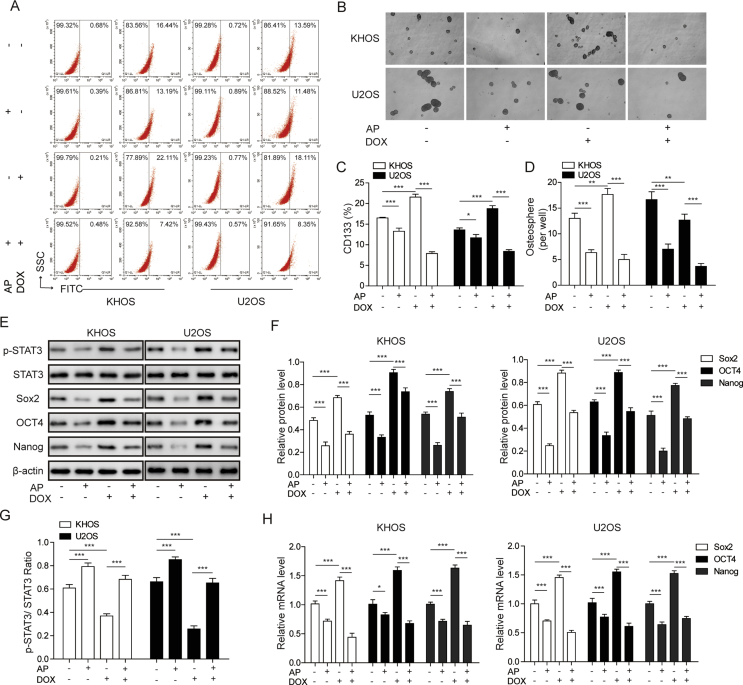
To further explore whether AP participates in the DOX-induced CSC-like properties in osteosarcoma cells, we assessed the proportion of CD133+ (a CSC marker of osteosarcoma) cells in the KHOS and U2OS cells treated with AP, DOX, or AP+DOX. The results showed that AP treatment significantly decreased the ration of CD133+ cells in the KHOS and U2OS cells, whereas DOX treatment remarkably increased the ration of CD133+ cells in the KHOS and U2OS cells compared with the control group. Compared with the DOX-alone group, the combination group of AP and DOX showed a significant lower ration of CD133+ cells in the KHOS and U2OS cells (Figure 3A and C). Moreover, in the sphere-formation assay, we found that the KHOS and U2OS cells showed a reduced sphere-formation capacity after AP treatment and an increased sphere-formation capacity after exposure to DOX (Figure 3B). The KHOS and U2OS cells exhibited a reduced sphere-formation capacity after exposure to the AP and DOX combination compared with DOX-alone exposure (Figure 3D). To better characterise the effects of AP and DOX on stemness, three CSC-related genes (Sox2, Oct4, and Nanog) were detected by western blot assay and quantitative real-time polymerase chain reaction in the KHOS and U2OS cells. The expression of p-STAT3 was found to be downregulated or upregulated in AP- or DOX-treated KHOS and U2OS cells, respectively, compared with the control group, and the DOX-induced upregulation of p-STAT3 could be reversed by the application of AP (Figure 3E, F and 3G). In addition, compared with the control group, the AP-alone group showed a significant downregulation of Sox2, Oct4, and Nanog, whereas the DOX-alone group showed a significant upregulation of Sox2, Oct4, and Nanog in the mRNA level (Figure 3H). Compared with the DOX-alone group, the combination group of AP and DOX exhibited a remarkable reduction of Sox2, Oct4, and Nanog in KHOS and U2OS cells. These results suggested that AP could reverse the enhancement of stemness of osteosarcoma cells induced by DOX.
Figure 3.
AP reversed DOX-induced cancer stemness of osteosarcoma cells. (A) The percentage of the CD133+ subpopulation of KHOS and U2OS cells treated with AP alone, DOX alone, or both drugs in combination was determined by flow cytometry analysis. (B) KHOS and U2OS cells cultured with AP, DOX, or AP+ DOX for 6 days were subjected to a sphere-formation assay. (C) The percentage of the CD133+ subpopulation of KHOS and U2OS cells treated with AP alone, DOX alone, or both drugs in combination was calculated. (D) The sphere numbers of the KHOS and U2OS cells were counted. (E–G) Western blot assay was performed to evaluate the protein expression of STAT3/P-STAT3, Sox2, Otc4, and Nanog in KHOS and U2OS cells treated with AP alone, DOX alone, or both drugs in combination. (H) Relative mRNA expression of Sox2, Otc4, and Nanog was assessed via qRT-PCR in KHOS and U2OS cells treated with AP alone, DOX alone, or both drugs in combination. Data are represented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001. AP = apatinib; DOX = doxorubicin; MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; STAT3 = signal transducer and activator of transcription 3; qRT-PCR = quantitative real-time polymerase chain reaction; SD = standard deviation.
Sox2 overexpression abolished the enhanced effects of AP on the sensitivity of osteosarcoma cells to DOX
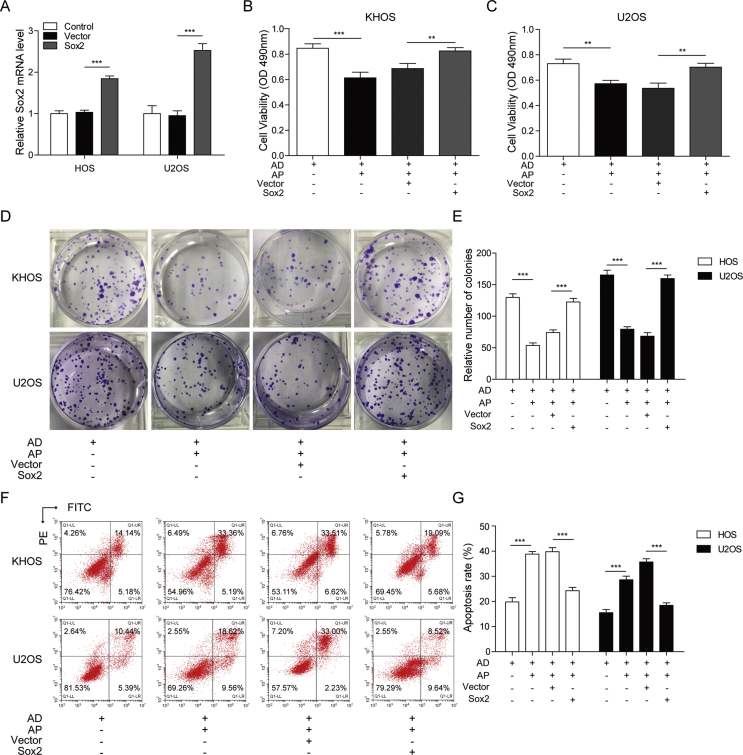
To investigate the role of Sox2 in the effects of AP on DOX-induced stemness of osteosarcoma cells, overexpression of Sox2 in KHOS and U2OS cells was studied by treating the cells with a lentiviral vector encoding the cDNA of Sox2. Relative mRNA expression of Sox2 was significantly increased in Sox2 vector–treated KHOS and U2OS cells compared with those cells treated with nothing and an empty vector (Figure 4A). In the MTT assay, we demonstrated that AP could exacerbate the DOX-induced downregulation of cell viability, and Sox2 overexpression abolished the effects of AP in KHOS and U2OS cells (Figure 4B and C). AP was demonstrated to enhance the inhibitory effects of DOX on colony formation, and we revealed that Sox2 overexpression remarkably reversed the enhanced effects of AP on DOX-induced inhibition of colony formation (Figure 4D and E). Moreover, the enhanced effects of AP on DOX-induced cell apoptosis of KHOS and U2OS cells were found to be abolished by overexpression of Sox2 (Figure 4F and G). These findings suggested that Sox2 overexpression could abolish the enhanced effects of AP on the sensitivity of osteosarcoma cells to DOX.
Figure 4.
Sox2 overexpression abolished the enhanced effects of AP on the sensitivity of osteosarcoma cells to DOX. Lentiviral vector encoding the cDNA of Sox2 were transfected into KHOS and U2OS cells (A) Relative mRNA expression of Sox2 in KHOS and U2OS cells were detected by qRT-PCR. (B)and (C) After treatment with DOX or AP+DOX, cell viability of Sox2 overexpressed KHOS and U2OS cells were evaluated by MTT assay. (D) and (E) Colony formation assay was performed in Sox2 overexpressed KHOS and U2OS cells to evaluate the effects of DOX or AP+DOX on cell proliferation. (F) and (G) After treatment with DOX or AP+DOX, the apoptosis of Sox2 overexpressed KHOS and U2OS cells were measured by flow cytometry. Data represent the means ± SD. ∗∗P<0.01, ∗∗∗P<0.001.
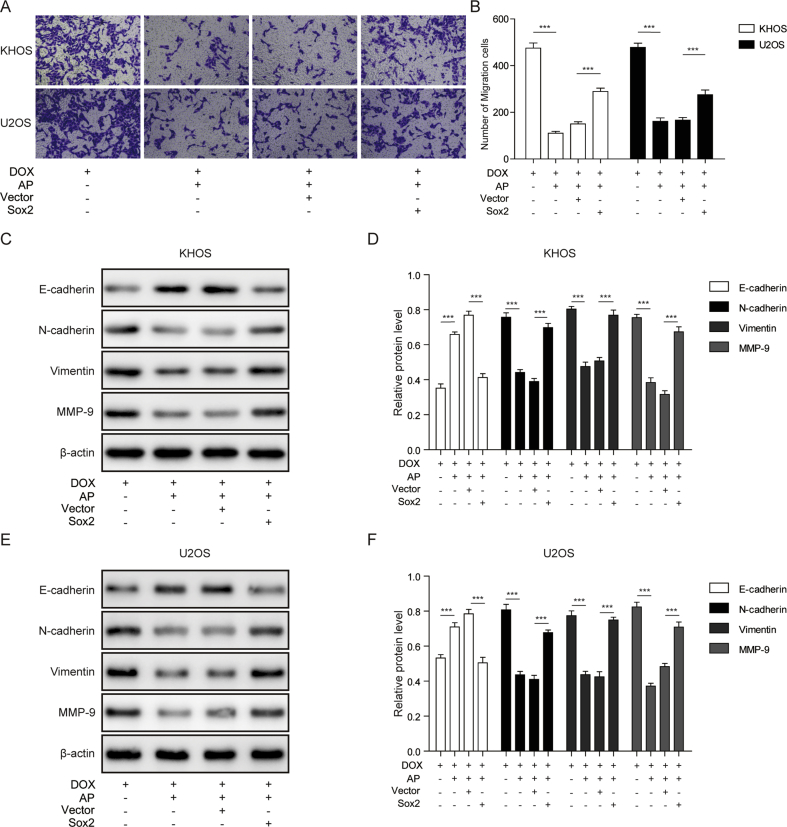
Sox2 overexpression abolished the inhibitory effects of AP on migration of DOX-induced osteosarcoma cells
To investigate the functions of Sox2 in the effects of AP on DOX-induced cell migration, transwell assay was performed, and migration-related proteins were detected by western blot assay. In the transwell assay, we revealed that AP abolished the DOX-induced cell migration of the KHOS and U2OS cells, and the Sox2 overexpression was revealed to partially block the effects of AP on DOX-induced cell migration (Figure 5A and B). To further confirm the roles of Sox2 in the influences of AP and DOX on cell migration, we evaluated the expression levels of E-cadherin, N-cadherin, vimentin, and MMP-9 in Sox2-overexpressed KHOS and U2OS cells with AP or DOX treatment. In Figure 5C–F, we showed that AP reversed the DOX-induced downregulation of E-cadherin and upregulation of N-cadherin, vimentin, and MMP-9; however, these effects were abolished in Sox2-overexpressed KHOS and U2OS cells. These results indicated that overexpression of Sox2 abolished the inhibitory effects of AP on DOX-induced migration in osteosarcoma cells.
Figure 5.
Sox2 overexpression abolishedthe inhibitory effects of AP on DOX-induced osteosarcoma cell migration. (A and B) Cell migration of DOX- or AP+DOX–treated KHOS and U2OS cells was measured by transwell assay after transfecting with Sox2. (C and D) Protein expression of E-cadherin, N-cadherin, vimentin, and MMP-9 was detected by western blot assay in Sox2-overexpressed KHOS cells treated with DOX or AP+DOX. (E and F) Protein expression of E-cadherin, N-cadherin, vimentin, and MMP-9 was detected by western blot assay in Sox2-overexpressed U2OS cells treated with DOX or AP+DOX. Data are represented as the mean ± SD. ***P < 0.001. AP = apatinib; DOX = doxorubicin; MMP = matrix metalloproteinase; SD = standard deviation.
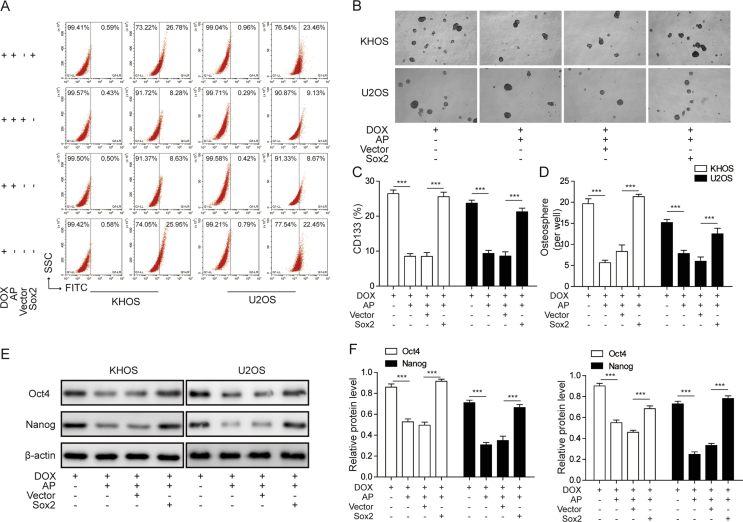
Sox2 overexpression abolished the inhibitory effects of AP on DOX-induced stemness of osteosarcoma cells
We then explored the roles of Sox2 in the effects of AP on DOX-induced stemness of osteosarcoma cells. In the flow cytometry analysis, we found that AP treatment could reverse the upregulation of CD133+ cells induced by DOX; however, these effects could be abolished by overexpressing Sox2 (Figure 6A and C). In the sphere-formation assay, we found that the inhibitory effects of AP on DOX-induced enhancement of sphere-formation capacity were blocked in Sox2-overexpressed KHOS and U2OS cells (Figure 6B and D). Moreover, western blot analysis of Oct4 and Nanog showed that the inhibitory effects of AP on DOX-induced upregulation of Oct4 and Nanog could be blocked by Sox2 overexpression (Figure 6E and F). These findings suggested that Sox2 overexpression abolished the inhibitory effects of AP on DOX-induced stemness of osteosarcoma cells.
Figure 6.
Sox2 overexpression abolished the inhibitory effects of AP on DOX-induced stemness of osteosarcoma cells. (A) Flow cytometry analysis of the CD133+ subpopulation of KHOS and U2OS cells treated with DOX, AP+DOX, AP+DOX+vector, or AP+DOX+Sox2. (B) After transfecting with the empty vector or Sox2 lentiviral vector and culturing with DOX or DOX+AP for 7 days, KHOS and U2OS cells were subjected to a sphere-formation assay. (C) The percentage of the CD133+ subpopulation of KHOS and U2OS cells treated with DOX, AP+DOX, AP+DOX+vector, or AP+DOX+Sox2 was calculated. (D) The sphere numbers of the KHOS and U2OS cells were counted. (E and F) Western blot analysis of Oct4 and Nanog expression of KHOS and U2OS cells treated with DOX, AP+DOX, AP+DOX+vector, or AP+ DOX+Sox2. Data are represented as the mean ± SD. ***P < 0.001. AP = apatinib; DOX = doxorubicin; SSC = Side SCatter; FITC = fluoresceine isothiocyanate; SD = standard deviation.
Discussion
In the present study, we showed that AP could increase the sensitivity of osteosarcoma cells to DOX by regulating Sox2 through the STAT3 signalling pathway in vitro. Moreover, we demonstrated that AP abolished the DOX-induced migration and stemness by inhibiting Sox2.
Despite multiple comprehensive therapies, including (neo)adjuvant chemotherapy, tumour surgical resection, and amputation, being used in osteosarcoma treatment, the morbidity and mortality of osteosarcoma remain high [26]. The combination therapy of surgery and multidrug chemotherapy is, currently, the standard therapeutic strategy for osteosarcoma, which significantly improved the cure rate of osteosarcoma [27]. The five-year survival rate of osteosarcoma has also been increased in the last several decades from 10 to around 70% under the present procedures of chemotherapy (multiple cycles with several of the most effective agents before and after surgery) [28]. Although combined therapy is widely used to treat osteosarcoma, recurrence still occurs in more than one-third of patients with osteosarcoma and localised disease and also occurs in more than three-fourths of patients with osteosarcoma and metastatic disease [28]. As one of the most effective antiosteosarcoma drugs, DOX was used as a front-line agent of adjuvant chemotherapy over the last 30 years [29]. However, drug resistance, almost inevitably during DOX-based chemotherapy, seriously limits the effects of DOX [2]. Previous studies have shown that the emergence of CSCs in osteosarcoma might cause the resistance of osteosarcoma cells to DOX-based chemotherapy [30]. CSCs could be produced from the oncogenic transformation of natural stem cells or acquisition of cancer stemness by non–stem cancer cells in response to microenvironmental stimulations [14]. DOX-based chemotherapy was revealed by several studies to induce CSC-like phenotypes in osteosarcoma cells [10], [14], which was consistent with our results. Cancer stemness can be regulated by transcription factors, such as Sox2, Oct4, and Nanog. Among these transcription factors, Sox2 was revealed to be increased in the isolated CSC-like population [31]. The important functions of Sox2 in keeping the properties of CSCs and the antiapoptosis feature of tumour cells have been extensively demonstrated [32]. Moreover, our results showed that overexpression of Sox2 in osteosarcoma cells significantly inhibited DOX-induced stemness of osteosarcoma cells, which was consistent with the conclusions by Maurizi et al [20], indicating that Sox2 might be a promising therapeutic target for osteosarcoma treatment.
AP is a selective receptor tyrosine kinase inhibitor that is usually used as a tumour inhibitor in multiple human cancers, such as gastric cancer, lung cancer, and pancreatic cancer [33], [34], [35]. Recently, AP was also reported to suppress invasion and migration of osteosarcoma cells by targeting STAT3 [25]. Moreover, Liu K et al. demonstrated that AP could promote autophagy and apoptosis in osteosarcoma cells via the vascular endothelial growth factor receptor 2/STAT3/B-cell lymphoma-2 (BCL-2) signalling pathway [36]. However, it has not been investigated whether AP participates in the induction of stemness of osteosarcoma. Herein, we first revealed that AP could ameliorate DOX-induced osteosarcoma cell stemness and migration capacity by regulating Sox2 through STAT3. However, the downstream pathways and targets of Sox2 in osteosarcoma cells are still unclear, which will be the direction of our future research.
In conclusion, our findings provided an effective therapeutic strategy and several targets to overcome the DOX-induced cancer stemness and migration in osteosarcoma cells, contributing to the clinical treatment of DOX. However, all the findings in the present study were revealed in in vitro experiments; further in vivo experiments should be performed to better characterise the effects of AP on DOX-induced stemness.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors, and no material support of any kind was received.
Conflict of interest
The authors have no conflicts of interest to disclose in relation to this article.
References
- 1.Moore D.D., Luu H.H. Osteosarcoma. Cancer Treat Res. 2014;162:65–92. doi: 10.1007/978-3-319-07323-1_4. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y., Yang J., Zhao N., Wang C., Kamar S., Zhou Y. Progress in the chemotherapeutic treatment of osteosarcoma. Oncol Lett. 2018;16(5):6228–6237. doi: 10.3892/ol.2018.9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyers P.A., Schwartz C.L., Krailo M., Kleinerman E.S., Betcher D., Bernstein M.L. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005;23(9):2004–2011. doi: 10.1200/JCO.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 4.Kager L., Zoubek A., Potschger U., Kastner U., Flege S., Kempf-Bielack B. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003;21(10):2011–2018. doi: 10.1200/JCO.2003.08.132. [DOI] [PubMed] [Google Scholar]
- 5.Otoukesh B., Boddouhi B., Moghtadaei M., Kaghazian P., Kaghazian M. Novel molecular insights and new therapeutic strategies in osteosarcoma. Cancer Cell Int. 2018;18:158. doi: 10.1186/s12935-018-0654-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyers P.A. Systemic therapy for osteosarcoma and Ewing sarcoma. Am Soc Clin Oncol Edu Book. 2015:e644–647. doi: 10.14694/EdBook_AM.2015.35.e644. [DOI] [PubMed] [Google Scholar]
- 7.Harrison D.J., Geller D.S., Gill J.D., Lewis V.O., Gorlick R. Current and future therapeutic approaches for osteosarcoma. Expert Rev Anticancer Ther. 2018;18(1):39–50. doi: 10.1080/14737140.2018.1413939. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Fernandez Y., Imbuluzqueta E., Zalacain M., Mollinedo F., Patino-Garcia A., Blanco-Prieto M.J. Doxorubicin and edelfosine lipid nanoparticles are effective acting synergistically against drug-resistant osteosarcoma cancer cells. Cancer Lett. 2017;388:262–268. doi: 10.1016/j.canlet.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Brown H.K., Tellez-Gabriel M., Heymann D. Cancer stem cells in osteosarcoma. Cancer Lett. 2017;386:189–195. doi: 10.1016/j.canlet.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Li Y., Xian M., Yang B., Ying M., He Q. Inhibition of KLF4 by statins reverses adriamycin-induced metastasis and cancer stemness in osteosarcoma cells. Stem Cell Report. 2017;8(6):1617–1629. doi: 10.1016/j.stemcr.2017.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawood S., Austin L., Cristofanilli M. Cancer stem cells: implications for cancer therapy. Oncology (Williston Park) 2014;28(12):1101–1107. 1110. [PubMed] [Google Scholar]
- 12.Vlashi E., Pajonk F. Cancer stem cells, cancer cell plasticity and radiation therapy. Semin Cancer Biol. 2015;31:28–35. doi: 10.1016/j.semcancer.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu L., Fan Z., Fang S., Yang J., Gao T., Simoes B.M. Cisplatin selects for stem-like cells in osteosarcoma by activating Notch signaling. Oncotarget. 2016;7(22):33055–33068. doi: 10.18632/oncotarget.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martins-Neves S.R., Paiva-Oliveira D.I., Wijers-Koster P.M., Abrunhosa A.J., Fontes-Ribeiro C., Bovee J.V. Chemotherapy induces stemness in osteosarcoma cells through activation of Wnt/beta-catenin signaling. Cancer Lett. 2016;370(2):286–295. doi: 10.1016/j.canlet.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Gong C., Liao H., Wang J., Lin Y., Qi J., Qin L. LY294002 induces G0/G1 cell cycle arrest and apoptosis of cancer stem-like cells from human osteosarcoma via down-regulation of PI3K activity. Asian Pac J Cancer Prev APJCP. 2012;13(7):3103–3107. doi: 10.7314/apjcp.2012.13.7.3103. [DOI] [PubMed] [Google Scholar]
- 16.Lee J.H., Yun C.W., Han Y.S., Kim S., Jeong D., Kwon H.Y. Melatonin and 5-fluorouracil co-suppress colon cancer stem cells by regulating cellular prion protein-Oct4 axis. J Pineal Res. 2018;65(4) doi: 10.1111/jpi.12519. e12519. [DOI] [PubMed] [Google Scholar]
- 17.Zhou L., Zhao L.C., Jiang N., Wang X.L., Zhou X.N., Luo X.L. MicroRNA miR-590-5p inhibits breast cancer cell stemness and metastasis by targeting SOX2. Eur Rev Med Pharmacol Sci. 2017;21(1):87–94. [PubMed] [Google Scholar]
- 18.Wang H., Liu B., Wang J., Li J., Gong Y., Li S. Reduction of NANOG mediates the inhibitory effect of aspirin on tumor growth and stemness in colorectal cancer. Cell Physiol Biochem. 2017;44(3):1051–1063. doi: 10.1159/000485405. [DOI] [PubMed] [Google Scholar]
- 19.Martins-Neves S.R., Corver W.E., Paiva-Oliveira D.I., van den Akker B.E., Briaire-de-Bruijn I.H., Bovee J.V. Cleton-jansen AM: osteosarcoma stem cells have active Wnt/beta-catenin and overexpress SOX2 and KLF4. J Cell Physiol. 2016;231(4):876–886. doi: 10.1002/jcp.25179. [DOI] [PubMed] [Google Scholar]
- 20.Maurizi G., Verma N., Gadi A., Mansukhani A., Basilico C. Sox2 is required for tumor development and cancer cell proliferation in osteosarcoma. Oncogene. 2018;37(33):4626–4632. doi: 10.1038/s41388-018-0292-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siveen K.S., Sikka S., Surana R., Dai X., Zhang J., Kumar A.P. Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochim Biophys Acta. 2014;1845(2):136–154. doi: 10.1016/j.bbcan.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Bak Y., Kwon T., Bak I.S., Hong J., Yu D.Y., Yoon D.Y. IL-32theta inhibits stemness and epithelial-mesenchymal transition of cancer stem cells via the STAT3 pathway in colon cancer. Oncotarget. 2016;7(6):7307–7317. doi: 10.18632/oncotarget.7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu C., Dong L., Sun Z., Wang L., Wang Q., Li H. Esculentoside A suppresses breast cancer stem cell growth through stemness attenuation and apoptosis induction by blocking IL-6/STAT3 signaling pathway. Phytother Res. 2018;32(11):2299–2311. doi: 10.1002/ptr.6172. [DOI] [PubMed] [Google Scholar]
- 24.Geng R., Song L., Li J., Zhao L. The safety of apatinib for the treatment of gastric cancer. Expert Opin Drug Saf. 2018;17(11):1145–1150. doi: 10.1080/14740338.2018.1535592. [DOI] [PubMed] [Google Scholar]
- 25.Zheng B., Ren T., Huang Y., Guo W. Apatinib inhibits migration and invasion as well as PD-L1 expression in osteosarcoma by targeting STAT3. Biochem Biophys Res Commun. 2018;495(2):1695–1701. doi: 10.1016/j.bbrc.2017.12.032. [DOI] [PubMed] [Google Scholar]
- 26.Li B., Wang S., Wang S. MiR-195 suppresses colon cancer proliferation and metastasis by targeting WNT3A. Mol Genet Genom. 2018;293(5):1245–1253. doi: 10.1007/s00438-018-1457-y. [DOI] [PubMed] [Google Scholar]
- 27.Xiao X., Wang W., Wang Z. The role of chemotherapy for metastatic, relapsed and refractory osteosarcoma. Paediatr Drug. 2014;16(6):503–512. doi: 10.1007/s40272-014-0095-z. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari S., Serra M. An update on chemotherapy for osteosarcoma. Expert Opin Pharmacother. 2015;16(18):2727–2736. doi: 10.1517/14656566.2015.1102226. [DOI] [PubMed] [Google Scholar]
- 29.Buondonno I., Gazzano E., Jean S.R., Audrito V., Kopecka J., Fanelli M. Mitochondria-targeted doxorubicin: a new therapeutic strategy against doxorubicin-resistant osteosarcoma. Mol Cancer Ther. 2016;15(11):2640–2652. doi: 10.1158/1535-7163.MCT-16-0048. [DOI] [PubMed] [Google Scholar]
- 30.Wang W., Chen D., Zhu K. SOX2OT variant 7 contributes to the synergistic interaction between EGCG and Doxorubicin to kill osteosarcoma via autophagy and stemness inhibition. J Exp Clin Cancer Res. 2018;37(1):37. doi: 10.1186/s13046-018-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen S., Xu Y., Chen Y., Li X., Mou W., Wang L. SOX2 gene regulates the transcriptional network of oncogenes and affects tumorigenesis of human lung cancer cells. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0036326. e36326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen S., Li X., Lu D., Xu Y., Mou W., Wang L. SOX2 regulates apoptosis through MAP4K4-survivin signaling pathway in human lung cancer cells. Carcinogenesis. 2014;35(3):613–623. doi: 10.1093/carcin/bgt371. [DOI] [PubMed] [Google Scholar]
- 33.Geng R., Li J. Apatinib for the treatment of gastric cancer. Expert Opin Pharmacother. 2015;16(1):117–122. doi: 10.1517/14656566.2015.981526. [DOI] [PubMed] [Google Scholar]
- 34.Zeng D.X., Lei W., Wang C.G., Huang J.A., Jiang J.H. Low dosage of apatinib monotherapy as rescue treatment in advanced lung squamous cell carcinoma. Cancer Chemother Pharmacol. 2019;83(3):439–442. doi: 10.1007/s00280-018-3743-0. [DOI] [PubMed] [Google Scholar]
- 35.Xu M.D., Liu L., Wu M.Y., Jiang M., Shou L.M., Wang W.J. The combination of cantharidin and antiangiogenic therapeutics presents additive antitumor effects against pancreatic cancer. Oncogenesis. 2018;7(11):94. doi: 10.1038/s41389-018-0102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu K., Ren T., Huang Y., Sun K., Bao X., Wang S. Apatinib promotes autophagy and apoptosis through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma. Cell Death Dis. 2017;8(8) doi: 10.1038/cddis.2017.422. e3015. [DOI] [PMC free article] [PubMed] [Google Scholar]