Abstract
Objective
To explore the expression of lubricin in the lumbar endplate and its association with Modic changes (MCs).
Methods
Human endplate specimens harvested from patients undergoing surgery for thoracolumbar spine fractures or lumbar interbody fusion were divided into two groups: MCs group and normal group. Lubricin expression was examined by immunohistochemistry, and differences between the groups were analysed using quantitative polymerase chain reaction (qPCR). Lubricin expression and differences between endplates with MCs and normal endplates were confirmed using a rabbit model. In a final experiment, rabbit endplate chondrocytes were cocultured with Propionibacteria acnes (P. acnes) supernatant, and the expression of lubricin and endplate degeneration related genes were evaluated. In addition, the expression of matrix metalloproteinase 1(MMP-1), A disintegrin-like and metalloproteinase with thrombospondin type 5 motif (ADAMTS5) and inflammatory factors (Interleukin- 1β (IL-1β) and Interleukin-6 (IL-6)) were evaluated after lubricin overexpression.
Results
Lubricin was found in human lumbar endplates and its expression was lower in the MCs group compared to the normal group. In the rabbit model, lubricin was also found in the endplate. In rabbits injected with P. acnes (the MCs group), lubricin expression of endplate decreased compared to the normal group. In the culture of rabbit endplate chondrocytes with P. acnes supernatant, the expression of lubricin, aggrecan, sox9 and collagen type-II decreased significantly, while that of MMP-1 and ADAMTS5 increased significantly. Moreover, lubricin overexpression could downregulate the expression of MMP-1, ADAMTS5 and inflammatory factors (IL-1β and IL-6) compared to negative control.
Conclusion
Lubricin is present in the lumbar endplate where it may have an anti-inflammatory role. P. acnes infection inhibits lubricin expression by cartilage endplate cells and this may facilitate the progression of MCs and endplate degeneration.
The translational potential of this article
Lubricin may have an anti-inflammatory role. P. acnes infection inhibits lubricin expression by cartilage endplate cells and this may facilitate the progression of MCs and endplate degeneration.
Keywords: Lubricin, Lumbar endplate, Modic changes, P. acnes
Introduction
Vertebral body endplates, which consist of a hyaline cartilage layer weakly bound to perforated cortical bone, have been identified as a pain source [1], and various endplate defects have been shown to be common in the lower lumbar spine [2], where they are closely associated with back pain [3]. Painful endplates are not easily identified in individual patients, but they could potentially be revealed by inflammatory-like ‘Modic’ changes (MCs), which are known to be associated with back pain [4,5].
Many studies of MCs have focused on the lumbar vertebral endplate and its pathology [[6], [7], [8], [9], [10], [11], [12], [13]]. Despite more than 2 decades of study, the pathogenic mechanisms resulting in MCs remain controversial, although Propionibacterium acnes is widely considered to be a factor that results in MCs in recent years. Researchers have found that P. acnes infection is highly correlated with the prevalence of MCs, meanwhile a double-blind randomised clinical trial showed that antibiotic treatment is more effective in those low back pain patients with MCs [[14], [15], [16]]. Our previous study, which involved injecting P. acnes into healthy rabbit discs, has confirmed that P. acnes can result in MCs [17].
The potential role of lubricin in the development of MCs has received little attention. Lubricin is a large proteoglycan encoded by the gene proteoglycan 4 (Prg4), which has been shown to play a vital role in the lubrication of synovial joints [18,19]. In addition, in vitro studies have shown that lubricin in saline buffer acts as a lubricant between various surfaces [[20], [21], [22], [23], [24]] as well as in synovial fluid, providing evidence that lubricin is a principal lubricating protein in all types of joint. In addition to its lubricating properties, lubricin has been shown to exert an antiadhesive action [25] and plays a role in strain energy dissipation, suggesting that it has a protective effect on underlying cells [26]. Furthermore, a previous report by Teeple et al [27] showed that lubricin also performed a mechanical function in the intervertebral disc, demonstrating a lack of lubricin can cause elevated apparent torsional moduli in Prg4-knockout mice.
Immunohistochemical analysis has demonstrated the presence of lubricin not only in joints but also in tendon [25], meniscus [28], ligament [29], muscle [29], skin [29], and intervertebral disc [30]. The vertebral endplate, which is comprises a hyaline cartilage endplate attached to a bony endplate of the perforated cortical bone, has a structure similar to the articular surface of a synovial joint where articular cartilage is bound to the subchondral bone. In synovial joints, lubricin has been shown to play an anti-inflammatory role in synovial fluid [31]. If present in the vertebral endplate, lubricin may have a similar role in preventing inflammation and, in this way, may protect endplates from the development of MCs. However, whether lubricin is expressed in the vertebral endplate remains unknown and hence its association with MCs has not been investigated.
The purpose of this study was to (1) explore whether lubricin is expressed in the vertebral endplate and (2) investigate whether decreased lubricin expression is associated with MCs.
Materials and methods
Human endplate tissue
Endplate specimens were harvested from patients undergoing surgery for thoracolumbar spine fractures, or lumbar interbody fusion, between August 2014 and August 2016. Two groups of patients were identified on the basis of preoperative spine MR imaging: a MCs group and a normal group (normal endplates mean “no Modic endplates”). The injured endplates whose adjacent discs which were classified as Grade 1 and 2 using the Pfirrmann grading system were also defined as ‘normal’ endplates Figure 1A). Endplates obtained from patients with thoracolumbar spine fractures were all ‘normal’ endplates. Endplate specimens from patients with lumbar interbody fusion included both normal and MCs specimens. Endplate specimens were divided into two parts: one part was fixed for immunohistochemistry (IHC) to examine whether lubricin was present, whereas the other was frozen at −80 °C for quantitative polymerase chain reaction (qPCR) analysis to evaluate lubricin expression. A total of 110 endplate specimens were enrolled; 62 normal endplates and 48 Modic endplates were used to evaluate lubricin expression by IHC and qPCR (about 50 mg tissue weight per patient for qPCR). There was no significant difference in age between the two groups (MCs group: 62 ± 5.2 yrs; normal group: 60 ± 6.1 yrs).
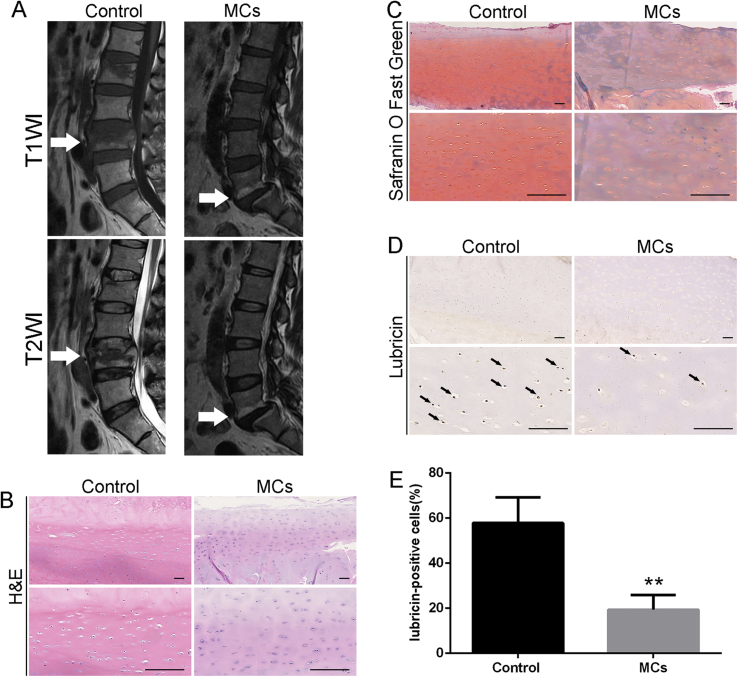
Figure 1.
(A) Representative MRI images of ‘normal’ and MCs discs. (B) In human endplate specimens, H&E staining showed homogenous extracellular matrix and small round chondrocytes in the control group versus fibrotic and sclerotic extracellular matrix and fewer chondrocytes in the MCs group. (C) Furthermore, a reduction in the glycosaminoglycan content was observed in the MCs group through Safranin O Fast Green staining, and (D and E) immunohistochemistry demonstrated reduced lubricin expression in the MCs group (Scale bar: 100 μm, *p < 0.05, **p < 0.01,***p < 0.005). H&E staining = haematoxylin and eosin staining MRI = magnetic resonance imaging.
Rabbit model
A rabbit model of MCs was generated according to our previous study [17] after which endplates with MCs and normal endplates were harvested. Ninety-six clean grade male New Zealand white rabbits with a mean age of 6.14 ± 0.91 months (range, 5–7months) and a mean body weight of 2.83 ± 0.59 kg (range, 2.12–3.38 kg) were used in this study. Specimens of endplates with MCs and normal endplates (20 samples each) from L4–5 or L5–6 were harvested. Lubricin expression was confirmed by IHC and qPCR, and differences between endplates with MCs and normal endplates were evaluated.
Endplate chondrocytes cocultured with P. acnes supernatant
Rabbit endplate chondrocytes were plated in 6-well plates at a density of 2 × 104 cells/well in triplicate. The chondrocytes were then treated with different concentrations of P. acnes supernatant (0%, 1%, 2%, and 4%) for 48 h (bacterial culture supernatant was collected after centrifugation and filtration from 1 × 107 Colony-Forming Units (CFU)/mL P. acnes suspension). qPCR was then performed to evaluate endplate degeneration-related genes (aggrecan, collagen type-II, sox9, MMP-1, and ADAMTS5). MMP-1, ADAMTS5, and inflammatory-related genes (IL-1β and IL-6) were also detected with or without overexpression of lubricin. qPCR was also used to assess lubricin expression in the endplate cells and, in this case, protein levels were also analysed using western blot assays.
Ethical approval was obtained from the Medical Ethics Committee of our hospital. The study approval number is SRRSH2013070901. In addition, all patients gave written informed consent for their information to be stored in the hospital's database and used for research.
DNA transfection
Endplate chondrocytes were plated into 6-well plates 1 day before transfection. One day later, cells were transfected with vector or lubricin plasmid using Lipofectamine 3000 (Invitrogen, USA) as described by the manufacturer. After 48 h, total proteins or RNAs were harvested from the transfected cells and subjected to western blot or qPCR analysis.
RNA isolation, reverse transcription, and quantitative polymerase chain reaction (qPCR)
Total RNA of endplate chondrocytes was extracted by Ultrapure RNA Kit (CW0581, CWBIO, China) according to the manufacturer's protocols. Quantity of total RNAs was measured by Nanodrop 2000. Reverse transcription was then performed using HiFiScript cDNA Synthesis kit (CW2569, CWBIO, China). The qPCR was performed using the UltraSYBR Mixture (CW0957, CWBIO, China) and the polymerase chain reaction cycling program included 95 °C for 10 min (pre-incubation); 40 cycles of 95 °C for 15 s, 60 °C for 60s, 72 °C for 20 s, 95 °C for 15 s (amplification), 60 °C for 60 s (melting curves), and 4 °C for 5 min (cooling). The amplification signals from target genes were normalised by the glyceraldehyde-3-phosphate dehydrogenase in the same reaction. Primer sequences of lubricin, aggrecan, Sox9, and collagen type-II, MMP-1, ADAMTS5, IL-1β, and IL-6 were summarised in Table 1. Col2a1: Collagen Type II Alpha 1 Chain.
Table 1.
Sequences of primers.
| Rat Gene | Primer sequences (5′-3′) | |
|---|---|---|
| Lubricin (human) | Forward | AAAGTCAGCACATCTCCCAAG |
| Reverse | GTGTCTCTTTAGCGGAAGTAGTC | |
| Lubricin (rabbit) | Forward | GCTGCTCCGACTACGAGAAA |
| Reverse | CAGAAACAGAGTGTTCTTCCGT | |
| Col2a1 (rabbit) | Forward | AAGGGACACCGAGGTTTCACTGG |
| Reverse | GGGCCTGTTTCTCCTGAGCGT | |
| Aggrecan (rabbit) | Forward | GCAGCACAGACACTTCAGGA |
| Reverse | CCCACTTTCTACAGGCAAGC | |
| Sox9 (rabbit) | Forward | GGATGTCAAAGCAACAGGCG |
| Reverse | ATGTGCGTTCTCTGGGACTG | |
| ADAMTS5 (rabbit) | Forward | AGTACAGTTTGCCTACCGCC |
| Reverse | GATTTGCCGTTAGGTGGGCA | |
| MMP-1 (rabbit) | Forward | TAGCTGGTTCAACTGCAGGAA |
| Reverse | AGGCTCACAGAATACATTGGG | |
| IL-1β (rabbit) | Forward | GGCACAACAGATCGCTTTGG |
| Reverse | GGACCATCGGCCTCAAAGAA | |
| IL-6 (rabbit) | Forward | ACTGGCGGAAGTCAATCTGC |
| Reverse | CAGCCCCGAAGTGATTCTCA | |
Western blotting analysis
Endplate chondrocytes were seeded (4 × 105 cells/well) in six-well plates. Cells were stimulated with or without indicated concentration (0, 1, 2 or 4%) of P. acnes supernatant for 48 h. Total proteins were then obtained from cultured cells using radio immunoprecipitation assay (RIPA) buffer (Solarbio, Beijing, China) supplemented with 100 mM phenylmethanesulfonyl fluoride (Beyotime, Zhengzhou, China), 100 × Phosphatase Inhibitor Cocktail (CWBIO, China), and Protease Inhibitor Cocktail (Millipore, USA). Supernatant was extracted after centrifugation at 12,000 rpm for 15 min. Total proteins were resolved on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred by electroblotting to polyvinylidene fluoride (PVDF) membranes (Millipore, USA). The membranes were blocked in 5% (w/v) non-fat dry milk in TBS with tween 20 (TBST) at RT for 45 min, and then incubated with Rabbit antibody lubricin (1:1000 dilution, Millipore, USA) at 4 °C overnight. Washed five times with TBST, bands were then incubated with the horseradish peroxidase (HRP)-conjugated goat anti-mouse/rabbit IgG (1:5000 dilution; Abcam). Bands were observed by Image Lab software (Bio-Rad, Hercules, CA). The images were quantified by Image J software (Bethesda, USA).
Immunohistochemistry
Human and rabbit cartilaginous endplate specimens were dehydrated with a graded series of ethanol, then embedded in paraffin, and cut into 4 μm sections. To examine the immunoreactivity of lubricin, sections were immunostained. Immunohistochemical analysis was performed using an SP Rabbit & Mouse HRP Kit (CW2069, CWBIO, China). Rabbit antilubricin (Santa Cruz, USA) and mouse antilubricin (Millipore, USA) polyclonal antibodies immunoglobulin G were used at a dilution of 1:100. The images were then obtained using a high-quality microscope. The semiquantification of lubricin was IHC performed, and three pathologists, who were blind to the clinical tissue data, were responsible for counting numbers of total cartilage endplate (CEP) cells and lubricin-positive CEP cells under high-power fields (magnification of 200×) for each of three sections in each specimen. The sections were recounted if the intraclass correlation coefficient was less than 0.8.
Statistical analysis
All data are expressed as mean ± SD. Student t tests, one-way analysis of variance, and least significant difference (LSD) and Chi-square tests were used to compare mean values and proportions, respectively. Statistical analyses were performed using Statistical program for social sciences (SPSS), version 18.0, software (PASW Statistics; IBM, USA), and values of p < 0.05 were considered significant (*p < 0.05, **p < 0.01, ***p < 0.005).
Results
Lubricin expression in the endplate
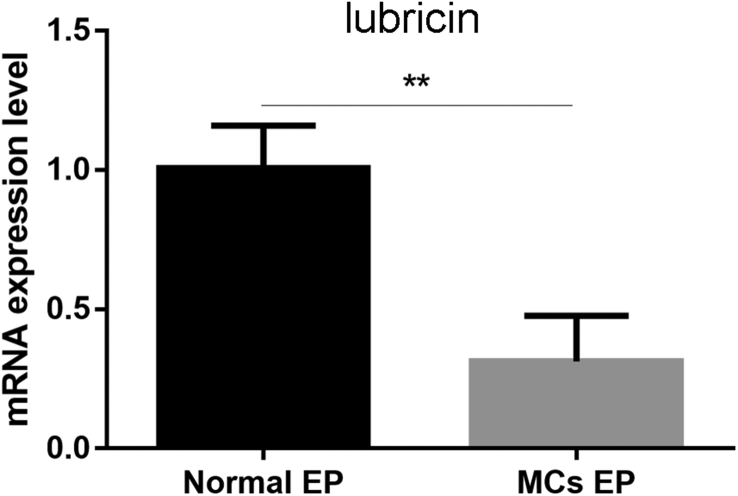
Representative magnetic resonance imaging images of ‘normal’ and MCs discs were shown in Figure 1A. In human endplate specimens, haematoxylin and eosin staining showed homogenous extracellular matrix and small round chondrocytes in the control group versus fibrotic and sclerotic extracellular matrix and fewer chondrocytes in the MCs group (Figure 1B). Furthermore, a reduction in the glycosaminoglycan content was observed in the MCs group through Safranin O Fast Green staining (Figure 1C), IHC demonstrated reduced lubricin expression in the MCs group (Figure 1D and E, p < 0.01). qPCR also revealed reduced gene expression of lubricin in the MCs group when compared with the normal group (p < 0.01, Figure 2).
Figure 2.
In the human cartilage endplate specimens, qPCR revealed reduced lubricin expression in the MCs group when compared with the normal group (*p < 0.05, **p < 0.01, ***p < 0.005).
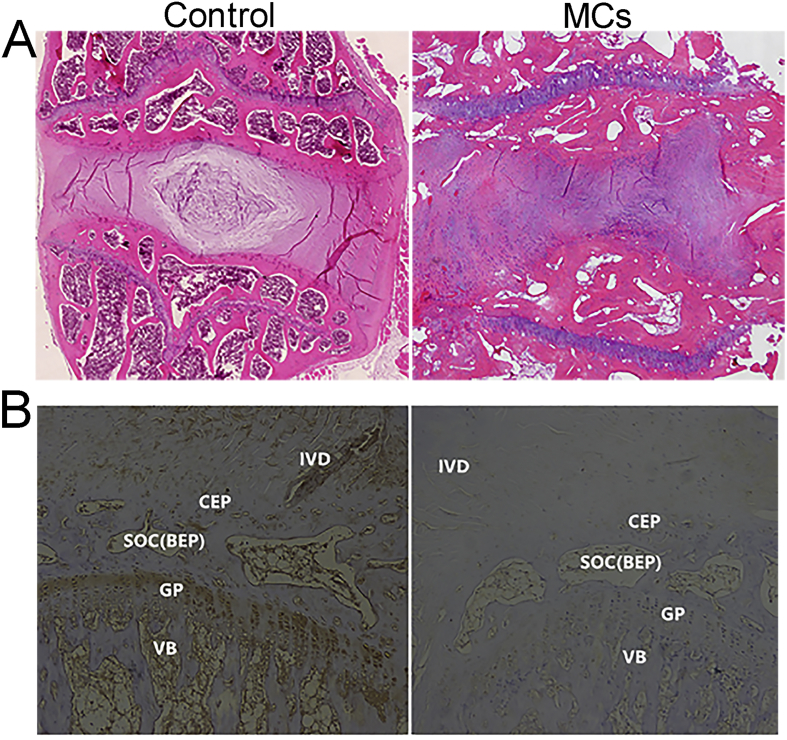
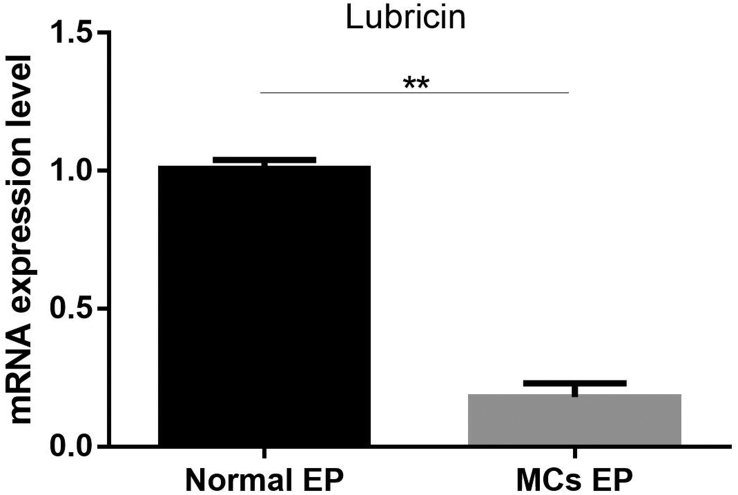
Similar to the findings in human endplates, lubricin could be found in the cartilage endplate and bony endplate of rabbits used in the animal model. Furthermore, lubricin expression was decreased in the MCs group compared with the normal group, as indicated by both IHC (Figure 3) and qPCR (p < 0.01, Figure 4).
Figure 3.
(A) In the rabbit cartilage endplate specimens, H&E staining showed disorganised endplate in the MCs group. (B) Similar to the findings in human endplates, lubricin was found in the endplate of rabbit. Furthermore, lubricin expression was decreased in the MCs endplate compared with the normal endplate (Scale bar: 100 μm). H&E staining = haematoxylin and eosin staining. IVD: Intervertebral disc; CEP: Cartilaginous endplate; SOC: Secondary ossification center; BEP: Bony endplate; SOC(BEP): SOC is equivalent to the BEP; GP: Growth plate; VB: Vertebral body.
Figure 4.
In the rabbit cartilage endplate specimens, qPCR revealed reduced lubricin expression in the MCs group when compared with the normal group (*p < 0.05, **p < 0.01,***p < 0.005).
Rabbit endplate chondrocytes cocultured with P. acnes supernatant
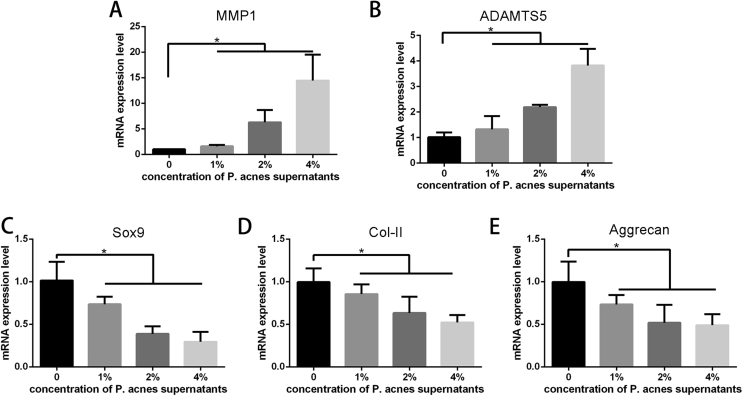
As shown in Figure 5, the expression of aggrecan, Sox9,and collagen type-II in endplate chondrocytes stimulated with P. acnes supernatant decreased significantly compared with controls (p < 0.05), and the reduction tended to be greater at the higher concentrations. The expression of matrix metalloprotease 1 (MMP-1) and ADAMTS5 was increased in a dose-dependent manner compared with controls (p < 0.05).
Figure 5.
(A–B) The expression of MMP 1(A) and ADAMTS5(B) in endplate chondroctyes stimulated with P. acnes supernatants was increased compared with controls. (C–E) The expression of Sox9(C), collagen type-II(D) and aggrecan(E) in endplate chondroctyes stimulated with P. acnes supernatants significantly decreased compared with controls, and the reduction tended to be greater at the higher concentrations. (*p< 0.05, ** p< 0.01,*** p< 0.005).
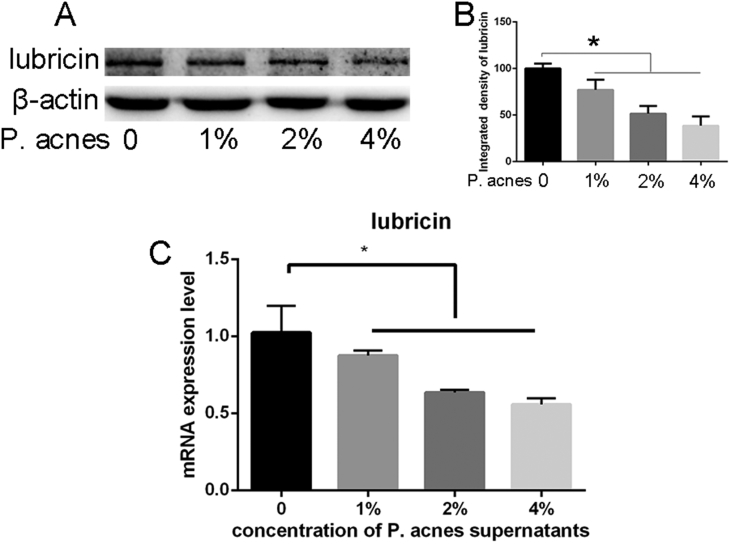
The results of lubricin expression are presented in Figure 6. qPCR and Western blot analysis demonstrated that lubricin decreased significantly after endplate chondrocytes were cocultured with P. acnes supernatant (p < 0.05), with greater reductions occurring at higher concentrations of P. acnes supernatant.
Figure 6.
(A–C) Western blot analysis (A and B) and qPCR(C) demonstrated that lubricin decreased significantly after endplate chondrocytes were co-cultured with P. acnes supernatant fluid, with greater reductions occurring at higher concentrations of P. acnes. (*p< 0.05, ** p< 0.01,*** p< 0.005).
Overexpression of lubricin
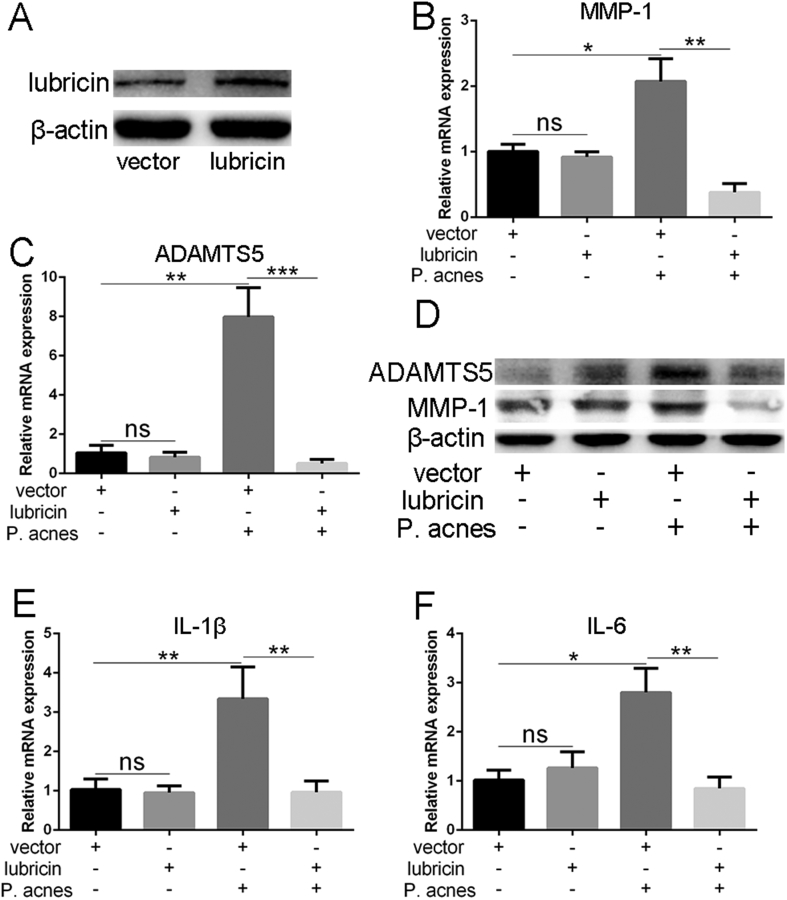
The results of lubricin overexpression are presented in Figure 7. The overexpression of lubricin was confirmed using Western blotting in Figure 7. As the chondrocytes were cocultured with P. acnes supernatant (at concentrations of 1%), lubricin overexpression could downregulate the expression of MMP-1, ADAMTS5, and inflammatory factors (IL-1β and IL-6) compared with negative control.
Figure 7.
(A) Lubricin overexpression are presented in endplate chondrocytes. (B–C) As the chondrocytes were co-cultured with P. acnes supernatant fluid (at concentrations of 1%), lubricin overexpression could downregulate the gene expression of MMP-1(B), ADAMTS5(C). (D) Western blot analysis showed lubricin overexpression could downregulate protein level of MMP-1 and ADAMTS5. (E–F) lubricin overexpression could downregulate the expression of IL-1β(E) and IL-6(F) compared with negative control (*p< 0.05, ** p< 0.01,*** p< 0.005).
Discussion
Summary of results
In this study, lubricin was found in the cartilage endplate of patients undergoing spine surgery, and its expression was lower in endplates with MCs than in normal endplates. These data were confirmed in a rabbit model where MCs were initiated by injecting P. acnes into the disc. When endplate chondrocytes were cocultured with P. acnes supernatant for 48 h, the expression of lubricin, aggrecan, Sox9,and collagen type-II was reduced significantly compared with that in controls, while that of matrix metalloproteinase 1 (MMP-1) and ADAMTS5 was increased. Lubricin overexpression could downregulate the expression of MMP-1, ADAMTS5, and inflammatory factors (IL-1β and IL-6) compared with negative control.
Strengths and weaknesses of the study
To our knowledge, this is the first demonstration of lubricin in the human endplate. Similar findings in the rabbit model suggest that lubricin plays some roles in maintaining normal function of the vertebral endplate. The effects of P. acnes infection on lubricin were evaluated not only by examining changes in gene expression but also by directly measuring changes in lubricin levels using Western blotting, and these results show that P. acnes inhibits the synthesis of lubricin by endplate cells. Lubricin overexpression could downregulate the expression of MMP-1, ADAMTS5, and inflammatory factors (IL-1β and IL-6) compared with negative control, which showed that lubricin may have an anti-inflammatory role in the development of MCs.
Our study also has some limitations. First, the human endplate specimens were difficult to obtain, resulting in a small sample size, while the difference between three types of MCs needs to be further investigated. Second, the study focused only on the association between lubricin and MCs; the anti-inflammatory effects of lubricin, which may act to protect the endplate and prevent the development of MCs, need to be further investigated.
Lubricin expression in the endplate
Lubricin, which was first identified as a molecule secreted by synovial fibroblasts and superficial zone articular chondrocytes [[32], [33], [34]], is a surface-active mucin glycoprotein that is attached to the articular surfaces providing chondroprotective and antiadhesive properties. Lubricin protects the cartilage surface by inhibiting synoviocyte overgrowth [35] and preventing wear of articular cartilage [36]. Consequently, loss of lubricin functionality is regarded as an adverse factor in degenerative arthritis. The vertebral endplate, which is composed of a hyaline cartilage endplate and a bony endplate, has a structure similar to the articular surface of a synovial joint where articular cartilage is bound to the subchondral bone. However, unlike cartilage in synovial joints, the cartilaginous endplate is not subjected to high shearing forces between joint surfaces so the lubricating effects of lubricin are unlikely to be of much benefit. The presence of lubricin in the vertebral endplate suggests it may have an alternative role which may be related to its anti-inflammatory effects, as reported previously [20].
P.acnes and MCs
A recent study showed that low-grade virulent bacterial infections are closely associated with MCs [[37], [38], [39], [40]]. P. acnes is checked out most frequently, accounting for a proportion of 85% [14,15]. P. acnes, a common skin organism, is an anaerobic, gram-positive bacterium characterised by slow growth. It is most notably recognised for its role in acne vulgaris [41]. A previous study [37] also showed that P. acnes may play a role in degenerative disc disease. Stirling's et al [14] study demonstrated that 53% of the patients with severe sciatica had gram-positive anaerobic microorganisms in their disc tissue, of which 84% were P. acnes. Albert et al. [16] indicated that antibiotics are an efficient treatment option for patients with low back pain(LBP)and Modic type 1 changes following lumbar disc herniation. Amoxicillin–clavulanate was chosen as the treatment option for such patients, based on the study by Stirling et al. [14]. Amoxicillin–clavulanate was shown to reduce the volume of MCs in endplate, suggesting that P. acnes infection may be involved in the development of MCs. We confirmed this hypothesis in a previous study which showed that MCs could be initiated in an animal model by injecting P. acnes into rabbit discs. The findings of the current study now suggest other detrimental effects of P. acnes infection which was shown to inhibit lubricin expression by cartilage endplate cells and may facilitate the progression of MCs and endplate degeneration.
Lubricin and MCs
Currently, the Toll-like receptor 2 (TLR-2) is the main receptor in the innate immune response that recognises P. acnes, which causes inflammatory responses through TLR-2 signalling [[42], [43], [44]]. Recent studies have shown that lubricin can inhibit TLR-2 activity [31,45], and this may be the mechanism by which lubricin elicits its anti-inflammatory effects in synovial fluid [31]. MCs are often regarded as an inflammatory process that occurs within the endplate, so we hypothesised that decreased lubricin expression would be associated with MCs. The current findings show that lubricin is expressed in chondrocytes in the vertebral endplate and that its expression is reduced in endplates with MCs. Our study also found that lubricin expression and lubricin production was decreased significantly in endplate chondrocytes when cocultured with P. acnes, suggesting that lubricin is synthesised and secreted by the endplate chondrocytes.
Beside, decreased lubricin might have negative impact to the mechanical environment of endplate, including direct effects like tissue mechanical properties defection, inadequate lubrication resulted tissue damage accumulation, and indirect effects like altered loading and motion of endplate caused by early-onset degenerative spondylosis. This may be another mechanism of lubricin dysregulation in the development of MCs.
Conclusions
Lubricin is present in both human and rabbit vertebral endplates where it may have an anti-inflammatory role. P. acnes infection inhibits lubricin expression by cartilage endplate cells, which may facilitate the progression of MCs and endplate degeneration.
Conflicts of interest
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Acknowledgement
The study was supported by The National Natural Science Foundation of China (project number 81672208 and 81802192).
Contributor Information
Junhui Liu, Email: ljhzju@zju.edu.cn.
Xiaoan Wei, Email: wxa951211@icloud.com.
Bao Huang, Email: huangbao@zju.edu.cn.
Hao Wu, Email: harwu@ucdavis.edu.
Xuyang Zhang, Email: jiurizhang8698@163.com.
Jian Chen, Email: cjian6352@163.com.
Zhi Shan, Email: 11418255@zju.edu.cn.
Shunwu Fan, Email: Fansw@srrsh.com.
Fengdong Zhao, Email: zhaofengdong@zju.edu.cn.
References
- 1.Peng B., Chen J., Kuang Z., Li D., Pang X., Zhang X. Diagnosis and surgical treatment of back pain originating from endplate. Eur Spine J: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2009;18:1035–1040. doi: 10.1007/s00586-009-0938-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y., Videman T., Battie M.C. Lumbar vertebral endplate lesions: prevalence, classification, and association with age. Spine. 2012;37:1432–1439. doi: 10.1097/BRS.0b013e31824dd20a. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y., Videman T., Battie M.C. ISSLS prize winner: lumbar vertebral endplate lesions: associations with disc degeneration and back pain history. Spine. 2012;37:1490–1496. doi: 10.1097/BRS.0b013e3182608ac4. [DOI] [PubMed] [Google Scholar]
- 4.Albert H.B., Manniche C. Modic changes following lumbar disc herniation. Eur Spine J: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2007;16:977–982. doi: 10.1007/s00586-007-0336-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kjaer P., Korsholm L., Bendix T., Sorensen J.S., Leboeuf-Yde C. Modic changes and their associations with clinical findings. Eur Spine J: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2006;15:1312–1319. doi: 10.1007/s00586-006-0185-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albert H.B., Kjaer P., Jensen T.S., Sorensen J.S., Bendix T., Manniche C. Modic changes, possible causes and relation to low back pain. Med Hypotheses. 2008;70:361–368. doi: 10.1016/j.mehy.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Beaudreuil J., Orcel P. Modic 1 discopathy. Joint, bone, spine. Revue du rhumatisme. 2009;76:4–6. doi: 10.1016/j.jbspin.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Jones A., Clarke A., Freeman B.J., Lam K.S., Grevitt M.P. The Modic classification: inter- and intraobserver error in clinical practice. Spine. 2005;30:1867–1869. doi: 10.1097/01.brs.0000173898.47585.7d. [DOI] [PubMed] [Google Scholar]
- 9.Karchevsky M., Schweitzer M.E., Carrino J.A., Zoga A., Montgomery D., Parker L. Reactive endplate marrow changes: a systematic morphologic and epidemiologic evaluation. Skelet Radiol. 2005;34:125–129. doi: 10.1007/s00256-004-0886-3. [DOI] [PubMed] [Google Scholar]
- 10.Kuisma M., Karppinen J., Haapea M., Lammentausta E., Niinimaki J., Tervonen O. Modic changes in vertebral endplates: a comparison of MR imaging and multislice CT. Skelet Radiol. 2009;38:141–147. doi: 10.1007/s00256-008-0590-9. [DOI] [PubMed] [Google Scholar]
- 11.Kuisma M., Karppinen J., Niinimaki J., Ojala R., Haapea M., Heliovaara M. Modic changes in endplates of lumbar vertebral bodies: prevalence and association with low back and sciatic pain among middle-aged male workers. Spine. 2007;32:1116–1122. doi: 10.1097/01.brs.0000261561.12944.ff. [DOI] [PubMed] [Google Scholar]
- 12.Schmid G., Witteler A., Willburger R., Kuhnen C., Jergas M., Koester O. Lumbar disk herniation: correlation of histologic findings with marrow signal intensity changes in vertebral endplates at MR imaging. Radiology. 2004;231:352–358. doi: 10.1148/radiol.2312021708. [DOI] [PubMed] [Google Scholar]
- 13.Vital J.M., Gille O., Pointillart V., Pedram M., Bacon P., Razanabola F. Course of Modic 1 six months after lumbar posterior osteosynthesis. Spine. 2003;28:715–720. doi: 10.1097/01.brs.0000051924.39568.31. discussion 721. [DOI] [PubMed] [Google Scholar]
- 14.Stirling A., Worthington T., Rafiq M., Lambert P.A., Elliott T.S. Association between sciatica and Propionibacterium acnes. Lancet (London, England) 2001;357:2024–2025. doi: 10.1016/s0140-6736(00)05109-6. [DOI] [PubMed] [Google Scholar]
- 15.Albert H.B., Lambert P., Rollason J., Sorensen J.S., Worthington T., Pedersen M.B. Does nuclear tissue infected with bacteria following disc herniations lead to Modic changes in the adjacent vertebrae? Eur Spine J: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2013;22:690–696. doi: 10.1007/s00586-013-2674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albert H.B., Sorensen J.S., Christensen B.S., Manniche C. Antibiotic treatment in patients with chronic low back pain and vertebral bone edema (Modic type 1 changes): a double-blind randomized clinical controlled trial of efficacy. Eur Spine J: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2013;22:697–707. doi: 10.1007/s00586-013-2675-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shan Z., Zhang X., Li S., Yu T., Mamuti M., Zhao F. The influence of direct inoculation of Propionibacterium acnes on modic changes in the spine: evidence from a rabbit model. J Bone Joint Surg Am. 2017;99:472–481. doi: 10.2106/jbjs.16.00146. [DOI] [PubMed] [Google Scholar]
- 18.Swann D.A., Hendren R.B., Radin E.L., Sotman S.L., Duda E.A. The lubricating activity of synovial fluid glycoproteins. Arthritis Rheum. 1981;24:22–30. doi: 10.1002/art.1780240104. [DOI] [PubMed] [Google Scholar]
- 19.Jay G.D., Torres J.R., Warman M.L., Laderer M.C., Breuer K.S. The role of lubricin in the mechanical behavior of synovial fluid. Proc Natl Acad Sci USA. 2007;104:6194–6199. doi: 10.1073/pnas.0608558104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radin E.L., Swann D.A., Weisser P.A. Separation of a hyaluronate-free lubricating fraction from synovial fluid. Nature. 1970;228:377–378. doi: 10.1038/228377a0. [DOI] [PubMed] [Google Scholar]
- 21.Swann D.A., Sotman S., Dixon M., Brooks C. The isolation and partial characterization of the major glycoprotein (LGP-I) from the articular lubricating fraction from bovine synovial fluid. Biochem J. 1977;161:473–485. doi: 10.1042/bj1610473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swann D.A., Slayter H.S., Silver F.H. The molecular structure of lubricating glycoprotein-I, the boundary lubricant for articular cartilage. J Biol Chem. 1981;256:5921–5925. [PubMed] [Google Scholar]
- 23.Jay G.D., Lane B.P., Sokoloff L. Characterization of a bovine synovial fluid lubricating factor. III. The interaction with hyaluronic acid. Connect Tissue Res. 1992;28:245–255. doi: 10.3109/03008209209016818. [DOI] [PubMed] [Google Scholar]
- 24.Zappone B., Ruths M., Greene G.W., Jay G.D., Israelachvili J.N. Adsorption, lubrication, and wear of lubricin on model surfaces: polymer brush-like behavior of a glycoprotein. Biophys J. 2007;92:1693–1708. doi: 10.1529/biophysj.106.088799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Funakoshi T., Schmid T., Hsu H.P., Spector M. Lubricin distribution in the goat infraspinatus tendon: a basis for interfascicular lubrication. J Bone Joint Surg Am. 2008;90:803–814. doi: 10.2106/jbjs.g.00627. [DOI] [PubMed] [Google Scholar]
- 26.Schaefer D.B., Wendt D., Moretti M., Jakob M., Jay G.D., Heberer M. Lubricin reduces cartilage--cartilage integration. Biorheology. 2004;41:503–508. [PubMed] [Google Scholar]
- 27.Teeple E., Aslani K., Shalvoy M.R., Medrano J.E., Zhang L., Machan J.T. Lubricin deficiency in the murine lumbar intervertebral disc results in elevated torsional apparent modulus. J Biomech. 2015;48:2210–2213. doi: 10.1016/j.jbiomech.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Y., Berger E.J., Zhao C., Jay G.D., An K.N., Amadio P.C. Expression and mapping of lubricin in canine flexor tendon. J Orthop Res: official publication of the Orthopaedic Research Society. 2006;24:1861–1868. doi: 10.1002/jor.20239. [DOI] [PubMed] [Google Scholar]
- 29.Sun Y., Berger E.J., Zhao C., An K.N., Amadio P.C., Jay G. Mapping lubricin in canine musculoskeletal tissues. Connect Tissue Res. 2006;47:215–221. doi: 10.1080/03008200600846754. [DOI] [PubMed] [Google Scholar]
- 30.Shine K.M., Simson J.A., Spector M. Lubricin distribution in the human intervertebral disc. J Bone Joint Surg Am. 2009;91:2205–2212. doi: 10.2106/jbjs.h.01344. [DOI] [PubMed] [Google Scholar]
- 31.Alquraini A., Garguilo S., D'Souza G., Zhang L.X., Schmidt T.A., Jay G.D. The interaction of lubricin/proteoglycan 4 (PRG4) with toll-like receptors 2 and 4: an anti-inflammatory role of PRG4 in synovial fluid. Arthritis Res Ther. 2015;17:353. doi: 10.1186/s13075-015-0877-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jay G.D., Britt D.E., Cha C.J. Lubricin is a product of megakaryocyte stimulating factor gene expression by human synovial fibroblasts. J Rheumatol. 2000;27:594–600. [PubMed] [Google Scholar]
- 33.Jay G.D., Tantravahi U., Britt D.E., Barrach H.J., Cha C.J. Homology of lubricin and superficial zone protein (SZP): products of megakaryocyte stimulating factor (MSF) gene expression by human synovial fibroblasts and articular chondrocytes localized to chromosome 1q25. J Orthop Res: official publication of the Orthopaedic Research Society. 2001;19:677–687. doi: 10.1016/s0736-0266(00)00040-1. [DOI] [PubMed] [Google Scholar]
- 34.Flannery C.R., Hughes C.E., Schumacher B.L., Tudor D., Aydelotte M.B., Kuettner K.E. Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and Is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem Biophys Res Commun. 1999;254:535–541. doi: 10.1006/bbrc.1998.0104. [DOI] [PubMed] [Google Scholar]
- 35.Rhee D.K., Marcelino J., Baker M., Gong Y., Smits P., Lefebvre V. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Investig. 2005;115:622–631. doi: 10.1172/jci22263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teeple E., Elsaid K.A., Fleming B.C., Jay G.D., Aslani K., Crisco J.J. Coefficients of friction, lubricin, and cartilage damage in the anterior cruciate ligament-deficient Guinea pig knee. J Orthop Res: official publication of the Orthopaedic Research Society. 2008;26:231–237. doi: 10.1002/jor.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ganko R., Rao P.J., Phan K., Mobbs R.J. Can bacterial infection by low virulent organisms be a plausible cause for symptomatic disc degeneration? A systematic review. Spine. 2015;40:E587–E592. doi: 10.1097/BRS.0000000000000832. [DOI] [PubMed] [Google Scholar]
- 38.Li B., Dong Z., Wu Y., Zeng J., Zheng Q., Xiao B. Association Between Lumbar Disc Degeneration and Propionibacterium acnes Infection: ClinicalResearch and Preliminary Exploration of Animal Experiment. Spine (Phila Pa 1976) 2016 Jul 1;41(13):E764–E769. doi: 10.1097/BRS.0000000000001383. [DOI] [PubMed] [Google Scholar]
- 39.Rigal J., Thelen T., Byrne F., Cogniet A., Boissière L., Aunoble S. Prospective study using anterior approach did not show association between Modic 1 changes and low grade infection in lumbar spine. Eur Spine J. 2016;25:1000–1005. doi: 10.1007/s00586-016-4396-5. [DOI] [PubMed] [Google Scholar]
- 40.Chen Z., Zheng Y., Yuan Y., Jiao Y., Xiao J., Zhou Z. Modic changes and disc degeneration caused by inoculation of Propionibacterium acnes inside intervertebral discs of rabbits: a pilot study. BioMed Res Int. 2016;2016 doi: 10.1155/2016/9612437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perry A.L., Lambert P.A. Propionibacterium acnes. Lett Appl Microbiol. 2006;42:185–188. doi: 10.1111/j.1472-765X.2006.01866.x. [DOI] [PubMed] [Google Scholar]
- 42.Kawai T., Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 43.Kim J., Ochoa M.T., Krutzik S.R., Takeuchi O., Uematsu S., Legaspi A.J. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535–1541. doi: 10.4049/jimmunol.169.3.1535. Baltimore, Md : 1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su Q., Grabowski M., Weindl G. Recognition of Propionibacterium acnes by human TLR2 heterodimers. Int J Med Microbiol : IJMM. 2017;307:108–112. doi: 10.1016/j.ijmm.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 45.Iqbal S.M., Leonard C., Regmi S.C., De Rantere D., Tailor P., Ren G. Lubricin/proteoglycan 4 binds to and regulates the activity of toll-like receptors in vitro. Sci Rep. 2016;6:18910. doi: 10.1038/srep18910. [DOI] [PMC free article] [PubMed] [Google Scholar]