Abstract
Objective
The magnitude of the therapeutic effects of intra-articular injection of platelet-rich plasma (PRP) on osteoarthritis (OA) is still under debate. The goal of this study that was a systematic review of randomised controlled trials of PRP injections for the treatment of OA was to elucidate the therapeutic efficacy of PRP.
Methods
Electronic databases of PubMed, CENTRAL, EMBASE, EBSCO, ClinicalTrials.gov, and International Clinical Trials Registry Platform were searched from inception to June 2018 for RCTs that compared PRP injections to controls in patients with OA. A random-effects approach was used to compile data and subgroups according to trial size (large trials versus small trials), patient profile (age and gender), and PRP preparation method was performed.
Results
Thirty trials met the inclusion criteria and were analysed. All results had unexplained statistical heterogeneity. Patients treated with PRP compared with control showed statistically relevant pain relief and function improvement at short term (standardised mean difference [SMD] = −0.62, 95% confidence interval [CI]: −0.98 to −0.27, P = 0.0006, SMD = −0.74, 95% CI: −1.11 to 0.36, P = 0.0001, respectively), medium term (SMD = −0.53, 95% CI: −0.83 to −0.23, P = 0.0006, SMD = −0.50, 95% CI: −0.75 to −0.25, P = 0.0006), and long term (SMD = −0.69, 95% CI: −1.08 to −0.30, P = 0.0006, SMD = −0.68, 95% CI: −0.1.09 to −0.27, P = 0.001, respectively). A subgroup analysis of the data from large trials and from trials composed of less than 50% female patients revealed that therapeutic effects of the treatment are insignificant.
Conclusions
According to the currently available data, PRP injections are beneficial for pain relief and function improvement in patients with OA. This meta-analysis, however, demonstrated that the efficacy of PRP is related to sample size and gender composition. Thus, more randomised controlled trials of high quality and larger patient size, also including gender aspects, are required to understand this phenomenon.
The translational potential of this article
The translation potential of this meta-analysis is that provided another perspective to analyse the treatment effect of PRP for OA. In future research, phenotypes subpopulation and gender difference of OA patient should be considered for PRP treatment.
Keywords: Meta-analysis, Osteoarthritis, Platelet-rich plasma, Randomised controlled trials
Abbreviations: PRP, platelet-rich plasma; OA, osteoarthritis; RCTs, randomised controlled trials; ICTRP, International Clinical Trials Registry Platform; FDA, the U.S. Food and Drug Administration; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; IA, intra-articular; SMD, standardised mean difference; CI, confidence intervals; LP, leucocyte-poor; LR, Leucocyte-rich; CCTs, clinical controlled trials; HA, hyaluronic acid
Introduction
Osteoarthritis (OA) is the most prevalent degenerative joint disorder characterised by pain, stiffness, limitation of movement, and disability [1], affecting approximately 18% of women and 10% of men older than 60 years of age [2]. Despite intensive laboratory and clinical investigations, unambiguously effective therapies targeting the underlying causes have not yet been developed [2]. Blood-derived products as a safe treatment could modify the biological microenvironment at different points in the disease process and might provide an opportunity to interfere with self-perpetuating mechanisms in OA [3]. One of the strategies to relieve symptoms of OA is the injection of platelet-rich plasma (PRP) into affected joints because it is safe, simple to use, and acceptable [3]. PRP is an autologous concentrate of human platelets isolated through centrifugation of the patient's blood, containing numerous components including growth factors, cytokines, and many other mediators. PRP injections have been shown to be able to promote healing of injured tendons, ligaments, muscles, and joints and can also be applied when various musculoskeletal problems occur [4]. Although intraarticular (IA) injections of PRP are legally available and offered in the United States to patients with OA in the clinic [5], meta-analysis published to date have not reached consistent conclusions and the American Academy of Orthopaedic Surgeons (AAOS) guideline mentions “we are unable to recommend for or against growth factor injections and/or PRP for patients with symptomatic OA of the knee” [[5], [6], [7], [8], [9]]. New randomised controlled trials have been conducted after the most recently published meta-analysis on PRP [[10], [11], [12], [13]]. In this study, we identified all randomised trials published to date and analysed all of which fulfilled the required quality standard to provide a statistically supported and updated insight into the efficacy of PRP in treating OA.
Methods
This meta-analysis was performed according to the Cochrane Handbook for Systematic Reviews of Interventions [14] and presented based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines [15]. The protocol for this meta-analysis is available in International Prospective Register of Systematic Reviews (PROSPERO) (CRD42018100067).
Data sources and searches
We identified studies that evaluated the efficacy of PRP for patients with OA by searching PubMed, the Cochrane Central Register of Controlled Trials, EMBASE, EBSCO, ClinicalTrials.gov, and International Clinical Trials Registry Platform databases from inception to June 12, 2018. The search terms used were “platelet rich plasma”, “platelet rich fibrin”, “PRP” combined with “osteoarthritis” and “osteoarthrosis”; Table S1 shows the search strategy details. We also extracted relevant articles that met the inclusion criteria for randomised trials which were included in previous systematic reviews or meta-analysis.
Eligibility criteria
Types of studies
Randomised controlled trials available as full-text articles were potentially eligible for inclusion.
Types of participants
Adult patients who were clinically diagnosed with OA based on the criteria described by the American College of Rheumatology or clinical and radiological information.
Types of interventions
Studies of interest were patients who received IA injections with PRP or closely related platelet-containing products [i.e., autologous blood, platelet–leucocyte gel, platelet concentrate, platelet gel, or plasma rich in growth factors (PRGF-Endoret)], which were compared with control treatments including saline, or no treatment, or another active treatment (e.g. nonsteroidal anti inflammatory drugs, hyaluronic acid [HA], or physical therapy). Further inclusion criteria were that platelet-rich therapy was the only treatment given or was delivered in addition to a standard of care treatment applied to all trial participants, which includes operative or nonoperative measures.
Types of outcomes
Studies reporting one or both of the following outcome measures were eligible for inclusion: (1) pain and (2) physical function, measured with standard medical instruments.
Data extraction
Two authors independently extracted the following information: authors, the year of publication, country, age distribution, gender proportion, study design, intervention condition, intervention period, and outcome measures. If there were disagreements between the two reviewers, a third author was consulted to decide for inclusion or exclusion of the study for the meta-analysis. If the trials permitted multiple comparisons, only the information and data of interest reported in the original articles were extracted. In case the necessary information on any data was unavailable but important for the study, the authors responsible for the published report were contacted and the data were obtained.
Risk of bias assessment
Studies that met the inclusion criteria were evaluated for methodological quality to assess the risk of bias using the Cochrane Collaboration's risk of bias tool as described in the Cochrane Handbook for Systematic Reviews of Interventions; each quality item was graded into three categories: low risk, high risk, or unclear risk [14]. The quality assessment covered the following domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other possible sources of bias. Review Manager 5.3 (Nordic Cochrane Centre) was used to present the results graphically. This assessment was performed independently by two reviewers and in the event of disagreements regarding the assessment of studies, a third reviewer was consulted.
Data synthesis and analysis
We used a prespecified cutoff of 50 randomly assigned patients per arm to distinguish between small-scale and large-scale trials and grouped the outcomes into three time points of assessment: short term (≤3 months), medium term (>3 months but ≤6 months), and long term (12 months).
Continuous outcomes were used for statistical efficacy analysis using Hedge's standardised mean difference (SMD) with 95% confidence intervals (CIs) with the random effects model for pooling estimates for each analysis. The significance of the pooled effects was evaluated by a Z-test, and a P value of less than 0.05 was considered significant. I2 statistic was used to examine overall heterogeneity between studies, and values higher than 50% were defined to have high heterogeneity [16].
Subgroup analyses were carried out according to trial size (large trials versus small trials), patient characteristics (age and gender), and PRP preparation method (PRP category, spinning approach, and activator).
All statistical analyses were performed using Review Manager 5.3 and Stata software (version 15.1; StataCorp, USA).
Results
Study identification and characteristics
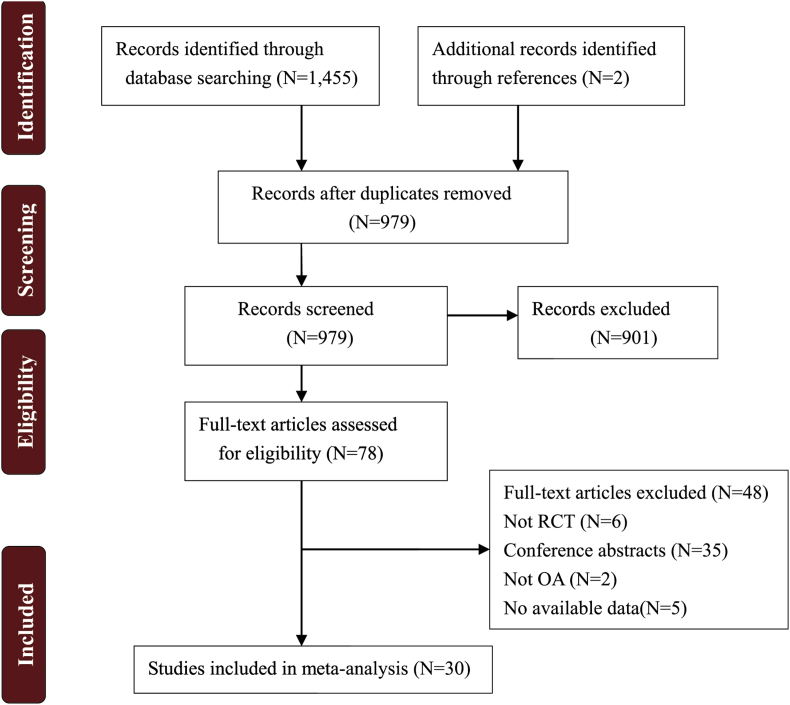
From the database search, 1455 potentially eligible records and two previously published meta-studies [17,18] were identified, from which 478 articles were duplicates. After a review of the abstract, 901 studies did not meet our inclusion criteria and were excluded. The remaining 78 full-text documents were analysed, however, only 30 randomised controlled trials (RCTs) [10,12,13,[17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43]] were ultimately included in this meta-analysis (Figure 1).
Figure 1.
Diagram of the study selection process for the systematic review and meta-analysis.
All studies were randomised and 12 were double-blind trials; 20 trials described an adequate random sequence generation process. The risk of bias of included studies is shown in Figure S1. The characteristics of the included data are summarised in Table 1. The 30 included trials were published between 2012 and 2018, with sample sizes ranging from 31 to 183 patients and a total of 2178 patients. The mean participant age ranged from 32.3 to 71.4 years.
Table 1.
Characteristics of included studies.
| Study | Country | PRP group |
Control group |
Outcome | Measurement time point (month) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample size | Age (year) | Female (%) | Category | Spinning approach | Activator | Sample size | Age (year) | Female (%) | Intervention | ||||
| Sánchez, 2012 [17] | Spain | 89 | 60.5 ± 7.9 | 52 | LP | Single | CaCl2 | 87 | 58.9 ± 8.2 | 52 | HA | WOMAC pain, stiffness, physical function, and adverse event | 6 |
| Spakova, 2012 [19] | Slovakia | 60 | 52.8 ± 12.4 | 45 | LP | Treble | NR | 60 | 53.2 ± 14.5 | 48 | HA | NRS and adverse event | 3 and 6 |
| Battaglia, 2013 [20] | Italy | 50 | 51.0 ± 12.0 | 40 | LR | Double | CaCl2 | 50 | 56.0 ± 12.0 | 34 | HA | VAS, HHS, and adverse event | 1, 3, 6, and 12 |
| Patel, 2013 [21] | India | 27 | 53.1 ± 11.6 | 59 | LP | Single | CaCl2 | 23 | 53.7 ± 8.2 | 74 | Saline | VAS | 6 |
| Vaquerizo, 2013 [18] | Spain | 48 | 62.4 ± 6.6 | 67 | LP | Single | CaCl2 | 48 | 64.8 ± 7.7 | 54 | HA | WOMAC pain, stiffness, physical function, and adverse event | 6 and 12 |
| Rayegani, 2014 [22] | Iran | 31 | 58.1 ± 9.0 | 94 | LR | Double | NR | 31 | 54.7 ± 10.8 | 94 | Unclear | WOMAC pain, stiffness, and physical function | 6 |
| Angoorani, 2015 [23] | Iran | 27 | 62.2 ± 12.1 | 82 | LR | Double | CaCl2 | 27 | 61.6 ± 8.1 | 93 | TENS+exercise | KOOS and adverse event | 1 and 2 |
| Duif, 2015 [25] | Germany | 24 | 64.1 ± 9.0 | 42 | LP | Single | NR | 34 | 64.3 ± 9.0 | 65 | Blank | Lysholm | 1.5, 6, and 12 |
| Filardo, 2015 [26] | Italy | 94 | 53.3 ± 13.2 | 36 | LR | Double | CaCl2 | 89 | 57.6 ± 11.8 | 42 | HA | KOOS, EQ-VAS, IKDC, and adverse event | 2, 6, and 12 |
| Hegab, 2015 [27] | Egypt | 25 | 39.0 ± 5.0 | 64 | LP | Single | NR | 25 | 38.2 ± 4.4 | 56 | HA | VAS, MMO, and adverse event | 12 |
| Kiliç, 2015 [24] | Turkey | 18 | 32.2 ± 14.3 | 88 | LR | Single | NR | 12 | 35.1 ± 14.8 | 93 | Blank | VAS, MMO, adverse event | 12 |
| Raeissadat, 2015 [28] | Iran | 77 | 56.9 ± 9.1 | 90 | LR | Double | NR | 62 | 61.1 ± 7.5 | 76 | HA | WOMAC pain, stiffness, and physical function | 12 |
| Forogh, 2016 [31] | Iran | 24 | 59.1 ± 7.0 | 71 | LR | Double | CaCl2 | 24 | 61.1 ± 6.7 | 63 | corticosteroid | VAS and KOOS | 2 and 6 |
| Kiliç, 2016 [29] | Turkey | 18 | 32.2 ± 14.3 | 89 | LR | Single | NR | 13 | 28.1 ± 11.1 | 77 | HA | VAS, MMO, and adverse event | 12 |
| Lana, 2016 [32] | USA | 36 | 60.9 ± 7.0 | 81 | LR | Double | thrombin | 36 | 60.0 ± 6.6 | 92 | HA | VAS, WOMAC pain, stiffness, and physical function | 1, 3, 6, and 12 |
| Mario, 2016 [34] | Mexico | 33 | 57.2 ± 8.1 | 67 | LP | Double | CaCl2 | 32 | 55.6 ± 11.4 | 62 | acetaminophen | VAS | 3 |
| Paterson, 2016 [33] | Australia | 11 | 49.9 ± 13.7 | 27 | LR | Double | Ultraviolet light | 10 | 52.7 ± 10.3 | 30 | HA | VAS, KOOS, and adverse event | 1 and 3 |
| Sante, 2016 [30] | Italy | 21 | 71.4 ± 6.0 | 48 | LP | Double | NR | 22 | 73.6 ± 7.9 | 59 | HA | VAS, WOMAC pain, stiffness, and physical function | 1 and 4 |
| Smith, 2016 [35] | USA | 15 | 53.5 ± 8.2 | 67 | LP | Single | NR | 15 | 46.6 ± 9.3 | 60 | Saline | WOMAC pain, stiffness, physical function, and adverse event | 0.25, 0.5, 2, 3, 6, and 12 |
| Cole, 2017 [36] | USA | 49 | 56.0 ± 10.4 | 43 | LP | Single | NR | 50 | 56.9 ± 10.5 | 60 | HA | WOMAC pain | 3, 6, and 12 |
| Doria, 2017 [37] | Italy | 40 | 67.3 ± 5.8 | Unclear | LR | Double | thrombin | 40 | 68.0 ± 4.6 | unclear | HA | VAS, WOMAC pain, stiffness, physical function, HHS, and adverse events | 6 and 12 |
| Duymus, 2017 [38] | Turkey | 33 | 60.4 ± 5.1 | 97 | LR | Single | NR | 34 | 60.3 ± 9.1 | 97 | HA | WOMAC pain, stiffness, and physical function | 1, 3, 6, and 12 |
| Görmeli, 2017 [39] | Turkey | 39 | 53.7 ± 13.1 | 59 | LR | Double | CaCl2 | 39 | 53.5 ± 14.0 | 56 | HA | IKDC and EQ-VAS | 6 |
| Jubert, 2017 [40] | Spain | 35 | 65.6 ± 8.6 | 66 | LP | Double | NR | 30 | 68.0 ± 7.2 | 80 | corticosteroid | VAS and KOOS | 1, 3, and 6 |
| Raeissadat, 2017 [41] | Iran | 36 | 57.0 ± 7.2 | 81 | LR | Trible | CaCl2 | 33 | 59.5 ± 7.5 | 82 | HA | VAS, WOMAC pain, stiffness, and physical function | 2 and 6 |
| Ahmad, 2018 [10] | Egypt | 45 | 56.2 ± 6.8 | 69 | LR | Single | NR | 44 | 56.8 ± 7.4 | 68 | HA | IKDC, NRS and VAS | 3 and 6 |
| Louis, 2018 [11] | France | 24 | 53.2 ± 11.7 | 42 | LP | Double | CaCl2 | 24 | 48.5 ± 11.5 | 54 | HA | VAS, WOMAC pain, stiffness, physical function, and adverse events | 1, 3, and 6 |
| Su, 2018 [12] | China | 25 | 54.2 ± 6.6 | 56 | LR | Double | CaCl2 | 30 | 53.1 ± 6.4 | 60 | HA | VAS, WOMAC pain, stiffness, physical function, and adverse events | 1, 3, 6, 12, and 18 |
| Wu, 2018 [42] | China | 20 | 63.3 ± 6.8 | 75 | LR | Single | NR | 20 | 63.3 ± 6.8 | 75 | Saline | WOMAC pain, stiffness, physical function, and adverse event | 0.5, 1, 3, and 6 |
| Zhang, 2018 [13] | China | 30 | 65.5 ± 5.8 | 53 | LR | Double | NR | 30 | 66.2 ± 4.9 | 60 | HA | VAS and Lysholm | 1 and 3 |
HA = hyaluronic acid; LP = leucocyte-poor; LR = leucocyte-rich; PRP = platelet-rich plasma; VAS = Visual Analogue Scale; WOMAC = the Western Ontario and McMaster Universities Osteoarthritis Index; IKDC = the International Knee Documentation Committee Subjective Knee Form; KOOS = the Knee Injury and Osteoarthritis Outcome Score; EQ-VAS = EuroQol Visual Analogue Scale; HHS = Household Hunger Scale; MMO = maximum mouth opening; NR = Not report; TENS = transcutaneous electrical nerve stimulation; Lysholm = the Lysholm score; NRS = numeric rating scale.
Outcome of meta-analysis
Detailed scale data that were included in the meta-analysis are summarised in Table S2.
Pain relief
Short-term follow-up
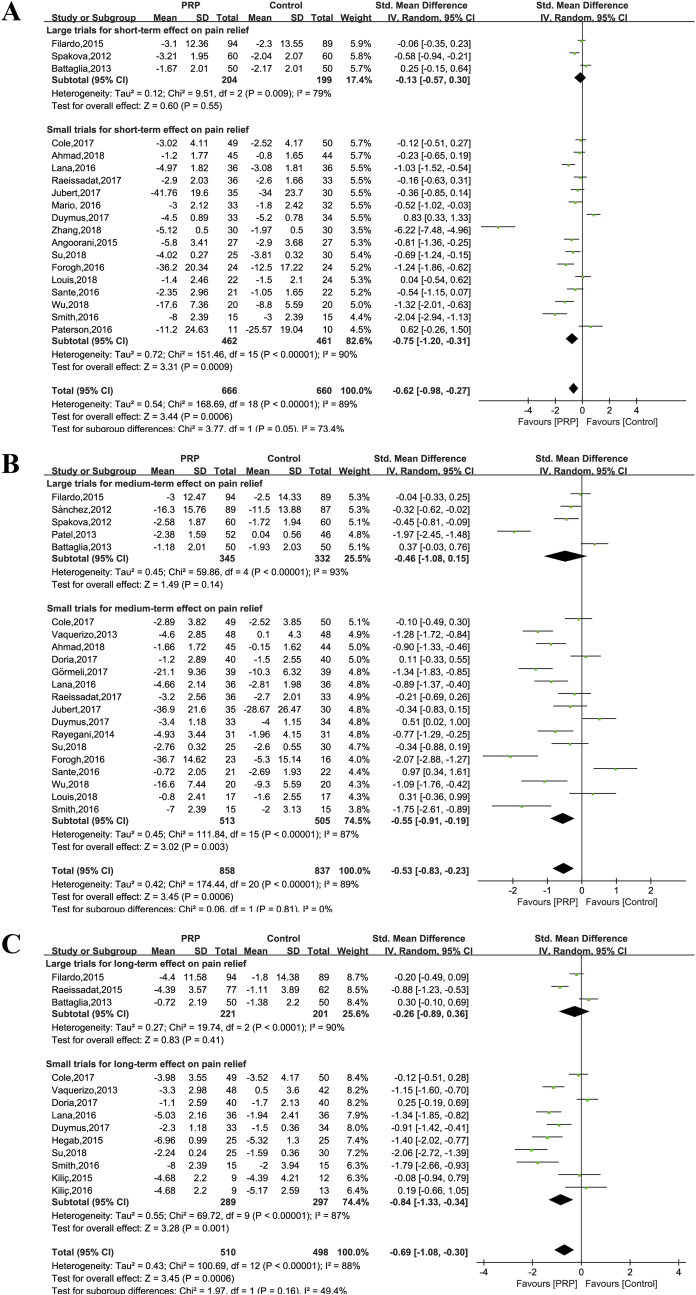
Data of nineteen trials (1326 patients) contributed to the metaanalysis of pain relief at short-term. Unexplained statistical heterogeneity was excessive (I2 = 89%), and we could not identify a particular trial causing this excess variability (Figure 2A). Data pooling when such a high degree of heterogeneity of unknown cause exists is not advisable [44]. If the data were pooled, a significant effect of PRP treatment on pain was observed (SMD = −0.62, 95% CI: −0.98 to −0.27, P = 0.0006). In contrast to this, pooling large trials showed no significant effect of the treatment on pain levels (SMD = −0.13, 95% CI: −0.57 to 0.30, P = 0.55), but both results were heterogeneous (I2 = 79%) (Figure 2A).
Figure 2.
Forest plot for effectiveness of PRP compared with controls for pain relief. (A). At short-term follow-up; (B) at medium-term follow-up (C) at long-term follow-up.
Medium-term follow-up
Twenty-one trials reported pain reduction in the treatment group (n = 859) relative to the control group (n = 838) at medium-term follow-up. Pooling showed that PRP injection had a benefit on pain reduction when compared with all controls (SMD = −0.53, 95% CI: −0.83 to −0.23, P = 0.0006) with an excessive degree of unexplained statistical heterogeneity (I2 = 89%) (Figure 2B). When pooling large trials comparing PRP with all controls (SMD = −0.46, 95% CI: −1.08 to 0.15, P = 0.14) (Figure 2B), results were inconclusive. One trial [30] was only followed for 4 months, in contrast to other studies, which were followed for 6 months. The exclusion of the 4-month trial did not statistically change the magnitude or direction of the overall obtained observations.
Long-term follow-up
Thirteen studies were available for analysis (510 intervention patients and 498 control patients). To ensure the consistency of results, data from a 12-month follow-up period were used instead of 18-month follow-up [12]. Pooling these studies showed a significant overall effect of PRP treatment being beneficial (SMD = −0.69, 95% CI: −1.08 to −0.30, P = 0.0006). However, no significant effect is observed when pooling large trials (SMD = −0.26, 95% CI: −0.89 to 0.36, P = 0.41) (Figure 2C).
Physical function improvement
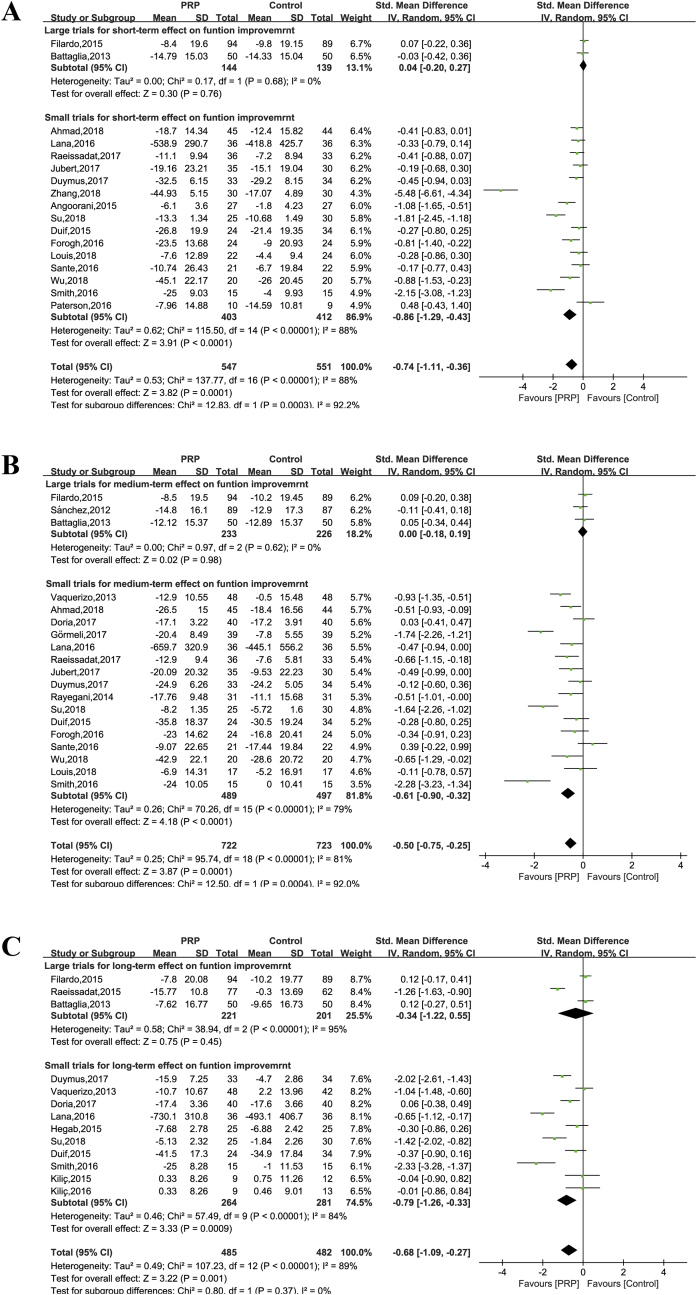
Seventeen trials reported a measure of joint function assessed after a short-term period (547 intervention patients and 551 control patients). Because different measurement systems were used in these trials, we calculated the standardised effect. Only two large trials were available [20,26], which showed no significant effect favouring PRP treatment for joint function improvement when their data were pooled (SMD = 0.04, 95% CI: −0.20 to 0.27, P = 0.76), and heterogeneity was acceptable (I2 = 0%) (Figure 3A). At medium-term and long-term follow-up, metaanalysis of both large trials could not demonstrate a significant effect of PRP treatment for both time periods. The overall SMD between the groups at short term was −0.74 (95% CI: −1.11 to 0.36, P = 0.0001) (Figure 3A), at medium term −0.50 (95% CI: −0.75 to −0.25, P = 0.0006) (Figure 3B), and at long term −0.68 (95% CI: −0.1.09 to −0.27, P = 0.001) (Figure 3C); the results of both were heterogeneous (I2 = 88%, 81%, and 84%, respectively).
Figure 3.
Forest plot for effectiveness of PRP compared with controls for function improvement. (A) At short-term follow-up; (B) at medium-term follow-up; (C) at long-term follow-up.
Subgroup analysis
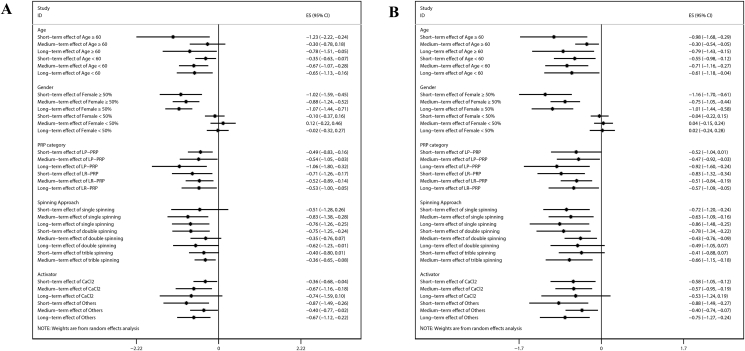
The meta-analysis revealed no significant effect for all follow-up periods regarding pain relief or joint function improvement (Figure 4) in the case when data were pooled with a proportion of women of less than 50%. While subgroup analyses showed that PRP treatments have consistently a supportive effect, no conclusion can be drawn about the treatment effects of different PRP protocols (category, spinning approach, and activator). Both, leucocyte-poor and Leucocyte-rich PRP preparations were shown to both have a significant effect on pain relief in all follow-up periods. In addition, a positive effect on pain relief and function improvement at short term and medium term but not in the long term was observed with CaCl2 as an activator.
Figure 4.
Subgroup analysis of meta-analysis. (A) Pain relief result; (B) function improvement result.
Discussion
The meta-analysis of the entire dataset of all randomised control PRP treatments fulfilling the selection criteria for this study demonstrated that the injection of PRP has only a supportive effect on pain relief and function improvement in patients with OA. A superior effect of PRP treatments was not observed in the subgroup of large trials, as no significant effect on pain relief or joint function improvement was observed at short-, medium-, and long-term follow-up time points. The same result was seen when data were analysed in which the proportion of female patients was less than 50%.
Several systematic reviews or meta-analysis investigating the effectiveness of PRP for OA have been published [[5], [6], [7], [8],[44], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [56]], but pooled RCTs and clinical controlled trials would increase the risk of selection bias [6,45,[48], [50], [51],55]. A meta-analysis was performed to compare outcomes between PRP injections versus HA or placebo for knee OA where PRP injections were shown to be more effective in reducing pain and improving functions, as measured by the scale stratified analysis. Another meta-analysis revealed that at 12-month post-injection, PRP was associated with superior pain relief and function improvement [47]. Similarly, a meta-analysis including ten RCTs also showed significantly higher outcome scores with PRP injections when compared with HA [7]. These results were similar to our overall meta-analysis. Interestingly, we observed that larger trials showed no statistical difference when comparing PRP treatments with controls, and our meta-analysis also indicates that gender composition of the patient group has a major impact. The data of treatment effects were drastically different in subgroups with less than 50% women, for which we observed no significant benefit of PRP treatments, suggesting that PRP may not be effective in male patients. One possible explanation is that the existence of different OA phenotypes, PRP may only be effective for a certain phenotype, so when the sample size is expanded, the direction would be changed. Similar conclusions were found as CR4056 is effective for metabolic OA phenotype and males but not for all population [57]. This conclusion requires further validation, possibly by conducting a study or studies focussing specifically on gender or specific phenotype. In addition, from the data of this meta-analysis, we could not draw conclusion of which PRP preparation method is the best one.
Our findings have important implications for clinical practice and further research. It becomes obvious that it is essential to find novel, highly efficient therapeutic strategies for OA treatment. Our meta-analysis demonstrated that PRP has a minor beneficial effect on pain relief and function improvement when the entire dataset was analysed. In contrast to this, no significant effects were observed in large trials. In addition, if the patient cohort was composed of a majority of male patients, no efficacy of the treatment could be shown. Both sample size and gender effects suggest that, it is essential to develop disease-modifying drugs in future research and to pay special attention to gender to validate our conclusion of this metastudy on the efficacy of PRP.
To the best of our knowledge, this meta-analysis is a comprehensive update that systematically and quantitatively evaluates the effectiveness of PRP for OA by including RCTs for a more accurate analysis. Our meta-analysis provided another perspective to analyse the treatment effect of PRP for OA by trial size. In addition, we used different time points for our analysis instead of an end time point, which also highlights the temporal effect of PRP efficacy.
Some limitations include the significant heterogeneity in each calculation and the variation of nonstandardised evaluation tools used across different studies. Besides, the OA grade also is an important factor that influences the efficacy of PRP and satisfaction of patients. However, owing to the limitations of the original research, we cannot obtain the efficacy data of different OA grades, which limits the guiding significance of our research for clinical treatment.
Conclusions
According to the currently available evidence, PRP injections are beneficial for pain relief and function improvement in OA. However, this meta-analysis demonstrated that the efficacy of PRP is related to sample size and gender composition. To fully evaluate the benefits of this treatment, it is obvious that more high-quality randomised controlled trials with larger patient numbers need to be conducted and the phenotypes subpopulation and gender difference should be considered. To advance the treatment or improve its efficacy, it is also necessary to understand the underlying cellular and molecular mechanisms occurring after PRP injections. This would ideally lead to the optimisation and standardisation of the PRP preparation method in the future.
Conflict of interest
The authors have no conflicts of interest to disclose in relation to this article.
Acknowledgements
The authors thank Lana Jose Fabio Santos Duarte (Lana JFSD) for providing us with the original data for analysis. This work was supported by the National Key Research and Development Program of China (2017YFA0104900); the National Natural Science Foundation of China (NSFC) grants (81630065, 31830029).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2019.10.002.
Contributor Information
Xiao-hui Zou, Email: zouxiaohui@zju.edu.cn.
Hongwei Ouyang, Email: hwoy@zju.edu.cn.
Appendix ASupplementary data
The following is/are the Supplementary data to this article:
References
- 1.Martel-Pelletier J., Barr A.J., Cicuttini F.M., Conaghan P.G., Cooper C., Goldring M.B. Osteoarthritis. Nat Rev Dis Primers. 2016;2:16072. doi: 10.1038/nrdp.2016.72. [DOI] [PubMed] [Google Scholar]
- 2.Glyn-Jones S., Palmer A.J., Agricola R., Price A.J., Vincent T.L., Weinans H. Osteoarthritis. Lancet. 2015;386(9991):376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 3.Andia I., Maffulli N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat Rev Rheumatol. 2013;9(12):721. doi: 10.1038/nrrheum.2013.141. [DOI] [PubMed] [Google Scholar]
- 4.Foster T.E., Puskas B.L., Mandelbaum B.R., Gerhardt M.B., Rodeo S.A. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37(11):2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 5.Jones I.A., Togashi R., Wilson M.L., Heckmann N., Vangsness C.T., Jr. Intra-articular treatment options for knee osteoarthritis. Nat Rev Rheumatol. 2019;15(2):77–90. doi: 10.1038/s41584-018-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang H.F., Wang C.G., Li H., Huang Y.T., Li Z.J. Intra-articular platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: a meta-analysis. Drug Des Dev Ther. 2018;12:445–453. doi: 10.2147/DDDT.S156724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Z., Luo J., Huang X., Wang B., Zhang J., Zhou A. Efficacy of platelet-rich plasma in pain and self-report function in knee osteoarthritis: a best-evidence synthesis. Am J Phys Med Rehabil. 2017;96(11):793–800. doi: 10.1097/PHM.0000000000000746. [DOI] [PubMed] [Google Scholar]
- 8.Kanchanatawan W., Arirachakaran A., Chaijenkij K., Prasathaporn N., Boonard M., Piyapittayanun P. Short-term outcomes of platelet-rich plasma injection for treatment of osteoarthritis of the knee. Knee Surg Sport Traumatol Arthrosc. 2016;24(5):65–1677. doi: 10.1007/s00167-015-3784-4. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z., Liu Y., Liu M. Platelet-rich plasma injection is not more effective than hyaluronic acid to treat knee osteoarthritis when using a random-effects model. Br J Sports Med. 2016;50(15):953–954. doi: 10.1136/bjsports-2015-095512. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad H.S., Farrag S.E., Okasha A.E., Kadry A.O., Ata T.B., Monir A.A. Clinical outcomes are associated with changes in ultrasonographic structural appearance after platelet-rich plasma treatment for knee osteoarthritis. Int J Rheum Dis. 2018;21(5):960–966. doi: 10.1111/1756-185X.13315. [DOI] [PubMed] [Google Scholar]
- 11.Louis M.L., Magalon J., Jouve E., Bornet C.E., Mattei J.C., Chagnaud C. Growth factors levels determine efficacy of platelets rich plasma injection in knee osteoarthritis: a randomized double blind noninferiority trial Compared with viscosupplementation. Arthroscopy. 2018;34(5):1530–1540.e2. doi: 10.1016/j.arthro.2017.11.035. [DOI] [PubMed] [Google Scholar]
- 12.Su K., Bai Y., Wang J., Zhang H., Liu H., Ma S. Comparison of hyaluronic acid and PRP intra-articular injection with combined intra-articular and intraosseous PRP injections to treat patients with knee osteoarthritis. Clin Rheumatol. 2018;37(5):1341–1350. doi: 10.1007/s10067-018-3985-6. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y. Platelet-rich plasma therapy in refractory knee osteoarthritis combined with infection. Int J Clin Exp Med. 2018;11(5):4801–4807. [Google Scholar]
- 14.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J. The Cochrane Collaboration; 2011. Cochrane handbook for systematic reviews of interventions version 5.1.0.http://trainingcochraneorg/handbook [Google Scholar]
- 15.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 16.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez M., Delgado D., Sánchez P., Fiz N., Azofra J., Orive G. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy. 2012;28(8):1070–1078. doi: 10.1016/j.arthro.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Vaquerizo V., Plasencia M.A., Arribas I., Seijas R., Padilla S., Orive G. Comparison of intra-articular injections of plasma rich in growth factors (PRGF-Endoret) versus Durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: a randomized controlled trial. Arthroscopy. 2013;29(10):1635–1643. doi: 10.1016/j.arthro.2013.07.264. [DOI] [PubMed] [Google Scholar]
- 19.Spakova T., Rosocha J., Lacko M., Harvanova D., Gharaibeh A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91(5):411–417. doi: 10.1097/PHM.0b013e3182aab72. [DOI] [PubMed] [Google Scholar]
- 20.Battaglia M., Guaraldi F., Vannini F., Rossi G., Timoncini A., Buda R. Efficacy of ultrasound-guided intra-articular injections of platelet-rich plasma versus hyaluronic acid for hip osteoarthritis. Orthopedics. 2013;36(12):e1501–e1508. doi: 10.3928/01477447-20131120-13. [DOI] [PubMed] [Google Scholar]
- 21.Patel S., Dhillon M.S., Aggarwal S., Marwaha N., Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356–364. doi: 10.1177/0363546512471299. [DOI] [PubMed] [Google Scholar]
- 22.Rayegani S.M., Raeissadat S.A., Taheri M.S., Babaee M., Bahrami M.H., Eliaspour D. Does intra articular platelet rich plasma injection improve function, pain and quality of life in patients with osteoarthritis of the knee? A randomized clinical trial. Orthop Rev. 2014;6(3):112–117. doi: 10.4081/or.2014.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angoorani H., Mazaherinezhad A., Marjomaki O., Younespour S. Treatment of knee osteoarthritis with platelet-rich plasma in comparison with transcutaneous electrical nerve stimulation plus exercise: a randomized clinical trial. Med J Islam Repub Iran. 2015;29:223. [PMC free article] [PubMed] [Google Scholar]
- 24.Cömert Kiliç S., Güngörmüs M., Sümbüllü M.A. Is arthrocentesis plus platelet-rich plasma superior to arthrocentesis alone in the treatment of temporomandibular joint osteoarthritis? A randomized clinical trial. J Oral Maxillofac Surg. 2015;73(8):1473–1483. doi: 10.1016/j.joms.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 25.Duif C., Vogel T., Topcuoglu F., Spyrou G., von Schulze Pellengahr C., Lahner M. Does intraoperative application of leukocyte-poor platelet-rich plasma during arthroscopy for knee degeneration affect postoperative pain, function and quality of life? A 12-month randomized controlled double-blind trial. Arch Orthop Trauma Surg. 2015;135(7):971–977. doi: 10.1007/s00402-015-2227-5. [DOI] [PubMed] [Google Scholar]
- 26.Filardo G., Di Matteo B., Di Martino A., Merli M.L., Cenacchi A., Fornasari P. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43(7):1575–1582. doi: 10.1177/0363546515582027. [DOI] [PubMed] [Google Scholar]
- 27.Hegab A.F., Ali H.E., Elmasry M., Khallaf M.G. Platelet-Rich Plasma injection as an effective treatment for temporomandibular joint osteoarthritis. J Oral Maxillofac Surg. 2015;73(9):1706–1713. doi: 10.1016/j.joms.2015.03.045. [DOI] [PubMed] [Google Scholar]
- 28.Raeissadat S.A., Rayegani S.M., Hassanabadi H., Fathi M., Ghorbani E., Babaee M. Knee osteoarthritis injection choices: platelet-rich plasma (PRP) versus hyaluronic acid (a one-year randomized clinical trial) Clin Med Insights Arthritis Musculoskelet Disord. 2015;8:1–8. doi: 10.4137/CMAMD.S17894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cömert Kiliç S., Güngörmüş M. Is arthrocentesis plus platelet-rich plasma superior to arthrocentesis plus hyaluronic acid for the treatment of temporomandibular joint osteoarthritis: a randomized clinical trial. Int J Oral Maxillofac Surg. 2016;45(12):1538–1544. doi: 10.1016/j.ijom.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Di Sante L., Villani C., Santilli V., Valeo M., Bologna E., Imparato L. Intra-articular hyaluronic acid vs platelet-rich plasma in the treatment of hip osteoarthritis. Med Ultrason. 2016;18(4):463–468. doi: 10.11152/mu-874. [DOI] [PubMed] [Google Scholar]
- 31.Forogh B., Mianehsaz E., Shoaee S., Ahadi T., Raissi G., Sajadi S. Effect of single injection of platelet-rich plasma in comparison with corticosteroid on knee osteoarthritis: a double-blind randomized clinical trial. J Sports Med Phys Fitness. 2016;56(7–8):901–908. [PubMed] [Google Scholar]
- 32.Lana J.F., Weglein A., Sampson S.E., Vicente E.F., Huber S.C., Souza C.V. Randomized controlled trial comparing hyaluronic acid, platelet-rich plasma and the combination of both in the treatment of mild and moderate osteoarthritis of the knee. J Stem Cells Regen Med. 2016;12(2):69–78. doi: 10.46582/jsrm.1202011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paterson K.L., Nicholls M., Bennell K.L., Bates D. Intra-articular injection of photo-activated platelet-rich plasma in patients with knee osteoarthritis: a double-blind, randomized controlled pilot study. BMC Muscoskelet Disord. 2016;17:67. doi: 10.1186/s12891-016-0920-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simental-Mendía M., Vílchez-Cavazos J.F., Peña-Martínez V.M., Said-Fernández S., Lara-Arias J., Martí3nez-Rodríguez H.G. Leukocyte-poor platelet-rich plasma is more effective than the conventional therapy with acetaminophen for the treatment of early knee osteoarthritis. Arch Orthop Trauma Surg. 2016;136(12):1723–1732. doi: 10.1007/s00402-016-2545-2. [DOI] [PubMed] [Google Scholar]
- 35.Smith P. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44(4):884–891. doi: 10.1177/0363546515624678. [DOI] [PubMed] [Google Scholar]
- 36.Cole B.J., Karas V., Hussey K., Pilz K., Fortier L.A. Hyaluronic acid versus platelet-rich plasma: a prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2017;45(2):339–346. doi: 10.1177/0363546516665809. [DOI] [PubMed] [Google Scholar]
- 37.Doria C., Mosele G.R., Caggiari G., Puddu L., Ciurlia E. Treatment of early hip osteoarthritis: ultrasound-guided platelet rich plasma versus hyaluronic acid injections in a randomized clinical trial. Joints. 2017;5(3):152–155. doi: 10.1055/s-0037-1605584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duymus T.M., Mutlu S., Dernek B., Komur B., Aydogmus S., Kesiktas F.N. Choice of intra-articular injection in treatment of knee osteoarthritis: platelet-rich plasma, hyaluronic acid or ozone options. Knee Surg Sport Traumatol Arthrosc. 2017;25(2):485–492. doi: 10.1007/s00167-016-4110-5. [DOI] [PubMed] [Google Scholar]
- 39.Görmeli G., Görmeli C., Ataoglu B., Çolak C., Aslantürk O., Ertem K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sport Traumatol Arthrosc. 2017;25(3):958–965. doi: 10.1007/s00167-015-3705-6. [DOI] [PubMed] [Google Scholar]
- 40.Joshi Jubert N., Rodriguez L., Reverte-Vinaixa M., Navarro A. Platelet-rich plasma injections for advanced knee osteoarthritis: a prospective, randomized, double-blinded clinical trial. Orthop J Sports Med. 2017;5(2) doi: 10.1177/2325967116689386. 2325967116689386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raeissadat S.A., Rayegani S.M., Ahangar A.G., Abadi P.H., Mojgani P., Ahangar O.G. Efficacy of intra-articular injection of a newly developed plasma rich in growth factor (PRGF) versus hyaluronic acid on pain and function of patients with knee osteoarthritis: a single-blinded randomized clinical trial. Clin Med Insights Arthritis Musculoskelet Disord. 2017;10 doi: 10.1177/1179544117733452. 1179544117733452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yung-Tsan W., Kao-Chih H., Tsung-Ying L., Cheng-Kuang C., Liang-Cheng C. Effects of platelet-rich plasma on pain and muscle strength in patients with knee osteoarthritis. Am J Phys Med Rehabil. 2018;97(4):248–254. doi: 10.1097/PHM.0000000000000874. [DOI] [PubMed] [Google Scholar]
- 43.Arrich J., Piribauer F., Mad P., Schmid D., Klaushofer K., Müllner M. Intra-articular hyaluronic acid for the treatment of osteoarthritis of the knee: systematic review and meta-analysis. CMAJ (Can Med Assoc J) 2005;172(8):1039–1043. doi: 10.1503/cmaj.1041203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bousnaki M., Bakopoulou A., Koidis P. Platelet-rich plasma for the therapeutic management of temporomandibular joint disorders: a systematic review. Int J Oral Maxillofac Surg. 2018;47(2):188–198. doi: 10.1016/j.ijom.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 45.Chang K.V., Hung C.Y., Aliwarga F., Wang T.G., Han D.S., Chen W.S. Comparative effectiveness of platelet-rich plasma injections for treating knee joint cartilage degenerative pathology: a systematic review and meta-Analysis. Arch Phys Med Rehabil. 2014;95(3):562–575. doi: 10.1016/j.apmr.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 46.Chung P.Y., Lin M.T., Chang H.P. Effectiveness of platelet-rich plasma injection in patients with temporomandibular joint osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;S2212-4403(18):31187–31188. doi: 10.1016/j.oooo.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 47.Dai W.L., Zhou A.G., Zhang H., Zhang J. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Arthroscopy. 2017;33(3):659–670.e1. doi: 10.1016/j.arthro.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 48.Khoshbin A., Leroux T., Wasserstein D., Wolfstadt J., Law P.W., Mahomed N. The efficacy of platelet-rich plasma in the treatment of symptomatic knee osteoarthritis: a systematic review with quantitative synthesis. Arthroscopy. 2013;29(12):2037–2048. doi: 10.1016/j.arthro.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 49.Lai L.P., Stitik T.P., Foye P.M., Georgy J.S., Patibanda V., Chen B. Use of platelet-rich plasma in intra-articular knee injections for osteoarthritis: a systematic review. Pharm Manag PM R. 2015;7(6):637–648. doi: 10.1016/j.pmrj.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Laudy A.B., Bakker E.W., Rekers M., Moen M.H. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49(10):657–672. doi: 10.1136/bjsports-2014-094036. [DOI] [PubMed] [Google Scholar]
- 51.Shen L., Yuan T., Chen S., Xie X., Zhang C. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Orthop Surg. 2017;12(1):16. doi: 10.1186/s13018-017-0521-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meheux C.J., McCulloch P.C., Lintner D.M., Varner K.E., Harris J.D. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32(3):495–505. doi: 10.1016/j.arthro.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 53.Riboh J.C., Saltzman B.M., Yanke A.B., Fortier L., Cole B.J. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med. 2016;44(3):792–800. doi: 10.1177/0363546515580787. [DOI] [PubMed] [Google Scholar]
- 54.Sadabad H.N., Behzadifar M., Arasteh F., Behzadifar M., Dehghan H.R. Efficacy of platelet-rich plasma versus hyaluronic acid for treatment of knee osteoarthritis: a systematic review and meta-analysis. Electron Physician. 2016;8(3):2115–2122. doi: 10.19082/2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Souzdalnitski D., Narouze S.N., Lerman I.R., Calodney A. Platelet-rich plasma injections for knee osteoarthritis: systematic review of duration of clinical benefit. Tech Reg Anesth Pain Manag. 2015;19(1/2):67–72. [Google Scholar]
- 56.Ye Y., Zhou X., Mao S., Zhang J., Lin B. Platelet rich plasma versus hyaluronic acid in patients with hip osteoarthritis: a meta-analysis of randomized controlled trials. Int J Surg. 2018;53:279–287. doi: 10.1016/j.ijsu.2018.03.078. [DOI] [PubMed] [Google Scholar]
- 57.Rovati L.C., Brambilla N., Blicharski T., Connell J., Vitalini C., Bonazzi A. Efficacy and safety of the first-in-class imidazoline-2 receptor ligand CR4056 in pain from knee osteoarthritis and disease phenotypes: a randomized, double-blind, placebo-controlled phase 2 trial. Osteoarthritis Cartilage. 2019 doi: 10.1016/j.joca.2019.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.