Abstract
Introduction
Long-term study evidence about the BioBall® adapter system is limited, especially in highly morbid elderly patients. Thus, we analysed the long-term outcome of revision hip arthroplasty using this system in highly morbid elderly patients.
Materials and methods
We included 19 patients undergoing revision hip arthroplasty after primary or secondary total hip arthroplasty dislocations between July 2002 and August 2004 and followed up their long-term outcome until 2015.
Results
The patients achieved a median of 17 points in the Merle d'Aubigné hip score in 2004 and a median of 18 points in 2011, and the 4 surviving patients in 2015 achieved 18 points. For the four 12-year survivors, the Merle d'Aubigné score was virtually stable over the complete observation period. The Harris Hip Score showed comparable results. The patients had a median Barthel index of 90 in 2004 and 100 in 2011, and the 4 survivors in 2015 had Barthel indices of 65, 95, 100, and 100, respectively, in 2015.
Conclusions
In multimorbid patients, using the BioBall® adapter system for total hip arthroplasty, revision due to dislocation results in good long-term outcome without impairment of quality of life.
Translational potential
Our study provides long-term evidence in a vulnerable patient population. It shows how the therapeutic concept of revision hip replacement with an adapter device translates into long-term outcome and quality of life in these patients. Thus, it adds important information for evaluation of therapeutic options in this field.
Keywords: BioBall® adapter system, Revision arthroplasty, THA dislocation, THA instability, Total hip replacement
Abbreviations: CHD, coronary heart disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; THA, total hip arthroplasty
Introduction
With constantly growing numbers of multimorbid elderly people worldwide, an increasing need for total hip arthroplasty (THA), e.g., due to hip fractures, remains an increasing challenge to most health care systems. In addition, elderly patients undergoing THA are considered to suffer from higher morbidity and also are at higher risk of complications such as THA dislocation, periprosthetic fractures, infection, and bleeding, resulting in a higher preoperative and postoperative morbidity than younger patients [1], [2], [3].
Nevertheless, some surgeons consider “total solutions” in THA revision surgery, i.e., exchanging all parts and replacing them by revision implants, as this is the only possible way in the long term to solve problems such as secondary THA dislocation even in the multimorbid ASA (American Society of Anesthesiologists) class IV patient. Poor clinical outcomes resulting from this clinical pathway are foreseeable [4]. Other surgeons focus on quality of life and prioritise on minimising potential adverse effects. These less invasive approaches may resolve hip instability without reducing the patients' quality of life in the mid- and long-term period. Notably, adapter systems developed in the early years of this century allow achieving this objective.
Our pilot study approach aims to evaluate the long-term reliability of the Merete BioBall® adapter system for primary or secondary revisions of THA. This adapter system is available in different lengths from −3.5 to +21 mm in neutral and 7.5° angulation. It can be used with modular metal or ceramic heads with 28- to 36-mm diameter and is compatible with stem systems from different manufacturers [5].
Previous studies have already evaluated the BioBall® system in relatively healthy patients and followed up patients for up to 7 years [5], [6], [7]. Our retrospective study analysed the outcome of revision hip arthroplasty using the BioBall® system in highly morbid elderly patients with a follow-up of 12 years.
Materials and methods
The study population included all revision procedures in our department between July 2002 and August 2004 plus revisions within the primary implantation procedure that required the use of an adapter system. Data were collected during routine clinical procedures in our university hospital, and patients were asked and agreed to give their informed consent for follow-up of this retrospective long-term observational clinical study at the University Surgical Clinic of Heidelberg. The study was approved by the Ethics Committee of the University of Heidelberg (S-105/2015). The study was conducted according to the Declaration of Helsinki, as revised in 2004.
The approach to the hip was the standard transgluteal approach according to Bauer et al [8]. The Merete BioBall® adapter system (Merete Medical GmbH, Berlin) was approved equivalent to European Medical Device Directive and had a CE mark before clinical use.
All patients received a Merete BioBall® adapter. The most frequently used adapters in the present study were of sizes XL and 2XL (Table 1). A total of 16 patients received a 28-mm head, the other 3 received a 32-mm head; 9 received a metal head, and 10 received ceramic heads.
Table 1.
Patient and implant characteristics.
| Patient number | Age | Gender | ASA status | Indication for surgery | Adapter | Revision | Comorbidities | Death after years |
|---|---|---|---|---|---|---|---|---|
| 1 | 53 | M | 3 | Aseptic necrosis of the femoral head | 2XL; 28 mm; ceramic | No | Renal transplant, hepatitis C, diabetes mellitus, thrombocytopenia, status post bladder fistula | 4 |
| 2 | 58 | F | 4 | Implant failure | 4XL; 28 mm; ceramic | No | Korsakoff syndrome, liver cirrhosis, diabetes mellitus, thrombocytopenia, status post alcohol abusus | — |
| 3 | 49 | F | 3 | Dislocation | 4XL; 28 mm; metal | No | Status post uterus carcinoma + chemotherapy | 12 |
| 4 | 91 | F | 4 | Dislocation | 2XL; 28 mm; metal | Yes | Depression, meningioma, struma multinodosa III, hypertension, glucose intolerance | 3 |
| 5 | 66 | F | 4 | Dislocation | 3XL; 28 mm; ceramic | No | Hypertension, hepatitis C, pACK IIb, status post cholecystectomy, status post struma resection | — |
| 6 | 56 | M | 4 | Fracture of the femoral neck | XL; 28 mm; ceramic | No | Aortic stenosis, diabetes mellitus, hypertension, cardiovascular disease, CKD | 8 |
| 7 | 73 | M | 4 | Fracture of the femoral neck | 2XL; 28 mm; metal | No | Parkinson's disease, cardiovascular disease | 1 |
| 8 | 71 | M | 4 | Coxarthrosis | XL; 28 mm; ceramic | No | Hypertension, CKD, left ventricular hypertrophy, COPD, subileus | 3 |
| 9 | 97 | M | — | Acetabulum fracture | XL; 32 mm head; metal | Yes | Congestive heart failure, coxarthrosis, depression, prostate adenoma, presbycusis | 1 |
| 10 | 73 | F | 3 | Dislocation | 3XL; 28 mm; ceramic | No | Pneumatosis coli, colon ischaemia, cholecystolithiasis, splenectomy, intermittent atrial fibrillation | 5 |
| 11 | 72 | F | 3 | Fracture of the femur | XL; 28 mm; ceramic | No | Supraventricular tachycardia, CHD, hypertension, COPD, status post myocardial infarction | 8 |
| 12 | 54 | F | Fracture of the femoral neck | XL; 28 mm; metal | No | Mamma carcinoma | 0.5 | |
| 13 | 62 | M | 3 | Fracture of the femoral neck | M; 28 mm; ceramic | No | Parkinson's disease, hypokalemia, hypertension, paranoid psychosis, chronic obstipation | 5 |
| 14 | 79 | M | 4 | Cup loosening | 5XL; 28 mm; metal | No | Cardiac defibrillator, hypertension, aortic stenosis, tricuspid valve insufficiency, hyperuricaemia | 4 |
| 15 | 77 | F | 3 | Dislocation | 4XL; 28 mm; metal | Yes | Osteoporosis, hypertension, CHD, COPD, CKD | 11 |
| 16 | 74 | M | 3 | Fracture of the femoral neck | 2XL; 28 mm; ceramic | No | Coronary artery disease | — |
| 17 | 56 | M | 4 | Implant loosening | 2XL; 32 mm; metal | No | Diabetes mellitus | — |
| 18 | 66 | F | 2 | Total hip replacement following cutting-out phenomenon in pertrochanteric fracture treated with the proximal femoral nail | XL; 28 mm; ceramic | No | Hypertension, multiple sclerosis | 8 |
| 19 | 74 | F | 4 | Implant loosening | 5XL; 32 mm; metal | No | Status post myocardial infarction | 19 |
CHD = coronary heart disease; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease. ASA = American Society of Anesthesiologists.
The THA stems used in combination with the Merete BioBall® adapter were Bicontact® (n = 10; Aesculap, Tuttlingen, Germany) and Helios® (n = 9; Biomet Merck, Berlin, Germany) .
All patients were clinically followed up in 2004 and 2011 for a routine medical checkup. For this study, a further long-term follow-up was conducted in 2015. For the long-term follow-up, the hip-related outcome was evaluated using the Merle d'Aubigné score [9] and Harris Hip Score (HHS) [10].
The Merle d'Aubigné score and the HHS are commonly used clinician-based outcome measures to assess the outcome of total hip replacement based on standardised questionnaires. Highest scores indicate optimal outcome. The Merle d'Aubigné score has been developed earlier and was used because it was the standard score at the time of surgery in Heidelberg. (The HHS was not available for all patients.) Validity and reliability of the HHS has been shown previously [11].
Because most patients were geriatric patients, quality of life was also evaluated using the Barthel index, which is an assessment tool for evaluation of the performance in activities of daily living specifically in elderly patients [12].
Statistical analysis
All data are provided as mean ± standard deviation or median/quartiles. The results of Merle d'Aubigné scores and HHSs were tested using the Kruskal–Wallis test for statistically significant differences (alpha level: 5%). We provide summary measures (Table 1, outcome scores) for our patient group and short case reports for the survivors. The probability of revision-free survival was determined by Kaplan–Meier analysis.
Results
The most frequent indications for revision hip arthroplasty were implant loosening and/or dislocation in 10 patients. A total of 6 patients had a femoral fracture that required revision hip arthroplasty; one had a necrosis of the femoral neck, one had an acetabulum fracture, and one had coxarthrosis.
At the time of revision hip arthroplasty, the patients were aged 67.9 ± 11.8 years (median: 71 years, quartiles: 57, 74). Nine patients were men, and 10, women. Most patients (N = 10) had an ASA status IV before the procedure, 6 had ASA status III, and 1 had ASA status II. Each patient had several comorbidities, the most frequent ones were hypertension (N = 7), diabetes mellitus (N = 5), and coronary artery disease (N = 4).
All revisions were successful. A total of 15 patients died during the 12-year observation period. The probability for 12-year revision-free survival was 86% (Kaplan–Meier analysis). A total of 2 patients needed further revision of hip arthroplasty within 3 years; one of these underwent another revision after 5 years. These two patients had 2XL and 4XL adapters, respectively, both with metal heads. All other patients did not require further revisions or survived until end of the study.
Patients receiving large adapters are usually at higher risk of complications and further revisions. However, in the present study, both patients with 5XL adapters died at the age of 93 and 82 years, surviving 11 and 3 years, respectively, without need for further revisions.
Follow-up
At the first follow-up in 2004, the patients achieved a median of 17 (quartiles: 13, 18) points in the Merle d'Aubigné hip score. In 2011, the remaining 9 patients achieved a median score of 18 (quartiles: 12, 18). In 2015, 4 patients were still alive. One achieved a Merle d'Aubigné score of 10; the other three achieved a score of 18. None of the differences between the three time points was statistically significant (Figure 1). For the four 12-year survivors, the Merle d'Aubigné score was virtually stable over the complete observation period. Similarly, there was no significant change in the HHS (Figure 1).
Figure 1.
Merle d'Aubigné scores, Harris Hip Score, and Barthel index. Boxplot showing median and quartiles, minimum and maximum. The scores were not significantly different between different years.
Quality of life was evaluated using the Barthel index. The patients achieved a mean Barthel index of 79 ± 26 in 2004 [median: 90 (65, 100)] and 89 ± 20 in 2011 [median 100 (95, 100)], and the 4 survivors' outcome was Barthel indices of 65, 95, 100, and 100, respectively, in 2015. No significant change was observed in the index within the study population during the observation period (Figure 1).
Case reports of the 12-year survivors
Because the number of survivors in our series of multimorbid patients was very low, we present a case series of the four 12-year survivors in addition to our data.
Case 1
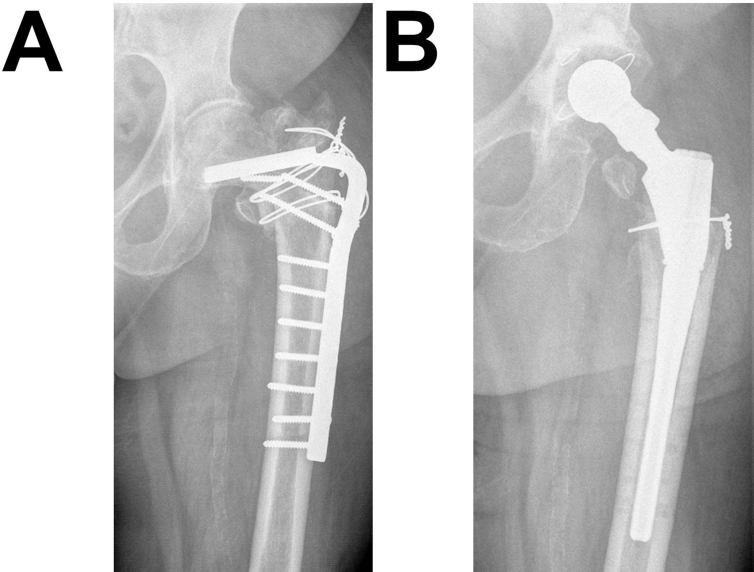
A 58-year-old woman was operated for THA after two previous failures of a femoral nail and a plate device, which were implanted after multiple fractures of the left femur (Figure 2A). At the time of THA, she had Korsakoff syndrome and liver cirrhosis, diabetes mellitus, thrombocytopenia, status post alcohol abusus, and adiposity. The patient received a 4XL BioBall® adapter with a 28-mm metal head.
Figure 2.
(A) Case 1, failure of an internal fixation plate. (B) Result after THA with BioBall® (4XL adapter). The patient remained stable during the whole duration of follow-up. THA, total hip arthroplasty.
After surgery and successful mobilisation of the patient (Figure 2B), the Merle d'Aubigné score (12) and HHS (68) were determined. Quality of life based on the Barthel index was low (35). Most importantly, the patient was virtually bedfast: she could only move for short distances and was not able to climb stairs. She had an adduction contracture, flexion contracture, and internal rotation contracture. Until 2015, the hip scores remained almost stable (Merle d'Aubigné: 10, HHS: 72) without need for further revisions. Although the patient is still dependent on a wheeled walker today, quality of life substantially improved (Barthel index: 65).
Case 2
A 66-year-old woman with hypertension, chronic kidney disease, and hepatitis C, status post cholecystectomy, and status post struma resection was scheduled for revision hip arthroplasty after dislocation of the previous implant (Figure 3A). For revision hip arthroplasty, a BioBall adapter 3XL, with a 28-mm ceramic head, was used.
Figure 3.
(A) Case 2, traumatic dislocation after THA. (B) Result after revision hip arthroplasty with BioBall® (3XL adapter). THA, total hip arthroplasty.
After the procedure (Figure 3B), further revisions were needed until today. After surgery and mobilisation of the patient, she achieved a Merle d'Aubigné score of 18 and a HHS of 84. The quality of life was good, indicated by a Barthel index of 85. The only handicaps were the need for assistance for climbing stairs and dressing. Eleven years later, at the age of 77 years, the patient was dependent on a wheeled walker and, therefore, needed help with climbing stairs and had limited mobility. Thus, she achieved a HHS of 65, but quality of life was good, as indicated by a Barthel index of 95.
Case 3
A 74-year-old man with coronary artery disease suffered a fracture of the femoral neck (Figure 4A). During THA, he received a BioBall® adapter 2 XL with a 28-mm ceramic head owing to considerable hip instability. The procedure was a successful (Figure 4B), and the patient had no reductions of the Merle d'Aubigné score or quality of life until today. The only recognisable impairment was a slight reduction of mobility of the joint, resulting in a HHS of 99 since 2011.
Figure 4.
(A) Case 3, femoral neck fracture. (B) Result after THA (BioBall® 2 XL adapter). THA, total hip arthroplasty.
Case 4
A 56-year-old man with diabetes mellitus was indicated for revision hip arthroplasty after 5 years owing to implant loosening (Figure 5A). The previous implant was changed to another stem using a BioBall® adapter 2XL with a 32-mm metal head owing to considerable instability during the procedure. After the procedure, the patient had no reductions of the Merle d'Aubigné score or quality of life until today. Since 2011, the mobility of the joint was slightly reduced, resulting in a HHS of 99.
Figure 5.
(A) Case 4, THA loosening after 5 years. (B) Result after revision hip arthroplasty using Bioball® (2 XL adapter). THA, total hip arthroplasty.
Discussion
General clinical aspects
Long-term outcome after revision hip arthroplasty with a modular head–neck adapter system is mainly reported in relatively healthy patients [5], [6], [7] but not in multimorbid patients, which we enrolled for our study. In multimorbid patients, the present study is the first of its kind with the Merete BioBall® system, covering a time span of up to 12 years. It demonstrates that the use of the Merete BioBall® adapter in an elderly and multimorbid population is associated with a comparable outcome as in healthier populations. In a similar study of Hoberg et al [6], most patients (95%) had ASA classification II or III before revision arthroplasty and can therefore be considered substantially healthier than multimorbid patients in our study with a majority (59%) classified as ASA status IV.
This fragile condition of our patients had been the reason to use the Merete BioBall® adapter whenever indicated to keep the extent of revision surgery as low as possible, thus reducing the risk of severe clinical complications such as bleeding, infection, hip dislocation, or periprosthetic fractures [13].
Our data were collected at a time when the adapter systems were newly developed and not yet used by the majority of surgeons. Therefore, the number of patients with long-term data is rather less, and patient characteristics were diverse (i.e., data are currently available for multimorbid patients only from the study by Hoberg et al [6] and our study). However, these data are important to verify long-term reliability of the adapter system. Today, adapter systems are clinically well-established products and are used on a daily basis [14]. Therefore, this pilot study should encourage more researchers to perform prospective long-term studies on mix-and-match combinations in hip arthroplasty. In general, the risks and benefits of a combination that is not specified as intended use of a device should be carefully evaluated, and local legal requirements for such combination should be respected. Nevertheless, our 12 years of experience provides clear first evidence for good patient safety and good clinical results when properly using the Merete BioBall® adapter system in multimorbid patients.
These data might also help surgeons to justify the use and perform the required risk-benefit assessment of adapter systems if they have to overcome medicolegal concerns against mix-and-match combinations. This is especially important in situations when such combinations are the only feasible option to mechanically stabilise an otherwise unstable THA articulation and they cannot get their patients' informed consent before surgery. There is an increasing focus on quality of life for multimorbid and elderly patients [15], which may be compromised by unwanted consequences of very prolonged surgeries. To prevent such consequences, such as postoperative loss of dexterity or cognitive decline, it might be justified to rely on mix-and-match approaches in selected cases [4].
To weigh the possible advantages of the Merete BioBall® adapter system of less invasive surgical procedures to solve recurrent THA instability and dislocation against its possible disadvantages of conus adapter problems including fretting and corrosion, a long-term prospective clinical study with large patient numbers would be required [6]. This should include stratified randomisation to have clinical subgroups according to ASA classification, implant type, and many more clinical characteristics.
Mix-and-match discussion
European medical directive (MDD) requirements on Class III medical devices such as hip implants in general and innovations such as the Merete BioBall® adapter systems require proof of clinical safety, clinical performance, risk-benefit analysis, and a gain in quality of life for the patient, displayed with the CE mark. Furthermore, clinical users (surgeons) must be instructed about proper use of the devices by the manufacturer. This information is summarised in the manufacturer's instructions for use and must be followed by surgeons. Any deviation from this document, especially when using medical devices in other indications than those indicated in the manufacturer's instructions, has to be considered an off-label use with all its legal consequences, especially with extra documentation and surgeons' liability when unexpected adverse effects occur.
In clinical routine, such an off-label use is established practice in many European countries, e.g., as documented in a study of the national joint registers of England and Wales, reporting more than 90,000 off-label use combinations [16]. In this study, the clinical follow-up of 79,117 patients with uncemented THA is reported. There were no increased revision rates associated with the use of 317 stems and 5906 cups as well as all mixed components (n = 152) from different manufacturers, even when used together off-label according to the definitions of the MDD. Both the long-term observational studies of Hoberg et al [6] and our data support and supplement these data about long-term clinical safety and quality of life when using the Merete BioBall® adapter system in combination with the two hip stems (Bicontact® and Helios®) in our study.
Despite solid clinical evidence and quality of life data for THA revision surgery in multimorbid patients, medicolegal experts may consider this treatment inappropriate based on their interpretation of formal legal aspects defined by the MDD. It is therefore mandatory for the surgeon to explain and also document the patient's consent and his or her considerations of the proven benefits (improvement of THA dislocation and good quality of life) versus the potential risks (long-term fretting and corrosion of the Merete BioBall® adapter system) in mixing and matching this adapter system with implants of other manufacturers.
Clinical outcome
The result of revision hip arthroplasty based on HHSs was similar to that of other studies: Shortly after the procedure, our patients started with a mean HHS of 74 and had reached a score of 84 at the end of the observation period. Previous studies reported a HHS of 80.9 [5], 54 [4], or 91 [17]. HHSs and Merle d'Aubigné scores in this study consistently showed no significant change over time.
The revision rate in our study was 12% after 12 years, which is comparable to rates reported by others (5–23% after 4–8 years) [5], [6], [17].
Quality of life
Our study also evaluated quality of life after surgery. Because most patients were geriatric patients, we used the Barthel index [12] for evaluation and found no change of the mean index over time. When taking into account the patients' multimorbid condition and high age, 3 of the four 12-year survivors still showed excellent outcome in their ability to perform activities of daily living.
Most important limitations of our observational study are the small sample size and the lack of a control group. Although we cannot draw firm, statistically significant conclusions, our results suggest that revision arthroplasty using the Merete BioBall® adapter can be performed with good long-term outcome in multimorbid patients.
In summary, our study showed that the Merete Bioball® system can be used in multimorbid elderly patients with good long-term outcome. Today, medicolegal concerns often interfere with the use of such systems, potentially overriding medical considerations of what is best for patients. Therefore, more research is required to improve evidence-based consideration of benefits and risks for the individual patient.
Conflict of interest
C.C. received the educational grant from Merete Medical GmbH, Berlin, Germany. The other authors declare that they have no other conflict of interest.
Acknowledgements
The authors would like to thank Merete Medical GmbH, Berlin, Germany, for supporting this study with an unrestricted educational grant. Medical writing assistance was provided by Aike Torben Schweda, PhD, DBM Wissen schafft GmbH, funded by Merete Medical GmbH. The authors are fully responsible for content and editorial decisions for this manuscript.
Contributor Information
Hans-Jürgen Kock, Email: hans-juergen.kock@median-kliniken.de.
Christopher Cho, Email: chrischodotcom@yahoo.com.
Klaus Buhl, Email: klaus.buhl@med.uni-heidelberg.de.
Joachim Hillmeier, Email: trauma@st-vincenz.de.
Franz X. Huber, Email: franz-xaver.huber@klinikum-ansbach.de.
References
- 1.Antapur P., Mahomed N., Gandhi R. Fractures in the elderly: when is hip replacement a necessity? Clin Interv Aging. 2011;6:1–7. doi: 10.2147/CIA.S10204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kannus P., Parkkari J., Sievänen H., Heinonen A., Vuori I., Järvinen M. Epidemiology of hip fractures. Bone. 1996;18(Suppl. 1):57S–63S. doi: 10.1016/8756-3282(95)00381-9. [DOI] [PubMed] [Google Scholar]
- 3.Kurtz S., Mowat F., Ong K., Chan N., Lau E., Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg. Am Vol. 2005;87(7):1487–1497. doi: 10.2106/JBJS.D.02441. [DOI] [PubMed] [Google Scholar]
- 4.Perka C., Fink B., Millrose M., Sentürk U., Wagner M., Schröder J. Revisionsendoprothetik. In: Claes L., Kirschner P., Perka C., editors. AE-Manual der Endoprothetik: Hüfte und Hüftrevision. 2012. Available from: [DOI] [Google Scholar]
- 5.Woelfle J.V., Fraitzl C.R., Reichel H., Wernerus D. Significantly reduced leg length discrepancy and increased femoral offset by application of a head-neck adapter in revision total hip arthroplasty. J Arthroplast. 2014;29(6):1301–1307. doi: 10.1016/j.arth.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 6.Hoberg M., Konrads C., Huber S., Reppenhagen S., Walcher M., Steinert A. Outcome of a modular head-neck adapter system in revision hip arthroplasty. Arch Orthop Trauma Surg. 2015;135(10):1469–1474. doi: 10.1007/s00402-015-2281-z. [DOI] [PubMed] [Google Scholar]
- 7.Vaishya R., Sharma M., Chaudhary R.R. Bioball universal modular neck adapter as a salvage for failed revision total hip arthroplasty. Indian J Orthop. 2013;47(5):519–522. doi: 10.4103/0019-5413.118211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer R., Kerschbaumer F., Poisel S., Oberthaler W. The transgluteal approach to the hip joint. Archives of orthopaedic and traumatic surgery. Archiv fur orthopadische und Unfall-Chirurgie. 1979;95(1–2):47–49. doi: 10.1007/BF00379169. [DOI] [PubMed] [Google Scholar]
- 9.Merle dÁubigné R., Postel M. M. Functional results of arthroplasty with acrylic prosthesis. J Bone Joint Surg. 1954;36-A:451–475. [PubMed] [Google Scholar]
- 10.Harris W.H. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end- result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed] [Google Scholar]
- 11.Södermann P., Malchau H. Is the Harris hip score system useful to study the outcome of total hip replacement? Clin Orthop Relat Res. 2001;384:189–197. doi: 10.1097/00003086-200103000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Mahoney F.I., Barthel D.W. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 13.Healy W.L., Iorio R., Clair A.J., Pellegrini V.D., Della Valle C.J., Berend K.R. Complications of total hip arthroplasty: standardized list, definitions, and stratification developed by the hip society. Clin Orthop Relat Res. 2016;474(2):357–364. doi: 10.1007/s11999-015-4341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weber P., Steinbrück A., Paulus A.C., Woicinski M., Schmidutz F., Fottner A. Partial exchange in total hip arthroplasty : what can we combine? Der Orthopäde. 2017;46(2):142–147. doi: 10.1007/s00132-016-3380-4. [DOI] [PubMed] [Google Scholar]
- 15.Navickas R., Petric V.K., Feigl A.B., Seychell M. Multimorbidity: what do we know? What should we do? J Comorbidity. 2016;6(1):4–11. doi: 10.15256/joc.2016.6.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tucker K., Pickford M., Newell C., Howard P., Hunt L.P., Blom A.W. Mixing of components from different manufacturers in total hip arthroplasty: prevalence and comparative outcomes. Acta Orthop. 2015;86:671–677. doi: 10.3109/17453674.2015.1074483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jack C.M., Molloy D.O., Walter W.L., Zicat B.A., Walter W.K. The use of ceramic-on-ceramic bearings in isolated revision of the acetabular component. Bone Joint J. 2013;95-B(3):333–338. doi: 10.1302/0301-620X.95B3.30084. [DOI] [PubMed] [Google Scholar]