Abstract
Background
Mesenchymal stem cells (MSCs) can be easily expanded without losing the ability of multilineage differentiation, including oesteogenic, chondrogenic and adipogenic differentiation. These characters make MSCs a promising cell resource for cartilage defect repair. MSCs could be recruited by inflammatory stimulation, then home to the injury tissues. However, its capacity of homing is extremely limited. Thus, it has become extremely necessary to develop an agent or a method, which can be used to enhance the efficiency of MSCs homing. This study investigates the effect of stearic acid methyl ester (SAME) on MSCs mobilisation and cartilage regeneration.
Methods
MSCs were isolated from femurs of Sprague-Dawley (SD) rats. MTT assay was used to detect effect of SAME on viability of MSCs. Transwell assay and wound healing assay were used to detect effect of SAME on migration of MSCs. RNA-seq, quantitative real-time PCR and western blot were performed to analyze the expression of RNAs and proteins. Colony forming assay and flow cytometry were used to evaluate the effect of SAME on MSCs mobilisation in vivo. A rat cartilage defect model was created to evaluate the effect of SAME on cartilage regeneration.
Results
We found that SAME could promote the migration of MSCs. Interestingly, we found SAME significantly increased the expression levels of Vav1 in MSCs. On the other hand, the enhanced migration ability of MSCs induced by SAME was retarded by Vav1 small interfering RNA (siRNA) and Rho-associated protein kinase 2 (ROCK2) inhibitor. In addition, we also checked the effect of SAME on mobilisation of MSCs in vivo. The results showed that SAME increased the number of MSCs in peripheral blood and enhanced the capacity of colony formation. Finally, using a cartilage defect model in rats, we found SAME could improve cartilage repair.
Conclusion
Our study demonstrates that SAME can enhance MSCs migration ability mainly through the Vav1/ROCK2 signaling pathway, which could contribute to the accelerated cartilage regeneration.
The translational potential of this article
These findings provide evidence that SAME could be used as a therapeutic reagent for MSCs mobilisation and cartilage regeneration.
Keywords: Cartilage, Mesenchymal stem cells, Stearic acid methyl ester, Vav1
Introduction
Arthritis is a crippling disease which affects people's life and health. It is characterised by the progressive loss of articular cartilage and chondrocytes within synovial joints, leading to symptoms such as pain and stiffness. Not only are the elderly but also young people commonly injured by this disease [1]. The cartilage is difficult to self-repair because of lack of blood vessels, nerves and nutritional sources [2,3]. And the management of cartilage lesions is still a big challenge for surgeons because of its limited regenerative ability. Generally, several treatments have been attempted to restore joint surface defects, such as osteochondral autograft transplant system and autologous chondrocyte implantation and microfracture [4]. Unfortunately, the cartilage formed by these procedures is frequently fibrocartilage instead of functional hyaline cartilage. The development of tissue engineering provides a new approach for cartilage repair. Mesenchymal stem cells (MSCs) are regarded as the most promising seed cell because of its multidirectional differentiation and self-renewal potential [5,6]. Preclinical studies with MSCs transplantation provide evidence that they have capacity of tissue repair such as bone nonunion and osteoarthritis [[7], [8], [9]]. However,MSCs transplantation has been hampered by several key problems,lack of targeting or poor cell migrates to injury sites and apoptosis of the implanted cells [10,11].
MSCs migration is one of the important factors which influences tissue repair. When stimulated by specific signals such as cytokines or chemokines, MSCs can be released into circulation and recruited to the target tissues. Then, MSCs differentiate into specific cells in situ and contribute to tissue regeneration [12]. These cells can potentially be mobilised into the circulation in response to injury signals and exert their reparative effects at the site of injury. Trafficking of MSCs to the injury site and their subsequent participation in the regenerative process is thought to be a natural healing response that can be imitated or augmented by enhancing the endogenous MSCs pool with exogenously administered MSCs [13]. Cell migration is precisely regulated by multiple signalling pathways of which Rho-associated protein kinase (ROCK) pathway plays an important role. ROCK, also known as Rho kinase, is the downstream target effector of RhoA that has been studied in a detailed manner currently. Inhibition of ROCK signalling pathway results in the inhibition of migration of haematopoietic stem cells, monocytes, neutrophils and myoblast [[14], [15], [16], [17]]. ROCK signalling pathway regulates rearrangements of the actomyosin cytoskeleton, which constitute a conductive loop of the contractile force to conduct contractile force and result in cell migration [18].
Some researchers have reported that chemotactic factors such as platelet derived growth factor (PDGF), transforming growth factor β3 (TGF-β3) loaded on bioadhesive materials enhanced endogenous MSCs recruitment and facilitated in situ articular cartilage regeneration [19,20]. These studies indicate that mobilisation of endogenous MSCs is beneficial to cartilage repair. Sufficient number of MSCs homing to sites of injury is an important factor in tissue repair. However, the capacity of MSCs homing to the injured site is extremely limited. Only a few MSCs can cross the endothelial cells and migrate to the injured site for repair [21]. To improve the homing efficiency, different strategies have been developed such as pretreatment of MSCs in culture, genetic modifications and cell surface engineering [22]. Results of the in vivo studies indicate that the combination of insulin-like growth factor 1 (IGF1) and Plerixafor octahydrochloride (AMD3100) could augment the bone growth by mobilisation of stem/progenitors from the bone marrow (BM) [23]. Drug therapy is a valuable method worthy spreading because of its simplicity, low toxicity and good effects compared with other treatments. Some studies have indicated that pretreatment of MSCs in culture by some herb extracts such as ligustrazine, icariin and ilexonin-A can enhance MSCs proliferation and migration [[24], [25], [26]]. Stearic acid methyl ester (SAME) can be extracted from some herbs such as safflower and plastrum testudinis, which has been proven to promote MSCs proliferation [27]. Safflower has long been used to promote blood circulation and remove blood stasis in China [28]. In China, the blood activating and stasis eliminating drugs are widely applied for the treatment of arthritis and obtain good clinical outcomes [29]. SAME is one of the main components in Safflower's volatile oil. Our previous study has shown that volatile oil of safflower can promote migration capacity of MSCs, while there is no report about the effect of SAME on MSCs migration. In this study, we found that SAME could promote the proliferation and migration of MSCs by activating the Vav1/ROCK signalling pathway. Furthermore, we demonstrated that SAME could mobilise endogenous MSCs to improve the healing of impaired cartilage in the knee cartilage defect model of Sprague-Dawley (SD) rats. These findings provide evidence that SAME can be used as a therapeutic reagent for MSCs mobilisation and cartilage regeneration.
Materials and methods
MSCs isolation, culture and characterisation
MSCs were harvested from femurs of SD rats (male, 4-weeks-old). In brief, the bone marrow of the bilateral femoral was flushed out and layered onto LymphoprepTM (1077 g/mL; Axis-Shield) for centrifugation at 400g for 25 min. The isolated mononuclear cells were suspended in low-glucose Dulbecco's modified Eagle's medium (DMEM, Gibco, USA, serial number: C11885500BT) containing 10% foetal bovine serum (FBS, Gibco, USA, serial number: 10099141) and 1% penicillin-streptomycin (Gibco, USA, serial number: 15140122) and incubated in air containing 5% CO2 at 37°C. The nonadherent cells were removed by phosphate buffered saline (PBS) washing and changing the medium 6d later. Then, medium was changed once every 2 days. When the adherent cells reached 80~; 90% confluence, they were passaged at a ratio of 1:3. Cells from passages 3 to 5 were used in the study. The cell surface markers (cluster of differentiation, CD90, CD45, CD44 and CD34) were confirmed by flow cytometry. The cells were induced for osteogenic and adipogenic differentiation in vitro to determine multipotency of MSCs, as described previously [30].
MTT assay
MSCs were inoculated at 2 × 104/mL in 96-well culture plates (approximately 2000 cells in 100 μL of medium per well). The cells were incubated at different concentrations of SAME (0, 1, 5, 10, 50 and 100 μg/mL) in DMEM. 10 ul MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5 diphenyl tetrazolium bromide, Beyotime, China) was added to each well after 24 h or 72 h, and cells were incubated for an additional 4 h at 37°C. 100 μL of Formazan lysis was added to each well and incubated for another 4 h at 37°C. The absorbance was recorded at a wavelength of 570 nm using a microplate reader to determine the level of proliferation in each well.
Cell migration assay
Cell migration ability was evaluated by transwell assay and wound healing assay. Transwell plates (Corning Costar, USA, serial number: 3422) with a pore size of 8 μm (Corning Costar) were used to transwell assay. Cells were pretreated by different condition including different concentrations of SAME, Y27632 (ROCK inhibitor) or transfection of Vav1 small interfering RNA (siRNA). Then, cells were digested and plated to upper chamber with 1.5 × 104 MSCs in 200 μl supplemented with 10% FBS. After culturing in an incubator for 2 h, the lower chamber was loaded with 700 μl DMEM supplemented with 10% FBS. The plates were incubated at 37°C in 5% CO2 for 10 h. The upper surface of the membrane was then gently scraped using a cotton swab to remove the nonmigrated cells and washed with PBS. The membrane was then fixed in 4% paraformaldehyde for 30 min, followed by staining with 0.5% crystal violet staining solution. Migrated cells were observed and photographed under a phase contrast microscope. The number of migrated cells was determined by averaging five random fields per well.
For wound healing assay, MSCs were incubated in 6 cm dish and cultured until 95% confluence. Then the medium was changed with 1% FBS to exclude the influence of cell proliferation. A scratch wound was created with a micropipette tip. The cells were photographed and counted under a phase contrast microscope.
RNA-seq and data analysis
MSCs were treated with 10 μg/ml SAME for 24 h.Total RNA was obtained from the control or SAME-treated MSCs using TRIzol Reagent (Takara, Dalian, China). The quality and integrity of total RNA samples were assessed using a 2100 Bioanalyzer or a 2200 TapeStation (Agilent Technologies) according to the manufacturer's instructions. The preparation of whole transcriptome libraries and deep sequencing were performed by the Annoroad Gene Technology Corporation (Beijing, China). The differentially expressed genes (above 2.0 folds) from RNAseq were analysed by DAVID bioinformatics tool for functional annotation enrichment and clustering.
Quantitative real-time PCR
Total RNA was extracted from MSCs using TRIzol reagent (Invitrogen, USA, serial number: 15596-026) according to the manufacturer's instructions. Then RNAs were treated with DNase I to remove the DNA contamination. The concentration and purity of RNA were measured using Nanodrop 2000. cDNA was synthesised with PrimeScript RT Master Mix (TaKaRa, Japan, serial number: RR036Q). Real-time quantitative RT-PCR was performed using the SYBR Premix Ex Taq II (TaKaRa, Japan, serial number: RR820A). β-actin was used as an internal parameter to determine the relative expression.
Primer sequences were as follows:
Vav1-forward: 5′-GGAGTCCAGCAGACTGAGG-3′and Vav1-reverse: 5′-CTGACTCCACCTGACTGCAA-3´.
ROCK2-forward: 5′-AACCAAGATTGCTTGATTTGGCA-3′and ROCK2-reverse: 5′- GCCCAGACAAACCTCTCCAT-3´.
β-actin-forward: 5′-CGTAAAGACCTCTATGCCAACA-3′and β-actin-reverse: 5′-CGGACTCATCGTACTCCTGCT -3´.
Western blot analysis
MSCs were lysed with radio immunoprecipitation assay (RIPA) buffer containing protease and phosphatase inhibitors (Beyotime). The protein concentration was measured by a bicinchoninic acid (BCA) Protein Assay kit (Beyotime). Equal amounts of protein were separated by sodium dodecylsulphate polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a polyvinylidene fluoride (PVDF) membrane, blocked in 5% milk, and immunoblotted with primary antibodies overnight at 4°C. The membranes were washed in tris buffered saline with tween 20 (TBST) and incubated with a corresponding secondary antibody for 1 h at room temperature. Protein bands were visualised using an enhanced chemiluminescence kit (Pierce). The following primary antibodies were used: Vav1 (1:1,000, Abcam, UK, serial number: ab97574); β-actin (1:1000; CST, USA, serial number: 3700S).
Gene silencing of Vav1
siRNAs targeting Vav1 and negative mismatched control were designed and synthesised from Genepharma (China). The sequences for siRNAs targeting Vav1 were as follows: siRNAs targeting Vav1 (siVav1)-1: GUGGGAGGGAAGAAAUACATT; siVav1-2: GUGGAGGUCAAGCAUAUUATT; siVav1-3: GAGGAAGACUAUUCUGAAUTT. The transfection was performed with lipofectamine 3000 solution (Invitrogen) according to the manufacturer's protocol. After the terminal transfection for 48 h, the cells were collected analysed.
Colony forming assay of peripheral blood
Peripheral blood was collected before and at the 7th day after treatment with heparin anticoagulation. Then 1 ml blood was transferred into a 15 ml conical centrifuge tube and treated with fresh cold red blood cell lysing solution. Then, the tubes were inverted or rocked for 10 min at room temperature until liquid was clear red. The cells were then centrifuged and cultured in MesenCult medium supplemented with mesenchymal stem cell stimulatory factors. The cells were allowed to grow for 10 days, and numbers of adherent cell colonies were enumerated in a phase contrast microscope.
Determine the number of MSCs in peripheral blood
To confirm the number of MSCs mobilised to peripheral blood, peripheral blood was collected by orbital blood collection before and at 6th day after treatment. The cell surface markers of peripheral blood mesenchymal stem cells (PBMSCs) were determined by flow cytometry. The cells were incubated for 30 min at room temperature with monoclonal antibodies for the stem cell markers CD90 and the haematopoietic marker CD45. Irrelevant identical isotypic antibody served as negative control. Cells were analysed using a BD (Becton, Dickinson and Company) Biosciences FACSCanto II flow cytometer.
Rat cartilage defect model
A rat cartilage defect model was created as previously described [31]. Briefly, the osteochondral defects in the femoral trochlear groove of SD rats under anesthesia as previously described. The 8-week-old SD rats were anesthetised by intraperitoneal injection of ketamine and xylazine. Skin tissue around the knee was disinfected, and then the right knee joints were exposed through a medial parapatellar approach. The knee remains fully flexed to expose the femoral condyle. Then a defect (diameter 1.5 mm and 1.5 mm in depth) was created in the centre of the groove using a dental drill. All debris was washed out of the defect. The joint capsule, subcutaneous tissues and skin were closed with sutures. The experimental group was divided into high-dose group (n = 3) and low-dose group (n = 3). High-dose group was injected with SAME at 0.05 mg/kg per day through intraperitoneal while low-dose group at 0.01 mg/kg per day. Control group (n = 3) received PBS supplemented with dimethylsulfoxide (DMSO). After 4 weeks, all rats were sacrificed, and femoral samples were collected for further study.
Safranin-O/Fast Green and immunohistochemical staining
Femoral samples were fixed in 10% buffered formalin and then decalcified in 10% buffered ethylenediaminetetraacetic acid (EDTA) (pH 7.4, Sigma-Aldrich, USA, serial number: E6758-500G) followed by the Safranin O (Sigma-Aldrich, USA, serial number: 84120-25G) and Fast Green (Sigma-Aldrich, USA, serial number: F7252-5G) and immunohistochemical staining with collagen type II antibody. Bone callus samples were fixed in 10% formaldehyde solution for 12 h, demineralised in 5% ethylenediaminetetraacetic acid for 1 month, dehydrated through a graded ethanol series, cleared in xylene, embedded in paraffin, and sliced into 5 μm thick sections. Sections were subjected to Safranin-O/Fast Green staining to evaluate the morphological changes in osteoblasts and trabecular bone and to determine the content of cartilage and collagen matrix within the callus. Sections were also incubated with the corresponding primary antibody and stained by the standard method. Colour development was performed using a diaminobenzidine (DAB) kit following the manufacturer's instructions. Each section was observed under a light microscope, and four visual fields were randomly selected for measurement of the rate of positive cells and the gray value, which were measured at the same light intensity. Histological sections of the repaired tissue were analysed using a grading system reported by other researchers [32]. Details of histological scores are provided in the Supplementary Table 1. The scoring was performed by three observers in a blinded manner.
Statistical analysis
Data are presented for each group as means ± standard deviation. Analysis was performed using SPSS16.0 software. Differences between groups were compared by t tests or one-way analysis of variance. A value of p < 0.05 was considered to be statistically significant.
Results
Characterisation of MSCs
MSCs were obtained from the femur bone marrow of SD rats. At passage 3, the cells were completely adherent and grew into uniform spindle-shaped (Figure 1A). Alizarin red staining showed that intracellular calcium nodule formation, indicating osteogenic differentiation ability (Figure 1B). Oil red staining showed the formation of red small droplets of oil in cell, indicating adipogenic differentiation ability (Figure 1C). MSCs surface markers were analysed by flow cytometry. FITC-CD44, FITC-CD90, FITC-CD45 and FITC-CD34 antibodies were used to stain MSCs and isotype IgG1 as negative control. The result showed that MSCs were positive expression of CD44 (99.24%), CD90 (97.03%) and negative for CD45 (0.06%), CD34 (0.20%) (Figure 1D)
Figure 1.
Characteristics of MSCs. (A) Morphology of MSCs in passage 3 presented uniform spindle-shaped. (B) Alizarin red staining showed the formation of intracellular calcium nodule. (C) Oil red staining showed the formation of red small droplets of oil in cell. (D) Flow cytometry suggested that MSCs were positive for CD44 (99.24%), CD90 (97.03%) and negative for CD45 (0.06%) and CD34 (0.20%). FITC, fluorescein isothiocyanate; FSC, forward scatter; MSC, mesenchymal stem cells.
Effect of SAME on the viability of MSCs
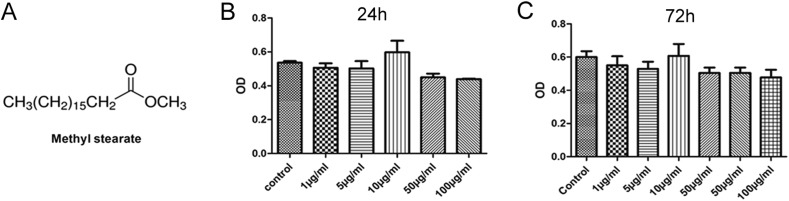
The molecular structure of SAME (methyl stearate) was shown in Figure 2A. To observe whether SAME affected the viability of MSCs, MSCs were treated with SAME at different concentration (1, 5, 10, 50 and 100 μg/ml) for 24 h and 72 h, followed by MTT assay. As shown in Figure 2B&C, SAME ranging from 1 μg/ml to 100 μg/ml did not cause any significant changes of the cell viability at 24 h and 72 h, which means that SAME had no cytotoxicity to MSCs.
Figure 2.
Effect of SAME on the viability of MSCs. (A) The chemical structure of SAME (methyl stearate). (B and C) The MSCs were incubated with different dosages of SAME for 24 and 72 h. Then the MTT solution was added to test the cell viability. Data are presented as mean ± SD (n = 6). There was no statistical difference between the drug groups and the control group (p > 0.05). MSC, mesenchymal stem cells; SAME, stearic acid methyl ester.
Effect of SAME on migration of MSCs
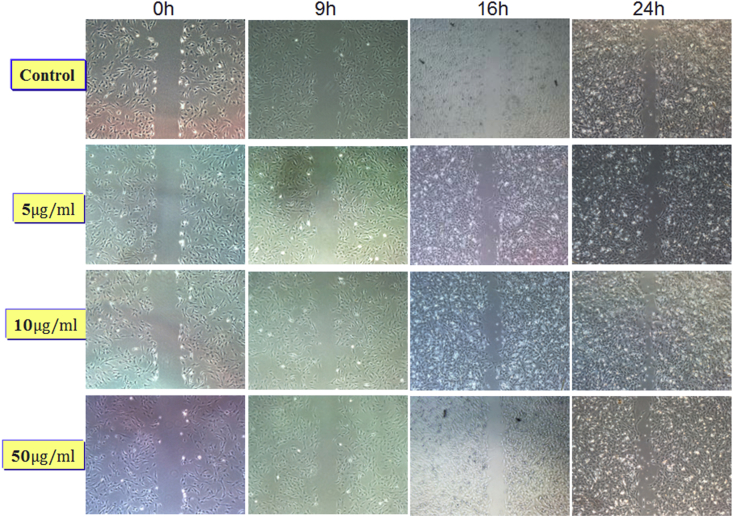
To observe the effect of SAME on migration of MSCs, we performed transwell assay and wound healing assay. For transwell assay, MSCs were pretreated by different dosages of SAME (5, 10, 50 μg/ml) for 24 h. And then the equal number of MSCs was added into upper chamber. The lower chamber contained DMEM supplemented with 10% FBS. After 10 h, migrated cells were counted after staining and photographing. The result showed that SAME with the concentrations of 5, 10 and 50 μg/ml could increase the MSCs migratory capacity compared with the control group, especially in the 10 μg/ml SAME group (Figure 3A–E). For wound healing assay, we observed the cell migration at 0 h, 9 h, 16 h, 24 h and 36 h. We obtained similar results that the concentrations of 5, 10 and 50 μg/ml could increase the MSCs migratory capacity compared with the control group. The results are shown on Supplementary Figure 1.
Figure 3.
Stimulatory effect of SAME on migration of MSCs. (A-D) Crystal violet staining was used to detect the MSCs migrated through the membrane. (E) The number of migrated cells were quantified by averaging five random fields per well under microscope. Data are presented as mean ± SD (n = 6, *p < 0.05 vs. the control group). MSC, mesenchymal stem cells; SAME, stearic acid methyl ester.
RNAseq analysis of SAME-treated MSCs
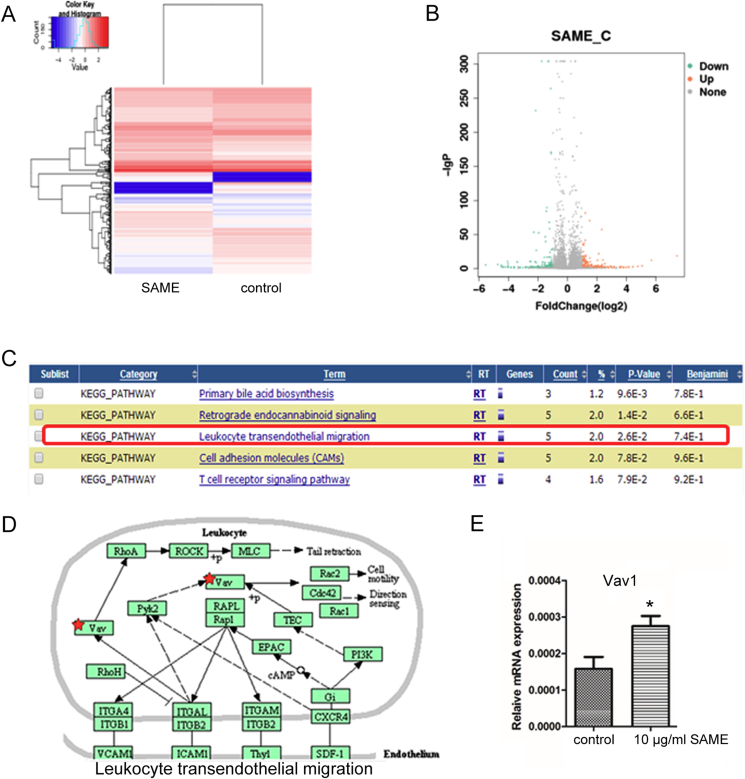
To analyse the underlying mechanism leading to the enhanced migration ability of MSCs by SAME, RNAseq was further performed to check the gene expression profiles of these MSCs. The Heatmap and volcano map were shown in Figure 4A and 4B. The Kyoto Encyclopedia of Genes and Genomes analysis revealed that several signalling pathways were enriched as shown in Figure 4C, among which we found that leucocyte transendothelial migration signalling was most attractive for further study (Figure 4D). One of the main components of this signalling pathway, Vav1, was significantly increased by SAME. And this finding was further confirmed by quantitative RT-PCR (Figure 4E).
Figure 4.
SAME activated Vav1/ROCK signalling pathway in MSCs. (A) Heatmap depicting expression levels of genes between control and SAME-treated MSCs. In total, 2800 genes were differentially expressed in SAME-treated MSCs. (B) Volcano map of the differentially expressed genes in control and SAME-treated MSCs. (C) Top 5 enriched signalling pathways analysed by the KEGG analysis. (D) Schematic depicts the enriched leukocyte transendothelial migration pathway in SAME-treated MSCs. (E) Upregulation of Vav1 was confirmed by quantitative RT-PCR in MSCs treated with 10 μg/ml of SAME. Data are presented as mean ± SD (n = 3, *p < 0.05 vs. the control group). KEGG = Kyoto Encyclopedia of Genes and Genomes; MSC, mesenchymal stem cells; SAME, stearic acid methyl ester.
ROCK inhibitor represses SAME's effect on migration
RhoA/ROCK signalling axis has been found to promote actin stress fibre formation and influence cell migration. To identify the mechanism underlying MSCs migration, we conducted kinase inhibition assays with the ROCK inhibitor Y27632. As the SAME in concentration 10 μg/ml had significant effects on MSCs migration ability with transwell chamber, we therefore chose the dose for the subsequent experiments. The combined use of SAME and Y27632 decreased the migration ability of MSCs, compared with the use of SAME alone. It demonstrated that inhibition of ROCK signalling pathway significantly suppressed the effect of SAME on migration capacity of MSCs (Figure 5).
Figure 5.
Stimulatory effect of SAME on migration was suppressed by ROCK inhibitor. (A–C) MSCs were treated with SAME or SAME and the ROCK inhibitor Y27632 for 48 h. Then the MSCs were seeded in the upper layer of migratory chamber to perform the transwell assay. Crystal violet staining was used to detect the MSCs migrated through the membrane. (D) The number of migrated cells were quantified by averaging five random fields per well under microscope. Data are presented as mean ± SD (n = 5, *p < 0.05). MSC, mesenchymal stem cells; SAME, stearic acid methyl ester.
Silencing endogenous Vav1 inhibits SAME's effect on migration
In previous studies, we found that the expression of Vav1 was significantly increased. We wondered whether Vav1, as the upstream of RhoA/ROCK, was involved in MSCs migration. Therefore, we verified its function by silencing the expression of Vav1 by transfection siRNA.
Three siRNAs were designed to target endogenous Vav1. The silencing efficiency of siRNAs was checked and the most effective one (siVav1-3) was chosen to use in the following experiments (Figure 6C). Then we further confirmed the protein level of Vav1 in MSCs treated by SAME was significantly increased, as compared with the control group (Figure 6A and 6B). We found that the effect of SAME on Vav1 expression was significantly suppressed by siRNA-Vav1 (Figure 6D and 6E). And the transwell assay also demonstrated that silencing Vav1 significantly suppressed the effect of SAME on migration capacity of MSCs (Figure 6F–I).
Figure 6.
Stimulatory effect of SAME on MSCs migration was suppressed by silencing endogenous Vav1. (A) Total proteins were extracted from MSCs treated with SAME (1 and 10 μg/ml) or control and then analysed by Western blot using indicated antibodies. β-actin was used as loading control. The experiments were repeated three times. (B) Quantification of the bands intensity using Image J software. The protein level was normalised to β-actin. (C) The siRNA targeting Vav1 was transfected into MSCs as mentioned in Materials and Methods. siVav1-3 showed the best knockdown efficiency. The data are expressed as mean ± SD (n = 3), *p < 0.05. (D) MSCs were transfected with siVav1 or negative control. Then the cells were treated with SAME or not. Total proteins were extracted from MSCs and analysed by Western blot using indicated antibodies. β-actin was used as loading control. The experiments were repeated three times. (E) Quantification of the bands intensity using Image J software. The protein level was normalised to β-actin. (F) Transwell assay. The number of migrated cells were quantified by averaging five random fields per well under microscope. Data are presented as mean ± SD (n = 5, *p < 0.05 vs. the control group). (G–I) Representative images of MSCs migrated cross the membrane stained with crystal violet. MSC, mesenchymal stem cells; SAME, stearic acid methyl ester.
Effect of SAME on mobilisation of MSCs
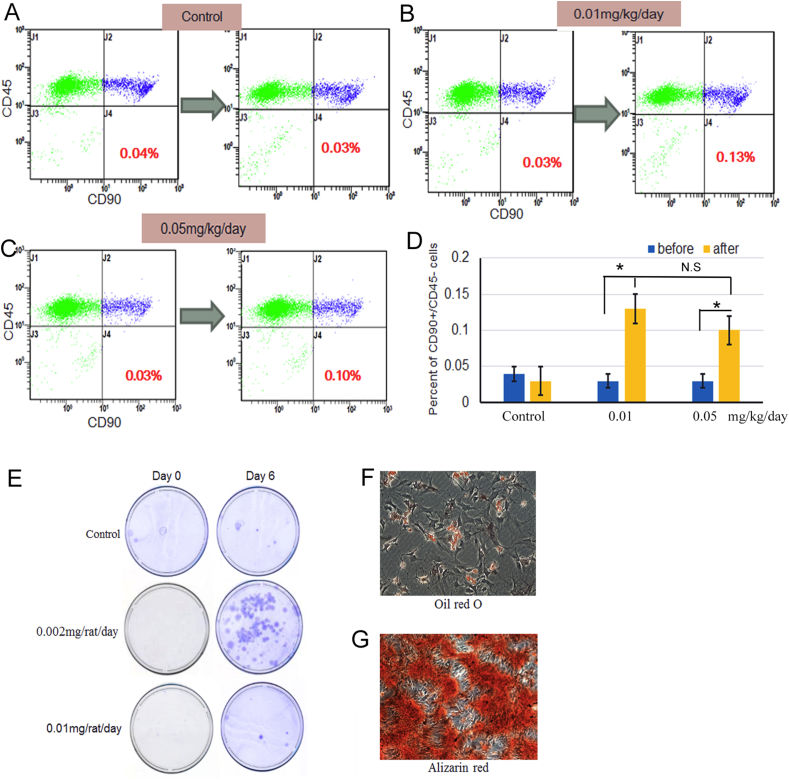
To confirm whether SAME can mobilise MSCs to peripheral blood, flow cytometry was used to detect the quantity of PBMSCs (defined as CD45-/CD90 + mononuclear cells). Compared with the control group (about 0.3%), the percentage of CD45-/CD90 + PBMSCs in rats treated with SAME was significantly increased in both low- and high-dose group (p < 0.05), indicating SAME could significantly mobilise MSCs to peripheral blood (Figure 7A–D). The colony formation assay showed that the colonies in the low-dose group were much bigger and larger, compared with the control group (Figure 7E). And the in vitro differentiation assay showed that circulating MSCs could be induced to differentiate into adipocytes and osteoblasts (Figure 7F and 7G).
Figure 7.
Effect of SAME on mobilisation of MSCs. (A–C) Representative images of flow cytometry showing the changes of circulating MSCs in rats administrated with SAME or control PBS. (D) The number of MSCs in peripheral blood in SAME treated group was significantly increased after treatment (*p < 0.05), especially in low dosage group. (E) The colony formation assay using isolated circulating MSCs in the control, low-dose and high-dose groups. The colonies were fixed and stained with crystal violet. (F) In vitro differentiation of circulating MSCs into adipocytes and stained with Oil Red O. (G) In vitro differentiation of circulating MSCs into osteoblasts, and the calcium deposits were stained with Alizarin Red S. MSC, mesenchymal stem cells; SAME, stearic acid methyl ester.
SAME accelerates cartilage defect repair
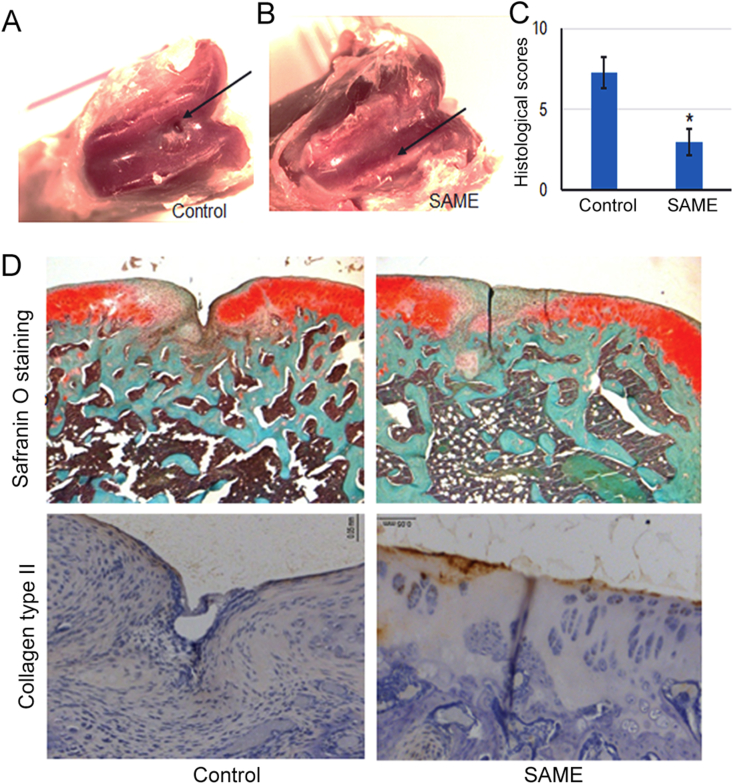
Finally, to determine the effect of SAME on cartilage regeneration, rat cartilage defect model was created and injected with SAME or PBS through intraperitoneal. After 4 weeks of treatment, the animals were killed, and the cartilages were harvested to perform histological examinations. Safranin-O/Fast Green staining of bone sections showed that there was a significant difference in the structure of articular cartilage. Compared with the control group, the defects were healed, accompanied by local fibrous cartilage connection and smoothing of cartilage surface (Figure 8A–D). The histologic grading scale for cartilage repair showed that the score of SAME-treated group was significantly improved (Figure 8C). As shown in Figure 8D, there was a remarkable increase in positive regions of cartilage marker protein-collagen type II expression, suggesting the promoting role of SAME in cartilage regeneration.
Figure 8.
SAME accelerated the healing of impaired cartilage. The osteochondral defect model is established and SAME or PBS was injected as described in the methods. (A–B) Macroscopic observation of cartilage defect at 4w after SAME treatment. For the control group, the defect area is not completely filled. (C) Histological scores for the cartilage defect after SAME treatment. Data are expressed as the mean ± SD (n = 3; *p < 0.05). (D) Histological observation of the cartilage defect area, including Safranin O staining and IHC staining using collagen type II antibody. IHC, immunohistochemistry; SAME, stearic acid methyl ester.
Discussion
In present study, we have shown that treatment of MSCs with SAME enhances the migration ability of MSCs and demonstrated the underlying mechanisms that activation of Vav1/Rock signalling pathway is accounted for the effect of SAME. Most importantly, we found SAME could be used to mobilise MSCs and accelerate cartilage defect repair. This is very interesting because the cartilage itself cannot heal well without additional treatment. While, SAME as a natural chemical found in many Chinese herbs, such as plastrum testudinis and safflower, has never been reported to help cartilage defect repair. Our findings provide strong evidence that SAME can be used as a therapeutic reagent for cartilage regeneration.
MSCs transplantation has been applied in bone and cartilage repair. However, it has been hampered by the poor capacity of migration towards pathologic tissues. In recent years, it is reported that preconditioning of MSCs could be a beneficial strategy to improve the therapeutic potential of MSCs transplantation by improving cell survival and homing to the lesion sites [11]. Thus, it has been increasingly necessary to devise an agent, which can be used to promote MSCs homing to pathologic tissues. The role of ROCK pathway in regulation of cell migration is well recognised [[33], [34], [35]].
However, there are no simple and effective ways to enhance MSCs migration by activating ROCK signalling pathway. In this study, we investigated the optimal conditions required for SAME to upregulate Vav1 and Rho-associated protein kinase 2 (ROCK2) to affect MSCs migration. Results showed that treatment of MSCs with 50ug/ml for 48 h produced the most optimal effects. In addition, we found that SAME had no cytotoxicity to MSCs in a broad dosages. At the dosage of 10 μg/ml, SAME could promote the MSCs proliferation at 48 h.
RhoA/ROCK axis is the main signalling pathway controlling the formation of stress fibres which consist of actin filaments and play important roles in various cellular functions, including cell adhesion, isometric contraction, and cell motility [36,37]. F-actin cytoskeleton networks are believed to be essential for the force generation necessary for cell migration [38]. ROCK affects F-actin cytoskeleton networks via myosin light chain (MLC) phosphorylation. Phosphorylated MLC can promote actin–myosin interaction and generate F-actin-dependent cytoskeletal contractile tension [39]. ROCK inhibits the activity of MLC phosphatase and increases phosphorylated MLC through phosphorylation of MYPT1. The phosphorylation of MLC enhances the cross-linking of myosin and actin, which enhances the contraction of actin and induces cell migration [40,41]. Our founding showed that the effect of SAME on MSCs migration was significantly suppressed by ROCK inhibitor (Y27263). SAME also improved F-actin cytoskeleton, which shown more noticeable stress fibres and elongated morphology.
To further investigate the mechanism of SAME promoting cell migration, we focused on the upstream molecules of ROCK pathway. Rho guanosine triphosphate hydrolases (ROCK) is regulated by upstream Rho GTPases [42]. Activation of Rho GTPases can activate ROCK to participate in cell adhesion, migration, proliferation, and differentiation [43]. Vav protein is a catalyst for Rho family GTPases, which can activate Rho GTPases by catalysing the exchange of guanosine diphosphate (GDP) and GTP [44]. In present study, we found that SAME significantly increased Vav1 expression. We also found that the migration ability of MSCs decreased by RNA interference knockdown of Vav1 expression and the inhibition of ROCK signalling. The above results suggested that SAME could promote MSCs migration by regulation of Vav1. This finding also confirmed that the Vav1 is an upstream factor in the ROCK signalling pathway.
MSCs migration is a highly integrated multistep process that involves the release of chemokines and mobilisation of MSCs into peripheral blood. Then, the MSCs rolls along the wall of vascular endothelial cells and finally passes through the endothelial cells to the site of injury [45,46]. It is reported that increased numbers of MSCs have been isolated from peripheral blood cells of injured mice compared with noninjured controls, which is evidence that MSCs participate in the repair of injury [47]. In the present study, the results of flow cytometry analysis shown that SAME significantly increase the number of CD45-/CD90 + circulating MSCs. Colony formation assay suggested that SAME treatment group had a significantly higher number of cultured MSCs colonies. The above results indicated that SAME could mobilise MSCs to the peripheral blood in vivo.
The ultimate goal of promoting MSCs migration is to repair damaged tissues. Therefore, we established a cartilage defect model to study whether SAME could promote cartilage defect regeneration. In our study, Safranin O & Fast green staining of bone sections showed that the defect surface of cartilage in the SAME treatment group was obviously repaired, accompanied by local fibrous cartilage connection and smoothing of cartilage surface, indicating that SAME improved cartilage healing.
To summarise, our study confirms that SAME can promote the migration of MSCs, and most importantly, it can mobilise MSCs to peripheral blood. Mechanistically, SAME robustly increased ROCK transcriptional level in MSCs via the Vav1/ROCK signalling pathway. Furthermore, we demonstrated that SAME could mobilise endogenous MSCs to improve the healing of impaired cartilage in knee cartilage defect model of SD rats. These findings provide evidence that SAME can be used as a therapeutic reagent for MSCs mobilisation and cartilage regeneration.
Conflict of interest
The authors have no conflicts of interest to disclose in relation to this article.
Ethics approval and consent to participate
This experiment was approved by the Animal Care and Use Committee of Guangzhou University of Chinese Medicine. The study has been carried out in accordance with the guidelines of the Chinese Medical Association.
Acknowledgements
This work was supported by the National Nature Science Foundation of China (81503593, 81273783, 81473699), Science and Technology Planning Project of Guangdong Province (2014A020221055), and Nature Science Foundation of Guangdong Province (2016A030313649, 2016A030310289, 2017A030313729).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2019.09.008.
Contributor Information
Yiwen Luo, Email: gzhlyw@gzucm.edu.cn.
Bin Wang, Email: wangbin1973@163.com.
Gang Li, Email: gangli@cuhk.edu.hk.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
References
- 1.Gelber A.C., Hochberg M.C., Mead L.A., Wang N.Y., Wigley F.M., Klag M.J. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med. 2000;133:321–328. doi: 10.7326/0003-4819-133-5-200009050-00007. [DOI] [PubMed] [Google Scholar]
- 2.Lubis A.M., Lubis V.K. Adult bone marrow stem cells in cartilage therapy. Acta Med Indones. 2012;44:62. [PubMed] [Google Scholar]
- 3.Armiento A.R., Stoddart M.J., Alini M., Eglin D. Biomaterials for articular cartilage tissue engineering: learning from biology. Acta Biomater. 2018;65:1–20. doi: 10.1016/j.actbio.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 4.Robert H., Bahuaud J., Kerdiles N., Passuti N., Capelli M., Pujol J.P. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation: a review of 28 cases. Rev Chir Orthopédique. 2007;93:701–709. doi: 10.1016/s0035-1040(07)73255-5. [DOI] [PubMed] [Google Scholar]
- 5.Lavrentieva A., Hatlapatka T., Neumann A., Weyand B., Kasper C. Potential for osteogenic and chondrogenic differentiation of MSC. Adv Biochem Eng Biotechnol. 2013;129:73–88. doi: 10.1007/10_2012_133. [DOI] [PubMed] [Google Scholar]
- 6.James A.W. Review of signaling pathways governing MSC osteogenic and adipogenic differentiation. Sci Tech Rep. 2013;2013:684736. doi: 10.1155/2013/684736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang J., Cui W., Song F., Zhai C., Hu H., Zuo Q. Effects of mesenchymal stem cells on interleukin-1beta-treated chondrocytes and cartilage in a rat osteoarthritic model. Mol Med Rep. 2015;12:1753–1760. doi: 10.3892/mmr.2015.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granero-Molto F., Weis J.A., Miga M.I., Landis B., Myers T.J., O'Rear L. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells. 2009;27:1887–1898. doi: 10.1002/stem.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qu Z., Fang G., Cui Z., Liu Y. Cell therapy for bone nonunion: a retrospective study. Minerva Med. 2015;106:315. [PubMed] [Google Scholar]
- 10.Amiri F., Jahaniannajafabadi A., Roudkenar M.H. In vitro augmentation of mesenchymal stem cells viability in stressful microenvironments : in vitro augmentation of mesenchymal stem cells viability. Cell Stress & Chaperones. 2015;20:237–251. doi: 10.1007/s12192-014-0560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu S.P., Wei Z., Wei L. Preconditioning strategy in stem cell transplantation therapy. Transl Stroke Res. 2013;4:76–88. doi: 10.1007/s12975-012-0251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Z.J., Zhuge Y., Velazquez O.C. Trafficking and differentiation of mesenchymal stem cells. J Cell Biochem. 2009;106:984–991. doi: 10.1002/jcb.22091. [DOI] [PubMed] [Google Scholar]
- 13.Fong E.L.S., Chan C.K., Goodman S.B. Stem cell homing in musculoskeletal injury. Biomaterials. 2011;32:395–409. doi: 10.1016/j.biomaterials.2010.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honing H., van den Berg T.K., van der Pol S.M., Dijkstra C.D., van der Kammen R.A., Collard J.G. RhoA activation promotes transendothelial migration of monocytes via ROCK. J Leukoc Biol. 2004;75:523–528. doi: 10.1189/jlb.0203054. [DOI] [PubMed] [Google Scholar]
- 15.Goetsch K.P., Snyman C., Myburgh K.H., Niesler C.U. ROCK-2 is associated with focal adhesion maturation during myoblast migration. J Cell Biochem. 2014;115:1299–1307. doi: 10.1002/jcb.24784. [DOI] [PubMed] [Google Scholar]
- 16.Fonseca A.-V., Freund D., Bornhäuser M., Corbeil D. Polarization and migration of hematopoietic stem and progenitor cells rely on the RhoA/ROCK I pathway and an active reorganization of the microtubule network. J Biol Chem. 2010;285:31661–31671. doi: 10.1074/jbc.M110.145037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niggli V. Rho-kinase in human neutrophils: a role in signalling for myosin light chain phosphorylation and cell migration. FEBS Lett. 1999;445:69–72. doi: 10.1016/s0014-5793(99)00098-8. [DOI] [PubMed] [Google Scholar]
- 18.Amano M., Nakayama M., Kaibuchi K. Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 2010;67:545–554. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J.M., Kim B.S., Lee H., Im G.I. In vivo tracking of mesechymal stem cells using fluorescent nanoparticles in an osteochondral repair model. Mol Ther J Am Soc Gene Ther. 2012;20:1434–1442. doi: 10.1038/mt.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Im G.I. Endogenous cartilage repair by recruitment of stem cells. Tissue Eng B Rev. 2016;22:160. doi: 10.1089/ten.TEB.2015.0438. [DOI] [PubMed] [Google Scholar]
- 21.Devine S.M., Cobbs C., Jennings M., Bartholomew A., Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- 22.De Becker A., Riet I.V. Homing and migration of mesenchymal stromal cells: how to improve the efficacy of cell therapy? World J Stem Cells. 2016;8:73–87. doi: 10.4252/wjsc.v8.i3.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar S., Ponnazhagan S. Mobilization of bone marrow mesenchymal stem cells in vivo augments bone healing in a mouse model of segmental bone defect. Bone. 2012;50:1012. doi: 10.1016/j.bone.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyata Y., Akamatsu N., Sugawara Y., Kaneko J., Yamamoto T., Suzuki H. Pharmacokinetics of a once-daily dose of tacrolimus early after liver transplantation: with special reference to CYP3A5 and ABCB1 single nucleotide polymorphisms. Ann Transplant. 2016;21:491. doi: 10.12659/aot.898358. [DOI] [PubMed] [Google Scholar]
- 25.Piatkov I., Caetano D., Assur Y., Lau S.L., Jones T., Boyages S.C. ABCB1 and ABCC1 single-nucleotide polymorphisms in patients treated with clozapine. Pharmacogenomics Personalized Med. 2017;10:235–242. doi: 10.2147/PGPM.S142314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan R.J., Lou T.T., Wu Y.F., Chen W.S. Single nucleotide polymorphisms of ABCB1 gene and response to etanercept treatment in patients with ankylosing spondylitis in a Chinese Han population. Medicine. 2017;96(5):e5929. doi: 10.1097/MD.0000000000005929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Megias-Vericat J.E., Montesinos P., Herrero M.J., Moscardo F., Boso V., Martinez-Cuadron D. Impact of single nucleotide polymorphisms of Abcb1 gene upon the effectiveness and toxicity of induction chemotherapy in patients with acute myeloid leukemia. Haematologica. 2016;101 375-375. [Google Scholar]
- 28.Yue S.J., Xin L.T., Fan Y.C., Li S.J., Tang Y.P., Duan J.A. Herb pair Danggui-Honghua: mechanisms underlying blood stasis syndrome by system pharmacology approach. Sci Rep. 2017;7:40318. doi: 10.1038/srep40318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei J., Wang Q. Current situation on the treatment of knee joint osteoarhritis by activating blood & dredging collateral. Chin J Ethnomed Ethnopharmacy. 2016;24:53–56. 59. [Google Scholar]
- 30.Xu L., Huang S., Hou Y., Liu Y., Ni M., Meng F. Sox11-modified mesenchymal stem cells (MSCs) accelerate bone fracture healing: sox11 regulates differentiation and migration of MSCs. FASEB J : Off Pub Fed Am Soc Exp Biol. 2015;29:1143–1152. doi: 10.1096/fj.14-254169. [DOI] [PubMed] [Google Scholar]
- 31.Lin S., Lee W.Y.W., Feng Q., Xu L., Wang B., Man G.C.W. Synergistic effects on mesenchymal stem cell-based cartilage regeneration by chondrogenic preconditioning and mechanical stimulation. Stem Cell Res Ther. 2017;8:221. doi: 10.1186/s13287-017-0672-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamaguchi S., Aoyama T., Ito A., Nagai M., Iijima H., Tajino J. The effect of exercise on the early stages of mesenchymal stromal cell-induced cartilage repair in a rat osteochondral defect model. PLoS One. 2016;11 doi: 10.1371/journal.pone.0151580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ridley A.J. Rho GTPases and cell migration. J Cell Sci. 2001;114:2713–2722. doi: 10.1242/jcs.114.15.2713. [DOI] [PubMed] [Google Scholar]
- 34.Ridley A.J. Rho GTPase signalling in cell migration. Curr Opin Cell Biol. 2015;36:103–112. doi: 10.1016/j.ceb.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Worthylake R.A., Burridge K. RhoA and ROCK promote migration by limiting membrane protrusions. J Biol Chem. 2003;278:13578–13584. doi: 10.1074/jbc.M211584200. [DOI] [PubMed] [Google Scholar]
- 36.Lv Z., Hu M., Ren X., Fan M., Zhen J., Chen L. Fyn mediates high glucose-induced actin cytoskeleton reorganization of podocytes via promoting ROCK activation in vitro. J Diabetes Res. 2016;2016:5671803. doi: 10.1155/2016/5671803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pellegrin S., Mellor H. Actin stress fibres. J Cell Sci. 2007;120:3491–3499. doi: 10.1242/jcs.018473. [DOI] [PubMed] [Google Scholar]
- 38.Popp D., Yamamoto A., Iwasa M., Maéda Y. Direct visualization of actin nematic network formation and dynamics. Biochem Biophys Res Commun. 2006;351:348–353. doi: 10.1016/j.bbrc.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 39.Zeng L., Xu H., Chew T.L., Eng E., Sadeghi M.M., Adler S. HMG CoA reductase inhibition modulates VEGF-induced endothelial cell hyperpermeability by preventing RhoA activation and myosin regulatory light chain phosphorylation. Faseb J Off Pub Fed Am Soc Exp Biol. 2005;19:1845–1847. doi: 10.1096/fj.05-4240fje. [DOI] [PubMed] [Google Scholar]
- 40.Shi J., Wu X., Surma M., Vemula S., Zhang L., Yang Y. Distinct roles for ROCK1 and ROCK2 in the regulation of cell detachment. Cell Death Dis. 2013;4:e483. doi: 10.1038/cddis.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mizuno Y., Isotani E., Huang J., Ding H., Stull J.T., Kamm K.E. Myosin light chain kinase activation and calcium sensitization in smooth muscle in vivo. Am J Physiol Cell Physiol. 2008;295:C358–C364. doi: 10.1152/ajpcell.90645.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hodge R.G., Ridley A.J. Regulating Rho GTPases and their regulators. Nat Rev Mol Cell Biol. 2016;17:496–510. doi: 10.1038/nrm.2016.67. [DOI] [PubMed] [Google Scholar]
- 43.Calzado-Martin A., Mendez-Vilas A., Multigner M., Saldana L., Gonzalez-Carrasco J.L., Gonzalez-Martin M.L. On the role of RhoA/ROCK signaling in contact guidance of bone-forming cells on anisotropic Ti6Al4V surfaces. Acta Biomater. 2011;7:1890–1901. doi: 10.1016/j.actbio.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 44.Turner M., Billadeau D.D. VAV proteins as signal integrators for multi-subunit immune-recognition receptors. Nat Rev Immunol. 2002;2:476–486. doi: 10.1038/nri840. [DOI] [PubMed] [Google Scholar]
- 45.Jacamo R., Chen Y., Wang Z., Ma W., Zhang M., Spaeth E.L. Reciprocal leukemia-stroma VCAM-1/VLA-4-dependent activation of NF-κB mediates chemoresistance. Blood. 2014;123:2691–2702. doi: 10.1182/blood-2013-06-511527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruster B., Gottig S., Ludwig R.J., Bistrian R., Muller S., Seifried E. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108:3938–3944. doi: 10.1182/blood-2006-05-025098. [DOI] [PubMed] [Google Scholar]
- 47.Wang C.H., Cherng W.J., Yang N.I., Kuo L.T., Hsu C.M., Yeh H.I. Late-outgrowth endothelial cells attenuate intimal hyperplasia contributed by mesenchymal stem cells after vascular injury. Arterioscler Thromb Vasc Biol. 2008;28:54–60. doi: 10.1161/ATVBAHA.107.147256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.