Abstract
Objective
The objective of this study was to assess the efficacy and safety of denosumab therapy in osteoporotic postmenopausal women who were previously treated with bisphosphonates.
Methods
Meta-analyses of four available randomised controlled trials that compared osteoporotic patients who switched to denosumab from bisphosphonates (n = 1416) and those who continued bisphosphonates therapy (n = 1411) were included.
Results
The increase in bone mineral density (BMD) of both the spine and hip was significantly higher in patients who shifted to denosumab than in those who continued bisphosphonates. Despite the incidence of adverse events (AEs) and fractures being comparable, treatment withdrawal owing to AEs was significantly less frequent in the denosumab group.
Conclusion
The outcomes and treatment compliance were improved in postmenopausal osteoporotic women who shifted to denosumab from bisphosphonates.
The translational potential of this article
The replacement of bisphosphonates with denosumab may lead to better therapeutic efficacy and fewer adherence barriers than those with continued usage of bisphosphonates, which in the future may guide the choice of drug therapy in clinics.
Keywords: Bisphosphonate, Denosumab, Meta-analysis, Osteoporosis, Postmenopausal osteoporosis, Randomised controlled trial
Introduction
Osteoporosis is a chronic and progressive condition characterised by decreased bone mass with microarchitectural deterioration, which increases bone fragility, compromises bone strength, and increases the risk of fractures [1]. Osteoporosis is an important contributor to decreased life span and impaired life quality of senile patients. The incidence of osteoporosis-related fracture, fracture-delayed union, and even nonunion has dramatically increased in recent years. Currently, the most commonly used pharmacologic therapies include bone resorption inhibitors (biphosphonates), bone anabolic factors (parathyroid hormone), and calcium supplements to promote bone mineralisation.
Bisphosphonates have a strong affinity to calcium crystals and can preferentially bind to calcium and inhibit the resorptive activities of osteoclasts in terms of number and function. Studies have shown that bisphosphonates can significantly reduce the risk of osteoporosis-related fractures [2]. Thus, bisphosphonates are now the recommended first-line drugs for treating postmenopausal osteoporosis (PMOP) [3].
However, the inconvenient dosing regimens and side effects of bisphosphonates often lead to poor compliance [4]. For example, alendronate is the most widely prescribed bisphosphonate which is available as an oral formulation with a once-weekly dosing schedule. The recognised side effects of alendronate include upper gastrointestinal symptoms and bowel disturbance. To ameliorate these side effects and ensure adequate and rapid absorption, alendronate should be taken after an overnight fast and at least 30 min before the first food or drink or any other oral medicinal products and supplements. Tablets should be swallowed whole with a glass of water, and the patient is required to sit or stand to keep an upright position. It is recommended that patients do not lie down for at least 30 min after taking the tablet. Therefore, poor adherence to bisphosphonate therapy is common, thus leading to poor outcomes. Most patients discontinue this therapy within the first year of treatment [5], [6]. Although extended dosing intervals can improve adherence, efficacy remains an influential determinant of patient preference for and adherence to osteoporosis medications [7], [8], [9]. Less frequent bisphosphonate dosing has been considered for patients intolerant to more frequently administered oral bisphosphonate treatment or who experience treatment failure. However, there is no evidence that switching these patients from a more to less frequently administered bisphosphonate regimen provides greater benefit [10], [11]. Besides, atypical femoral fractures (AFFs), as one of the adverse effects resulting from low bone turnover after long-term bisphosphonate treatment, are a kind of low-energy femoral fracture located typically in the area of distal to the lesser trochanter to proximal to the supracondylar flare of the distal femoral metaphysis [12], [13]. The nonunion and delayed union rates of AFFs were high in clinical cases, leading to great concern among doctors [14], [15].
In addition, suboptimal outcomes or patient dissatisfaction with treatment is commonly encountered in clinical practice. Despite no recommendation currently to such guidelines, transitioning to other therapies should be considered if BMD does not remain stable or increase, bone turnover markers (BTMs) are not maintained or decreased, or new fractures occur after the osteoporosis therapy. In this situation, it is of great importance to fully consider which therapeutic regimen is more suitable as an alternative therapy.
Denosumab (a novel bone resorption inhibitor) is the first approved biologic agent for osteoporosis therapy in clinics [16]. The mechanism underlying the effect of denosumab differs from that of bisphosphonates. It is a monoclonal antibody that prevents the binding of the receptor activator of nuclear factor-kappa B (RANK) with its ligand RANKL. RANK is expressed by preosteoclasts and osteoclasts, while RANKL is a key mediator of the differentiation, activation, and survival of osteoclasts [17]. By inhibiting the binding of RANKL with RANK, denosumab suppresses bone resorption, augments BMD, and increases bone strength [17].
In previous studies, denosumab therapy for 2 years was shown to significantly increase the BMD at the lumbar spine, hip, and distal radius and thus significantly reduce the risk of vertebral and nonvertebral fractures (i.e., hip) compared with placebo [18], [19], [20], [21]. Besides, the convenient dosage schedule of denosumab (subcutaneous injection, once every six months) is likely to improve patient compliance. In addition, denosumab was better tolerated than bisphosphonates. The transient and mild side effects of denosumab include skeletal and muscle pain, oedema, infection, sciatica, constipation, and eczema. Of note, the risk of hypocalcaemia and osteonecrosis of the jaw is 5%–10% and 1%–2%, respectively [22].
To date, most clinical studies pertaining to denosumab and bisphosphonates therapy has just involved comparison between their outcomes and safety. However, only a few studies have investigated patients with osteoporosis who shift from bisphosphonates to denosumab. Of note, no meta-analysis has synthesised the available evidence on this aspect. In this study, we performed a systematic review of published literature to assess the efficacy and safety of denosumab in postmenopausal women with osteoporosis who have been previously treated with bisphosphonates based on available published work. Our findings may provide a preliminary theoretical basis for therapeutic decision-making to improve the outcomes of osteoporosis.
Materials and methods
Ethical statements
This study was conducted in accordance with ethical approvals from our institution with informed consents from patients, if any.
Identification of eligible studies
Inclusion criteria were as follows: randomised controlled trials (RCTs); participants were postmenopausal women with osteoporosis; patients in both groups were pretreated with bisphosphonates, and the study group was treated with denosumab, while the control group was treated with the same or another bisphosphonates. We excluded non-RCTs, retrospective and observational studies, or conference abstracts in which complete data were not available.
Literature search and study selection criteria
The biomedical databases PubMed, Embase, Cochrane library, Wanfang Data, and CBM were systematically searched for relevant studies published before April 2018 in either Chinese or English using combinations of the following keywords: osteoporosis, diphosphonate, minodronate, alendronate, zoledronate, risedronate, ibandronate, and denosumab. The titles, abstracts, and main headings of the articles retrieved on database search were screened to exclude duplicate publications, irrelevant studies, and studies that did not fulfil the inclusion and exclusion criteria. The reference lists of the included studies were also manually screened to identify additional relevant studies.
Data extraction
Data were extracted by two reviewers independently using a customised database. Disagreements, if any, were resolved by consensus. Data pertaining to the following variables were collected for each study: journal, first author, year of publication, study design, sample size, age of patients, intervention, duration of follow-up, ratio of patients lost to follow-up, and outcomes (mean and standard deviation of continuous variables, relative risk of dichotomous outcomes). The primary outcome assessed was the percentage change of BMD in two skeletal sites, that is, lumbar spine and total hip. Other outcomes included incidence of adverse reactions, serious adverse reactions, and withdrawal of patients from the study. Two authors independently assessed the risk of bias in the included studies using the Cochrane Collaboration's tool for risk of bias [23]. The content of the assessment included the following elements: randomisation, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting. Based on the information available for the included studies, each item was recorded as “yes (low risk of bias)”, “no (high risk of bias)”, or “unclear (lack of information or unknown risk of bias)”.
Statistical methods
This meta-analysis was performed with a significance threshold of P ≤ 0.05. Heterogeneity among the included studies was assessed with Chi-square test and I-square statistic. When I2 < 50% or P >0.10, a fixed-effect model was used; otherwise, a random-effect model was used. If I2 and P contradicted each other, P was chosen as the standard and a P < 0.05 was assumed to be statistically significant. In the event of significant heterogeneity among the RCTs for which no obvious reason could be found, we used descriptive statistics method rather than meta-analysis. Relative risks with 95% confidence intervals (CIs) were calculated for dichotomous outcomes. Differences in mean values with 95% CIs were calculated for continuous outcomes if the metric used was the same; otherwise, standardised difference in mean with 95% CIs was calculated. Cochrane software, RevMan 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014), was used to perform all analyses.
Results
Search results
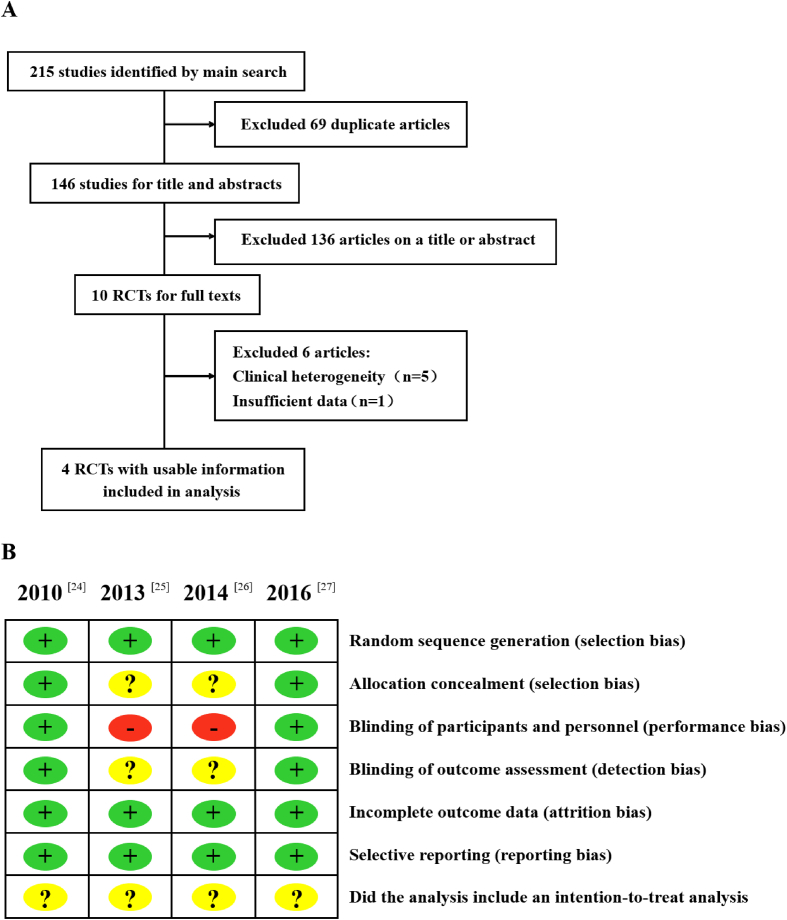
A total of 215 RCTs of bisphosphonates and denosumab for treatment of osteoporosis were identified. Of these, 69 articles were eliminated owing to duplicate publications. Of the remaining 146 articles, 136 were excluded after a review of titles and abstracts. Full text of the remaining 10 articles was reviewed. After further excluding 3 studies pertaining to osseous metastasis, 2 studies on rheumatoid disease–induced osteoporosis, and 1 article without extractable data and safety discussion, 4 RCTs were included in this meta-analysis [24], [25], [26], [27]. A schematic illustration of literature search is presented as Fig. 1A. The characteristics of the included trials are shown in Table 1. All 4 RCTs were multicentre, open-labelled trials with parallel treatment arms. The combined study population was 2827 patients (mean age: >65 years); 1416 switched to denosumab (subcutaneously administered every 6 months), while 1411 continued bisphosphonate therapy. All studies included a follow-up period of 1 year. At the beginning of the respective studies, all patients had received bisphosphonates therapy for more than 1 month or had stopped treatment for less than 6 months. In addition to the intervention drugs, all patients received basic supplements that included at least 500 mg of calcium and 800 IU of vitamin D.
Figure 1.
(A) Schematic illustration of literature search and study selection criteria; (B) quality analysis. RCTs = randomised controlled trials.
Table 1.
Basic characteristics of studies included in the analysis.
| First author (year) | Intervention | Number | Age (years) | Supplements | Follow-up (months) | Withdraw (%) |
|---|---|---|---|---|---|---|
| Kendler [24] 2010 | Denosumab 60 mg Q6M SC | 243 | 67.6 | 1000 mg Ca+ | 12 | 4.6 |
| Alendronate 70 mg QW PO | 238 | 400 IU Vit D QD | ||||
| Recknor [25] 2013 | Denosumab 60 mg Q6M SC | 417 | 67.2 | 500 mg Ca+ | 12 | 9.6 |
| Ibandronate 150 mg QM PO | 416 | 66.2 | 800 IU Vit D QD | |||
| Roux [26] 2014 | Denosumab 60 mg Q6M SC | 435 | 67.7 | 1000 mg Ca+ | 12 | 5.3 |
| Risedronate 150 mg QM PO | 435 | 800 IU Vit D QD | ||||
| Miller [27] 2016 | Denosumab 60 mg Q6M SC | 321 | 68.5 | 1000 mg Ca+ | 12 | 2.8 |
| Zoledronic acid 5 mg Q12M IV | 322 | 69.5 | 800 IU Vit D QD |
IV = intravenous; PO = oral; QD = once a day; QW = once a week; QM = once a month; Q6M = every 6 months; Q12M = once a year; SC = subcutaneous injection.
Study quality
The methodological quality of the included studies was evaluated independently by two reviewers using the Cochrane Collaboration's tool for assessing the risk of bias [23]. Results of the quality assessment are shown in Fig. 1B. All 4 studies were RCTs. Among these, 2 studies had described the method of random sequence generation [24], [27]. These 2 studies were double-blind trials wherein the care providers and the evaluators were blinded to the group identity [24], [27]. The allocation of concealment was not clear in 2 studies [25], [26]. All these studies had incomplete outcome data and selective reporting. The intention-to-treat analysis of the 4 studies was unclear. Overall, the results indicate that the included studies have a low risk of bias and are of good quality. Identical conclusions of these four studies were reported.
Meta-analysis
Increase in BMD
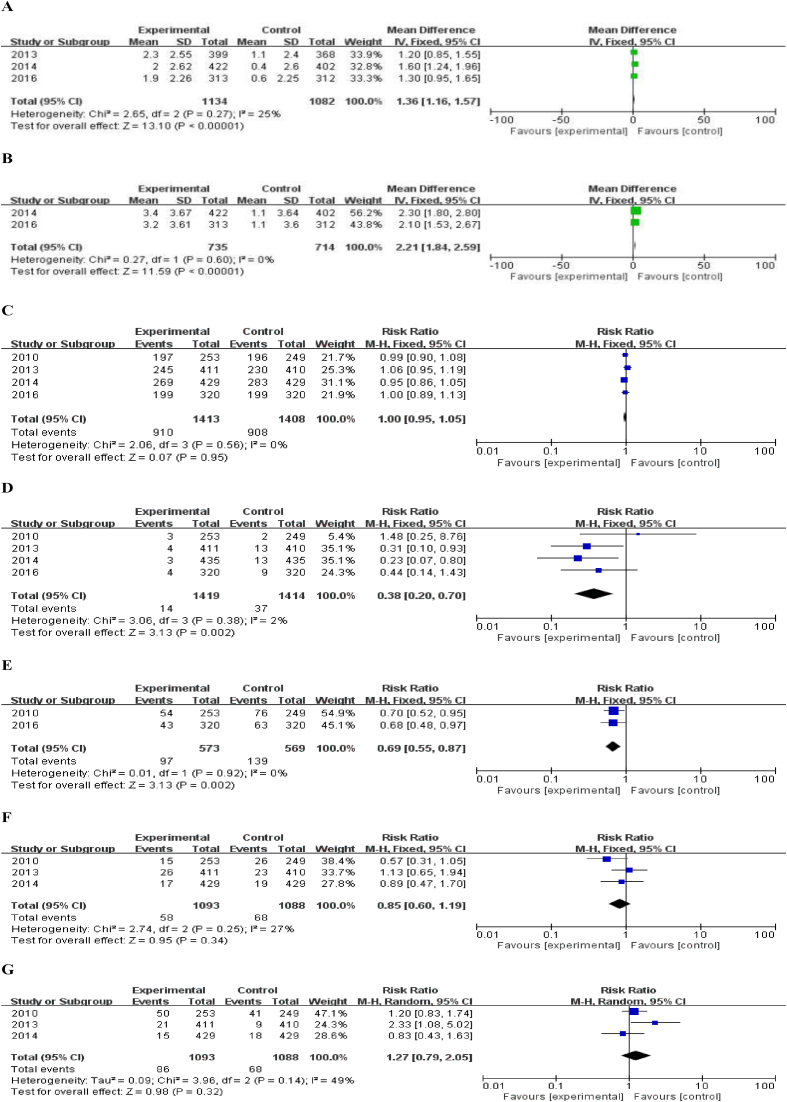
In the efficacy analysis, the authors compared the increase of BMD in the total hip and lumbar vertebrae after 12 months of treatment. Three studies had reported the increases in total hip BMD after 12-month treatment [24], [25], [26]. As shown in Fig. 3, there was low heterogeneity among these 3 studies (I2 = 25%, P = 0.27); therefore, the fixed-effect model was used for analysis. The results indicated that the increase of BMD in the denosumab treatment group was 1.36% higher than that in the bisphosphonates group after a follow-up period of 1 year, and the between-group difference was statistically significant (95% CI: 1.16%–1.57%) (Fig. 2A). Data pertaining to the increase of BMD in lumbar vertebrae after 12-month treatment were available for two studies [26], [27]. No significant heterogeneity was observed among these two studies (I2 = 0%, P = 0.60); therefore, again, the fixed-effect model was used for analysis. The results showed that increase of BMD in the lumbar vertebrae in the denosumab treatment group was 2.21% higher than that of the bisphosphonates treatment group and the between-group difference was statistically significant (95% CI: 1.84%–2.59%) (Fig. 2B).
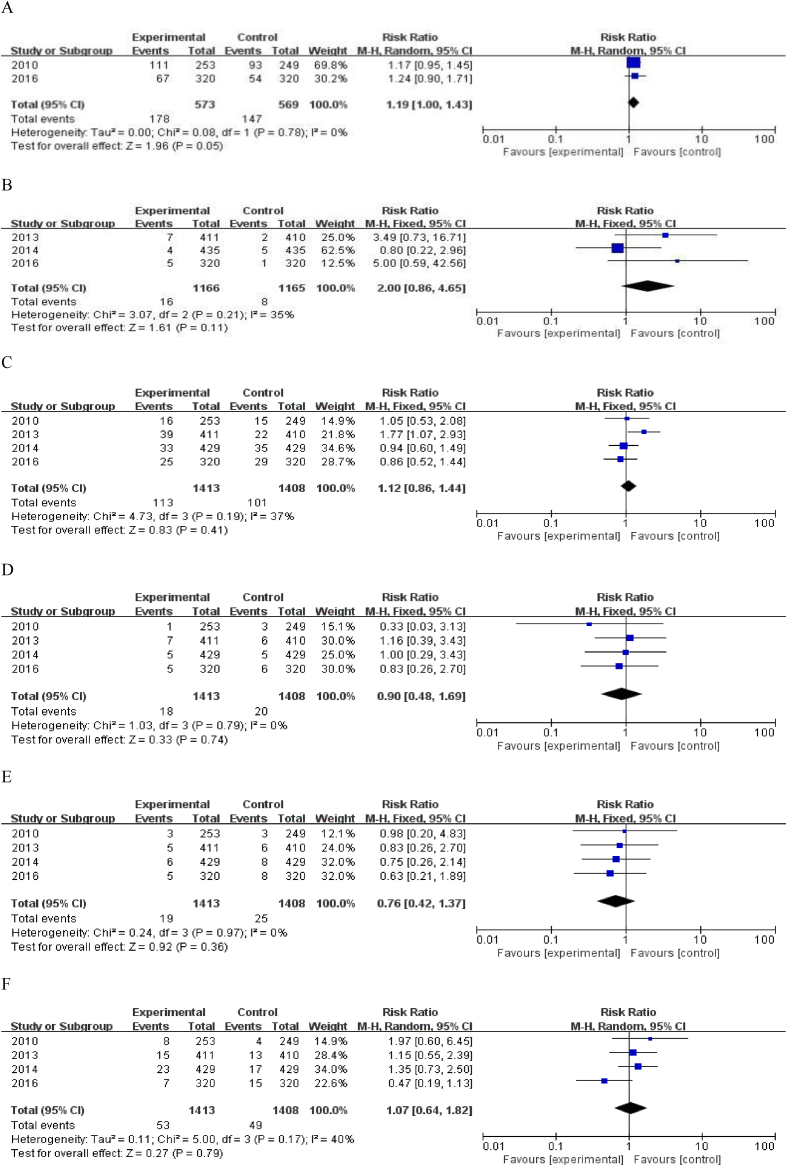
Figure 3.
Comparison of the (A) infection rates; (B) eczema; (C) severe adverse reactions; (D) severe infection; (E) malignant tumours; and (F) fracture. CI = confidence interval.
Figure 2.
(A) Meta-analysis of the percent increase in total hip density; (B) the percent increase in vertebral density; (C) comparison of the incidence of all adverse reactions; (D) withdrawal from the study caused by adverse reactions; (E) pain in the musculoskeletal system; (F) joint pain; (G) upper respiratory tract infection. CI = confidence interval; SD = standard deviation.
Adverse events
For safety analysis, we performed a meta-analysis of the total number of adverse reactions, serious adverse reactions, and withdrawal of patients from the study due to the adverse reactions within one year. For these four studies, the relative risk (RR) value of total adverse reactions was 1.00 (95% CI: 0.95–1.05), P = 0.95 (Fig. 2C). The RR of patients withdrawing from the study due to adverse reactions was 0.38 (95% CI: 0.20–0.70), P < 0.01 (Fig. 2D).
In these four studies, the dose of denosumab used in all 4 RCTs was 60 mg every 6 months. Musculoskeletal system pain and upper respiratory tract infection were the most common complications. Data pertaining to musculoskeletal system pain such as joint pain, back pain, and limb pain were available in 2 studies [24], [27]; the RR of musculoskeletal system pain for patients treated with denosumab (vs. bisphosphonate) was 0.69 (95% CI: 0.55–0.87), P < 0.01 (Fig. 2E). Three of these four studies [24], [25], [26] have reported the number of patients with joint pain, and the RR in the denosumab group (vs. bisphosphonate) was 0.85 (95% CI: 0.60–1.19), P = 0.34 (Fig. 2F). The incidence of upper respiratory tract infection such as nasopharyngitis and bronchitis was higher than the other adverse events in the two treatment groups. Three studies [25], [26], [27] reported incidence rates of upper respiratory tract infection with an RR of 1.27 (95% CI: 0.79–2.05), P = 0.32 (Fig. 2G). The incidence of infection of the respiratory system, urinary system, and digestive system was calculated in two studies [24], [27] with an RR of 1.19 (95% CI: 1.00–1.43), P = 0.05 (Fig. 3A). In addition, the RR of eczema was 2.00 (95% CI: 0.86–4.65), P = 0.11 (Fig. 3B).
Incidence of serious adverse events (SAEs) was reported for all the studies. The RR of SAEs in the denosumab group (vs. bisphosphonate) was 1.12 (95% CI: 0.86–1.44), P = 0.41 (Fig. 3C). The RR of severe infection was 0.90 (95% CI: 0.48–1.69), P = 0.74 (Fig. 3D), while that for malignant tumours was 0.76 (95% CI: 0.42–1.37), P = 0.36 (Fig. 3E).
None of the included four studies were designed to assess the therapeutic efficacy in terms of prevention of fracture; instead, fracture was counted as an adverse event. The RR of fracture (not including atypical fractures of the proximal femur) in the denosumab group (vs. bisphosphonate) was 1.27 (95% CI: 0.79–2.05), P = 0.32 (Fig. 3F).
The aforementioned results show that the total adverse events, incidence of SAEs, fracture and joint pain, infection, upper respiratory tract infection, eczema, severe infection, and the risk of malignant tumours showed no significant difference between the denosumab and bisphosphonate groups. The incidence of withdrawal of patients from the study due to adverse effects and the incidence of musculoskeletal pain in the denosumab group were significantly lower than those of the bisphosphonate group.
Discussion
Here, we discuss the typical clinical scenarios encountered during treatment of osteoporosis. In clinical settings, most patients with osteoporosis may have received bisphosphonates treatment. However, about half of the patients discontinue this drug in the first year, owing to adverse effects [5], [6]. Studies have shown that patients with low satisfaction level with respect to oral antiosteoporosis drugs have a 37% higher probability of discontinuation or replacement of therapy as compared with patients with high overall treatment satisfaction [28]. Given that patients with high treatment compliance have a lower relative risk of fractures [25], there is an urgent need to revise the treatment plan. In such cases, it is recommended to shift to another drug with weaker effect against bone resorption or to another drug of the same class that has better efficacy or to shift to a different class of drugs (that is, shift from drugs that prevent bone resorption to the ones that promote bone formation).
Denosumab is a kind of anthropogenic IgG2 monoclonal antibody type of biological agent that targets factors that modulate osteoclastic activity. It exhibits high specificity and affinity for activation of nuclear factor κB ligand (RANKL) receptor. The development of this drug followed the discovery of osteoprotegerin (OPG)/RANK/RANKL signal pathway. The transmembrane protein RANK which is expressed in osteoclast precursor cells, together with its ligand RANKL expressed by osteoblasts, can promote maturation of osteoclast precursor cells and activate osteoclasts. Osteoprotegerin is the decoy receptor of RANKL, which competitively inhibits the maturation and activation of osteoclasts and induces their apoptosis. The reduction of oestrogen level in postmenopausal women promotes the expression of RANKL. By binding with OPG, the level of OPG in the body will be decreased, and the activity of osteoclasts will eventually increase, thus accelerating bone absorption [17].
As a RANKL-targeted monoclonal antibody, denosumab mimics the endogenous mechanism of OPG and competitively blocks the combination of RANKL and RANK, consequentially leading to the inhibition of bone absorption. As drugs against bone resorption, denosumab and bisphosphonates however do not share the same mechanism of action. The main difference and advantages lie in the following: (1) Reversibility: As against bisphosphonates, which combine with hydroxyapatite and bone matrix, denosumab acts only on RANKL and does not affect the bone mineralisation process. Thus, it does not irreversibly interfere with the physiological activity of bone cells after dosing interval or discontinuation. (2) Denosumab not only inhibits the activity and survival of mature osteoclasts but also acts on the precursors of osteoclasts and inhibits their maturation and activation before their attachment to the bone matrix [24]. (3) There are no obvious gastrointestinal adverse effects or acute phase reactions after treatment with denosumab. (4) Only two subcutaneous injections in a year are required. Denosumab also offers an advantage over bisphosphonates, both with respect to mode of administration and the dosing interval. (5) As against bisphosphonates, denosumab is not excreted by renal metabolism but is metabolised in the body through the reticular endothelial system. Therefore, it has no renal toxicity and can be used in patients with renal insufficiency. In 2009, the Food and Drug Administration of the United States approved denosumab for treating osteoporosis in postmenopausal women. The 2010 guidelines of the American Society of Clinical Endocrinologists recommend denosumab as the first-line drug for treatment of osteoporosis in postmenopausal women [29]. In 2010, the European Commission also approved denosumab injection for treatment of PMOP and for hormone inhibition of bone loss in patients with prostate cancer.
Common adverse effects of denosumab include infection in the respiratory tract and urinary tract, muscle pain (typically back pain), joint pain, sciatica, hypercholesterolaemia, constipation, and rash. However, most adverse effects are mild and transient. In terms of serious complications, the incidence of hypocalcaemia is 5%–10%, while necrosis of mandible bone is relatively rare (1%–2%) [22]. Some scholars have recommended using daily supplements of calcium and vitamin D during denosumab treatment to prevent some of the aforementioned adverse effects [30]. Due diligence is advisable during denosumab therapy in patients who are prone to develop hypocalcaemia such as patients with hypoparathyroidism, a history of thyroid surgery, parathyroid surgery, malabsorption syndrome, and a history of intestinal resection [30].
In this study, we analysed the treatment efficacy and safety in patients who transitioned from bisphosphonates to denosumab therapy. These 4 RCTs were comparable with respect to study design and baseline characteristics of patients. The number of patients who were lost to follow-up and those who exited the study was also reported; the quality of the included studies was high. In terms of efficacy indicators, BMD is an important indicator of bone quality and reflects the degree of osteoporosis and predicts fracture risk [31]. Three studies reported data on changes in hip BMD, while change in lumbar vertebral BMD was reported by two studies. However, all studies had graphically illustrated the trend of bone turnover markers (BTMs), and the original source data were not accessible. Therefore, only the percentage change in lumbar vertebral and hip BMD from baseline was used as an indicator of therapeutic efficacy.
Meta-analysis showed that the improvement in BMD of the denosumab group was significantly better than that of the bisphosphonate group, which is consistent with the results of efficacy indicators in all the four articles. In 3 RCTs [25], [26], [27], minimum significant change in BMD values for individual patients (i.e., minimal BMD change, which helped clinical doctors conclude that the increase in BMD of each part) in the denosumab group was higher than that in the bisphosphonates group, suggesting the superior therapeutic efficacy of denosumab. However, owing to the lack of relevant data, the efficacy of denosumab in improving BMD of other body parts (e.g., one-third of the distal radius, femoral) and its effect on BTMs could not be assessed in this meta-analysis. Further studies are required in this respect.
Regarding treatment safety, we found no significant difference between the bisphosphonate and denosumab groups with respect to the incidence of total adverse events, common adverse effects (such as joint pain, infection, upper respiratory tract infection, constipation, eczema, allergies, fracture), and severe adverse reactions (such as severe infection and malignant tumours). This shows that the transition to denosumab therapy does not increase the incidence of adverse events. In the denosumab group, the number of patients who were unable to tolerate the adverse effects and dropped out of the study was significantly lower than that in the bisphosphonates group. This suggests that patients who discontinue bisphosphonates owing to adverse effects or those who exhibit poor treatment compliance may benefit from shifting to denosumab. Unfortunately, although the incidence of fracture is one of the most important indicators used to evaluate the effect of antiosteoporosis drugs, the studies included in this research did not include fracture rate as a measure of curative effect but only as an adverse event. Besides, the follow-up period of the 4 RCTs was only 12 months, so we could not evaluate long-term adherence and the potential long-term complications such as AFFs, which are particularly important in the treatment of chronic, multifactorial disease such as PMOP. For example, in intravenous zoledronic acid, which is administered once a year, the adherence at one year is 100%. Thus, more rigorous large multicentre studies with long-term follow-up are still expected to assess the effect of denosumab on fracture rates, long-term adverse events, and adherence.
To the best of our knowledge, this is the first meta-analysis of a clinically relevant topic on replacement of bisphosphonates with RANKL monoclonal antibody denosumab for treatment for PMOP. Therefore, there are several limitations of this analysis that should be considered: First, only 4 studies were eligible and included in the meta-analysis owing to paucity of relevant RCTs. Second, the type and dosage of the bisphosphonates adopted in the experimental group and the regional source of patients was different, which may have affected our results. Third, the follow-up period of the studies was not enough to assess the long-term adherence and complications of this treatment option. Furthermore, none of the included studies were designed to assess the clinical efficacy of drugs in reducing the fracture risk. Therefore, we could not evaluate the impact of drug replacement on fracture risk. Finally, all 4 RCTs included in the analysis were sponsored by the pharmaceutical industry. Therefore, due diligence is warranted with some conclusions of this study. We hope that future RCTs will provide more detailed original data and incorporate homogeneity to inform more credible clinical guidelines.
Conclusion
This meta-analysis shows that replacement of bisphosphonates with RANKL monoclonal antibody denosumab for treatment for PMOP leads to better therapeutic efficacy in improving BMD than that with continued usage of bisphosphonates in short-term follow-up. We found no significant increase in side effects after transition from bisphosphonates to denosumab. Although this meta-analysis has provided evidence for guiding the decision, more rigorous multicentre RCTs with larger sample size and long-term follow-up are still expected.
Conflicts of interest
The authors have no conflicts of interest to disclose in relation to this article.
Acknowledgements
The authors would like to thank Professor Ling QIN of the Chinese University of Hong Kong for his constructive suggestions on improving the quality of the manuscript. The present study was partially supported by the National Natural Science Foundation of China (81802152 and 81271619), Shantou Science and Technology Project (0022352630820022), and the start-up of Postdoctoral Science Foundation.
References
- 1.Kawate H., Takayanagi R. Efficacy and safety of bazedoxifene for postmenopausal osteoporosis. Clin Interv Aging. 2011;6:151–160. doi: 10.2147/CIA.S15711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kling J.M., Clarke B.L., Sandhu N.P. Osteoporosis prevention, screening, and treatment: a review. J Women's Health. 2014;23:563–572. doi: 10.1089/jwh.2013.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Compston J., Bowring C., Cooper A., Cooper C., Davies C., Francis R. Diagnosis and management of osteoporosis in postmenopausal women and older men in the UK: National Osteoporosis Guideline Group (NOGG) update 2013. Maturitas. 2013;75:392–396. doi: 10.1016/j.maturitas.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Sambrook P., Cooper C. Osteoporos Lancet. 2006;367:2010–2018. doi: 10.1016/S0140-6736(06)68891-0. [DOI] [PubMed] [Google Scholar]
- 5.Yeaw J., Benner J.S., Walt J.G., Sian S., Smith D.B. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm. 2009;15:728–740. doi: 10.18553/jmcp.2009.15.9.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverman S.L., Gold D.T. Compliance and persistence with osteoporosis therapies. Curr Rheumatol Rep. 2008;10:118–122. doi: 10.1007/s11926-008-0021-x. [DOI] [PubMed] [Google Scholar]
- 7.Cotte F.E., Fardellone P., Mercier F., Gaudin A.F., Roux C. Adherence to monthly and weekly oral bisphosphonates in women with osteoporosis. Osteoporos Int. 2010;21:145–155. doi: 10.1007/s00198-009-0930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Recker R.R., Gallagher R., MacCosbe P.E. Effect of dosing frequency on bisphosphonate medication adherence in a large longitudinal cohort of women. Mayo Clin Proc. 2005;80:856–861. doi: 10.4065/80.7.856. [DOI] [PubMed] [Google Scholar]
- 9.Cramer J.A., Gold D.T., Silverman S.L., Lewiecki E.M. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18:1023–1031. doi: 10.1007/s00198-006-0322-8. [DOI] [PubMed] [Google Scholar]
- 10.Orwoll E.S., Miller P.D., Adachi J.D., Brown J., Adler R.A., Kendler D. Efficacy and safety of a once-yearly i.v. Infusion of zoledronic acid 5 mg versus a once-weekly 70-mg oral alendronate in the treatment of male osteoporosis: a randomised, multicenter, double-blind, active-controlled study. J Bone Miner Res. 2010;25:2239–2250. doi: 10.1002/jbmr.119. [DOI] [PubMed] [Google Scholar]
- 11.McClung M., Recker R., Miller P., Fiske D., Minkoff J., Kriegman A. Intravenous zoledronic acid 5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronate. Bone. 2007;41:122–128. doi: 10.1016/j.bone.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Zheng N.Y., Tang N., Qin L. Atypical femoral fractures and current managements. J Orthop Transl. 2016;7:7–22. doi: 10.1016/j.jot.2016.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian L., Deng N., Ngai T., Wu C., Huang L., Yuan Y.C. Hybrid fracture fixation systems developed for orthopaedic applications, a general review. J Orthop Transl. 2018;16:1–13. doi: 10.1016/j.jot.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schilcher J., Koeppen V., Aspenberg P., Michaelsson K. Risk of atypical femoral fracture during and after bisphosphonate use. N Engl J Med. 2014;10:974–976. doi: 10.1056/NEJMc1403799. [DOI] [PubMed] [Google Scholar]
- 15.Jamal S.A., Dion N., Ste-Mane L.G. Atypical femoral fractures and bone tumover. N Engl J Med. 2011;13:1261–1262. doi: 10.1056/NEJMc1107029. [DOI] [PubMed] [Google Scholar]
- 16.Bonnick S.L., Harris S.T., Kendler D.L., Mcclung M.R., Silverman S.L. Management of osteoporosis in postmenopansal women: 2010 position statement of the North American Menopause Society. Menopause. 2010;17:25–54. doi: 10.1097/gme.0b013e3181c617e6. [DOI] [PubMed] [Google Scholar]
- 17.Tsourdi E., Rachner T.D., Rauner M., Hamann C., Hofbauer L.C. Denosumab for bone diseases: translating bone biology into targeted therapy. Eur J Endocrinol. 2011;165:833–840. doi: 10.1530/EJE-11-0454. [DOI] [PubMed] [Google Scholar]
- 18.Lewiecki E.M., Miller P.D., McClung M.R., Cohen S.B., Bolognese M.A., Liu Y. Two-year treatment with denosumab (AMG 162) in a randomised phase 2 study of postmenopausal women with low BMD. J Bone Miner Res. 2007;22:1832–1841. doi: 10.1359/jbmr.070809. [DOI] [PubMed] [Google Scholar]
- 19.Miller P.D., Bolognese M.A., Lewiecki E.M., McClung M.R., Ding B., Austin M. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomised blinded phase 2 clinical trial. Bone. 2008;43:222–229. doi: 10.1016/j.bone.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Bone H.G., Bolognese M.A., Yuen C.K., Kendler D.L., Wang H., Liu Y. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96:972–980. doi: 10.1210/jc.2010-1502. [DOI] [PubMed] [Google Scholar]
- 21.McClung M.R., Lewiecki E.M., Cohen S.B., Bolognese M.A., Woodson G.C., Moffett A.H. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354:821–831. doi: 10.1056/NEJMoa044459. [DOI] [PubMed] [Google Scholar]
- 22.Adler R.A., Gill R.S. Clinical utility of denosumab for treatment of bone loss in men and women. Clin Interv Aging. 2011;6:119–124. doi: 10.2147/CIA.S14565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins J.P.T., Altman D.G. Chapter 8: assessing rick of bias in included studies. In: Higgins J.P.T., Green S., editors. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; Chichester: 2008. pp. 190–194. [Google Scholar]
- 24.Kendler D.L., Roux C., Benhamou C.L., Brown J.P., Lillestol M., Siddhanti S. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res. 2010;25:72–81. doi: 10.1359/jbmr.090716. [DOI] [PubMed] [Google Scholar]
- 25.Recknor C., Czerwinski E., Bone H.G., Bonnick S.L., Binkley N., Palacios S. Denosumab compared with ibandronate in postmenopausal women previously treated with bisphosphonate therapy: a randomised open-label trial. Obstet Gynecol. 2013;121:1291–1299. doi: 10.1097/AOG.0b013e318291718c. [DOI] [PubMed] [Google Scholar]
- 26.Roux C., Hofbauer L.C., Ho P.R., Wark J.D., Zillikens M.C., Fahrleitner-Pammer A. Denosumab compared with risedronate in postmenopausal women suboptimally adherent to alendronate therapy: efficacy and safety results from a randomised open-label study. Bone. 2014;58:48–54. doi: 10.1016/j.bone.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Miller P.D., Pannacciulli N., Brown J.P., Czerwinski E., Nedergaard B.S., Bolognese M.A. Denosumab or zoledronic acid in postmenopausal women with osteoporosis previously treated with oral bisphosphonates. J Clin Endocrinol Metab. 2016;101:3163–3170. doi: 10.1210/jc.2016-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrett-Connor E., Wade S.W., Do T.P., Satram-Hoang S., Stewart R., Gao G. Treatment satisfaction and persistence among postmenopausal women on osteoporosis medications: 12-month results from POSSIBLE US. Osteoporos Int. 2012;23:733–741. doi: 10.1007/s00198-011-1620-3. [DOI] [PubMed] [Google Scholar]
- 29.Boroojeny S.B., Fard M.M. Effect of oral clonidine on acute intraocular pressure rise after cataract extraction under general anaesthesia. J Pak Med Assoc. 2012;62:1285–1288. [PubMed] [Google Scholar]
- 30.Sun J.T., Wei J.W., Sui G.S., Guan Z.P. Denosumab: a review of its use in postmenopausal woman with osteoporosis. Orthop J China, 2015;23:1007–1010. [In Chinese, English abstract] [Google Scholar]
- 31.Rizzoli R. How does teriparatide compare with alendronate for the treatment of glucocorticoid-induced osteoporosis? Nat Clin Pract Endocrinol Metab. 2008;4:372–373. doi: 10.1038/ncpendmet0838. [DOI] [PubMed] [Google Scholar]