Abstract
Allopregnanolone is synthesized in the central nervous system either de novo from cholesterol or from steroid hormone precursors like progesterone and pregnenolone. Over the past 30 years, direct and rapid, non-genomic actions of allopregnanolone and its derivatives via GABAA receptors have been demonstrated. Changes in brain levels of allopregnanolone during pregnancy and in the postpartum period, or during exposure to protracted stress appear to play a crucial role in the pathophysiology of mood disorders. The discovery that allopregnanolone at low (nanomolar) concentrations elicits marked anxiolytic, anti-stress and antidepressant effects by facilitating allosterically the action of GABA at extrasynaptic GABAA receptors has provided new perspectives for the discovery of novel drugs useful for the treatment of mood disorders. These findings have led to the seminal clinical studies that recently demonstrated that treatment with allopregnanolone (i.e., brexanolone) can dramatically and rapidly improve the symptoms of postpartum depression in many patients.
Keywords: Postpartum depression, GABAA receptors, Neurosteroids, Allopregnanolone, Brexanolone, Zulresso, Mood disorders
1. Neurosteroids: the concept
It is well recognized that the cytosolic steroid hormone receptors, after binding with their ligand, translocate to the nucleus where they may alter gene expression via chromatin remodeling mechanisms (McEwen et al., 1976). Over the past 30 years, direct and rapid membrane actions of steroid hormones and their metabolites have also been demonstrated. One of the clearest examples of a rapid, non-genomic steroid action on a membrane receptor is the demonstration that the natural metabolites of progesterone and deoxycorticosterone (DOC); i.e., allopregnanolone and allotetrahydroDOC (THDOC), respectively, exert their main actions as powerful endogenous positive allosteric modulators of the inhibitory neurotransmitter γ-aminobutyric acid (GABA) at both synaptic and extrasynaptic GABAA receptors (Majewska et al., 1986, Harrison et al., 1987, Puia et al., 1990; Robel and Baulieu, 1994; Brickley and Mody, 2012). These steroids are termed neurosteroids and sometimes referred to as neuroactive steroids. As proposed by Baulieu, the term neurosteroid “applies to those steroids that are both synthesized in the nervous system, either de novo from cholesterol or from steroid hormone precursors like progesterone that accumulate in the nervous system” (Robel and Baulieu, 1994). Neuroactive steroids, a term coined by Paul and Purdy (1992) refers to “any natural or synthetic steroid that rapidly alters neuronal excitability via non-genomic mechanisms.”
In a historical perspective, herein, we review findings of seminal preclinical and clinical studies that recently have led to the demonstration that treatment with allopregnanolone (i.e., brexanolone, marketed as Zulresso™) can dramatically and rapidly improve symptoms in many women with postpartum depression (PPD).
The authors dedicate this article to the memory of Erminio Costa, a true pioneer in the field of neurosteroids and GABAA receptors, our scientific mentor, collaborator and friend.
2. The brain as a neuroendocrine organ
To answer the question of whether allopregnanolone is synthesized in brain, Purdy et al. (1991) using a sensitive radioimmunoassay measured allopregnanolone in rat brain and serum both before and following swim stress. Levels of allopregnanolone increased in brain and serum after acute swim stress and demonstrable brain levels persisted even after adrenalectomy and ovariectomy (Purdy et al., 1991). Subsequently Cheney et al. (1995) measured allopregnanolone, 5α-dihydro-progesterone (5α-DHP), and progesterone in brain, plasma and liver of control and adrenalectomized mice using gas chromatography-mass spectrometry (GC/MS) with unsurpassed molecular selectivity and specificity (Uzunov et., 1996; Pinna et al., 2000; for an update, see Locci and Pinna, 2019). In this study, allopregnanolone remained elevated in brain also 15 days after adrenalectomy. At this time, the level of progesterone was greatly reduced. These two studies suggest that the allopregnanolone present in brain is synthesized, at least in part, independently of the control of the pituitary on peripheral endocrine tissues (Purdy et al., 1992; Cheney et al., 1995).
3. Allopregnanolone: biosynthesis and metabolism in mammalian brain
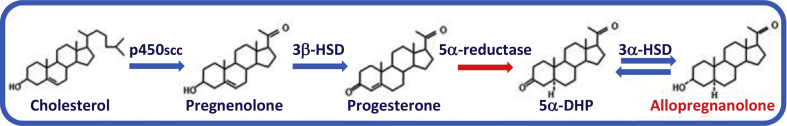
The biosynthesis of allopregnanolone starts from cholesterol (Fig. 1). To this end, it became essential to identify the location of the metabolic steps and enzymes that control the recruitment and metabolism of cholesterol to a specific small pool that can be used for progesterone and allopregnanolone biosynthesis. The first step of neurosteroid biosynthesis is the transport of cholesterol from the outer into the inner mitochondrial membrane primarily in glial cells. Costa and colleagues discovered that the transport of cholesterol to the inner mitochondrial membrane is dependent on the presence and activation of a small 18 kD protein named “translocator protein” (TSPO), initially known as the peripheral benzodiazepine receptor (Sprengel et al., 1989; Mukhin et al., 1989) because of its ability to bind benzodiazepines and an endogenous peptide called diazepam binding inhibitor (DBI) (Costa and Guidotti, 1991). TSPO is expressed in glial cells and not in neurons and when occupied by 2-aryl-indole-acetamide derivatives (Romeo et al., 1994), or by N-benzyl-N-ethyl-2-(7-methyl-8-oxo-2-phenylpurin-9-yl)acetamide (AC-5216, XBD173) (Rupprecht et al., 2009) elicits a non-sedative, anxiolytic, and antipanic action via activation of neurosteroid biosynthesis and enhancement of GABAA receptor activity. Inside mitochondria, cholesterol is initially metabolized to pregnenolone by the action of P450scc and to progesterone by 3β-hydroxysteroid dehydrogenase (3β-HSD).
Fig. 1.
Biosynthesis of allopregnanolone.
Neurons can synthesize allopregnanolone de novo starting from cholesterol, which is recruited and translocated into the inner mitochondrial membrane preferentially in glial cells. Here, P450SCC metabolizes cholesterol into pregnenolone, the precursor of all neurosteroids. Pregnenolone is then taken up from neurons and is further metabolized into progesterone by 3β-HSD and progesterone can be further converted by the rate-limiting step enzyme, 5α-reductase type I into 5α-dihydroprogesterone (5α-pregnan-3,20-dione, 5α-DHP). Finally, 5α-DHP can be reduced into allopregnanolone (3α-hydroxy-5α-pregnan-20-one or 3α,5α-tetrahydroprogesterone) by the 3α-hydroxysteroid dehydrogenase (3α-HSD) enzyme. Allopregnanolone can eventually be reconverted into 5α-DHP.
Using in situ hybridization and immunohistochemistry it was possible to define the neuronal and glial expression and location of the enzymes required for allopregnanolone biosynthesis in rodents (Agis-Balboa et al., 2006). Allopregnanolone and THDOC are synthesized in the brain from progesterone or deoxycorticosterone, respectively, by the sequential action of two enzymes: 5α-reductase (5α-R) type I and 3α-hydroxysteroid dehydrogenase (3α-HSD). These studies demonstrated that 5α-R type I and 3α-HSD, the rate-limiting step enzymes for allopregnanolone biosynthesis (Dong et al., 2001), colocalize in cortical, hippocampal, basolateral amygdala and olfactory bulb glutamatergic principal neurons and in some output neurons of the lateral amygdala and thalamus (Agis Balboa et al., 2006, 2007). Neither 5α-R type I nor 3α-HSD mRNAs, however, are expressed in S100β- or glial fibrillary acidic protein-positive glial cells. Using glutamic acid decarboxylase 67/65 antibodies as a marker for GABAergic neurons, it appears that there is no detectable 5α-R type I and 3α-HSD in cortical and hippocampal GABAergic interneurons (Agis-Balboa et al., 2006). However, 5α-R type I and 3α-HSD are expressed in GABAergic output neurons, such as striatal neurons, reticular thalamic nucleus, and cerebellar Purkinje neurons (Agís-Balboa et al., 2007). A similar distribution and cellular location of these neurosteroidogenic enzymes was observed in human brain (Agis-Balboa et al., 2014). More recently, using a specific antibody against allopregnanolone, Morrow and colleagues confirmed the neuronal localization of this neurosteroid in the rodent brain (Cook et al., 2014).
Taken together, these data suggest that allopregnanolone and THDOC, which can be synthesized in principal output neurons, modulate GABA's action at GABAA receptors, either in an autocrine or paracrine manner or by accessing GABAA receptor intracellular sites through lateral diffusion in the neuronal membrane (Pinna et al., 2008). In this regard, it is also remarkable that TSPO is present in glial cells and not in neurons (Papadopoulos et al., 2006).
4. Allopregnanolone and GABAA receptor modulation
Allopregnanolone acts at low nanomolar concentrations as an endogenous modulator of GABA action at GABAA receptors (Majewska et al., 1986; Puia et al., 1990; Paul and Purdy, 1992; Lambert et al., 1995; Gee et al., 1995). Electrophysiological studies with different combinations of transfected GABAA receptor subunits in 293 kidney tumor cell lines, as well as native GABAA receptors in cerebellar granule cells indicate that allopregnanolone and its congeners bind to specific sites on GABAA receptors (Morrow et al., 1987, 1990; Hosie et al., 2006), that differ from those at which the benzodiazepines bind (Puia et al., 1990, 1993; Lambert et al., 1995; Zhu and Vicini, 1997). For example, benzodiazepines increase the potency of GABA only when α1, α2, or α3 subunits are assembled with β and γ2 subunits in the canonical pentameric structure of GABAA receptors (Schweizer et al., 2003). In contrast, allopregnanolone will regulate the function of GABAA receptors containing the δ subunit in place of the γ subunit (Morrow et al., 1990; Seeburg et al., 1990; Breier and Paul, 1990; Puia et al., 1990). These δ subunit-containing GABAA receptors are presumably extrasynaptic and extremely sensitive to GABA and allopregnanolone but relatively insensitive to the action of benzodiazepines (Maguire and Mody, 2008; Maguire et al., 2005; reviewed in Locci and Pinna, 2017). By this mechanism, endogenously produced allopregnanolone plays a pivotal neurophysiological role in the fine-tuning of GABAA receptors to GABAmimetic drugs, agonists and allosteric modulators, and may as well regulate emotional behavior in response to stress (Pinna et al., 2000; Guidotti et al., 2001).
5. Allopregnanolone and the pathophysiology of depression
Decreases in the brain levels of allopregnanolone during the postpartum period, or during exposure to protracted severe stress appear to play a crucial role in controlling neuronal excitability via local modulation of extrasynaptic GABAA receptors. Marked fluctuations in both the brain and blood concentrations of allopregnanolone strongly correlate with fluctuations of GABAA receptor function and plasticity. In addition to pregnancy (mentioned above) there are several other interesting pathophysiological conditions that alter the function and plasticity of GABAA receptors by modifying the brain level of allopregnanolone. They include the postpartum period in human (Luisi et al., 2000), mice and rats (Concas et al., 1998), social isolation stress in mice (Matsumoto et al., 1999; Pinna et al., 2004), and major depression in humans (Uzunova et al., 1998; Romeo et al., 1998).
The increase in brain levels of allopregnanolone during pregnancy and the sudden decrease immediately following parturition have been shown to alter the expression/function of specific GABAA subunits in the cortico-limbic structures (Concas et al., 1998; see also Grobin and Morrow, 2001). These data suggest that alterations in GABAA receptor expression, especially extrasynaptic GABAA receptors, may be related to psychiatric disorders associated with changes in neurosteroid levels, i.e., those associated with pregnancy and the menstrual cycle in women. These findings could help to provide potential new treatments for these disabling hormone-dependent syndromes.
The discovery that allopregnanolone and THDOC elicit marked anxiolytic and anti-stress effects and selectively facilitate GABA-mediated neurotransmission (Crawley et al., 1986; Matsumoto et al., 2007; Girdler et al., 2001; Zorumski et al., 2013), has also provided new perspectives for our understanding of the pathophysiology and neurobiology of stress and anxiety. Protracted exposure of mice to the stress of social isolation results in a marked decrease of 5α-R type I and a 50% decrease in brain allopregnanolone levels (Matsumoto et al., 1999; and Guidotti et al., 2001; Pibiri et al., 2008). Treatment with the antidepressant fluoxetine normalizes this stress-induced decrease in allopregnanolone (Pinna et al., 2004; reviewed in 2006). The exact mechanism responsible for the decreased brain allopregnanolone levels in the brain of socially isolated mice is not known but it is possible that it involves epigenetic regulation of the genes encoding one or more of the enzymes involved in neurosteroid biosynthesis or metabolism (discussed in Pinna et al., 2008). Interestingly, in socially isolated mice, fluoxetine and analogs stereospecifically normalize the decrease of allopregnanolone biosynthesis and improve behavioral dysfunction by a mechanism that is independent of 5-HT reuptake inhibition (Pinna et al., 2004, 2006).
The evidence that downregulation of allopregnanolone biosynthesis in brain results in downregulation of GABA receptor-mediated tonic inhibition in patients with major depression and that this can be reversed by indirectly increasing the brain content of allopregnanolone with fluoxetine, supports the view that depression, stress, neurosteroids, and the function of GABAA receptors are intimately related.
Fluoxetine or paroxetine, two selective serotonin reuptake inhibitors (SSRIs), when administered to rats, increase the brain content of allopregnanolone without altering the brain content of other neurosteroids (Uzunov et al., 1996). As mentioned above allopregnanolone binds with high affinity to δ subunit-containing GABAA receptor subtype and augments the action of GABA at these receptors, which control tonic inhibition in the brain (Puia et al., 1990; Mody, 2012). We hypothesized that the increased brain content of allopregnanolone induced by treatment with SSRIs could contribute to alleviating the symptoms of anxiety and depression associated with major depression (Guidotti and Costa, 1998). Further, we measured allopregnanolone content in the cerebrospinal fluid (CSF) before and 8–10 weeks after treatment with fluoxetine or fluvoxamine in 15 patients with major depression (Uzunova et al., 1998). The concentration of allopregnanolone (approximately 40 fmol/ml in each CSF fraction of three control subjects) was about 60% lower in patients with major depression compared to matched non-depressed controls. Moreover, in the same patients, fluoxetine or fluvoxamine treatment normalized CSF allopregnanolone concentrations. Importantly, a statistically significant correlation (r = 0.58; P < 0.023; n = 15) between improvement in depressive symptoms (Hamilton Rating Scale for Depression scores) and the increase in CSF allopregnanolone following fluoxetine or fluvoxamine treatment was observed. The CSF concentration of pregnenolone and progesterone remained unaltered after treatment and did not correlate with the SSRI-induced increase of CSF allopregnanolone or improvement in depression (Uzunova et al., 1998). The normalization of CSF allopregnanolone concentration in depressed patients could at least in part mediate some of the anxiolytic and antidepressant actions of fluoxetine or fluvoxamine indirectly via their effects on neurosteroid biosynthesis and thus the positive allosteric modulation of GABAA receptors (discussed in Guidotti and Costa, 1998; Pinna et al., 2006). New molecules that stimulate TSPO and elevate allopregnanolone levels, including XBD173 and etifoxine induce anxiolytic effects in animal models and anxious patients (Rupprecht et al., 2009, Rupprecht et al., 2010; Schüle et al., 2011).
6. Allopregnanolone and postpartum depression
PPD is the most common complication of pregnancy and affects between 10 and 20% of women during or just following childbirth. PPD negatively affects not only the suffering mother but her entire family and it has been estimated that 20% of postpartum deaths are due to suicide secondary to PPD. Given the early data demonstrating that serum and brain levels of progesterone and allopregnanolone rise during pregnancy, reaching a peak in the third trimester, and then fall precipitously following parturition (Luisi et al., 2000), it has long been speculated that PPD might in part be due to these rapid changes in neurosteroid levels that occur during pregnancy and the immediate postpartum period. While this area has been comprehensively reviewed by Walton and Maguire (2019), we will highlight just a few of the salient data that strongly implicate this neuroactive steroid as the trigger for PPD (at least in many women), and which has led directly to the successful development of a proprietary formulation of allopregnanolone as the first effective treatment for PPD (Meltzer-Brody et al., 2018).
While decreased CSF levels of allopregnanolone have been implicated in major depression as reviewed above and while some groups have reported lower and even higher serum allopregnanolone levels during pregnancy in women who subsequently develop PPD, the relationship between serum allopregnanolone levels and PPD per se has been inconsistent at best across studies and thus it is difficult to assign a specific role for alterations in allopregnanolone levels in the etiology or pathophysiology of PPD. However, more recent animal models of PPD have been informative and strongly suggest that the adaptive changes in GABAA receptor expression that occur in response to increased brain levels of allopregnanolone during pregnancy and specifically a downregulation of extrasynaptic GABAA receptors that control tonic inhibition may underlie the pathophysiology of PPD. Mice that lack or have diminished numbers of δ subunit-containing extrasynaptic GABAA receptors develop depression-like behaviors and marked deficits in maternal care mimicking PPD. Using this animal model of PPD, Maguire and Mody (2008) demonstrated that drugs that enhance extrasynaptic GABAA receptor-mediated tonic inhibition were effective at improving these PPD-like behaviors. These data, together with all of the early work implicating allopregnanolone and GABAA receptors in depression, anxiety and sleep disorders (all prominent symptoms of PPD) led to the seminal clinical studies by scientists and clinicians at Sage Therapeutics which have demonstrated that treatment with allopregnanolone (i.e., brexanolone) can dramatically improve the signs/symptoms of PPD in many women.
The development of allopregnanolone for PPD began with a small open label proof-of-concept study by Meltzer-Brody and colleagues and progressed sequentially into formal double blind and placebo-controlled phase 2 and phase 3 clinical trials (Kanes et al., 2017; Meltzer-Brody et al., 2018). Each of these studies showed that intravenous infusion of allopregnanolone/brexanolone at levels mimicking those that occurs during late pregnancy resulted in marked and rapid improvement in measures of depression, anxiety and sleep in the majority (but not all) of women with PPD. Most of these patients had moderate or severe PPD that did not respond adequately to other traditional antidepressant drugs. Importantly, the therapeutic benefit of brexanolone infusion over 60 h appeared to be quite durable as patients remained euthymic or free of depressive symptoms 30 days following treatment (Kanes et al., 2017; Meltzer-Brody et al., 2018). Given the efficacy of brexanolone in improving the debilitating symptoms of PPD, its relative safety profile and thus very positive benefit/risk ratio, brexanolone was approved by the FDA on March 19, 2019 and is now marketed in the U.S., as Zulresso™.
Conclusions
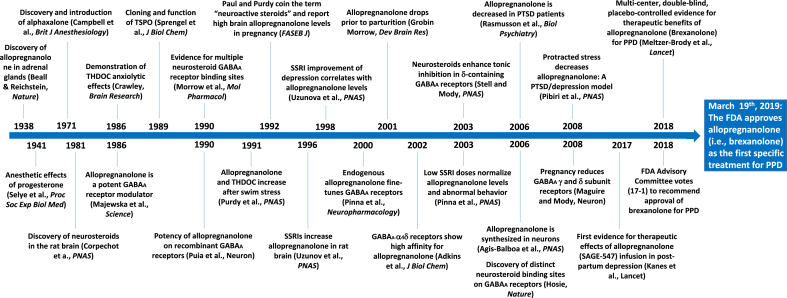
The discovery of the potent actions of the neurosteroid, allopregnanolone on GABAA receptors has catalyzed much good work by scientists across the globe leading to the successful development of the first targeted treatment for PPD. In our view, this represents one of the best examples of rationale drug discovery in neuropsychiatry, based on solid scientific evidence and clinical data (see Fig. 2 for a timeline of major neuroactive steroids discoveries leading to the development of brexanolone for PPD). Hopefully, other effective novel neuroactive steroid medicines for treating serious mood and anxiety disorders will follow.
Fig. 2.
Timeline of Major Neuroactive Steroid Discoveries leading to the Development of Brexanolone for Post-Partum Depression.
CRediT authorship contribution statement
Steven M Paul: Writing - original draft, Writing - review & editing. Graziano Pinna: Writing - original draft, Writing - review & editing. Alessandro Guidotti: Writing - original draft, Writing - review & editing.
Declaration of competing interest
Graziano Pinna has a patent application that was published in December 2018 on allopregnanolone analogs in the treatment of neuropsychiatric disorders. Steven Paul is a co-founder, board member and shareholder of Sage Therapeutics.
Acknowledgement
This study was supported by the United States Department of Defense Grant W81XWH-15-1-0521 to G. Pinna.
Contributor Information
Steven M Paul, Email: steve@karunatx.com.
Alessandro Guidotti, Email: aguidott@uic.edu.
References
- Agis-Balboa R.C., Guidotti A., Pinna G. Allopregnanolone biosynthesis is downregulated in the prefrontal cortex/Brodmann's area 9 (BA9) of depressed patients. Psychopharmacology. 2014;231(17):3569–3580. doi: 10.1007/s00213-014-3567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agis-Balboa R.C., Pinna G., Zhubi A., Veldic M., Costa E., Guidotti A. Location and expression of brain enzymes catalyzing neurosteroid biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agís-Balboa R.C., Pinna G., Pibiri F., Kadriu B., Costa E., Guidotti A. Downregulation of 5α-reductase type I mRNA expression in cortico-limbic glutamatergic neurons in socially-isolated mice. Proc. Natl. Acad. Sci. U.S.A. 2007;104:18736–18741. doi: 10.1073/pnas.0709419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier A., Paul S.M. The GABAA/benzodiazepine receptor: implications for the molecular basis of anxiety. J. Psychiatr. Res. 1990;24(Suppl. 2):91–104. doi: 10.1016/0022-3956(90)90040-w. Review. No abstract available. [DOI] [PubMed] [Google Scholar]
- Brickley S.G., Mody I. Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron. 2012 Jan 12;73(1):23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney D.L., Uzunov D., Costa E., Guidotti A. Gas chromatographic-mass fragmentographic quantitation of 3 alpha-hydroxy-5 alpha-pregnan-20-one (allopregnanolone) and its precursors in blood and brain of adrenalectomized and castrated rats. J. Neurosci. 1995 Jun;15(6):4641–4650. doi: 10.1523/JNEUROSCI.15-06-04641.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concas A., Mostallino M.C., Porcu P., Follesa P., Barbaccia M.L., Trabucchi M., Purdy R.H., Grisenti P., Biggio G. Role of brain allopregnanolone in the plasticity of γ‐aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J.B., Dumitru A.M., O'Buckley T.K., Morrow A.L. Ethanol administration produces divergent changes in GABAergic neuroactive steroid immunohistochemistry in the rat brain. Alcohol Clin. Exp. Res. 2014;38:90–99. doi: 10.1111/acer.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa E., Guidotti A. Diazepam binding inhibitor (DBI): a peptide with multiple biological actions. Life Sci. 1991;49(5):325–344. doi: 10.1016/0024-3205(91)90440-m. Review. [DOI] [PubMed] [Google Scholar]
- Crawley J.N., Glowa J.R., Majewska M.D., Paul S.M. Anxiolytic activity of an endogenous adrenal steroid. Brain Res. 1986 Nov 29;398(2):382–385. doi: 10.1016/0006-8993(86)91500-3. [DOI] [PubMed] [Google Scholar]
- Dong E., Matsumoto K., Uzunova V., Sugaya I., Takahata H., Nomura H., Watanabe H., Costa E., Guidotti A. Brain 5alpha-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc. Natl. Acad. Sci. U. S. A. 2001 Feb 27;98(5):2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee K.W., McCauley L.D., Lan N.C. A putative receptor for neurosteroids on the GABAA receptor complex: the pharmacological properties and therapeutic potential of epalons. Crit. Rev. Neurobiol. 1995;9(2–3):207–227. [PubMed] [Google Scholar]
- Girdler S.S., Straneva P.A., Light K.C., Pedersen C.A., Morrow A.L. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol. Psychiatr. 2001;49:788–797. doi: 10.1016/s0006-3223(00)01044-1. [DOI] [PubMed] [Google Scholar]
- Grobin A.C., Morrow A.L. 3Alpha-hydroxy-5alpha-pregnan-20-one levels and GABA(A) receptor-mediated 36Cl(-) flux across development in rat cerebral cortex. Brain Res Dev Brain Res. 2001 Nov 26;131(1–2):31–39. doi: 10.1016/s0165-3806(01)00242-5. [DOI] [PubMed] [Google Scholar]
- Guidotti A., Dong E., Matsumoto K., Pinna G., Rasmusson A.M., Costa E. The socially-isolated mouse: a model to study the putative role of allopregnanolone and 5α-dihydroprogesterone in psychiatric disorders. Brain Res. Rev. 2001;37:110–115. doi: 10.1016/s0165-0173(01)00129-1. [DOI] [PubMed] [Google Scholar]
- Guidotti A., Costa E. Can the antidysphoric and anxiolytic profiles of selective serotonin reuptake inhibitors be related to their ability to increase brain 3 alpha, 5 alpha-tetrahydroprogesterone (allopregnanolone) availability? Biol. Psychiatr. 1998 Nov 1;44(9):865–873. doi: 10.1016/s0006-3223(98)00070-5. Review. [DOI] [PubMed] [Google Scholar]
- Harrison N.L., Majewska M.D., Harrington J.W., Barker J.L. Structure-activity relationships for steroid interaction with the gamma-aminobutyric acidA receptor complex. J. Pharmacol. Exp. Therapeut. 1987 Apr;241(1):346–353. [PubMed] [Google Scholar]
- Hosie A.M., Wilkins M.E., da Silva H.M., Smart T.G. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006 Nov 23;444(7118):486–489. doi: 10.1038/nature05324. Epub 2006 Nov 15. [DOI] [PubMed] [Google Scholar]
- Kanes S., Colquhoun H., Gunduz-Bruce H., Raines S., Arnold R., Schacterle A., Doherty J., Epperson C.N., Deligiannidis K.M., Riesenberg R., Hoffmann E., Rubinow D., Jonas J., Paul S., Meltzer-Brody S. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017 Jul 29;390(10093):480–489. doi: 10.1016/S0140-6736(17)31264-3. [DOI] [PubMed] [Google Scholar]
- Lambert J.J., Belelli D., Hill-Venning C., Peters J.A. Neurosteroids and GABAA receptor function. Trends Pharmacol. Sci. 1995 Sep;16(9):295–303. doi: 10.1016/s0165-6147(00)89058-6. Review. [DOI] [PubMed] [Google Scholar]
- Locci A., Pinna G. Stimulation of PPAR-α by N-palmitoylethanolamine engages allopregnanolone biosynthesis to modulate emotional behavior. Biol. Psychiatr. 2019;85:1036–1045. doi: 10.1016/j.biopsych.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Locci A., Pinna G. Neurosteroid biosynthesis downregulation and changes in GABAA receptor subunit composition: a biomarker axis in stress-induced cognitive and emotional impairment. Br. J. Pharmacol. 2017;174:3226–3241. doi: 10.1111/bph.13843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luisi S., Petraglia F., Benedetto C., Nappi R.E., Bernardi F., Fadalti M., Reis F.M., Luisi M., Genazzani A.R. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J. Clin. Endocrinol. Metab. 2000 Jul;85(7):2429–2433. doi: 10.1210/jcem.85.7.6675. [DOI] [PubMed] [Google Scholar]
- Maguire J.L., Stell B.M., Rafizadeh M., Mody I. Ovarian cycle‐linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat. Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Maguire J., Mody I. GABAAR plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska M.D., Harrison N.L., Schwartz R.D., Barker J.L., Paul S.M. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986 May 23;232(4753):1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Uzunova V., Pinna G., Taki K., Uzunov D.P., Mienville J.M., Watanabe H., Guidotti A., Costa E. Permissive role of brain allopregnanolone content in the regulation of pentobarbital-induced righting reflex loss. Neuropharmacology. 1999;38:955–963. doi: 10.1016/s0028-3908(99)00018-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Puia G., Dong E., Pinna G. GABAA receptor neurotransmission dysfunction in a mouse model of social isolation-induced stress: possible insights into a non-serotoninergic mechanism of action of SSRIs in mood and anxiety disorders. Stress. 2007;10:3–12. doi: 10.1080/10253890701200997. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., de Kloet R., Wallach G. Interactions in vivo and in vitro of corticoids and progesterone with cell nuclei and soluble macromolecules from rat brain regions and pituitary. Brain Res. 1976 Mar 19;105(1):129–136. doi: 10.1016/0006-8993(76)90928-8. [DOI] [PubMed] [Google Scholar]
- Meltzer-Brody S., Colquhoun H., Riesenberg R., Epperson C.N., Deligiannidis K.M., Rubinow D.R., Li H., Sankoh A.J., Clemson C., Schacterle A., Jonas J., Kanes S. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018 Sep 22;392(10152):1058–1070. doi: 10.1016/S0140-6736(18)31551-4. [DOI] [PubMed] [Google Scholar]
- Mody I. Plasticity of GABAA receptors relevant to neurosteroid actions. In: Noebels J.L., Avoli M., Rogawski M.A., Olsen R.W., Delgado-Escueta A.V., editors. Jasper's Basic Mechanisms of the Epilepsies [Internet] fourth ed. National Center for Biotechnology Information (US); Bethesda (MD): 2012. [PubMed] [Google Scholar]
- Morrow A.L., Pace J.R., Purdy R.H., Paul S.M. Characterization of steroid interactions with γ-aminobutyric acid receptor-gated chloride ion channels: evidence for multiple steroid recognition sites. Mol. Pharmacol. 1990;37:263–270. [PubMed] [Google Scholar]
- Morrow A.L., Suzdak P.D., Paul S.M. Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur. J. Pharmacol. 1987;142:483–485. doi: 10.1016/0014-2999(87)90094-x. [DOI] [PubMed] [Google Scholar]
- Mukhin A.G., Papadopoulos V., Costa E., Krueger K.E. Mitochondrial benzodiazepine receptors regulate steroid biosynthesis. Proc. Natl. Acad. Sci. U. S. A. 1989 Dec;86(24):9813–9816. doi: 10.1073/pnas.86.24.9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V., Baraldi M., Guilarte T.R., Knudsen T.B., Lacapère J.J., Lindemann P., Norenberg M.D., Nutt D., Weizman A., Zhang M.R., Gavish M. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006 Aug;27(8):402–409. doi: 10.1016/j.tips.2006.06.005. Epub 2006 Jul 5. [DOI] [PubMed] [Google Scholar]
- Paul S.M., Purdy R.H. Neuroactive steroids. Faseb. J. 1992 Mar;6(6):2311–2322. [PubMed] [Google Scholar]
- Pibiri F., Nelson M., Costa E., Guidotti A., Pinna G. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: a model relevant for posttraumatic stress disorder. Proc. Natl. Acad. Sci. U.S.A. 2008;105:5567–5572. doi: 10.1073/pnas.0801853105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G., Uzunova V., Matsumoto K., Puia G., Mienville J.M., Costa E., Guidotti A. Brain allopregnanolone regulates the potency of the GABAA receptor agonist muscimol. Neuropharmacology. 2000;39:440–448. doi: 10.1016/s0028-3908(99)00149-5. [DOI] [PubMed] [Google Scholar]
- Pinna G., Costa E., Guidotti A. Fluoxetine and norfluoxetine stereospecifically facilitate pentobarbital sedation by increasing neurosteroids. Proc. Natl. Acad. Sci. U. S. A. 2004;101(16):6222–6225. doi: 10.1073/pnas.0401479101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G., Costa E., Guidotti Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses inactive on 5-HT reuptake. Psychopharmacology. 2006;186:362–372. doi: 10.1007/s00213-005-0213-2. [DOI] [PubMed] [Google Scholar]
- Pinna G., Agis-Balboa R., Pibiri F., Nelson M., Guidotti A., Costa E. Neurosteroid biosynthesis regulates sexually dimorphic fear and aggressive behavior in mice. Neurochem. Res. 2008;33:1990–2007. doi: 10.1007/s11064-008-9718-5. [DOI] [PubMed] [Google Scholar]
- Puia G., Santi M.R., Vicini S., Pritchett D.B., Purdy R.H., Paul S.M., Seeburg P.H., Costa E. Neurosteroids act on recombinant human GABAA receptors. Neuron. 1990 May;4(5):759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- Puia G., Ducić I., Vicini S., Costa E. Does neurosteroid modulatory efficacy depend on GABAA receptor subunit composition? Recept. Channel. 1993;1(2):135–142. [PubMed] [Google Scholar]
- Purdy R.H., Morrow A.L., Moore P.H., Jr., Paul S.M. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc. Natl. Acad. Sci. U. S. A. 1991 May 15;88(10):4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy R.H., Moore P.H., Jr., Morrow A.L., Paul S.M. Neurosteroids and GABAA receptor function. Adv. Biochem. Psychopharmacol. 1992;47:87–92. [PubMed] [Google Scholar]
- Robel P., Baulieu E.E. Neurosteroids biosynthesis and function. Trends Endocrinol. Metabol. 1994 Jan-Feb;5(1):1–8. doi: 10.1016/1043-2760(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Romeo E., Cheney D.L., Zivkovic I., Costa E., Guidotti A. Mitochondrial diazepam-binding inhibitor receptor complex agonists antagonize dizocilpine amnesia: putative role for allopregnanolone. J. Pharmacol. Exp. Therapeut. 1994 Jul;270(1):89–96. [PubMed] [Google Scholar]
- Romeo E., Strohle A., Spalletta G., di Michele F., Hermann B., Holsboer F., Pasini A., Rupprecht R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am. J. Psychiatr. 1998;155:910–913. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Papadopoulos V, Rammes G, Baghai T.C., Fan J, Akula N, Groyer G, Adams D, Schumacher M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev. Drug Discov. 2010 Dec;9(12):971–988. doi: 10.1038/nrd3295. [DOI] [PubMed] [Google Scholar]
- Rupprecht R., Rammes G., Eser D., Baghai T.C., Schüle C., Nothdurfter C., Troxler T., Gentsch C., Kalkman H.O., Chaperon F., Uzunov V., McAllister K.H., Bertaina-Anglade V., La Rochelle C.D., Tuerck D., Floesser A., Kiese B., Schumacher M., Landgraf R., Holsboer F., Kucher K. Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science. 2009 Jul 24;325(5939):490–493. doi: 10.1126/science.1175055. [DOI] [PubMed] [Google Scholar]
- Schüle C., Eser D., Baghai T.C., Nothdurfter C., Kessler J.S., Rupprecht R. Neuroactive steroids in affective disorders: target for novel antidepressant or anxiolytic drugs? Neuroscience. 2011 Sep 15;191:55–77. doi: 10.1016/j.neuroscience.2011.03.025. Epub 2011 Mar 23. Review. [DOI] [PubMed] [Google Scholar]
- Schweizer C., Balsiger S., Bluethmann H., Mansuy I.M., Fritschy J.M., Mohler H., Lüscher B. The gamma 2 subunit of GABA(A) receptors is required for maintenance of receptors at mature synapses. Mol. Cell. Neurosci. 2003 Oct;24(2):442–450. doi: 10.1016/s1044-7431(03)00202-1. [DOI] [PubMed] [Google Scholar]
- Seeburg P.H., Wisden W., Verdoorn T.A., Pritchett D.B., Werner P., Herb A., Lüddens H., Sprengel R., Sakmann B. The GABAA receptor family: molecular and functional diversity. Cold Spring Harbor Symp. Quant. Biol. 1990;55:29–40. doi: 10.1101/sqb.1990.055.01.006. [DOI] [PubMed] [Google Scholar]
- Sprengel R., Werner P., Seeburg P.H., Mukhin A.G., Santi M.R., Grayson D.R., Guidotti A., Krueger K.E. Molecular cloning and expression of cDNA encoding a peripheral-type benzodiazepine receptor. J. Biol. Chem. 1989 Dec 5;264(34):20415–20421. [PubMed] [Google Scholar]
- Uzunov D.P., Cooper T.B., Costa E., Guidotti A. Fluoxetine-elicited changes in brain neurosteroid content measured by negative ion mass fragmentography. Proc. Natl. Acad. Sci. U. S. A. 1996 Oct 29;93(22):12599–12604. doi: 10.1073/pnas.93.22.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V., Sheline Y., Davis J.M., Rasmusson A., Uzunov D.P., Costa E., Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc. Natl. Acad. Sci. U.S.A. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton N., Maguire J. Allopregnanolone-based treatments for postpartum depression: why/how do they work? Neurobiol Stress. 2019 Oct 24;11:100198. doi: 10.1016/j.ynstr.2019.100198. eCollection 2019 Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W.J., Vicini S. Neurosteroid prolongs GABAA channel deactivation by altering kinetics of desensitized states. J. Neurosci. 1997 Jun 1;17(11):4022–4031. doi: 10.1523/JNEUROSCI.17-11-04022.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski C.F., Paul S.M., Izumi Y., Covey D.F., Mennerick S. Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci. Biobehav. Rev. 2013 Jan;37(1):109–122. doi: 10.1016/j.neubiorev.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]