Abstract
A new school of thought in evolutionary developmental biology, combined with research in the neurobiology of stress, suggest that early exposure to stressful circumstances may be a cause of dyslexia. A balance between epigenetic, stress-induced and cognitive-growth genetic programs modulates the brain's cellular, regional, and network homeostasis. This balance is essential for adaptability to the normative range of everyday stress. However, even mild chronic stress exposition may overactivate the hypothalmic-pituitary-adrenal stress axis, upsetting the homeostatic balance between these programs, and exposing the brain to harmful levels of stress hormones. A protective strategy to sustained disequilibrium precociously advances maturation at the cost of neuroplasticity, which blunts stress axis reactivity but also compromises learning potential in the prefrontal cortex and networks associated with dyslexia. Stress exceeding an individual's range of resilience: (1) reduces levels of TFEB and BDNF, gene regulatory factors prolonging maturation and neuroplasticity; (2) interferes with the insular cortex, amygdala and hippocampus in coordinating afferent visceral signals with cognitive performance; (3) over-recruits the brain's Default Mode network; and (4) amplifies release from the Locus coeruleus/norepinephrine system which impairs the entrainment of oscillations in the lower phonological frequencies of speech. Evidence supporting a stress-growth imbalance is preliminary, but holds promise for reconceptualizing the neurobiology of dyslexia and reducing its prevalence.
Keywords: Dyslexia, Stress, Evolution, Epigenetics, Neuroplasticity
Highlights
-
•
Reading requires stress system homeostasis at the cellular, region, and network levels.
-
•
Dyslexia may be a positive evolutionary adaptation to challenges to stress homeostasis.
-
•
Dyslexia may be caused by a tradeoff between epigenetic, stress and growth gene programs.
-
•
Stress in dyslexia involves the HPA axis, LC/NE system, and neural network interactions.
1. Introduction
1.1. An evolutionary framework
Revisions to the conventional neo-Darwinian account of human biological evolution mandate a new understanding in children's neurocognitive development, of natural selection, adaptation, and the role of genes in maturation (e.g., Michel et al., 2018). This reinvigored approach to evolution is called the Extended Evolutionary Synthesis (EES). The combined integration of the EES framework with: (1) current research in evolutionary developmental biology or “Evo-Devo” (Jablonka, 2017); (2) stress system reactivity to every day human living (FeldmanHall et al., 2019); and (3) the high incidence of anxiety in children with learning disorders (Haft et al., 2019) has transformational implications for how we conceptualize the evolution and development of literacy and, more specifically, the high prevalence of children's difficulties in acquiring beginning and fluent reading skills, i.e., dyslexia (Zuk et al., 2019).
Developmental dyslexia is a hereditary, neurocognitive-based learning difficulty, usually encountered in primary education when young children struggle to acquire proficiency in beginning reading skills. Important corollaries of an Evo-Devo perspective are: (1) dyslexia represents the lower ranges of a normal distribution of the emerging evolution of reading skills in literate societies (Pennington and Bishop, 2009); and (2) dyslexia is independent of general intellectual performance (Tanaka et al., 2011). Depending on the skill level cut-off point that various clinicians and researchers have used, dyslexia estimates have ranged from 5% to as high as 20% (Pugh et al., 2013). However, no reliable differences in neurobiological or cognitive processes have ever been demonstrated across the levels of reading disability (cf. Siegel, 1989). Therefore, we use an inclusive definition of dyslexia. We consider poor readers to be dyslexic if they are below-grade in their reading ability, despite having normal IQs, adequate educational opportunities, and without a history of emotional problems (cf. Zuk et al., 2019).
An Evo-Devo reorientation requires a break from the traditional view of brain evolution as an ancient, time-bound process in our ancestral genetic lineage. The traditional view of evolution has little or no relevance to the dynamics of ongoing neurocognitive change. This conceptual shift creates exciting new opportunities, but complex challenging issues, for parenting and early childhood education. At the fountainhead of this shift is an unprecedented knowledge explosion in neuroscience and education (e.g., Kershner, 2020). Interdisciplinary research supports a vast potential for the brain to undergo functional and morphological adaptations in response to environmental circumstances. Beginning at conception and throughout the lifespan, multiple time windows exist for activity-evoked, neuronal systems to undergo fundamental reprogramming. These changes are viewed as heritable, evolutionarily conserved positive adaptations, with life-altering consequences affecting children's education, behavior and vocational success. This movement is gaining momentum and having a noticeable effect on research. Over the last decade, in child development an increasing number of studies have been performed from an Evo-Devo perspective (Bjorklund, 2018).
Stress, from this orientation is a physiological response cultured by evolution to insure the survival of the species.. Moderate levels of stress, within an individual's range of tolerance, serve counterbalanced developmental and evolutionary functions: adaptation strategies, that are essential to successfully managing the tensions that we all experience in coping with life's everyday needs and challenges. On the one hand, the brain must be in an optimal state of attentional arousal and alertness for learning to take place. Concurrently, the same attentional control mechanisms, via extended neuronal interactions with the brain's stress system, are poised to recruit “flight or fight” response tendencies, should the stress level become excessive. Thus, evolutionary selection has nourished attentional and stress management systems capable of simultaneously maximizing both (1) learning ability for cognitive growth and (2) avoidance behaviors in unsettling and potential life-threatening circumstances.
Recently, dyslexia has been associated with the expression of stress-related genes (Zakopaulou et al., 2019), and with dysregulation of the hypothalamic-pituitary-adrenal (HPA) stress axis (Espin et al., 2019). To put such fundamental neurophysiological findings in dyslexia in perspective, Evo-Devo has been recognized as a theoretical framework for (1) a better understanding of dyslexia (Benitez-Burraco and Murphy, 2019), and (2) conceptualizing the stress/dyslexia connection (Kershner, 2019). This paper is the first to argue for the validity of conceptualizing the stress/dyslexia connection within an evolutionary theoretical framework. Simply put, prenatal and early childhood stress may be a dyslexia risk factor, and dyslexia may be the natural outcome of an evolutionarily-conserved adaptive response to stress.
However, early adversity as a putative cause of reading disability is not a new revelation. Large-sample studies have firmly established an association between low socioeconomic status, home literacy environment, and family turmoil, to poor academic performance, including reading (Brito et al., 2017; Dilnot et al., 2016; Hamilton et al., 2016; Noble et al., 2006; Ozernov-Palchik et al., 2018). Notably, multiple studies have documented that stress is capable of altering the brain regions affected by the stress hormones known to be involved in dyslexia (Gilmore et al., 2018; McGowan and Matthew, 2018; Van den Bergh, 2011; Vogel and Schwabe, 2016). In addition, maternal stress, presumably by altering the intrauterine environment, can have detrimental effects on the reading ability of their offspring (Li et al., 2013; D'Sousa et al., 2016) For example, D'Sousa et al. (2016) in a longitudinal study compared the later reading ability of 500 children born from mothers who were stressed vs non-stressed during late cycle pregnancy. With controls for sex, birth weight, and maternal education, the study revealed that children carrying a homozygous variant of the K1AA0319 dyslexia risk gene who were exposed to high maternal stress were significantly poorer readers during adolescence. Clearly, dyslexia in some children has its origin in stress system dysregulation, related to suboptimal environmental circumstances. The Evo-Devo perspective is consistent with these studies, but adds a comprehensive evolutionary overview proposing adaptation to stress as a cause of dyslexia, identifying the underlying cortico-limbic mechanisms of the stress response dysfunction, and pointing a pathway to prevention.
To summarize, new currents in evolutionary developmental biology (Evo-Devo), combined with research in stress and dyslexia, provide a novel theoretical platform for understanding dyslexia. Compared to main-stream dyslexia studies, this more naturalistic orientation has a different fundamental logic and range of predictions. From this perspective, dyslexia is seen as the non-pathological outcome of an interactive evolutionary process in child development between stress system reactivity and the emerging neurobiological substrates involved in reading acquisition.
1.2. Stress vs growth genetic programmes
Foundational to a working Evo-Devo framework in children's neurocognitive growth is the formative influence of human neoteny (Gould, 1997). Neoteny refers to the prolongation of high cortical metabolism and synaptic plasticity in humans compared to other primates. Throughout the human life span, cortical association areas show increased expression of the genes that regulate neuroplasticity (Bufill et al., 2011; Liu et al., 2012; Miller et al., 2012; Somel et al., 2009, 2011). Moreover, this inherited evolutionary bias is orchestrated by a dynamic balance between epigenetic, stress-induced and cognitive-growth gene expression programs (e.g., Lopez-Maury et al., 2008; Pryluk et al., 2019; Schultz et al., 2019). As an example of the tradeoff between cognition and aversive emotions, Pryluk et al. (2019) compared single unit recordings of neural firing patterns from the amygdala and prefrontal cingulate cortex in humans and monkeys (Macaca fascicularis). Both regions have evolved extensively and, together, form a dense reciprocal synaptic complex of interconnecting neurons (Ghashghaei et al., 2007). The amygdala evolved to subserve defensive behaviors, and is central to coping with modern-day socio-emotional events. The cingulate cortex evolved to support complex cognition and adaptive learning. Pryluk et al. (2019) devised a measure of neural coding efficiency called Contrast Entropy which pitted signal complexity, implicating neuroplasticity with high energy requirements, against the maximum capacity of a neuron/pathway (for a different definition of entropy, see Peters et al., 2017). This measure of entropy is based on the assumption that the net amount of entropy (channel capacity) is unaffected by stress. Rather, emotional activation has a relative influence on (1) signal complexity or the amount of capacity used efficiently and (2) robustness or speed of response and synchrony between neurons. Efficient coding provides greater Contrast Entropy, increasing the ability to adapt to uncertainty and changing environments. The results showed that humans coded more efficiently in both regions, and the cingulate in both species was more efficient than the amygdala. Firing patterns in the amygdala were less complex and highly predictable. These findings corroborate the view that evolution seeks to advance cognition, but at the same time protects the stability and reliability of response to threats. The main takeaway for our thesis is that evolutionary advances in learning ability succeed best in a complimentary stress/growth environment. But, undue or chronic stress can enhance robustness at the expense of the neuroplasticity essential for cognitive growth. A stress imbalance, while potentially damaging to general cognitive growth, would be expected to take a bigger toll on the actively evolving neural regions and networks that are in an emerging state, supporting the acquisition of relatively new cognitive abilities such as literacy (Benitez-Burraco and Murphy, 2019; Gollo et al., 2018; Wagner et al., 1997). The important upshot of this is that reading ability has a lower threshold for stress-induced environmental compromise than general intelligence. Hence dyslexia can result from relatively lower intensities of stress, with moderate stress system dysregulation, and at all IQ levels (Tanaka et al., 2011).
As the stress/growth balance plays out in daily living, the expression of both genetic programmes is regulated largely by epigenetics. Epigenetic biochemical processes control the transcriptional factors that bind to promoter regions of the genome next to a gene, or to messenger RNAs. Throughout development they regulate protein synthesis and the expression of germ-line genes. In humans, progressive evolution depends on the primacy of enrichment-related genetic programs to promote rapid cognitive growth by prolonging maturation and neuroplasticity. At the same time, unstable and emotionally stressful environments also are well-established as central operational features of adaptive evolution (Le Rouzic et al., 2013). In every-day common living, the brain's stress-growth programs are counter-poised, in msec. to msec. timing, to respond spontaneously to feelings of anxiety and threat, while preserving the potential for cognitive development.
As children mature, moderate levels of stress and a responsive stress axis prime the sensory-motor systems subserving arousal, attention, and motivation, and also play an essential role in neuronal synaptic plasticity and learning (Murray et al., 2004). However, excessive stress-induced cellular metabolic levels, resulting from early traumatic events, may upset the balance between the stress and growth programs. When this happens, the bioenergetic resources needed for extended neotenous brain growth are redirected to stress protection: an adaptive response to environmental adversity or deprivation (Teicher et al., 2016). As the normal range of homeostatic balance is exceeded by traumatic experiences maintained over time, an excess of glucocorticoids (cortisol in humans) is released from the hypothalamic-pituitary-adrenal axis (HPA). This release has potentially toxic effects on the hippocampus, amygdala, and prefrontal cortex (PFC), all areas coactivated by socio-emotional events and cognitive processing. If unchecked, excessive cortisol also becomes a risk factor in adult poor health outcomes (McGowan and Matthews, 2018; Raymond et al., 2018). Fortunately, to offset extensive cellular metabolic damage, individuals have the adaptive capacity to accelerate maturation (Callaghan and Tottenham, 2016). Faster development is an evolutionary strategy to increase survival and fitness in uncertain and threatening environmental contexts (Krugers et al., 2017). Precocious maturation reduces neuroplasticity which dampens the stress response system, but unfortunately is associated with decreased performance in cognitive functions (Burns et al., 2018). For example, continuous harsh parenting at 3 years is related at 10 years-of-age to below-average stress system reactivity (Jaffe et al., 2015). Early chronic stress appears to upend the developmental balance between the stress and cognitive-growth programs, eventually resulting in widespread losses of neuroplasticity. Mutual antagonism between these programs, responding to the degree of environmental adversity vs enrichment, acts respectively to shorten or extend the maturational neuroplasticity essential for learning and memory. Thus, the relative degree of stress, tempered by its nature, onset, and duration, is recognized as an epigenetic trigger guiding the timing of neuroplasticity during development and cross-generational evolutionary change (Badyaev, 2005; McGowan and Matthews, 2018). In short, prolonged stress reactivity is antithetical to learning.
The purpose of the present paper is to present an Evo-Devo perspective of dyslexia. The review proposes an adaptive tradeoff between the stress and cognitive-growth programs as a root cause. To come to terms with this hypothesis, we need to consider multiple sources of supporting evidence. The second section is a brief overview of evolution, differentiating the EES and Evo-Devo from the traditional neo-Darwinian view. The third section reviews the effects of levels of stress on behavior and brain maturation. Section four discusses the brain's stress response axis, and how early adversity, via epigenetic mechanisms, upsets homeostasis and down-regulates neuroplasticity in reading-related brain circuitry. Five, is a summary discussion emphasizing the beneficial human and socioeconomic consequences of an Evo-Devo perspective of dyslexia, and suggesting research to test directly the stress-growth imbalance hypothesis.
2. EES and Evo-Devo
In the neo-Darwinian view of evolution, the course of child development is portrayed as the unfolding of a predetermined program of instructions coded in the child's genotype. With the successful mapping and detailed investigations of the human genome, fractures began occurring in the neo-Darwinian account. Cross-species comparisons revealed that human-subhuman primate differences in protein-coding genes were extremely rare at about 3.42%; and a majority of the human genome was dominated by noncoding DNA variants (Hartwell et al., 2011; Lui et al., 2019). It appears that complex phenotypic trait variance (e.g., reading ability) is mediated largely by epigenetic factors which mediate the complex interaction between coding genes and their behavioral outcomes (i.e., D'Souza et al., 2016). These discoveries implicate intermediary networks of environmentally-activated, gene regulatory processes in heritability and in shaping the course of neurocognitive development (Campbell and Wood, 2019; Grigorenko et al., 2016).
In other words, in contrast to neo-Darwinism, human uniqueness and children's individual behavioral traits are to be found to a significant extent in the noncoding regions of our genome. Thus, the modern science of epigenetics was born: commonly defined as environmentally-induced, heritable metabolic and cellular processes, regulating gene expression while conserving the gene's structural neucleotide sequence, i.e. not a genetic mutation (Champagne, 2016). The epigenome serves the dual functions of (1) facilitating cellular differentiation during embryonic development and (2) sustaining the neuroplasticity needed throughout development in coping with ever-changing environments. This breakout from the priority of genes to the epigenome is incompatible with the received neo-Darwinian view, and forms the conceptual core of the Evo-Devo portrayal of child development.
The gene-centric traditional account of evolution understates the formative role of plasticity in neurological development, which subserves children's ability to alter patterns of brain activity in accommodating to novelties in every-day living experiences. In the neo-Darwinian view, evolutionary change is seen as accumulated genetic baggage from the past, and as a future predetermined by the survival advantage of random gene mutations. According to this view, population adaptation and fitness are set genetically by “natural selection”, the late-stage survival advantage of competing alternative genetic alleles. Hence, evolution in the present, when considered in early childhood education, has been limited to the semi-automatic playout of instructions contained in the genetic code's DNA sequence; a species altering process that occurred in the distant past. In the EES and Evo-Devo reformulation, the well-spring of evolutionary change is in the present (Jablonka, 2017; Sultan, 2017). Evo-Devo gives educational efforts by caretakers, parents, and teachers a new sense of empowerment. Before natural selection can act, it is preempted by (1) stress threshold behaviors between mutually inhibiting, stress vs cognitive-growth gene expression programs and (2) inherited and environmentally-induced epigenetic variations, which together orchestrate the reprogramming of neuroplasticity (Burns et al., 2018). Although natural selection interacts on many levels with developmental selection, phenotypic flexibility in early childhood is the main unit of selection for evolutionary change.
Thus, in a significant revision of the “gene mutation” traditional view, Evo-Devo highlights child initiated developmental selection, given agency by the epigenome, as a primary source of advances in evolution. The behavioral decisions made by children in seeking a comfort zone or ecological niche come before genetic assimilation. In effect, the child's environmental context becomes imbedded in patterns of brain structure and function. At the same time, these non-random, goal-directed neural patterns serve each child's needs in coping with uncertainty and life's unexpected challenges. An evolutionary adaptation to prolonged duress accelerates brain maturation at the expense of plasticity, while nourishing environmental surroundings slow brain maturation, which prolongs plasticity, encourages inquisitiveness, and facilitates learning ability.
3. Stress levels, behavior, and brain maturation
We encounter multiple stressful circumstances in everyday living, defined as physical and social-emotional events that challenge metabolic cellular homeostasis. Stressors that have the potential to overload the stress system are more common than we might imagine. According to surveys in the U.S. and Europe, one half of all adults have experienced at least one form of early adversity (Bellis et al., 2014; Felitti et al., 1998). These studies included common early trauma, ranging from moderate to severe: emotional abuse; neglect; environmental disaster; parental separation; witnessing violence, death, or mental illness; and bullying.
Mild anxiety helps in navigating a wide variety of daily experiences, work and play activities, and social interactions, and is essential to the neurocognitive functions recruited for learning and memory (McEwen, 2007). However, as more intense exposure overrides the stabilizing influence of favorable aspects of the environment, the beneficial flow of stress can turn negative, referred to as “allostatic overload” (Burns et al., 2018). This gradual break with equilibrium supports the view that stressors are on a natural continuum from beneficial and mild to severely traumatizing (Grigorenko et al., 2016), which parallels the population distribution of cognitive abilities and the incidence and severity of dyslexia (Pugh et al., 2013).
Numerous studies with normal, nonclinical samples have documented significant correlations between environmental stress and poor neurocognitive functions (e.g., Moore et al., 2016). In addition, it has been suggested that such negative environmental effects may be common to all socioeconomic levels, and that they may be regulated epigenetically (Hackman et al., 2010). Taking a broader Evo-Devo view, an incremental scaling of negative environmental events would be expected across the general population, accompanied by alterations in the timing of brain maturation. Indeed, this prediction has been supported by the first large-scale research into the effects of traumatic environmental stress. The study involved a healthy and nonclinical sample of 9498 youths, aged 8–21 years, representing a range of socioeconomic levels (Gur et al., 2019). The investigation tested the effects of low socioeconomic status (L.SES) and traumatic stressful events (TSEs) on distress spectrum behaviors, cognitive skills, puberty, and brain maturation. The study calculated the incidence of TSE's for each participant including exposure to natural disasters or bad accidents; someone close hurt badly; witnessing a killing or beating; and personally experiencing assault. L.SES was defined primarily by parental educational attainment. The outcome, stress spectrum behaviors were aligned on a continuum of increasing severity from mood-anxiety, to fear, psychosis, and externalizing behaviors. Cognitive measures were episodic memory, complex cognition, and social cognition. Pubertal stage was indexed by the onset of secondary sex characteristics. Brain maturation was measured using fMRI whole brain imaging.
The first somewhat surprising result was that the incidence of TSEs was similar across socioeconomic levels. Overall, socioeconomic status yielded smaller effect sizes, so L.SES was also less informative than a count of the actual stressful events experienced by each participant. Secondly, the effect of TSEs (which ranged from 0 to 8) on stress behaviors showed that 2 or greater TSEs compared to 0–1 was associated with an increase in mood-anxiety distress in girls and psychosis in boys. Most importantly to our interest, 2 or greater TSEs was related to lower scores in executive functioning and complex reasoning. And finally, both TSEs and L.SES were related to advanced puberty and accelerated brain maturation, mainly affecting the fronto-limbic stress axis pathways.
These results demonstrate the deleterious effects of early life adversity on children's emotional well-being and general cognitive abilities. Such findings provide strong support for the Evo-Devo model of child development: increasing exposure to stressful events was shown to upset the homeostatic balance needed to prolong maturation and brain neuroplasticity, which leads to increased capacity for learning over an extended period-of-time. Hence, Evo-Devo reconceptualizes stress in terms of environmental events that influence the homeostatic balance essential for cognitive growth. Finding that stressful events advanced puberty is also consistent with Evo-Devo. A stress-induced switch from a relatively nonstressed growth strategy, to faster maturation, accelerating the age of puberty, is an adaptation for earlier reproductive success under difficult survival conditions. In this sense, whether it will confer a long-term advantage depends on the broader envelope of sociocultural and socioeconomic circumstances. In any event, stress-related early puberty appears to compromise neuroplasticity and learning potential.
4. Epigenetics, the stress axis, and reading disability
4.1. Epigenetics and the HPA stress axis
Epigenetic gene expression programs play a key role in normal development by maintaining a cellular and neural circuit, metabolic balance between stress protection and cognitive growth. The epigenetic “marks” or mechanisms recruited to maintain this balance when faced with the challenges of early life environments are DNA methylation, histone post-translational modifications, and noncoding RNAs (e.g., Burns et al., 2018). Once activated by environmental stress, alterations in these mechanisms have an influence, via the amygdala, on neurocognition by engaging the stress responsive, hypothalamic-pituitary-adrenal axis (HPA) (Griffiths and Hunter, 2014; Raymond et al., 2018).
In coping with life's everyday anxieties, the amygdala initiates an endocrine cascade by: (1) stimulating the hypothalamic paraventricular nucleus (PVN) to release corticotropic-releasing hormone (CRH); which (2) triggers the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary; which (3) causes the adrenal cortex to release glucocorticoids (GC). Corticosteroids (mainly cortisol) from the adrenal glands then cross the blood-brain barrier to (1) directly modulate activity outside of the HPA in the amygdala, hippocampus, and prefrontal cortex (PFC) and (2) inhibit the HPA via return pathways to the HPA from the hippocampus and PFC (McGowan and Matthews, 2018). In particular, the hippocampus, which is rich in GC receptors, has a major role in sustaining a negative feedback loop of the axis. Under mild stress, this negative feedback loop serves to moderate homeostatic balance, simultaneously buffering against the potential negative cortisol effects of strong emotion while facilitating neuroplasticity in higher cortical regions via reciprocal PFC connectivity. However, HPA release of excessive glucocorticoids eventually will suspend cortical plasticity (cf. Peters et al., 2017).
In addition to the HPA, two stress subsystems feedback to the brain. The Sympathetic Nervous System (SNS) allocates glucose, targeted to support specific extra energy needs. And, the Locus coeruleus releases norepinephrine (LC/NE system) which interacts with local glutamate levels, the brain's primary excitatory neurotransmitter, to synchronize brain oscillations (Peters et al., 2017). Thus, stress activates three systems that have powerful influences on higher cognitive functions. However, the HPA, regulated in large measure by epigenetic factors, has a direct effect on the PFC, controlling neuroplasticity and the protein synthesis required for learning.
The PFC feeds top-down inhibitory input to the HPA and also is the primary bottom-up target for variations in HPA activity to promote or repress cognitive growth. For instance, in typical adults, early childhood trauma has been associated with increased DNA methylation and a diminished cortisol stress response, thought to repress plasticity of the PFC (Houtepen et al., 2016). In general, stress effects on behavior occur via epigenetic regulation of neuroplasticity. The PFC lies at the junction of the brain's large-scale neurocognitive networks and includes the anterior cingulate and anterior insular cortices, major frontal lobe paralimbic relay stations propagating signals to and from the HPA (Seeley et al., 2007; Uddin, 2015). Moreover, it has rich interconnections throughout the brain's higher cortical and subcortical regions, evolved relatively recently, and continues to mature into the 30s. Developmental changes in the PFC play a crucial role in higher cognition, personality, and executive control (Numata et al., 2012). Thus, maintaining an HPA-PFC balance insures children's emotional well-being, while nurturing their capacity for learning (Fisher et al., 2016). The ease of learning something new is enhanced by a sense of psychological and physiological security.
However, as disturbing and threatening events increase in number, intensity, and duration, and especially in postnatal periods, if they are beyond the child's capacity for understanding or control, the HPA system goes into allostatic overload (Burns et al., 2018). At that tipping point, stress methylation produces a decrease in hippocampal GC receptor expression, which reverses the negative feedback loop by enhancing a positive feedback function and subsequent overactivity of the HPA (Burns et al., 2018). HPA dominance overrides system homeostasis, advancing maturation of the HPA and suppressing the PFC cognitive-growth pathways (e.g., Pryluk et al., 2019). The resulting stress-growth imbalance would be expected to impact homeostatic tradeoffs at three interconnected levels of neuronal function: cell to cell; region to region; and network to network. Dyslexia has been linked to an imbalance at all three levels.
4.2. Cellular imbalances
At the cellular level, the transcriptional factors TFEB and BDNF are master stress-related regulators of the cognitive-growth gene expression programs (Gray et al., 2013; Levine and Kroemer, 2018). During normal development, TFEB serves two functions. TFEB, a noncoding RNA transcription factor, governs the precise temporal and regional specificity of myelinating the brain's interconnecting axons (Sun et al., 2018). The developmental timing of an axon's myelin sheath must be age-synchronized for efficient signal propagation. Secondly, TFEB maintains a fine cytoprotective balance between the removal of damaging cellular cargo, without degrading essential cargo (Levine and Kroemer, 2018). A buildup of intracellular toxins will induce what is known as endoplasmic reticular stress (ER), interfering with neuronal protein homeostasis, cell membrane trafficking, and signal transduction (Di Meo et al., 2016). To keep this stress-growth balance in check, TFEB regulates a system of 16–20 stress-induced autophagy (ATG) or “housekeeping genes”. Excessive environmental stress can negatively impact both homeostatic functions by depleting TFEB, with resulting losses in neuroplasticity. More specifically, reduced TFEB causes premature myelination of the brain's white matter connectivity. This puts a brake on pathway plasticity (Sun et al., 2018), and at the same time induces cell-body ER stress, which reduces the computational plasticity of individual neurons (Levine and Kroemer, 2018). Of particular significance is that TFEB has an early effect on maturation of the corpus callosum, which has been shown over numerous replications to be myelinated atypically in dyslexia (for a review, see Kershner, 2019).
BDNF is a neurotropic factor linked inversely to stress-activated DNA methylation, and is concentrated in the amygdala, PFC, and hippocampus (Reus et al., 2013). In parallel with TFEB, BDNF also plays an essential role in regulating synaptic plasticity in response to stress (Gray et al., 2013; Grigorenko et al., 2016). A rat model linking BDNF with infant stress and prefrontal plasticity found: (1) childhood maltreatment yielded elevated DNA methylation and a reduction of BDNF mRNA and protein levels in the PFC; (2) reduced BDNF persisted into adulthood and (3) it was inherited by offspring of abused females (Roth et al., 2009). Similarly, maternal separation after weening (for 3 h per day over 10 days), of male rat pups, was shown to impair memory, cognitive flexibility, and caused a significant reduction in hippocampal BDNF (Menezes et al., 2020). Moreover, in a large sample of individuals with borderline personality disorder, the number of early traumatic events was associated with increased plasma DNA methylation and reduced levels of BDNF (Perroud et al., 2013).
It appears that BDNF-mediated synaptic plasticity may be essential for acquiring new knowledge, defined as high-energy functional and structural change between neurons (Goyal et al., 2014). Such energy intensive modifiability, even in the absence of environmental adversity, produces its own metabolic intracellular stressors that BDNF keeps within limits to maintain plasticity while avoiding damage. The high metabolic activity needed for learning generates free radicals and other reactive oxygen (ROS) and nitrogen species. Within normal limits, the ROS activate the HPA to children's benefit (Di Meo et al., 2016; Massaad and Klann, 2011). However, under the added weight of environmental adversities, which increases methylation and depletes BDNF, an increasing concentration of ROS can reach a saturation point, beyond the containment of available antioxidants. (Balmus et al., 2016). When ROS saturation occurs, the resulting oxidative stress hypermobilizes the HPA and suppresses growth neuroplasticity in higher cortical regions at the level of the individual neuron. Thus, the net effect of prolonged endogenous and exogenous stress can override TFEB and BDNF homeostasis by depleting both factors, resulting in an allostatic overload: a faster pace of maturation and reduced learning potential.
Reduced neuroplasticity in the neural circuitry involved in reading, implying a cellular homeostatic imbalance, has been shown to be characteristic of individuals struggling with dyslexia (e.g., Hancock et al., 2017; Huber et al., 2018; Malins et al., 2018). Our understanding of the pivotal role of the stress-reactive transcriptional factors TFEB and BDNF in sustaining such plasticity in white and gray matter, suggests a causal link to dyslexia. This is a compelling theoretical probability. Clearly, dyslexia is a learning disability related to compromised growth neuroplasticity at the level of the individual neuron. But whether dyslexia is associated with a stress-level imbalance correlated with reduced TFEB remains to be tested. However, a recent study confirmed lower levels of plasma BDNF in a Canadian sample of 28 boys and 14 girls (6–12 yrs old) with dyslexia compared to age-matched, average readers (Elhadidy et al., 2019). The results were unambiguous. There was no overlap between groups in BDNF levels, with the test showing high sensitivity and specificity. BDNF levels ranged in the dyslexic group from .86 to 1.34 ng/ml with a mean of 1.10; while the control group ranged from 1.60 to 2.40 ng/ml with a mean of 2.00.The authors suggest the appropriateness of plasma BDNF testing as a biomarker for dyslexia. Of course, we need a replication and more research prior to such an endorsement, but the results are indeed promising and may lead to a valid means of early identification.
4.3. Regional imbalances
The amygdala and hippocampus, which are enriched with high levels of BDNF, are midstream gatekeepers maintaining homeostasis, via dense reciprocal connectivity between the HPA and higher cortical regions (e.g., Gray et al., 2013; Pryluk et al., 2019). When the engagement of stress-related epigenetic marks upsets this regional balance, stress axis dominance, via disrupted signaling from the amygdala and hippocampus, accelerates maturation of the HPA and depresses activity in the PFC. Added to its inhibitory role in HPA function, the hippocampus is the postnatal brain's main source of neurogenesis, proliferating neural progenitor cells to sustain plasticity (Gage, 2019; Moreno-Jimenez et al., 2019). Stress suppresses the hippocampus, which compromises both functions, diminishing neuroplasticity throughout the brain's higher cognitive circuity. In tandem with the hippocampus, the amygdala coordinates emotion with cognition, via rich interconnectivity between the HPA (Raymond et al., 2018) and the anterior insula and cingulate (Pryluk et al., 2019; Thomas et al., 2018). Stress increases activation of the amygdala, fueling HPA dominance and altering the amygdala's communication to frontal cortical regions (Berry et al., 2019; Teicher et al., 2016). Key to this sequence of events is that homeostasis fails because stressed amygdala and hippocampus dysregulation initially overactivates the HPA and depresses the PFC (Kim et al., 2013; Raymond et al., 2018).
An association of such HPA-PFC regional imbalance implicating the hippocampus and amygdala with dyslexia is consistent with research showing (1) an atypical HPA response to stress in children with dyslexia (Espin et al., 2019), and (2) reduced white matter thickness in the arcuate fasciculus bilaterally, which connects the PFC with posterior areas, in children with familial risk of dyslexia (Wang et al., 2017). In addition, research into everyday trait anxiety suggests a more specific prefrontal pathway for the putative HPA-PFC imbalance in dyslexia. Studies of these brain regions in a resting state and under acute situational stress have shown stronger connectivity of HPA pathways asymmetrically to the right PFC (Berry et al., 2019; Wang et al., 2005). A regional tradeoff of the HPA with the right hemisphere is compatible with recent studies demonstrating poor auditory encoding of the low frequencies in speech in the right hemisphere as an underlying neurobiological risk factor for dyslexia (Goswami, 2019). Thus, an asymmetrical imbalance between the HPA and right PFC, although speculative, is attractive theoretically as a stress pathway that may be implicated in dyslexia.
Finally, after much controversy, it has become widely accepted that trauma-induced neuronal epigenetic alterations can be passed on to future generations (Curry, 2019). Nothing is more fundamentally at variance to the neo-Darwinian view than the proposition that parental neurological change can be forwarded to children and grandchildren by germline inheritance. Indeed, ground-breaking research in the nematode Caenorhabditis elegans has identified stress-related noncoding RNAs as an epigenetic mechanism capable of moving adaptive brain changes to the germline (Posner et al., 2016). C. elegans is capable of complex learning, making it an ideal organism to study basic biological mechanisms. This study is the first to present firm evidence of a molecular process demonstrating how stress-induced, parental learned behaviors can be passed to progeny.
Directly pertinent to the current review, an epigenetic stress-growth imbalance, propagated by parents before fertilization, has been shown to affect offspring during embryogenesis or early postnatal development. For a recent example, next generation rats of parents bred for low interest in learning, showed increased DNA methylation postnatally in the amygdala and increased anxiety; those bred for high anxiety produced offspring with low novelty response (McCoy et al., 2019). Indeed, maternal or paternal adversity before conception in humans can reset parental HPA, which may increase their children's survival at birth, but can have profound effects on their HPA, later behavior, and cognition (McGowan and Matthews, 2018; Vaiserman et al., 2018).
An epigenetic pattern of stress system inheritance has far-reaching consequences for dyslexia. Such transgenerational inheritance of putative neurobiological risk factors for dyslexia, and specifically HPA regional imbalance, if verified by future research, will call for a remodeling of the genetic basis of the disorder. It appears that a child's risk of dyslexia from a reprogrammed HPA can be inherited from the stressful experiences of previous generations without the child ever encountering a traumatic level of environmental stress (e.g., Nishikawa and Kingo, 2018).
In summary, indirect evidence arguably supports the hypothesis that epigenetic, stress axis regional imbalance may be related to dyslexia, and may be acquired by stressful events during development or experienced by parents and inherited. In any event, HPA stress reprogramming and regional imbalance has negative consequences for children's cognitive performance.
4.4. Network imbalances
4.4.1. Introduction to the stress system networks
Cellular and regional stress effects are embedded in higher-level brain circuitry, at play in coordination among the brain's major networks. Key to this macroscale neuronal collaboration under stress is the central core of the Default Mode network (DMN). The central core is made up of the medial PFC, precuneus, and posterior cingulate cortex, and is an extension of the brain's stress axis (Andrews-Hanna et al., 2010; Utevsky et al., 2014). The DMN central core, largely independent of sensory input, is thought to engage in self-referential processing, high-level emotion, and autobiographical memory (Dixon et al., 2018). In typical development, a stress-growth homeostatic balance is maintained between the DMN on the one hand, and the Salience (SN) and Frontoparietal networks (FPN), which are right-hemisphere lateralized and control executive behavior and higher cognitive processes (Spagna et al., 2018; Wu et al., 2019). Controlling hubs of the SN are the right frontal insular cortex (rFIC) and right frontal cingulate cortex (rFCC). Main cortical hubs of the FPN are the right dorsolateral PFC and right posterior parietal cortex.
The prevailing view of the brain's attentional control circuitry implicated in stress involves hierarchical interactions among these three networks (FeldmanHall et al., 2019; Satpute and Lindquest, 2019; Schultz et al., 2019; Xuan et al., 2016). In a study of networks and reading, Bailey et al. (2018) applied an fMRI, resting state data base of 11,406 studies to the reading-related 7-cortical networks identified by Yeo et al. (2011). All of the networks across the brain showed patterns of activation. However, the configuration of reading-related networks with the highest activations were the FPN and its subsystems, the Dorsal and Ventral Attention networks, which, together accounted for 56% of the net activation. Hence, a majority of the working networks of the reading brain appear to be domain-general networks involved in attention and executive functions. More generally, the FPN and SN which work together, are frequently anti-correlated with the DMN (Horowitz-Kraus et al., 2015; Utevsky et al., 2014; Zhou et al., 2018). In fluent reading, the DMN, with sparse global connectivity, may extract self-centered meaning from the text, while the FPN leads in comprehending the narrative flow of story-line events.
4.4.2. How the networks function
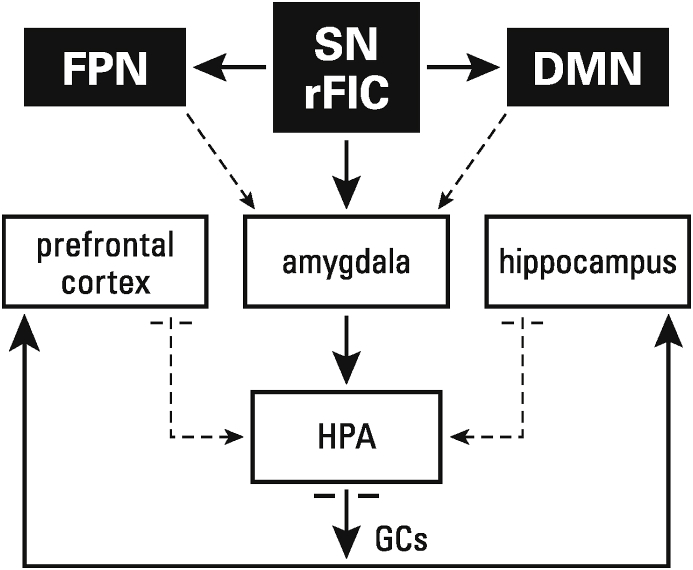
At the top of the hierarchy, the rFIC of the SN receives and coordinates somatosensory, interoceptive, and volitional control inputs (Critchly and Harrison, 2013; Mai et al., 2019). The rFIC is a major outflow hub, and first to respond to swings in afferent temperment, with significant downstream excitatory effects, via the amygdala, on the HPA. Thus, the stress-induced HPA cascade initiated by the amygdala has its origin in top-down signals from the rFIC-FPN-DMN circuitry. Following the integration of somatosensory, interoceptive and volitional control signals, the rFIC (1) activates the HPA and (2) modulates the relative balance in activation of the DMN vs the FPN (Seeley et al., 2007; Uddin et al., 2011; Uddin, 2015; Wu et al., 2019; Zhou et al., 2018). In overly stressful circumstances, the rFIC favors preferential recruitment of the DMN, which has low global connectivity (Schultz et al., 2019). Sustained engagement of the rFIC-DMN pathway is the first-line trigger, combined with rFIC-amygdala enhanced connectivity, for stress-induced overactivation of the HPA (see Fig. 1).
Fig. 1.
Diagram of the activation and inhibition pathways of the Cortico-Limbic Stress System. The right frontal insular cortex (rFIC) of the Salience Network (SN) maintains balance between the frontoparietal (FPN) and Default Mode (DMN) networks. All three serve to interact with the amygdala, which mediates between the networks and the hypothalamic-pituitary-adrenal (HPA) axis. To maintain homeostasis, the HPA production of glucocorticoids (GCs) is modulated by negative feedback loops to the HPA, via the hippocampus and prefrontal cortex. Dysregulation occurs when the SN/DMN/amygdala circuit becomes dominant, over the SN/FPN/amygdala circuit, which suppresses the hippocampus and prefrontal cortex, and incites the HPA to produce excess GCs.
Fig. 1 is a simplified diagram of the major players in this complex and fluid interaction meant to portray the system In periods of stress system stasis. For instance, reading, in the absence of excessive stress would find the three networks in a state of equilibrium, and the negative feedback loops modulated by the PFC and hippocampus maintaining homeostasis of the HPA and extended pathways.
However, in stressful circumstances or if the system is unstable, DMN dominance curtails and underserves the cognitive-growth functions of the FPN and PFC. Thus, the stress-activated rFIC-DMN circuit reallocates processing resources from cognitive-growth to DMN stress protection (Maron-Katz et al., 2016). Notably, this large-scale adaptation to stress of the brain's networks has been reported in situations of acute stress in adults, and in 2-month-old infants living in poverty-related stressful family circumstances (Maron-Katz et al., 2016; Turesky et al., 2019). Thus, such network reprogramming may reflect a fundamental whole-brain strategy to facilitate adaptive response to adversity, while simultaneously maintaining neuroplasticity and openness to new learning. When the equilibrium of this system fails, evolution favors stress management.
4.4.3. Network links to dyslexia
Research by Bailey et al. (2018) demonstrating the connectivity of interactions among the brain's reading networks, suggests that dyslexia can be characterized by a network imbalance. More specifically, an allostatic overload favoring the DMN has the potential for a direct effect on learning to read by overriding the FPN's attentional and executive functions. A stress compromised FPN in dyslexia is consistent with (1) the FPN's role in visuospatial attentional processes (Dixon et al., 2018), and (2) studies demonstrating atypical low frequency speech encoding by the right hemisphere in dyslexia (Goswami, 2011 & 2019). Taken together, these studies suggest an impairment of the right hemisphere frontoparietal attention networks as a putative cause of the phonological processing deficit that is a signature feature of dyslexia across languages (for a review, see Kershner, 2020).
The latter possibility (i.e. stress) would add a new dimension of support for Goswami's (2011 & 2019) temporal sampling theory (TST) of dyslexia. Goswami was the first to recognize impaired neural coding of low-frequency delta/theta oscillatory rhythms in speech in the right hemisphere as an etiological factor in dyslexia. Indeed, it has been proposed that the entrainment to the oscillatory code of the speech envelope may be a human-specific, evolving process that makes reading possible (Murphy and Benitez-Burroco, 2018). It follows, that dyslexia may be described as resulting from an ongoing evolutionary process, principally involving a stress system disorder of encoding species-specific, patterns of brain rhythms.
According to the TST, atypical phase-locking of amplitude rise-time in the speech envelope (stimulus onset to maximum peak amplitude) prevents the assimilation of the phonological primitives needed for subsequent phonemic processing of text by the left hemisphere. Morerover, a stress-oscillation origin of dyslexia is supported further by the finding that stress enhances Locus coeruleus/norepinephrine (LC/NE) drive, which gates the entrainment of slow-wave oscillations in the speech envelope (Mather et al., 2016). Individuals with dyslexia oversample the low frequencies in speech by entraining to higher gamma rhythms (Power et al., 2013). Therefore, stress can have a formative effect on the beginning stages of learning to read by modulating the FPN's early role in tracking low-frequency oscillations. Indeed, such a network imbalance in favor of DMN dominance in dyslexia has been supported by fMRI recordings of individuals with dyslexia, aged 11–20 years. Schurz et al., 2015 found that connectivity between the DMN and reading-related areas was atypically stronger in individuals with dyslexia compared to non-impaired readers.
In summary, substantial theoretical and empirical evidence suggests that a stress network imbalance may be an etiological factor in dyslexia. However, the actual stressors that may be involved remain unknown. And, to go beyond theoretical plausibility, we need direct investigations testing the hypothesis of a stress connection to the atypical frequency entrainment in dyslexia.
5. Discussion
The timeliness of this review is underscored by the currency of topical revisions in Evo-Devo pointing-up (1) the malleability of childhood neuronal reprogramming and (2) the applicability to children's neurological development of adaptive tradeoffs between stress-induced and cognitive-growth genetic programs. An Evo-Devo perspective of child development, combined with the research reviewed, presents a theoretical framework for understanding dyslexia in some children as a normally occurring, positive adaptation to early stressful environments. Early stress axis offsets may be transitory, but can presage later effects on socio-emotional and cognitive performance (Burns et al., 2018; Raymond et al., 2018). Prenatal and childhood traumas are known to cause cognitive, socio-emotional, and behavioral impairments (including ADHD, autism, conduct disorder, and schizophrenia), and are well-established as serious public health issues (Burns et al., 2018). Only a few studies, however, have linked the effects of early adversity to brain functioning and reading disability. An Evo-Devo perspective is consistent with the behavioral negative effects of early stress documented by these studies, but adds evolutionary principles of child development and underlying genetic/epigenetic explanatory mechanisms. Most significantly, this review suggests a direct causal pathway from stress system dysregulation to the brain's reading circuitry and dyslexia.
The research reviewed outlines a theoretical framework for how the stress-hierarchical organization of the brain's fronto-limbic stress response system may lead to reading problems. To summarize, at the top of the hierarchy of a compromised or overwrought stress system, the rFIC of the SN (1) reallocates processing resources from the FPN to the DMN and (2) engages the amygdala, which prioritizes hyperactivation of the HPA over the stabilizing and cognitive-growth functions of the hippocampus and PFC. This outflow of signals initiated by the rFIC is intended to offset the destabilizing behavioral and cellular consequences of stress, but also negatively impacts the cognitive-growth functions of the FPN, with deleterious effects on reading acquisition. Concurrent with hyperactivity of the HPA, stress drives the LC/NE system to desynchronize the FPN's control of the slow-wave oscillatory entrainment underlying the phonological lexicon.
Multiple studies have identified the right hemisphere's atypical encoding or entrainment of the low speech frequencies in dyslexia as a primary cause (Cutini et al., 2016; Di Liberto et al., 2018; Hamalainen et al., 2012; Lehangre et al., 2011; Lizarazu et al., 2015; Molinaro et al., 2016; Power et al., 2013). The study by Cutini et al. (2016) was able to localize the right hemisphere impairment to regions of the supramarginal gyrus and angular gyrus, directly implicating the attentional controls of the right FPN. It is generally agreed that such amplitude modulation and ongoing resetting of the low speech frequencies necessary for entrainment are a core ingredient of selective attention (e.g., Obleser and Kayser, 2019), and are controlled predominantly by the right frontoparietal attention networks (Daitch et al., 2013; Galumbic et al., 2012; Marshall et al., 2015; Szcepanski et al., 2014). In response to an allostatic overload and occurring in early postnatal development, such regional and network reprogramming would have a forward effect on the later phonemic processing capabilities of the left hemisphere reading network (Meyer, 2017). We assume that these effects will be associated with increased norepinephrine, and reduced TFEB and BDNF in the amygdala, PFC and hippocampus, downregulating cognitive-growth plasticity in the FPN and upsetting the stress-growth balance at the cellular, regional, and network levels. Direct tests of the Evo-Devo framework will require rigorous experiments addressing this pattern of brain organization in dyslexia.
In humans, plasma BDNF can be tested for DNA methylation and protein levels, and TFEB levels can be inferred from white matter structural imaging. Longitudinal designs with observations of early stress beginning prenatally would test directly the stress-growth hypothesis. These studies should include measures of BDNF; estimates of TFEB; HPA response and methylation; amygdala, hippocampal, and rFIC regional and network connectivity; and reading-related tests as the dependent measures. Transcranial magnetic stimulation coupled with EEG recordings can be used to measure the connectivity of critical neural structures involved in interoceptive and cognitive-emotional processes. In addition, research is needed to determine the individual threshhold at which stress turns negative at the cellular level. We assume that dyslexia occurs at marginal stress levels, or in individuals with a narrow range of stress resilience, because it is typically defined as not resulting from low general intelligence, or prior emotional disturbance. The specificity of the disorder suggests that the breaking point leading to dyslexia will be short of extreme trauma. Dyslexia risk is likely to be associated with complex genetic/epigenetic interactions triggered by the kind of stress, its onset, duration, and intensity, and each individual's comfort zone for stress tolerance.
However these issues turn out, identifying early adversity as a root cause of dyslexia raises the possibility that we may be able to reduce the high incidence of reading failure or its severity through efforts to improve the quality of children's environment in early development. This is easier said than done, but awareness is an essential first step. Also, once identified, reducing stress in the remedial educational environment would follow logically. Preventing an inherited stress imbalance of course is impossible, but inheritance by the epigenome is an optimistic change from the neo-Darwinian gene-centric account. Without question, prevention or correction of a stress imbalance in parents would stop dyslexia at that point from transgenerational passage.
The human and socioeconomic consequences of acknowledging an environmental cause of reading failure cannot be overstated. A national testing program of students in grades 4–12 in over 8000 schools in the U.S. revealed that only 35% were proficient in reading (NAEP, 2015). Individuals with dyslexia are less likely to complete high school, have lower incomes and fewer chances for employment, and are over-represented in the juvenile justice system and prison populations (McLaughlin et al., 2012). A comparison of graduate students without criminal records and offenders in a medium security prison found that the lower-level literacy skills associated with dyslexia were significant predictors of the prison population (Baker and Ireland, 2007).
Evo-Devo presents a rational basis for understanding why so many children cannot easily learn to read. The hypothesis of a stress-growth imbalance as a primary cause of dyslexia addresses a long-standing frustration in early childhood education and cognitive science. But, most importantly, if confirmed by future research, it points the way to reducing its prevalence. As with any major paradigm shift, an Evo-Devo framework for dyslexia invites criticism and debate, and calls for rigorous experimental protocols to test its validity.
6. Conclusion
New insight into the currency of evolutionary processes in child development, merged with recent findings on the importance of stress-reactive cellular, regional, and network homeostasis for cognitive growth, suggest that dyslexia may be a positive adaptation to inherited, prenatal, or childhood environmental challenges to stress system homeostasis.
Declaration of competing interest
None.
References
- Andrews-Hanna J., Reidler J., Sepulcre J., Paulin R., Buckner R. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65(4):550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badyaev A. Stress-induced variation in evolution: from behavioral plasticity to genetic assimilation. Proc. Biol. Sci. 2005;277:877–886. doi: 10.1098/rspb.2004.3045. http://royalsocietypublihing-org.myaccess.libray.utoronto.ca/doi/10.1098/r...1/1/2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey S., Aboud K., Nguyen T., Cutting L. Applying a network framework to the neurobiology of reading and dyslexia. J. Neurodev. Disord. 2018;10(37):1–20. doi: 10.1186/s11689-018-9251-z. Advance online publication. DOI: 1186/s11689-018-9251-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S., Ireland J. The link between dyslexic traits, executive functioning, impulsivity, and social self-esteem among an offender and non-offender sample. Am. J. Law Psychiatr. 2007;30:492–503. doi: 10.1016/j.ijlp.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Balmus I., Ciobica A., Antioch J., Dubrin R., Timofte D. Advance online publication; 2016. Oxidative stress implications in the affective disorders: main biometrics, animal models, genetic perspectives, and antioxidant approaches; pp. 1–60. (Oxidative Mediums and Cell Longevity). Aug. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellis M., Hughes K., Leckenby N., Jones L., Baban A., Kachaeva M. vol. 92. Bulletin of the World Health Organization; 2014. (Adverse Childhood Experiences and Associations with Health-Harming Behaviors in Young Adults: Surveys in Eight European Countries). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez-Burroco A., Murphy E. Why brain oscillations are improving our understanding of language. Front. Behav. Sci. 2019;13(190):1–10. doi: 10.3389/fnbeh.2019.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry A., White R., Furman D., Naskolnakorn J., Shah V., D'Esposito M., Jagust W. J. Neurosci. 2019;39(14):2735–2744. doi: 10.1523/JNEUROSCI.2382-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund D. How children invented humanity. Child Dev. 2018;89(5):1462–1466. doi: 10.1111/cdev.13020. [DOI] [PubMed] [Google Scholar]
- Brito N., Piccolo I., Noble K. Associations between cortical thickness and neurocognitive skills during childhood vary by family socioeconomic factors. Brain Cognit. 2017;116:54–62. doi: 10.1016/j.bandc.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufill E., Agusti J., Blesa R. Human neoteny revisited: the case of synaptic plasticity. J. Human Biol. 2011;23:729–739. doi: 10.1002/ajhb.21225. [DOI] [PubMed] [Google Scholar]
- Burns S., Szyszkowicz J., Luheshi G., Lutz P., Turecki G. Plasticity of the epigenome during early-life stress. Semin. Cell Dev. Biol. 2018;77:115–132. doi: 10.1016/j.semcdb.2017.09.033. [DOI] [PubMed] [Google Scholar]
- Callaghan B., Tottenham N. The stress acceleration hypothesis: effects of early-life adversity on emotion circuits and behavior. Curr. Opin. Behav. Sci. 2016;7:76–81. doi: 10.1016/j.cobeha.2015.11.018. https://sciencedirect.com/sciencearticle/pii/S2352154615001588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R., Wood M. How the epigenome integrates information and reshapes the synapse. Nat. Rev. Neurosci. 2019;20:133–147. doi: 10.1038/s41583-019-0121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F. Epigenetic legacy of parental experience: dynamic and interacting pathways of inheritance. Dev. Psychopathol. 2016;28:1219–1228. doi: 10.1017/S0954579416000808. [DOI] [PubMed] [Google Scholar]
- Critchley H., Harrison N. Visceral influences on brain and behavior. Neuron. 2013;77(4):624–638. doi: 10.1016/j.neuron.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Curry A. A painful legacy. Science. 2019;365(7450):212–215. doi: 10.1126/science.365.6450.212. July. [DOI] [PubMed] [Google Scholar]
- Cutini S., Szucs D., Mead N., Huss M., Gosswami U. Atypical right hemisphere response to slow temporal modulation in children with developmental dyslexia. Neuroimage. 2016;143:40–49. doi: 10.1016/j.neuroimage.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daitch A., Sharma M., Roland J., Astafiev S., Bundy D., Gaona C. Frequency in specific mechanisms links human brain networks for spatial skills. Proc. Natl. Acad. Sci. Unit. States Am. 2013;110(48):19585–19590. doi: 10.1073/pnas.1307947110. www.pnas.org/egi/doi/10.1073pnas.130794110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Liberto G., Peters V., Kalashikova M., Goswami U., Burnham D., Laler E. Atypical cortical entrainment to speech in the right hemisphere underpins phonemic deficits in dyslexia. Neuroimage. 2018;175:70–79. doi: 10.1016/j.neuroimage.2018.03.072. https://doi/10.1016/j.neuroimage.2018.03.072 [DOI] [PubMed] [Google Scholar]
- Di Meo S., Reed T., Venditti P., Victor V. Role of ROS and RNS sources on physiological and pathological conditions. Oxid. Med. Cell. Longev. 2016;1235049:1–44. doi: 10.1155/2016/1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilnot J., Hamilton I., Maughan B., Snowling M. vol. 22. Advance online publication; 2016. Child and environmental risk factors predicting readiness for learning in children at high risk of dyslexia; pp. 1–10. (Developmental Psychopathology). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon M., De La Vega A., Mills C., Andrews-Hanna J., Spreng R., Cole M., Christoff K. Hererogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proc. Natl. Acad. Sci. U.S.A., Feb. 13. 2018;115(7):E1598–E1607. doi: 10.1073/pnas.1715766115. www.pnas.org/cgi/doi/10.1073.1715766115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza S., Baskhouse-Smith A., Thompson J., Slykerman R., Marlow G., Wall C. Association between the K1AA0319 dyslexia susceptibility gene variants, antenatal maternal stress, and reading ability in a longitudinal birth cohort. Dyslexia. 2016;22:379–393. doi: 10.1002/dys.1534. [DOI] [PubMed] [Google Scholar]
- Elhadidy M., Gebril O., Hashish A., Kilany A., Nashaat N., Abdelraouf E. Brain-derived factor and coenzyme Q10 levels in blood of children with LD. J. Arab. Soc. Med. Res. 2019;14(2):124–129. [Google Scholar]
- Espin L., Garcia I., Sanchez M., Roman F., Salvador A. Effects of psychosocial stress on the hormonal and affective response in children with dyslexia. Trends Neurosci. Educ. 2019;15:1–9. doi: 10.1016/j.tine.2019.03.001. [DOI] [PubMed] [Google Scholar]
- FeldmanHall O., Glimcher P., Baker A., Nyu Prospec Collaboration, Phelps E. The functional role of the amygdala and prefrontal cortex in processing uncertainty. J. Cognit. Neurosci. 2019;32(11):1742–1754. doi: 10.1162/jocn_a_01443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti V., Anda R., Nordenberg D., Williams D., Spitz A., Edwards V. Relationship of childhood abuse and household dysfunction to many of the leading causes of death. Am. J. Prev. Med. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Fisher P., Beauchamp K., Roos L., Noll L., Flannery J., Delker B. The neurobiology of intervention and prevention in early adversity. Annu. Rev. Clin. Psychol. 2016;12:331–357. doi: 10.1146/annurev-clinpsy-032814-112855. [DOI] [PubMed] [Google Scholar]
- Gage F. Adult neurogenesis in mammals. Science. 2019;364(May 31):827–828. doi: 10.1126/science.aav6885. [DOI] [PubMed] [Google Scholar]
- Ghashghaei H., Hilgetag C., Barbas H. Sequence of information processing in emotions based on dialogue between prefrontal cortex and amygdala. Neuroscience. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore J., Knickmeyer R., Gao W. Imaging structural and functional brain development in early childhood. Nat. Rev. Neurosci. 2018;19(123):123–137. doi: 10.1038/nrn.2018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollo L., Roberts J., Cropley V., Di Biase M., Pantelis C., Zalesky A., Breakspear M. Fragility and volatility of structural hubs in the human connectome. Nat. Neurosci. 2018;21:1107–1116. doi: 10.1038/s41593-018-0188-z. [DOI] [PubMed] [Google Scholar]
- Golumbic E., Poeppel D., Schroder C. Temporal context in speech processing and attentional stream selection: a behavioral and neural perspective. Brain Lang. 2012;122:1151. doi: 10.1016/j.bandl.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami U. A temporal sampling framework for developmental dyslexia. Trends Cognit. Sci. 2011;15(1):3–10. doi: 10.1016/j.tics.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Goswami U. A neural oscillation perspective on phonological developmental and phonological processing in developmental dyslexia. LanguageLinguistic Compass. 2019;13 doi: 10.1111/lnc3.12328. e12328, 1-21. [DOI] [Google Scholar]
- Gould S. Harvard University Press; Cambridge, MA: 1997. Ontogeny and Phylogeny. [Google Scholar]
- Goyal M., Hawrylycz M., Miller J., Snyder A., Raichle M. Aerobic glycolysis in the human brain is associated with developmental and neotenous gene expression. Cell Metabol. 2014;19:49–57. doi: 10.1016/j.cmet.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J., Milner T., McEwen B. Dynamic plasticity: the role of glucocorticoids, brain-derived neurotropic factor and other tropic factors. Neuroscience. 2013;239:214–227. doi: 10.1016/j.neurocience.2112.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorenko E., Kornilov S., Naumova O. Epigenetic regulation of cognition: a circumscribed review of the field. Dev. Psychopathol. 2016;28:1285–1304. doi: 10.1017/S0954579416000857. [DOI] [PubMed] [Google Scholar]
- Griffiths B., Hunter R. Neuroscience forefront review neuroepigenetics of stress. Neuroscience. 2014;275:420–435. doi: 10.1016/j.neuroscience.2014.06.041. [DOI] [PubMed] [Google Scholar]
- Gur R., Moore T., Rosen A., Barzilay R., Roalf D., Calkins M. Burden of environmental adversity associated with psychopathology, maturation, and brain behavioral parameters in youth. J. Am. Med. Assoc. 2019;May:E1–E10. doi: 10.1001/jamapsychiatry.2019.0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman D., Farah M., Meaney J. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat. Rev. Neurosci. 2010;11:651–659. doi: 10.1038/nrn2897. www.nature.com/reviews/neuro [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haft S., Duong P., Ho T., Hendren R., Hoeft F. Anxiety and attentional bias in children with specific learning disorders. J. Abnorm. Child Psychol. 2019;47:487–497. doi: 10.1007/210802-018-0458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton L., Hayiou-Thomas M., Hulme C., Snowling M. The home literacy environment A predictor of the early development of children a family risk of dyslexia. Sci. Stud. Read. 2016;20(5):401–419. doi: 10.1080/10888438.2016.1213266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R., Pugh K., Hoeft F. Neural noise hypothesis of developmental dyslexia. Trends Cogn. Neurosci. 2017;21(6):434–448. doi: 10.1016/j.tics.2017.03.008. http://dx.soi/org/10.1016/j.tics.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L., Hood L., Goldberg M., Reynolds A., Silver L. fourth ed. McGraw-Hill; New York:NY: 2011. Genetics. [Google Scholar]
- Horowitz-Kraus T., Toro-Serey C., DiFrancesco M. Increased resting-state functional connectivity in the cingulo-opercular cognitive-control network after intervention in children with reading difficulties. PloS One. 2015;10(7):1–24. doi: 10.1371/journal.pone.0133762. e0133762. Advance online publication, July 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtepen L., Vinkers C., Carrillo-Roa T., Hiemsta M., van Lier P., Branje M. Genome-wide DNA methylation levels and altered cortisol reactivity in humans. Nat. Commun. 2016;7(10967):1–59. doi: 10.1038/ncomms10967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber E., Donnelly P., Rokem A., Yeatmen J. Rapid and widespread white matter plasticity during an intensive reading intervention. Nat. Commun. 2018;9(2260):1–13. doi: 10.1038/s41467-018-04627-5. https://www.nature.com/articles/ncomms10967 Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonka E. The evolutionary implications of epigenetic inheritance. Interface Focus. 2017;7:20160135. doi: 10.1098/refs.2016.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe S., McFarquhar T., Stevens S., Ouellet-Morin I., Melhuish E., Belsky J. Interactive effects of early and recent exposure to stressful contexts on cortisol reactivity in middle childhood. JCPP (J. Child Psychol. Psychiatry) 2015;56(2):138–140. doi: 10.1111/jcpp.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershner J. Neurobiological systems in dyslexia. Trends Neurosci. Educ. 2019;14:11–24. doi: 10.1016/j.tine.2018.12.001. [DOI] [PubMed] [Google Scholar]
- Kershner J. Neuroscience and education: cerebral lateralization of networks and oscillations in dyslexia. Laterality. 2020;25(1):109–125. doi: 10.1080/1357650X.2019.1606820. https://soi.org/10.1080/135765X.2019.1606820 [DOI] [PubMed] [Google Scholar]
- Kim P., Evans G., Angstadt M., Ho S., Sripada C., Swain J. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc. Natl. Acad. Sci. Unit. States Am. 2013;110(46):18442–18447. doi: 10.1073/pnas.1308240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugers H., Arp J., Xiong H., Kanatsou S., Lesuis S., Korosi A. Early life adversity: lasting consequences for emotional learning. Neurobiol. Stress. 2017;6:14–21. doi: 10.1016/j.ynstr.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Rouzic A., Alverez-Castrol J., Hansen T. The evolution of canalization and evolvability in stable and fluctuating environments. Evol. Biol. 2013;40:317–340. [Google Scholar]
- Lehongre K., Ramus F., Villiermet N., Schwartz D., Giraud A. Altered low-gamma sampling in auditory cortex accounts for the three main facets of dyslexia. Neuron. 2011;72:1080–1090. doi: 10.1016/j.neuron.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Levine B., Kroemer G. Biological functions of autophagy genes: a disease perspective. Cell. 2018;176:11–42. doi: 10.1016/j.cell.2018.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Robinson M., Malcova E., Jacoby P., Foster J., van Eekelen A. Maternal life stress events in pregnancy link to children's school achievement at age 10 years. J. Pediatr. 2013;162:483–489. doi: 10.1016/j.jpeds.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Liu X., Somel M., Tang L., Yan Z., Jiang X., Guo S. Extension of cortical synaptic development distinguishes human from chimpanzees and macaques. Genome Res. 2012;22:611–622. doi: 10.1101/gr.127324.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Li Y., Pritchard J. Trans effects on gene expression can drive omnigene inheritance. Cell. 2019;177 doi: 10.1016/j.call.2019.04.014. 1022-1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizarazu M., Lallier M., Molinaro N., Bourguignon M., Carreiras M. Developmental evaluation of atypical auditory sampling in dyslexia: functional and structural evidence. Hum. Brain Mapp. 2015;36:4986–5002. doi: 10.1002/hbm.22986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Maury L., Marguerat S., Bahler J. Tuning gene expression to changing environments: from rapid response to evolutionary adaptation. Nat. Rev. Genet. 2008;9:583–593. doi: 10.1038/nrg2398. [DOI] [PubMed] [Google Scholar]
- Mai S., Braun J., Probst V., Kammen T., Pollatos O. Changes in emotional processing following interoceptive network stimulation with rTMS. Neuroscience. 2019;406:405–419. doi: 10.1016/j.neuroscience.2019.03.014. [DOI] [PubMed] [Google Scholar]
- Malins J., Pugh K., Buis B., Frost S., Hoeft F., Landi N. Individual differences in reading skill are related to trial-by-trial neural activation in the reading network. J. Neurosci. 2018;38(12):2981–2989. doi: 10.1523/JNEUROSCI.0907-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron-Katz A., Vaisvaser S., Lin T., Hendler T., Shamir R. A large-scale perspective on stress-induced alterations in resting-state networks. Sci. Rep. 2016;6(21503):1–43. doi: 10.1038/srep21503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaad C., Klann E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxidants Redox Signal. 2011;14(10):2013–2054. doi: 10.1089/ars.2010.3208. http://www.ncbi.nlm.nih.gov/pmc/article/PMC3078504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M., Clewett D., Sakaki M., Harley C. Norepinephrine ignites local hotspots of neuronal excitation: how arousal amplifies selectivity in perception and memory. BBS (Behav. Brain. Sci.) 2016:1–75. doi: 10.1017/S0140525X15000667. Advance online publication, Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy C., Glover M., Flynn L., Simmons R., Cohen J., Ptacek T. Altered DNA methylation in the developing brain of rats genetically prone to high anxiety versus low anxiety. J. Neurosci. 2019;39(16):3144–3158. doi: 10.1523/JNEUROSCI.1157-15.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. Physiological and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McGowen P., Matthews S. Prenatal stress, glucocorticoids, and developmental programming of the stress response. Endocrinology. 2018;159(1):69–82. doi: 10.1210/en.2017-00896. https://doi-org.myaccess.libray.utoronto.ca/10.1220/en.2017.00896 [DOI] [PubMed] [Google Scholar]
- McLaughlin M., Speirs K., Shenassa E. Reading disability and adult attained education and income: evidence from a 30-year longitudinal study of a population-based sample. J. Learn. Disabil. 2012;47(4):374–386. doi: 10.1177/0022219412458323. [DOI] [PubMed] [Google Scholar]
- Menezes J., Souto des Neves B., Goncalves R., Benetti F., Mello-Carpes P. Maternal deprivation impairs memory and cognitive flexibility, effect that is avoided by environmental enrichment. Behav. Brain Res. 2020;381(112468):1–9. doi: 10.1016/j.bbr.2020.112468. [DOI] [PubMed] [Google Scholar]
- Meyer L. The neural oscillation of speech processing and language comprehension: state of the art and emerging mechanisms. Eur. J. Neurosci. 2017;48(7):1–52. doi: 10.1111/ejn.13748. https://doi-org.myaccess.library.utoronto.ca/10.1177/00222.19412458323 [DOI] [PubMed] [Google Scholar]
- Michel G., Babik I., Nelson E., Campbell J., Marcinowski E. Evolution and development of handedness: an Evo-Devo approach. Prog. Brain Res. 2018;238:347–368. doi: 10.1016/bs.pbr.2018.06.007. [DOI] [PubMed] [Google Scholar]
- Miller D., Duka T., Stimpson C., Schapiro S., Baze W., Mc Arthur M. Prolonged myelination in human neocortical evolution. Proc. Natl. Acad. Sci. Unit. States Am. 2012;109(41):16480–16485. doi: 10.1073/pnas.1117943109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinaro N., Lizarazu M., Lallier M., Bourguigon M., Carreiras M. Out-of-Synchrony speech entrainment in developmental dyslexia. Hum. Brain Mapp. 2016;37:2767–2783. doi: 10.1002/hbm.23206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T., Martin I., Gur O., Jackson C., Scott J., Calkins M. Characterizing social environmental associations with neurocognition using census and crime data linked to the Philadelphia Neurodevelopmental Cohort. Psychol. Med. 2016;46:599–610. doi: 10.1017/S0033291715002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Jimenez E., Flor-Garcia M., Torreros J., Raano A., Cafini F., Pallas-Bazarra N. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in Alzheimer's disease. Nat. Med. 2019;25:554–560. doi: 10.1038/s41591-019-0375-9. https://www.nature-com.myaccess.library.utoronto.ca/articles/s41591-019-03...6/4/2019 [DOI] [PubMed] [Google Scholar]
- Murphy E., Benitez-Burraco A. Toward the language oscillogenome. Front. Psychol. 2018:1–74. doi: 10.3389/fpsyg.2018.01999. Published online, October 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J., Whitfield M., Trinklein N., Meyers R., Brown P., Botstein D. Diverse and specific gene expression responses to stresses in cultured human cells. Mol. Biol. Cell. 2004;15:2361–2374. doi: 10.1091/mbc.E03-11-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National assessment of educational progress (NAEP) National Center for Educational Statistics; Washington:DC: 2015. [Google Scholar]
- Nishikawa K., Kingo A. Mechanisms of evolution by genetic assimilation: equivalence and independence of genetic mutation and epigenetic mechanisms in phenotypic expression. Biophysiol. Rev. 2018;10:667–676. doi: 10.1007/s12551-018-0403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K., Wolmetz M., Ochs I., Farah M., McCandless B. Brain-behavior relationships in reading acquisition are modulated by socio-economic factors. Dev. Sci. 2006;9(6):642–654. doi: 10.1111/j.1467-7687.2006.00542.x. [DOI] [PubMed] [Google Scholar]
- Numata S., Ye T., Hyde T., Guitart-Navarro X., Tao R., Wininger M. DNA methylation signature in development and ageing of the human prefrontal cortex. Am. J. Hum. Genet. 2012;90:260–272. doi: 10.1016/j.ajhg.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obleser J., Kayser C. Neural entrainment and attentional selection in the listening brain. Trends Cognit. Sci. 2019;9 doi: 10.1016/j.tics.2019.004. Published online, October. 1-52. [DOI] [PubMed] [Google Scholar]
- Ozernov-Palchik O., Norton E., Wang Y., Beach S., Zuk J., Wolf M. The relationship between socioeconomic status and white matter microstructure in pre-reading children: a longitudinal investigation. Hum. Brain Mapp. 2018;1:1–2. doi: 10.1002/hbm.24407. Advance online publication, Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington B., Bishop D. Relations among speech, language, and reading disorder. Am. Rev. Psychol. 2009;60:283–306. doi: 10.1146/annurev.psych.60.110707.163548. [DOI] [PubMed] [Google Scholar]
- Perroud N., Salzman A., Prada P., Nicastro R., Hoeppli M.-E., Furrer S. Response to psychotherapy in borderline personality disorder and methylation status of the BDNF gene. Transl. Psychiatry. 2013;3(e207):1–33. doi: 10.1038/tp.2012.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A., McEwen B., Friston K. Uncertainty and stress: why it causes diseases and how it is mastered by the brain. Prog. Neurobiol. (Oxf.) 2017;156:14–188. doi: 10.1016/j.pneurobio.2017.05.004. Sept. [DOI] [PubMed] [Google Scholar]
- Posner R., Toker I., Antonova O., Star E., Anava S., Azmon E. Neuronal small RNAs control behavior transgenerationally. Cell. 2016;177:1814–1826. doi: 10.1016/j.cell.2019.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power A., Mead N., Barnes L., Goswami U. Neural entrainment to rhythmic speech in children with developmental dyslexia. Front. Hum. Neurosci. 2013;7(777):1–23. doi: 10.3389/fnhum.2013.00777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryluk R., Kfir Y., Gelbard-Sagiv H., Fried I., Paz R. A tradeoff in the neural code across regions and species. Cell. 2019;176:597–609. doi: 10.1016/j.cell.2018.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh K., Landi N., Preston J., Mencl W., Austin A., Sibley D. The relationship between phonological and auditory processing and brain organization in beginning readers. Brain Lang. 2013;125:173–183. doi: 10.1016/j.bandl.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond C., Marin M., Majeur D., Lupien S. Early child adversity and psychopathology in adulthood: HPA axis and cognitive dysregulation as potential mechanisms. Prog. Neuro Psychopharmacol. 2018;85:152–160. doi: 10.1016/j.pnpbp.2017.07.015. [DOI] [PubMed] [Google Scholar]
- Reus G., Dos Santos M., Abelaira H., Riberiro K., Petronilho F., Vuelo F. Impramine reverses alterations in cytokines and BDNF levels induced by maternal deprivation in adult rats. Behav. Brain Res. 2013;242:40–46. doi: 10.1016/j.bbr.2012.11.044. [DOI] [PubMed] [Google Scholar]
- Roth T., Lubin F., Funk A., Sweatt J. Lasting epigenetic influence of early life adversity on the BDNF gene. Biol. Psychiatr. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpute A., Lindquest K. The default mode network's role in discrete emotion. Trends Cognit. Sci. 2019;23(10):851–864. doi: 10.1016/j.tics.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz D., Ito T., Solomyak L., Chen R., Mill R., Anticevic A., Cole M. Global connectivity of the fronto-parietal cognitive control networks is related to depression symptoms in the general population. Netw. Neurosci. 2019;3(1):107–123. doi: 10.1162/netn_a_00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurz M., Wimmer H., Richlan F., Ludersdorfer P., Klackl J., Kronbichler M. Resting-state and task-based functional brain connectivity in developmental dyslexia. Cerebr. Cortex. 2015;25:3502–3514. doi: 10.1093/cercor/bhu184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W., Menon V., Schatzberg A., Keller J., Glover G., Kenna H. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel L. IQ is irrelevant of the definition of learning disability. J. Learn. Disabil. 1989;22:469–478. doi: 10.1177/002221948902200803. [DOI] [PubMed] [Google Scholar]
- Somel M., Franz H., Yan Z., Lorenc A., Guo S., Giger T. Transcriptional neoteny in the human brain. Proc. Natl. Acad. Sci. Unit. States Am. 2009;106(14):5743–5748. doi: 10.1073/pnas.0900544106. www.pnas.org/cgi/cs/doi/10.1073/pnas.090054106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somel M., Liu X., Tang L., Zheng Y., Hu H., Guo S. PLoS Biol. 2011;9(12) doi: 10.1371/journal.pbio.1001214. www.plosbiology.org s1001214, 1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagna E., Kim T., Wu T., Fan J. Advance online publication; Cortex: 2018. Right Hemisphere Superiority for Executive Cerebral Control of Attention; pp. 1–14. Dec. [Google Scholar]
- Sultan S. Developmental plasticity: re-conceiving the genotype. Interface Focus. 2017;7(2017000):1–11. doi: 10.1098/refs.2017.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Mulinyawe S., Collins H., Ibrahim A., Li Q., Simon D., Barres B. Spatiotemporal control of CNS myelination by oligodendrocyte programmed cell death through TFEB-PUMA axis. Cell. 2018;175:11–42. doi: 10.1016/j.cell.2018.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanski S., Crone N., Kuperman R., Auguste K., Parvisi J., Knoght T. Dynamic changes in phase-amplitude coupling facilitates spatial attention control in fronto-parietal cortex. PLoS Biol. 2014;12(8):1–14. doi: 10.1371/journal.pbio.1001936. e1001096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H., Black J., Hulme C., Stanley L., Kesler S., Whitfiels-Gabrieli S. The brain basis of the phonological deficit in dyslexia is independent of IQ. Psychol. Sci. 2011;22(11):1447–1451. doi: 10.1177/0956797611419521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M., Samson J., Anderson C., Ohashi K. The effects of childhood maltreatment on brain structure, function, and connectivity. Nat. Rev. Neurosci. 2016;17(8):652–666. doi: 10.1038/nrn.2016.111. [DOI] [PubMed] [Google Scholar]
- Thomas E., Buss C., Rasmussen J., Entringer S., Ramirez J., Marr M. Newborn amygdala connectivity and emerging fear. Dev. Cogn. Neurosci. 2018:1–12. doi: 10.1016/j.dcn.2018.12.002. Advance online publication, December. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turesky T., Jensen S., Yu X., Kumer S., Wang Y., Silva D. The relationship between biological and psychosocial risk factor and resting state functional connectivity in 2-month-old Bangladeshi infants. Dev. Sci. 2019:1–17. doi: 10.1111/desc.12841. 00, e12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udden L. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 2015;16:55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- Udden L., Supekar K., Ryali S., Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J. Neurosci. 2011;31(50):18578–18589. doi: 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utevsky A., Smith D., Huettel S. Precuneus is a functional core of the default-mode network. J. Neurosci. 2014;34(3):932–940. doi: 10.1523/JNEUROSCI.4227-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaiserman A., Koliada A., Lushchak O. Developmental programming of ageing trajectory. Ageing Res. Rev. 2018;47:105–122. doi: 10.1016/j.arr.2018.07.007. [DOI] [PubMed] [Google Scholar]
- Van den Bergh B. Developmental programming of early brain and behavioral development and mental health: a conceptual framework. Dev. Med. Child Neurol. 2011;53:19–23. doi: 10.1111/j.1469-8749.2011.04057.x. [DOI] [PubMed] [Google Scholar]
- Vogel S., Schwabe L. Stress in the zoo: tracking the impact of stress on memory formation over time. Psychoneuroendocrinology. 2016;71:64–72. doi: 10.1016/j.psyneuen.2016.04.027. [DOI] [PubMed] [Google Scholar]
- Wagner G., Booth G., Bagheri-Chaichian H. A population genetic theory of canalization. Evolution. 1997;51(2):329–347. doi: 10.1111/j.1558-5646.1997.tb02420.x. [DOI] [PubMed] [Google Scholar]
- Wang J., Rao H., Wetmore G., Furlan P., Korczykowski M., Dinges D., Detre J. Perfusion functional MRI reveals cerebral blood patterns under psychological stress. Proc. Natl. Acad. Sci. Unit. States Am. 2005;49:17804–17809. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Mauer M., Raney T., Peysakhovich B., Bexter B., Silva D., Graab N. Development of tract-specific white matter pathways during early reading development in at-risk children and typical children. Cerebr. Cortex. 2017;27(4):2469–2485. doi: 10.1093/cercor/bhw095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Wang X., Wu Q., Spagna E., Yang J., Yuan C. Anterior insular cortex is a bottleneck of cognitive control. Neuroimage. 2019;195:490–504. doi: 10.1016/j.neuroimage.2019.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan B., Mackie M.-A., Spagna A., Wu T., Tian Y., Hof P., Fan J. The activation of interactive attentional networks. Neuroimage. 2016;129:308–319. doi: 10.1016/j.neuroimage.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B., Krieman F., Sepulcre J., Sabuncu M., Lashkar D., Hollinshead M. The organization of the brain cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakopoulou V., Vlailou A., Darsinou M., Papadopoulou Z., Theodoridou D., Papageorgiou K. Linking early life Hypothalmic-Pituitary-Adrenal Axis function, brain asymmetries, and personality traits in dyslexia. Front. Hum. Neurosci. 2019:1–26. doi: 10.3389/fnhum.2019.00327. Advance online publication, Oct. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Friston K., Zeidman P., Chen J., Shu L., Razi A. He hierarchical organization of the default and salience networks in adolescents and young adults. Cerebr. Cortex. 2018;28:726–737. doi: 10.1093/cercor/bhx307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk J., Dunstan J., Norton E., Yu X., Ozernov-Palchik O., Wang Y. Multifactorial pathways facilitate resilience among kindergarteners at risk for dyslexia: a longitudinal behavioral and neuroimaging study. Cold Springs Harbor Laboratory. 2019:1–7. doi: 10.1111/desc.12983. Bio Rxiv, Advance online publication, April 26. [DOI] [PMC free article] [PubMed] [Google Scholar]