Abstract
The presence of side effects during pharmacological treatment is unfortunately a quite common problem. In this review, we focused our attention on adverse events related to 5 alpha-reductase (5α-R) inhibitors (i.e., finasteride and dutasteride), approved for the treatment of benign prostatic hyperplasia and androgenetic alopecia (AGA).
Although these drugs are generally well tolerated, many reports described adverse effects in men during treatment, such as sexual dysfunction and mood alteration. In addition, it has been also reported that persistent side effects may occur in some AGA patients. This condition, termed post-finasteride syndrome (PFS) is characterized by sexual side effects (i.e., low libido, erectile dysfunction, decreased arousal and difficulty in achieving orgasm), depression, anxiety and cognitive complaints that are still present despite drug withdrawal. Indeed, some national agencies (e.g., Swedish Medical Products Agency, the Medicines and Healthcare Products Regulatory Agency of UK and the U.S. Food and Drug Administration) required to include multiple persistent side effects within the finasteride labels.
As here reported, these observations are mainly based on self-reporting of the symptomatology by the patients and few clinical studies have been performed so far. In addition, molecular mechanisms and/or genetic determinants behind such adverse effects have been poorly explored both in patients and animal models. Therefore, results here discussed indicate that PFS is an emerging clinical problem that needs to be further elucidated.
Keywords: Finasteride, 5alpha-reductase, Neuroactive steroids, Sexual dysfunction, Depression, Androgenetic alopecia
1. Introduction
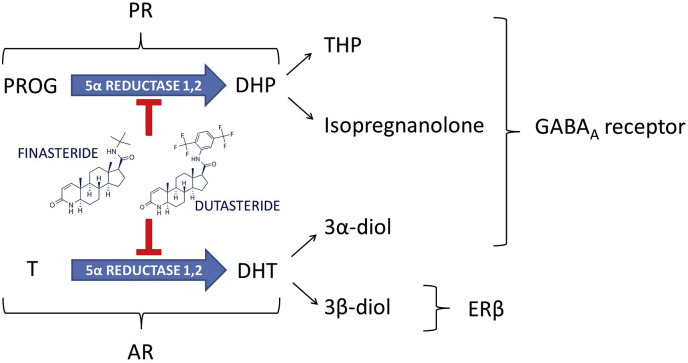
The enzyme 5alpha-reductase (5α-R) exerts a key role in the activation of neuroactive steroids, such as testosterone (T) and progesterone (PROG). Indeed, T and PROG are metabolized by 5α-R into dihydrotestosterone (DHT) and dihydroprogesterone (DHP), respectively. These neuroactive steroids (i.e., steroids synthesized from peripheral glands as well as directly in the nervous system) are then further converted by the action of 3α-hydroxysteroid oxidoreductase (3α-HSOR) or 3β-hydroxysteroid oxidoreductase (3β-HSOR) into further metabolites, such as 5α-androstane-3α,17β-diol (3α-diol) or 5α-androstane-3β,17β-diol (3β-diol) in case of DHT and tetrahydroprogesterone (THP, also known as allopregnanolone) or isopregnanolone in case of DHP. All these neuroactive steroids, together with their precursors (i.e., pregnenolone, PREG, and dehydroepiandrosterone, DHEA) interacting with classical (e.g., progesterone, PR, androgen, AR, and estrogen receptors, ER) and non-classical (e.g. neurotransmitter and membrane steroid receptors) steroid receptors represent important physiological modulators of the nervous function (Giatti et al., 2019; Melcangi et al., 2016). As reported in Fig. 1, finasteride (e.g., Propecia or Proscar) is an inhibitor of two isoforms of the 5α-R (i.e., type 1 and 2), although it has higher affinity for the type 2 in humans (Finn et al., 2006; Traish et al., 2015). This drug, proved to be highly effective in the control of DHT levels and of the progression of benign prostatic hyperplasia (BPH), was approved for this use in 1992. This inhibitor was then, in 1997, approved for the treatment of androgenetic alopecia (AGA). Finasteride at 1 mg/day has been shown to lead to a significant reduction in the progression of the baldness and to a stimulation of new hair growth (Kaufman et al., 1998). Dutasteride (e.g., Avodart) inhibits both 5α-R type 1 and 2 (Fig. 1) with greater potency than finasteride (Frye et al., 1998), and has similar efficacy to this latter drug on BPH symptoms. 5α-R inhibitors have generally been described as well-tolerated and relatively safe drugs; however, recent observations have led to a more critical re-evaluation of these concepts. Indeed, as will be here discussed, 5α-R inhibitors not only induced side effects during the treatment, but, as reported in case of finasteride, they may also persist after drug discontinuation inducing the so called post-finasteride syndrome (PFS).
Fig. 1.
Metabolism of progesterone and testosterone into 5alpha-reduced metabolites and their mechanisms of action: effects of finasteride and dutasteride. AR: androgen receptor; DHP: dihydroprogesterone; DHT: dihydrotestosterone; ERβ: estrogen receptor beta; PR: progesterone receptor; PROG: progesterone; T: testosterone; THP: tetrahydroprogesterone.
1.1. Side effects of 5α-reductase inhibitors and post-finasteride syndrome: what patients tell us
Finasteride or dutasteride treatment has been associated with the onset of different side effects. Mainly, the affected domains are related to sexual function. Indeed, sexual adverse events (AEs) in BPH patients treated with finasteride (Bruskewitz et al., 1999; Edwards and Moore, 2002; Fwu et al., 2014; Marberger, 1998; Nickel et al., 1996; Traish et al., 2015; Wilton et al., 1996) or dutasteride (Clark et al., 2004; Desgrandchamps et al., 2006; Kaplan et al., 2012; Roehrborn et al., 2002) have been reported. Moreover, observational studies as well as clinical reports indicated similar complaints in male subjects treated with these inhibitors for AGA (Belknap et al., 2015; Choi et al., 2016; Kaufman et al., 1998; Tsunemi et al., 2016). Indeed, analyzing the American Food and Drug Administration Adverse Event Reporting System (FAERS), Gupta and collaborators reported an increased risk to develop sexual dysfunction with finasteride (Gupta et al., 2017) or dutasteride (Gupta et al., 2018) use in comparison to the baseline risk assessed for all the other drugs. More recently, FAERS database was also queried in order to evaluate which cluster of symptoms have been described after finasteride use at the dose of 1 mg (to treat alopecia) and 5 mg (to treat BPH). Baas and colleagues identified three main areas of AEs, classified as sexual, psychological and physical domains (Baas et al., 2018). In all domains, a significant higher number of cases were present in patients treated with finasteride at the lower dose (1 mg) in comparison to those with the higher dose (5 mg). In particular, within the sexual domain, an increased sexual dysfunction, decreased or loss of libido, disorders of ejaculation, erectile dysfunction, testicular atrophy, orgasmic disorders and hypogonadism were reported by 1 mg finasteride users, while in the psychological domain, the same dose was associated to an increased self-harm, slow cognition, psychological pathology, change in emotional affect and sleep disturbances, compared to the higher dose. Finally, regarding the physical domain, significantly increased reports about skin rush and metabolic abnormalities were indicated by low dose finasteride users. In addition, gynecomastia was more frequently reported by 5 mg finasteride users, although this increase was not statistically significant (Baas et al., 2018). Interestingly, Motofei and colleagues reported differences in sexual complains between right-handed and left-handed subjects taking finasteride, suggesting that lateralization of functions might be relevant in these processes (Motofei et al., 2013, 2016, 2017). In particular, right-handed users presented an overall decreased International Index of Erectile Function (IIEF) score compared to the left-handed users, that have, on the other side, an improved orgasmic function compared to the right-handed finasteride users (Motofei et al., 2013, 2016, 2017). In contrast with these findings, Haber and collaborators reported no sexual dysfunction associated to finasteride treatment for alopecia in a single-centre, controlled study of 762 subjects (Haber et al., 2019). In addition, other reports, including randomized studies, showed no incidence of sexual impairment after finasteride use (Liu et al., 2016; Narasimhalu, 2015; Tosti et al., 2001, 2004).
Overall, these results indicate no consensus with respect to the presence of sexual side effects during treatment with 5α-R inhibitors.
However, recent observations also demonstrated that, in case of AGA, side effects associated with finasteride may also persist despite the drug suspension (i.e., inducing the PFS). Indeed, Irwig and Kolukula interviewed 71 subjects who reported sexual dysfunction despite they stopped finasteride use by at least three months (Irwig and Kolukula, 2011). They described low libido, erectile dysfunction, decreased arousal and difficulty in achieving an orgasm (Irwig and Kolukula, 2011). Interestingly, these adverse events did not resolve in the majority of subjects after 9 and 16 months of withdrawal from the drug (Irwig, 2012b). Another report collected 131 web-based surveys of patients lamenting new symptoms onset after finasteride use. Subjects were recruited from those seeking medical help after treatment or visiting Propeciahelp.com website (Ganzer et al., 2015). Authors divided symptoms collected from patients in five categories: physical, sexual libido, disorder of penis and testes, cognitive disorders and psychological disorders. From each category, the higher percentage of cases regarded: gynecomastia (70% of cases in physical category), decreased sex drive (93% of cases in sexual libido category), diminished semen volume and force (82% of cases in disorder of penis and testes category), mental cloudiness or brain fog (75% of cases in cognitive disorders category) and elevated anxiety (74% of cases in psychological disorders category) (Ganzer et al., 2015). This survey also highlighted the high number of subjects reporting suicidal ideation (63%), due to the negative thoughts and the inability to live a life with such symptoms (Ganzer et al., 2015). This latter result was in line with previous findings (Irwig, 2012a).
Observations based on a recent questionnaire-based survey performed in 54 PFS patients have been also published (Giatti et al., 2018). As shown, 22% of patients reported lack of connection between the brain and penis during treatment, and this percentage increased to 59% at interview time (i.e., at least three months after finasteride discontinuation). This side effect was not reported by the patients before the finasteride treatment. Similarly, the loss of libido and sex drive was reported in 24% of the patients during treatment and increased to 56% at interview time. This side effect was reported in 4% of patients before the treatment. Difficulty in achieving an erection was reported in 17% of patients during inhibitor administration and increased to 61% at interview time. Finally, genital numbness or paresthesia was reported in 17% of patients during treatment and increased to 37% of cases at interview time (Giatti et al., 2018). Beside sexual problems, PFS patients also reported psychological complaints (such as decreased self-confidence, irritability or easily flying into a rage, nervousness, agitation, inner restlessness, depression, hopelessness, feelings of worthlessness, suicidal thoughts, anxiety, panic attacks, sleep problems), muscular problems (tics, muscle spasms and fasciculation, tremors, involuntary muscle tension and contraction, chronic fatigue, weakness, ataxia, joint pain and muscular ache), physical alterations (dizziness, headache, migraine, head pressure, decreased body temperature) and cognitive complains (decreased initiative and difficulty in concentration, mental confusion, forgetfulness or loss of short-term memory, losing train of thought or reasoning, slurred speech or stumbling over words) (Melcangi et al., 2017).
“Propeciahelp.com” forum has been also considered as a source. Indeed, Walf and colleagues analyzed 224 discussions on this web site and collected self-reported symptoms of PFS patients (Walf et al., 2018). The symptoms were divided into four categories: anti-androgenic effects (such as demasculinizing events consequent to DHT deprivation on sexual organs), estrogenic effects (i.e., feminizing features related to the increased aromatization of testosterone), central/brain effects (linked to the alteration of neurosteroid synthesis and metabolism or to thromboembolitic events) and, finally, nonspecific/severe AEs (collecting other severe effects that could not be classified in the above categories but are considered serious) (Walf et al., 2018). The analysis indicated antiandrogenic effects in the 32%, central effects in the 30%, estrogenic effects in the 19%, and nonspecific/severe AEs in the 5% of cases (Walf et al., 2018).
Persistent sexual side effects possibly due to finasteride treatment have been also analyzed by Healy and colleagues based on the self-reported alerts into the RxISK.org website (i.e., an independent drug safety website aiming to collect data on all drug-related AEs) (Healy et al., 2018). A total of 24 male subjects completed the report indicating different symptoms related to sexual functions that persisted after finasteride use. In line with previous reports, 92% of patients claimed erectile dysfunction and loss of libido, 44% genital anesthesia and 32% watery ejaculation and testicular atrophy. In this survey, other infrequent symptoms, such as reduced sense of taste (8%) and of smell (4%), were reported (Healy et al., 2018).
Criticism about the existence of PFS has been also raised. In particular, subject selection in the study design, lack of adequate control groups, possible nocebo effect and the use of retrospective questionnaires may introduce important biases, leading to poor quality of the studies performed (Baas et al., 2018; Haber et al., 2019; Mondaini et al., 2007).
Nevertheless, it is important to highlight that the so called PFS is a rare condition, that could be not adequately investigated in larger cohort studies. In particular, many of these studies lack of long follow-up of patients, and specific symptoms (e.g., sexual dysfunction or psychological problems) may not be investigated in detail (Belknap et al., 2015). On the other hand, objective measurements and deeper clinical assessments are imperative to describe PFS symptomatology.
1.2. What has been so far clinically demonstrated about the existence of PFS
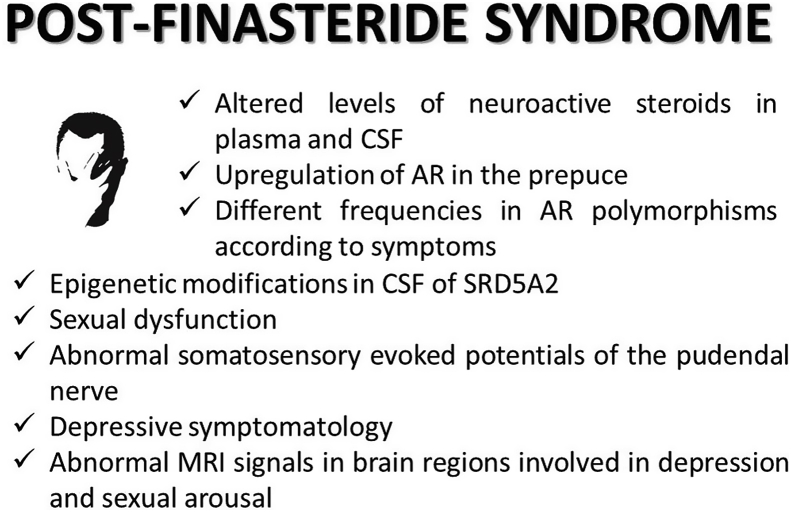
As mentioned in section 2.1, the observations present in literature are mainly based on symptoms self-reported by patients. Indeed, only few papers have rigorously investigated these aspects so far (Fig. 2). In this context, one of the markers taken in consideration has been the levels of neuroactive steroids in PFS patients. The rational was based on the following reasons: 1) finasteride is able to block a fundamental step in the activation of neuroactive steroids (i.e., 5α-R) (Finn et al., 2006; Traish et al., 2015); 2) finasteride in AGA patients induced, during treatment, changes in the plasma levels of neuroactive steroids (i.e., decrease in DHT and increase in T and androstenedione) (Duskova et al., 2010); 3) neuroactive steroids are important key regulators of the nervous functions (Giatti et al., 2019; Melcangi et al., 2016); 4) some neuroactive steroids, like for instance THP, isopregnanolone and 3α-diol are able to modulate GABA-A receptors (Lambert et al., 2003); 5) altered levels in plasma and cerebrospinal fluid (CSF) of GABA as well as of neuroactive steroids are associated with depression in several human studies (Melcangi et al., 2014, 2016; Zorumski et al., 2013). In addition, a subset of post-finasteride patients with persistent symptomatology showed a decline in their alcohol consumption (Irwig, 2013). This is very interesting, because a relationship between GABAergic neuroactive steroids and ethanol consumption is well ascertained (Kumar et al., 2009). On the basis of this rationale, in three different studies based on three (Melcangi et al., 2013), seven (Caruso et al., 2015) and fourteen (Melcangi et al., 2017) PFS patients, the plasma and CSF levels of different neuroactive steroids (i.e., PREG, DHEA, PROG and its metabolites, T and its metabolites) have been assessed by liquid chromatography-tandem mass spectrometry. Data obtained indicate that finasteride treatment has broad consequences on the levels of these molecules in plasma and particularly in CSF (Caruso et al., 2015; Melcangi et al., 2013, 2017). Thus, finasteride treatment did not only affect the 5α-reduced metabolites of PROG and T. Indeed, as observed in the study with the larger group of patients, lower levels of PREG, PROG, DHP, DHT and 17β-E and higher levels DHEA, T and 3α-diol were reported in the CSF of PFS patients in comparison with those observed in healthy patients (Melcangi et al., 2017). The results obtained in this latter study showed small differences in comparison to the previous ones (Caruso et al., 2015; Melcangi et al., 2013). This, together with the presence of a heterogeneous symptomatology, suggests that PFS patients are not a homogenous pathological group. Interestingly, the pattern in plasma did not exactly reflect what observed in CSF. For instance, at variance to what observed in CSF, the plasma levels of PREG were significantly increased and those of PROG and T metabolites, such as DHT, 3α-diol and 17β-E, were unaffected. In addition, the levels of THP that were unaffected in CSF, showed a significant decreased in plasma. These findings were not surprising because, as demonstrated in various physiological or pathological experimental models, neuroactive steroid changes occurring in plasma did not reflect exactly what occurs in CSF and in the nervous system (Caruso et al., 2013; Melcangi et al., 2014, 2016). As demonstrated, not only neuroactive steroid levels themselves but also their mechanisms of action may be altered by finasteride. For instance, an upregulation of AR occurred in the prostate of patients treated with finasteride for BPH (Hsieh et al., 2011) as well as in the prepuce of AGA patients showing persistent side effects (i.e., PFS patients) (Di Loreto et al., 2014). In addition, two polymorphisms in AR gene, (CAG) rs4045402 and (GGN) rs318869, have been reported to be more frequent among AGA and PFS patients (Cecchin et al., 2014). As further demonstrated, short and/or long (CAG)n and (GGN)n repeats in the AR gene have also different frequencies according to the symptomatology reported by PFS patients (Cauci et al., 2017).
Fig. 2.
Clinical features reported in patients affected by post-finasteride syndrome. For details, see text. AR: androgen receptor; CSF: cerebrospinal fluid; MRI: magnetic resonance imaging; SRD5A2: 5 alpha reductase type 2 gene.
Epigenetic modifications seem to be also involved (Melcangi et al., 2019). Indeed, as recently demonstrated, methylation analysis of the promoter genes coding for type 1 (i.e., SRD5A1) and type 2 (i.e., SRD5A2) 5α-R performed in plasma and CSF of 16 PFS patients, indicated that SRD5A2 promoter was more frequently methylated in CSF of PFS patients compared to healthy controls (56.3% versus 7.7%). Importantly, this is a tissue-specific methylation. Indeed, SRD5A2 promoter methylation has not been observed in plasma. Interestingly, both in plasma and CSF, SRD5A1 promoter was not methylated (Melcangi et al., 2019).
Basaria and coworkers (Basaria et al., 2016) observed impaired sexual function, assessed by IIEF and Male Sexual Health Questionnaire, in 25 PFS patients. These observations were confirmed in another study performed in 16 PFS patients, reporting that 10 of them showed a severe erectile dysfunction, while 6 patients a mild-moderate one (Melcangi et al., 2017). As demonstrated by ultrasonography scan, erectile dysfunction in these patients was not associated with altered testicular volume or alterations in ejaculatory ducts. Interestingly, an objective evidence of neuropathy involving the peripheral neurogenic control of erection was reported. Indeed, abnormal somatosensory evoked potentials of the pudendal nerve were observed in 25% of these PFS patients (Melcangi et al., 2017).
Depressive symptomatology is related with alteration in neuroactive steroid levels (Melcangi et al., 2014, 2016; Zorumski et al., 2013). In addition, the expression levels of 5α-R type 1 enzyme are downregulated in prefrontal cortex Brodmamn's area 9 of depressed patients (Agis-Balboa et al., 2014). Indeed, as reported in two different studies, using PHQ-9 depression scale, Beck Depression Inventory and Hamilton Depression Scale 17 (Basaria et al., 2016) or K-10, Mini-International Neuropsychiatric Interview and Beck Depression and Anxiety Inventories (Melcangi et al., 2017), the presence of DSM-IV major depressive disorder was confirmed in PFS patients. Interestingly, functional MRI in PFS patients confirmed abnormalities in brain regions implicated in depression and sexual arousal, such as nucleus accumbens and prefrontal cortex (Basaria et al., 2016). In addition, further studies showed that more than half of the 150 PFS patients considered had a pre-existing medically confirmed psychiatric diagnosis (Ganzer and Jacobs, 2016; Ganzer et al., 2015).
1.3. What experimental models tell us
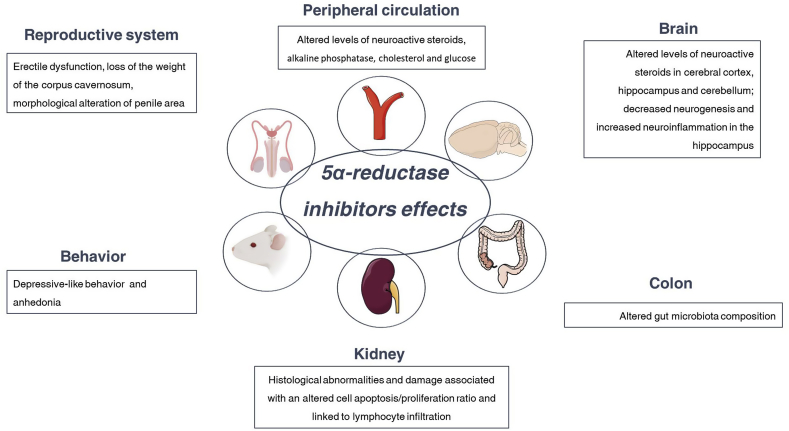
As mentioned in sections 2.1 and 2.2, observations so far obtained in AGA patients have indicated that both during finasteride treatment (Duskova et al., 2010) and after its suspension (i.e., in PFS patients) (Caruso et al., 2015; Melcangi et al., 2013, 2017) the levels of neuroactive steroids are affected. Observations obtained in male adult rats have confirmed these observations (Giatti et al., 2016). Interestingly, 20 days of finasteride treatment and its withdrawal (i.e., one month of suspension), not only alter the levels of neuroactive steroids in plasma and CSF, but also those in the central nervous system areas such as cerebral cortex, cerebellum and hippocampus (Giatti et al., 2016). Importantly, finasteride treatment differently influenced the neuroactive steroid levels in the brain areas considered. For instance, PROG levels were increased in the hippocampus but not in the cerebral cortex and cerebellum. In addition, levels of isopregnanolone and 3α-diol were increased and decreased respectively in the cerebellum; these changes did not occur in the cerebral cortex and hippocampus (Giatti et al., 2016). At the withdrawal, some of the changes observed persisted, such as the increase in DHP in the cerebellum, and new changes occurred, such as an increase and a decrease of PREG levels in the cerebellum and hippocampus, respectively and a decrease of THP in the cerebral cortex. In addition, T levels were increased while the levels of its metabolite, DHT, were decreased in the cerebellum (Giatti et al., 2016) (Fig. 3).
Fig. 3.
Side effects of treatment with 5 alpha-reductase inhibitors in experimental models. For details, see text.
Moreover, not only the levels of neuroactive steroids but also the expression of their receptors, are altered by finasteride treatment in the brain areas considered. Indeed, an upregulation of AR in rat cerebral cortex occurred after the chronic treatment as well as at the withdrawal (Giatti et al., 2016). This is particularly interesting, because it indicates that the expression of this receptor is not only affected in the periphery, as observed in AGA patients (Di Loreto et al., 2014), but also in the nervous system. In addition, the expression levels of one GABA-A subunit, such as the beta 3, were also upregulated in the cerebellum after finasteride treatment (Giatti et al., 2016). At the withdrawal, an upregulation of the isoform alpha and a downregulation of the isoform beta of ER were reported in the cerebral cortex. In addition, the expression of alpha 4 and beta 3 subunits of GABA-A receptors was downregulated in the cerebral cortex (Giatti et al., 2016).
As mentioned above, depressive symptomatology has been reported in PFS patients (Basaria et al., 2016; Melcangi et al., 2017). Concerning observations in animal models, controversial results have been collected so far. For instance, finasteride treatment in adult male rat showed an increased time of immobility in forced swim test. This effect occurred after one month of withdrawal, but not immediately after the treatment (Diviccaro et al., 2019) (Fig. 3). On the contrary, as recently reported in adult male rats by forced swim test, finasteride administration induces, immediately after the treatment, a depression-like phenotype (Sasibhushana et al., 2019). Differences in the experimental protocol, like for instance animal strain (Sprague-Dawley rat vs Wistar rat), finasteride dose (i.e., 3 mg/kg vs 30 mg/kg), time schedule (20 days of treatment vs 6 days) might be responsible for this discrepancy of effect.
Interestingly, anhedonia or decreased pleasure-seeking behavior have been also explored by Sasibhushana and colleagues (Sasibhushana et al., 2019). Results obtained by the splash test indicate that finasteride treatment decreased the grooming duration and increased the latency to groom, which indicates that the drug affected the motivational behavior in a negative way (Sasibhushana et al., 2019).
Not only alterations in neuroactive steroid levels (Melcangi et al., 2014, 2016; Zorumski et al., 2013) but also changes in hippocampal neurogenesis have been related with depressive-like behaviors (Santarelli et al., 2003; Snyder et al., 2011). Altered hippocampal morphology and reduced hippocampal neurogenesis have been also reported in depressed patients (Hercher et al., 2009; Stockmeier et al., 2004). Interestingly, 6 days of finasteride administration in male C57BL/6N mice were able to decrease the hippocampal neurogenesis (Romer and Gass, 2010; Romer et al., 2010). Recent observations in a rat model have shown that 20 days of treatment induced a decrease of granule cell density in the granular layer of the dentate gyrus in rats showing depressive-like behavior one month after the end of finasteride treatment (Diviccaro et al., 2019) (Fig. 3).
In humans and experimental models, depression has been demonstrated to be associated with neuroinflammation (Yirmiya et al., 2015). Treatment with finasteride also affects this parameter (Fig. 3). Indeed, a significant increase in the number of astrocytes (i.e., cellular mediators of the inflammatory response) in the hilus of the dentate gyrus has been demonstrated one month after the end of the finasteride treatment (Diviccaro et al., 2019). This effect might be ascribed to the elevated levels of DHP observed one month after the end of the finasteride treatment in male rat hippocampus (Giatti et al., 2016). Indeed, in other experimental models, DHP has been demonstrated to increase GFAP gene expression in astrocytes (Melcangi et al., 1996). Reactive gliosis is associated with increased levels of proinflammatory molecules in the brain (Hostenbach et al., 2014; Wang et al., 2018). In agreement, finasteride treatment increased, immediately after the treatment, the expression of TNF-α in male rat hippocampus and, at the withdrawal, that of IL-1β (Diviccaro et al., 2019). It is important to note that both cytokines are increased in plasma of depressed patients and in the brain of animal models for depression (Cheng et al., 2018; Li et al., 2017; Todorovic and Filipovic, 2017; Wang et al., 2018). Dysregulation of the dopamine system may also contribute to major depressive disorders (Belujon and Grace, 2017; Felger, 2017; Grace, 2016). Therefore, in this context it is important to recall that androgens regulate the dopaminergic system in the brain (de Souza Silva et al., 2009; Kindlundh et al., 2004; Mitchell and Stewart, 1989). Interestingly, finasteride treatment (25 and 50 mg/kg for 14 days) inhibited open-field behaviors and reduced contents of dopamine and its metabolites in central nervous system (Li et al., 2018). In addition, finasteride treatment was also able to impair the signaling of dopamine (Devoto et al., 2012; Frau et al., 2016).
Androgens are also involved in the physiological regulation of erection. Few preclinical observations have been obtained on the effects exerted by 5α-R inhibitors. For instance, a 5α-R inhibitor, such as 17 beta-testosterone carboxylic acid, significantly weakened the stimulatory effect exerted by T propionate on erection in castrated rats, but not the mating behavior (Bradshaw et al., 1981). Similarly, another study performed with finasteride in castrated rats also reported a reduction of erectile response, although receiving T replacement (Park et al., 1999). Pinsky and colleagues demonstrated a detrimental effect of dutasteride on erectile function as well as in the gene expression of nitric oxide synthase in intact adult male rats (Pinsky et al., 2011). In addition, another study demonstrated that oral administration of finasteride for one month was not able to affect the erectile response to electric stimulation of the cavernous nerve, but resulted in a loss of the weight of the corpus cavernosum (Zhang et al., 2012). Furthermore, a further study performed in Wistar–Kyoto adult rats showed that treatment with finasteride or dutasteride reduced cross-sectional penile area (Da Silva et al., 2018). In addition, in the same study, dutasteride, but not finasteride treatment, reduced smooth muscle in the corpus cavernosum (Da Silva et al., 2018). To our knowledge, only one preclinical study has explored so far possible persistent erectile dysfunction induced by 5α-R inhibitors (Sung et al., 2019). In particular, it seems that the recovery from erectile dysfunction depends on the duration of treatment. Indeed, after two weeks of withdrawal the erectile dysfunction persisted in rats treated with dutasteride for more than 8-weeks, but not for 4-weeks (Sung et al., 2019) (Fig. 3). In this context, it is also important to recall that, dopamine, a neurotransmitter that, as reported above, is affected by finasteride treatment (Devoto et al., 2012; Frau et al., 2016; Li et al., 2018), also modulates male sexual behavior in humans as well as in animal models (Peeters and Giuliano, 2008; Will et al., 2014).
Finally, as recently demonstrated, finasteride treatment also leads to kidney damage (Baig et al., 2019) as well as alterations in gut microbiota (Diviccaro et al., 2019). Indeed, an experimental study performed on sexually mature male Wistar rats, confirmed not only that finasteride treatment altered the steroid hormone levels in plasma, but also the expression of AR and intracellular junction proteins in the kidney was impaired (Fig. 3). These alterations are associated with an altered cell apoptosis/proliferation ratio, and linked to lymphocyte infiltration and to an increase of IL-6 (Baig et al., 2019). Finasteride treatment is also able to affect, not only immediately after treatment but also after one month of discontinuation of the drug, the gut microbiota composition (Diviccaro et al., 2019). Indeed, immediately after drug treatment an increase in Bacteroidetes phylum as well as in Prevotellaceae family was reported; after discontinuation of the finasteride, a decrease in Ruminococcaceae family, Oscillospira and Lachnospira genus was observed (Diviccaro et al., 2019) (Fig. 3). Changes in gut microbiota could be ascribed to different factors, such as changes induced by finasteride in plasma and/or brain neuroactive steroid levels (Giatti et al., 2016). Indeed, gonadectomy and hormone replacement have a clear effect on gut bacteria in rodents (Fields et al., 2017; Harada et al., 2016; Jasarevic et al., 2016; Moreno-Indias et al., 2016; Org et al., 2016; Tetel et al., 2018; Yurkovetskiy et al., 2013). For instance, Ruminococcaceae are significantly affected by orchidectomy in mice (Org et al., 2016). In addition, the existence of a gut microbiota-brain axis has been proposed (Lerner et al., 2017; Mayer, 2011; Sharon et al., 2016). On the other hand, although steroidogenesis has not been evaluated in detail in gut microbiota, intestinal epithelial cells seem to be able to synthesize glucocorticoids (Cima et al., 2004) and microbial species, like for instance Clostridium scindens, converts glucocorticoids into androgens (Ridlon et al., 2013). Thus, an alternative possibility is a direct action of finasteride on gut microbiota. Results obtained on rat gut microbiota population after finasteride treatment are also interesting since very similar changes have been observed in patients with major depressive disorder (Jiang et al., 2015; Lin et al., 2017; Naseribafrouei et al., 2014) as well as in animal models of depression. For instance, rats with depressive-like behavior show an increase in Bacteroidetes (Yu et al., 2017).
1.4. What about finasteride therapy and PFS in women?
Finasteride has been approved by the US FDA for the treatment of alopecia only in male subjects. However, this drug is increasingly used also in women to treat female pattern hair loss (FPHL) and frontal fibrosing alopecia (FFA) (Camacho-Martinez, 2009; Hu et al., 2019).
With particular regard to female alopecia, finasteride is considered both effective (Boersma et al., 2014; Oliveira-Soares et al., 2013; Won et al., 2018) and ineffective (Kim et al., 2012; Price et al., 2000; van Zuuren et al., 2016; Varothai and Bergfeld, 2014). Moreover, indications for finasteride dosage are confusing. Indeed, studies reporting dose of 1 mg, 1.25 mg, 2.5 mg or 5 mg have been published (Boersma et al., 2014; Kim et al., 2012; Mervis et al., 2018; Price et al., 2000).
Also in case of female finasteride users, side effects have been reported during treatment. In particular, Wu and colleagues discussed information about finasteride side effects in female patients, collected in the FAERS database (Wu et al., 2016). The top ten AEs reported were: abortion induction, abortion spontaneous, paternal drug affecting fetus, uterine cervix stenosis, menstruation irregularity, menorrhagia, endometrial hypertrophy, phalangeal agenesis, fatigue and arthritis (Wu et al., 2016). Regarding the adverse events on reproductive features, it is important to recall that finasteride is forbidden in pregnant women, therefore, careful contraception is strongly suggested during finasteride treatment.
Other data described, in women treated for alopecia with 5 mg of finasteride, headache, menstrual irregularities, dizziness and increased body hair growth as the most common complaints (Shum et al., 2002; Valsecchi et al., 2004; Yeon et al., 2011). Moreover, decreased libido and gastrointestinal discomfort were also described (Shum et al., 2002; Thai and Sinclair, 2002; Valsecchi et al., 2004; Yeon et al., 2011). A drug-gene network analysis, in patients treated with finasteride, also revealed that “oocyte meiosis” and “progesterone-mediated oocyte maturation” pathways were affected by finasteride treatment (Wu et al., 2016). Few studies evaluated finasteride toxicity in female animal models. Of note, a paper by Alkahtane and collaborators showed altered serum biochemical parameters (e.g., alkaline phosphatase, cholesterol, glucose), increased DNA damage and histological abnormalities in the liver, spleen, kidney and heart of female Swiss albino mice treated with finasteride (0.5 or 1.5 mg/kg/day) (Alkahtane et al., 2019) (Fig. 3).
Also in female patients, criticism about the possible side effects induced by 5α-R inhibitors has been raised. Indeed, in FPHL and FFA, very few side effects or AEs related to sexual function in women treated with finasteride have been reported (Seale et al., 2016). In agreement, Mervis and collaborators, by analyzing 24 clinical studies (a total of 521 women), reported a decrease of libido or headache in 1.9% and 2.2% of cases respectively (Mervis et al., 2018). All these side-effects were present during the finasteride treatment, but not after its suspension, suggesting, on the basis of the literature available so far, that PFS is present only in males.
2. Conclusions and perspectives
As here reported, finasteride or dutasteride treatment in male is associated with the onset of different side effects (i.e., mainly on sexual functions as well as on mood, even if other complaints have been described). In agreement, rodent models treated with these drugs present different degree of alterations in sexual function and mood regulation. Interestingly, some of these side effects has been reported also in female. On the other hand, reports observing no adverse events have been published as well, raising doubts about the real presence of such symptoms and the quality of the studies performed so far.
Another important point, recently emerged, is the persistence of these side effects after withdrawal of the therapy. This condition, named as PFS, seems to be present in male patients, with an incidence on the total population that is still unclear. Results obtained in animal models confirm the presence of long-term effects of finasteride or dutasteride treatment on sexual function and mood, such as depression and anxiety. However, also in this case, concerns about PFS existence have been raised. Overall, these data indicate the urgent need to high quality clinical trials, with long-term follow up, specifically addressing sexual function and mood disorders. In addition, it is imperative to study in detail the molecular mechanisms that cause this condition, trying to identify a possible genetic predisposition, in order to limit the burden of PFS. Finally, therapeutic strategies, like for instance neuroactive steroid treatment, able to relief or to cure this condition are also urgently needed.
Funding
This work was supported from MIUR “Progetto Eccellenza”; Post-Finasteride Foundation to RCM and by PRIN [grant n.2017ZFJCS3] from MIUR to SG.
Author contributions section
Silvia Diviccaro: Conceptualization; Writing-original draft; Writing-review & editing. Roberto Cosimo Melcangi: Conceptualization; Writing-original draft; Writing-review & editing; Funding acquisition; Supervision. Silvia Giatti: Conceptualization; Writing-original draft; Writing-review & editing.
Declaration of competing interest
Authors received funding from the Post-Finasteride Foundation in order to study PFS symptomatology of patients and in experimental models.
Acknowledgements
Authors acknowledge P. Motolese for graphical assistance.
References
- Agis-Balboa R.C., Guidotti A., Pinna G. 5alpha-reductase type I expression is downregulated in the prefrontal cortex/Brodmann's area 9 (BA9) of depressed patients. Psychopharmacology (Berlin) 2014;231:3569–3580. doi: 10.1007/s00213-014-3567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkahtane A.A., Albasher G., Al-Sultan N.K., Alqahtani W.S., Alarifi S., Almeer R.S., Alghamdi J., Ali D., Alahmari A., Alkahtani S. Long-term treatment with finasteride induces apoptosis and pathological changes in female mice. Hum. Exp. Toxicol. 2019;38:762–774. doi: 10.1177/0960327119842195. [DOI] [PubMed] [Google Scholar]
- Baas W.R., Butcher M.J., Lwin A., Holland B., Herberts M., Clemons J., Delfino K., Althof S., Kohler T.S., McVary K.T. A review of the FAERS data on 5-alpha reductase inhibitors: implications for postfinasteride syndrome. Urology. 2018;120:143–149. doi: 10.1016/j.urology.2018.06.022. [DOI] [PubMed] [Google Scholar]
- Baig M.S., Kolasa-Wolosiuk A., Pilutin A., Safranow K., Baranowska-Bosiacka I., Kabat-Koperska J., Wiszniewska B. Finasteride-induced inhibition of 5alpha-reductase type 2 could lead to kidney damage-animal, experimental study. Int. J. Environ. Res. Public Health. 2019;16 doi: 10.3390/ijerph16101726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basaria S., Jasuja R., Huang G., Wharton W., Pan H., Pencina K., Li Z., Travison T.G., Bhawan J., Gonthier R., Labrie F., Dury A.Y., Serra C., Papazian A., O'Leary M., Amr S., Storer T.W., Stern E., Bhasin S. Characteristics of men who report persistent sexual symptoms after finasteride use for hair loss. J. Clin. Endocrinol. Metab. 2016;101:4669–4680. doi: 10.1210/jc.2016-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap S.M., Aslam I., Kiguradze T., Temps W.H., Yarnold P.R., Cashy J., Brannigan R.E., Micali G., Nardone B., West D.P. Adverse event reporting in clinical trials of finasteride for androgenic alopecia: a meta-analysis. JAMA Dermatol. 2015;151:600–606. doi: 10.1001/jamadermatol.2015.36. [DOI] [PubMed] [Google Scholar]
- Belujon P., Grace A.A. Dopamine system dysregulation in major depressive disorders. Int. J. Neuropsychopharmacol. 2017;20:1036–1046. doi: 10.1093/ijnp/pyx056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma I.H., Oranje A.P., Grimalt R., Iorizzo M., Piraccini B.M., Verdonschot E.H. The effectiveness of finasteride and dutasteride used for 3 years in women with androgenetic alopecia. Indian J. Dermatol. Venereol. Leprol. 2014;80:521–525. doi: 10.4103/0378-6323.144162. [DOI] [PubMed] [Google Scholar]
- Bradshaw W.G., Baum M.J., Awh C.C. Attenuation by a 5 alpha-reductase inhibitor of the activational effect of testosterone propionate on penile erections in castrated male rats. Endocrinology. 1981;109:1047–1051. doi: 10.1210/endo-109-4-1047. [DOI] [PubMed] [Google Scholar]
- Bruskewitz R., Girman C.J., Fowler J., Rigby O.F., Sullivan M., Bracken R.B., Fusilier H.A., Kozlowski D., Kantor S.D., Johnson E.L., Wang D.Z., Waldstreicher J. Effect of finasteride on bother and other health-related quality of life aspects associated with benign prostatic hyperplasia. PLESS Study Group. Proscar Long-term Efficacy and Safety Study. Urology. 1999;54:670–678. doi: 10.1016/s0090-4295(99)00209-5. [DOI] [PubMed] [Google Scholar]
- Camacho-Martinez F.M. Hair loss in women. Semin. Cutan. Med. Surg. 2009;28:19–32. doi: 10.1016/j.sder.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Caruso D., Abbiati F., Giatti S., Romano S., Fusco L., Cavaletti G., Melcangi R.C. Patients treated for male pattern hair with finasteride show, after discontinuation of the drug, altered levels of neuroactive steroids in cerebrospinal fluid and plasma. J. Steroid Biochem. Mol. Biol. 2015;146:74–79. doi: 10.1016/j.jsbmb.2014.03.012. S0960-0760(14)00083-1 [pii] [DOI] [PubMed] [Google Scholar]
- Caruso D., Pesaresi M., Abbiati F., Calabrese D., Giatti S., Garcia-Segura L.M., Melcangi R.C. Comparison of plasma and cerebrospinal fluid levels of neuroactive steroids with their brain, spinal cord and peripheral nerve levels in male and female rats. Psychoneuroendocrinology. 2013;38:2278–2290. doi: 10.1016/j.psyneuen.2013.04.016. S0306-4530(13)00154-6 [pii] [DOI] [PubMed] [Google Scholar]
- Cauci S., Chiriaco G., Cecchin E., Toffoli G., Xodo S., Stinco G., Trombetta C. Androgen receptor (AR) gene (CAG)n and (GGN)n length polymorphisms and symptoms in young males with long-lasting adverse effects after finasteride use against androgenic alopecia. Sex. Med. 2017;5:e61–e71. doi: 10.1016/j.esxm.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchin E., De Mattia E., Mazzon G., Cauci S., Trombetta C., Toffoli G. A pharmacogenetic survey of androgen receptor (CAG)n and (GGN)n polymorphisms in patients experiencing long term side effects after finasteride discontinuation. Int. J. Biol. Mark. 2014;29:e310–316. doi: 10.5301/jbm.5000095. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Desse S., Martinez A., Worthen R.J., Jope R.S., Beurel E. TNFalpha disrupts blood brain barrier integrity to maintain prolonged depressive-like behavior in mice. Brain Behav. Immun. 2018;69:556–567. doi: 10.1016/j.bbi.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi G.S., Kim J.H., Oh S.Y., Park J.M., Hong J.S., Lee Y.S., Lee W.S. Safety and tolerability of the dual 5-alpha reductase inhibitor dutasteride in the treatment of androgenetic alopecia. Ann. Dermatol. 2016;28:444–450. doi: 10.5021/ad.2016.28.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cima I., Corazza N., Dick B., Fuhrer A., Herren S., Jakob S., Ayuni E., Mueller C., Brunner T. Intestinal epithelial cells synthesize glucocorticoids and regulate T cell activation. J. Exp. Med. 2004;200:1635–1646. doi: 10.1084/jem.20031958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R.V., Hermann D.J., Cunningham G.R., Wilson T.H., Morrill B.B., Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J. Clin. Endocrinol. Metab. 2004;89:2179–2184. doi: 10.1210/jc.2003-030330. [DOI] [PubMed] [Google Scholar]
- Da Silva M.H.A., Costa W.S., FJ B.S., De Souza D.B. The corpus cavernosum after treatment with dutasteride or finasteride: a histomorphometric study in a benign prostatic hyperplasia rodent model. Asian J. Androl. 2018;20:505–510. doi: 10.4103/aja.aja_28_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza Silva M.A., Mattern C., Topic B., Buddenberg T.E., Huston J.P. Dopaminergic and serotonergic activity in neostriatum and nucleus accumbens enhanced by intranasal administration of testosterone. Eur. Neuropsychopharmacol. 2009;19:53–63. doi: 10.1016/j.euroneuro.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Desgrandchamps F., Droupy S., Irani J., Saussine C., Comenducci A. Effect of dutasteride on the symptoms of benign prostatic hyperplasia, and patient quality of life and discomfort, in clinical practice. BJU Int. 2006;98:83–88. doi: 10.1111/j.1464-410X.2006.06241.x. [DOI] [PubMed] [Google Scholar]
- Devoto P., Frau R., Bini V., Pillolla G., Saba P., Flore G., Corona M., Marrosu F., Bortolato M. Inhibition of 5alpha-reductase in the nucleus accumbens counters sensorimotor gating deficits induced by dopaminergic activation. Psychoneuroendocrinology. 2012;37:1630–1645. doi: 10.1016/j.psyneuen.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Loreto C., La Marra F., Mazzon G., Belgrano E., Trombetta C., Cauci S. Immunohistochemical evaluation of androgen receptor and nerve structure density in human prepuce from patients with persistent sexual side effects after finasteride use for androgenetic alopecia. PLoS One. 2014;9 doi: 10.1371/journal.pone.0100237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diviccaro S., Giatti S., Borgo F., Barcella M., Borghi E., Trejo J.L., Garcia-Segura L.M., Melcangi R.C. Treatment of male rats with finasteride, an inhibitor of 5alpha-reductase enzyme, induces long-lasting effects on depressive-like behavior, hippocampal neurogenesis, neuroinflammation and gut microbiota composition. Psychoneuroendocrinology. 2019;99:206–215. doi: 10.1016/j.psyneuen.2018.09.021. [DOI] [PubMed] [Google Scholar]
- Duskova M., Hill M., Starka L. Changes of metabolic profile in men treated for androgenetic alopecia with 1 mg finasteride. Endocr. Regul. 2010;44:3–8. doi: 10.4149/endo_2010_01_3. [DOI] [PubMed] [Google Scholar]
- Edwards J.E., Moore R.A. Finasteride in the treatment of clinical benign prostatic hyperplasia: a systematic review of randomised trials. BMC Urol. 2002;2:14. doi: 10.1186/1471-2490-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger J.C. The role of dopamine in inflammation-associated depression: mechanisms and therapeutic implications. Curr Top Behav Neurosci. 2017;31:199–219. doi: 10.1007/7854_2016_13. [DOI] [PubMed] [Google Scholar]
- Fields C.T., Chassaing B., Paul M.J., Gewirtz A.T., de Vries G.J. Vasopressin deletion is associated with sex-specific shifts in the gut microbiome. Gut Microb. 2017;1:13. doi: 10.1080/19490976.2017.1356557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn D.A., Beadles-Bohling A.S., Beckley E.H., Ford M.M., Gililland K.R., Gorin-Meyer R.E., Wiren K.M. A new look at the 5alpha-reductase inhibitor finasteride. CNS Drug Rev. 2006;12:53–76. doi: 10.1111/j.1527-3458.2006.00053.x. CNS53 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frau R., Mosher L.J., Bini V., Pillolla G., Pes R., Saba P., Fanni S., Devoto P., Bortolato M. The neurosteroidogenic enzyme 5alpha-reductase modulates the role of D1 dopamine receptors in rat sensorimotor gating. Psychoneuroendocrinology. 2016;63:59–67. doi: 10.1016/j.psyneuen.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye S.V., Bramson H.N., Hermann D.J., Lee F.W., Sinhababu A.K., Tian G. Discovery and development of GG745, a potent inhibitor of both isozymes of 5 alpha-reductase. Pharm. Biotechnol. 1998;11:393–422. doi: 10.1007/0-306-47384-4_17. [DOI] [PubMed] [Google Scholar]
- Fwu C.W., Eggers P.W., Kirkali Z., McVary K.T., Burrows P.K., Kusek J.W. Change in sexual function in men with lower urinary tract symptoms/benign prostatic hyperplasia associated with long-term treatment with doxazosin, finasteride and combined therapy. J. Urol. 2014;191:1828–1834. doi: 10.1016/j.juro.2013.12.014. [DOI] [PubMed] [Google Scholar]
- Ganzer C.A., Jacobs A.R. Emotional consequences of finasteride: fool's gold. Am. J. Men's Health. 2016 doi: 10.1177/1557988316631624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzer C.A., Jacobs A.R., Iqbal F. Persistent sexual, emotional, and cognitive impairment post-finasteride: a survey of men reporting symptoms. Am. J. Men's Health. 2015;9:222–228. doi: 10.1177/1557988314538445. 1557988314538445 [pii] [DOI] [PubMed] [Google Scholar]
- Giatti S., Diviccaro S., Panzica G., Melcangi R.C. Post-finasteride syndrome and post-SSRI sexual dysfunction: two sides of the same coin? Endocrine. 2018;2:180–193. doi: 10.1007/s12020-018-1593-5. [DOI] [PubMed] [Google Scholar]
- Giatti S., Foglio B., Romano S., Pesaresi M., Panzica G., Garcia-Segura L.M., Caruso D., Melcangi R.C. Effects of subchronic finasteride treatment and withdrawal on neuroactive steroid levels and their receptors in the male rat brain. Neuroendocrinology. 2016;103:746–757. doi: 10.1159/000442982. 000442982 [pii] [DOI] [PubMed] [Google Scholar]
- Giatti S., Garcia-Segura L.M., Barreto G.E., Melcangi R.C. Neuroactive steroids, neurosteroidogenesis and sex. Prog. Neurobiol. 2019;176:1–17. doi: 10.1016/j.pneurobio.2018.06.007. [DOI] [PubMed] [Google Scholar]
- Grace A.A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 2016;17:524–532. doi: 10.1038/nrn.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A.K., Carviel J., Gupta M.A., Shear N.H. Assessing dutasteride-associated sexual dysfunction using the U.S. Food and drug administration adverse event reporting system. J. Eur. Acad. Dermatol. Venereol. 2018;32:1373–1376. doi: 10.1111/jdv.14728. [DOI] [PubMed] [Google Scholar]
- Gupta A.K., Carviel J., MacLeod M.A., Shear N. Assessing finasteride-associated sexual dysfunction using the FAERS database. J. Eur. Acad. Dermatol. Venereol. 2017;31:1069–1075. doi: 10.1111/jdv.14223. [DOI] [PubMed] [Google Scholar]
- Haber R.S., Gupta A.K., Epstein E., Carviel J.L., Foley K.A. Finasteride for androgenetic alopecia is not associated with sexual dysfunction: a survey-based, single-centre, controlled study. J. Eur. Acad. Dermatol. Venereol. 2019;33:1393–1397. doi: 10.1111/jdv.15548. [DOI] [PubMed] [Google Scholar]
- Harada N., Hanaoka R., Hanada K., Izawa T., Inui H., Yamaji R. Hypogonadism alters cecal and fecal microbiota in male mice. Gut Microb. 2016;7:533–539. doi: 10.1080/19490976.2016.1239680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy D., Le Noury J., Mangin D. Enduring sexual dysfunction after treatment with antidepressants, 5alpha-reductase inhibitors and isotretinoin: 300 cases. Int. J. Risk Saf. Med. 2018;29:125–134. doi: 10.3233/JRS-180744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercher C., Turecki G., Mechawar N. Through the looking glass: examining neuroanatomical evidence for cellular alterations in major depression. J. Psychiatr. Res. 2009;43:947–961. doi: 10.1016/j.jpsychires.2009.01.006. S0022-3956(09)00016-8 [pii] [DOI] [PubMed] [Google Scholar]
- Hostenbach S., Cambron M., D'Haeseleer M., Kooijman R., De Keyser J. Astrocyte loss and astrogliosis in neuroinflammatory disorders. Neurosci. Lett. 2014;565:39–41. doi: 10.1016/j.neulet.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Hsieh J.T., Chen S.C., Yu H.J., Chang H.C. Finasteride upregulates expression of androgen receptor in hyperplastic prostate and LNCaP cells: implications for chemoprevention of prostate cancer. The Prostate. 2011;71:1115–1121. doi: 10.1002/pros.21325. [DOI] [PubMed] [Google Scholar]
- Hu A.C., Chapman L.W., Mesinkovska N.A. The efficacy and use of finasteride in women: a systematic review. Int. J. Dermatol. 2019;58:759–776. doi: 10.1111/ijd.14370. [DOI] [PubMed] [Google Scholar]
- Irwig M.S. Depressive symptoms and suicidal thoughts among former users of finasteride with persistent sexual side effects. J. Clin. Psychiatry. 2012;73:1220–1223. doi: 10.4088/JCP.12m07887. [DOI] [PubMed] [Google Scholar]
- Irwig M.S. Persistent sexual side effects of finasteride: could they be permanent? J. Sex. Med. 2012;9:2927–2932. doi: 10.1111/j.1743-6109.2012.02846.x. [DOI] [PubMed] [Google Scholar]
- Irwig M.S. Decreased alcohol consumption among former male users of finasteride with persistent sexual side effects: a preliminary report. Alcohol Clin. Exp. Res. 2013;37:1823–1826. doi: 10.1111/acer.12177. [DOI] [PubMed] [Google Scholar]
- Irwig M.S., Kolukula S. Persistent sexual side effects of finasteride for male pattern hair loss. J. Sex. Med. 2011;8:1747–1753. doi: 10.1111/j.1743-6109.2011.02255.x. [DOI] [PubMed] [Google Scholar]
- Jasarevic E., Morrison K.E., Bale T.L. Sex differences in the gut microbiome-brain axis across the lifespan. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016;371:20150122. doi: 10.1098/rstb.2015.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Ling Z., Zhang Y., Mao H., Ma Z., Yin Y., Wang W., Tang W., Tan Z., Shi J., Li L., Ruan B. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Kaplan S.A., Chung D.E., Lee R.K., Scofield S., Te A.E. A 5-year retrospective analysis of 5alpha-reductase inhibitors in men with benign prostatic hyperplasia: finasteride has comparable urinary symptom efficacy and prostate volume reduction, but less sexual side effects and breast complications than dutasteride. Int. J. Clin. Pract. 2012;66:1052–1055. doi: 10.1111/j.1742-1241.2012.03010.x. [DOI] [PubMed] [Google Scholar]
- Kaufman K.D., Olsen E.A., Whiting D., Savin R., DeVillez R., Bergfeld W., Price V.H., Van Neste D., Roberts J.L., Hordinsky M., Shapiro J., Binkowitz B., Gormley G.J. Finasteride in the treatment of men with androgenetic alopecia. Finasteride male pattern hair loss study group. J. Am. Acad. Dermatol. 1998;39:578–589. doi: 10.1016/s0190-9622(98)70007-6. S0190-9622(98)70007-6 [pii] [DOI] [PubMed] [Google Scholar]
- Kim W.J., Song M., Ko H.C., Kim B.S., Kim M.B. Efficacy of finasteride 1.25 mg on female pattern hair loss; pilot study. Ann. Dermatol. 2012;24:370–372. doi: 10.5021/ad.2012.24.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindlundh A.M., Rahman S., Lindblom J., Nyberg F. Increased dopamine transporter density in the male rat brain following chronic nandrolone decanoate administration. Neurosci. Lett. 2004;356:131–134. doi: 10.1016/j.neulet.2003.11.040. [DOI] [PubMed] [Google Scholar]
- Kumar S., Porcu P., Werner D.F., Matthews D.B., Diaz-Granados J.L., Helfand R.S., Morrow A.L. The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berlin) 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J.J., Belelli D., Peden D.R., Vardy A.W., Peters J.A. Neurosteroid modulation of GABAA receptors. Prog. Neurobiol. 2003;71:67–80. doi: 10.1016/j.pneurobio.2003.09.001. S0301008203001539 [pii] [DOI] [PubMed] [Google Scholar]
- Lerner A., Neidhofer S., Matthias T. The gut microbiome feelings of the brain: a perspective for non-microbiologists. Microorganisms. 2017;5 doi: 10.3390/microorganisms5040066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Kang Y.X., Ji X.M., Li Y.K., Li S.C., Zhang X.J., Cui H.X., Shi G.M. Finasteride inhibited brain dopaminergic system and open-field behaviors in adolescent male rats. CNS Neurosci. Ther. 2018;24:115–125. doi: 10.1111/cns.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Li C., Yu H., Cai X., Shen X., Sun X., Wang J., Zhang Y., Wang C. Lentivirus-mediated interleukin-1beta (IL-1beta) knock-down in the hippocampus alleviates lipopolysaccharide (LPS)-induced memory deficits and anxiety- and depression-like behaviors in mice. J. Neuroinflammation. 2017;14:190. doi: 10.1186/s12974-017-0964-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P., Ding B., Feng C., Yin S., Zhang T., Qi X., Lv H., Guo X., Dong K., Zhu Y., Li Q. Prevotella and Klebsiella proportions in fecal microbial communities are potential characteristic parameters for patients with major depressive disorder. J. Affect. Disord. 2017;207:300–304. doi: 10.1016/j.jad.2016.09.051. [DOI] [PubMed] [Google Scholar]
- Liu L., Zhao S., Li F., Li E., Kang R., Luo L., Luo J., Wan S., Zhao Z. Effect of 5alpha-reductase inhibitors on sexual function: a meta-analysis and systematic review of randomized controlled trials. J. Sex. Med. 2016;13:1297–1310. doi: 10.1016/j.jsxm.2016.07.006. [DOI] [PubMed] [Google Scholar]
- Marberger M.J. Long-term effects of finasteride in patients with benign prostatic hyperplasia: a double-blind, placebo-controlled, multicenter study. PROWESS Study Group. Urology. 1998;51:677–686. doi: 10.1016/s0090-4295(98)00094-6. [DOI] [PubMed] [Google Scholar]
- Mayer E.A. Gut feelings: the emerging biology of gut-brain communication. Nat. Rev. Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcangi R.C., Caruso D., Abbiati F., Giatti S., Calabrese D., Piazza F., Cavaletti G. Neuroactive steroid levels are modified in cerebrospinal fluid and plasma of post-finasteride patients showing persistent sexual side effects and anxious/depressive symptomatology. J. Sex. Med. 2013;10:2598–2603. doi: 10.1111/jsm.12269. [DOI] [PubMed] [Google Scholar]
- Melcangi R.C., Casarini L., Marino M., Santi D., Sperduti S., Giatti S., Diviccaro S., Grimoldi M., Caruso D., Cavaletti G., Simoni M. Altered methylation pattern of the SRD5A2 gene in the cerebrospinal fluid of post-finasteride patients: a pilot study. Endocr Connect. 2019;8:1118–1125. doi: 10.1530/EC-19-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcangi R.C., Giatti S., Calabrese D., Pesaresi M., Cermenati G., Mitro N., Viviani B., Garcia-Segura L.M., Caruso D. Levels and actions of progesterone and its metabolites in the nervous system during physiological and pathological conditions. Prog. Neurobiol. 2014;113:56–69. doi: 10.1016/j.pneurobio.2013.07.006. S0301-0082(13)00068-3 [pii] [DOI] [PubMed] [Google Scholar]
- Melcangi R.C., Giatti S., Garcia-Segura L.M. Levels and actions of neuroactive steroids in the nervous system under physiological and pathological conditions: sex-specific features. Neurosci. Biobehav. Rev. 2016;67:25–40. doi: 10.1016/j.neubiorev.2015.09.023. S0149-7634(15)30099-3 [pii] [DOI] [PubMed] [Google Scholar]
- Melcangi R.C., Riva M.A., Fumagalli F., Magnaghi V., Racagni G., Martini L. Effect of progesterone, testosterone and their 5 alpha-reduced metabolites on GFAP gene expression in type 1 astrocytes. Brain Res. 1996;711:10–15. doi: 10.1016/0006-8993(95)01302-4. [DOI] [PubMed] [Google Scholar]
- Melcangi R.C., Santi D., Spezzano R., Grimoldi M., Tabacchi T., Fusco M.L., Diviccaro S., Giatti S., Carra G., Caruso D., Simoni M., Cavaletti G. Neuroactive steroid levels and psychiatric and andrological features in post-finasteride patients. J. Steroid Biochem. Mol. Biol. 2017;171:229–235. doi: 10.1016/j.jsbmb.2017.04.003. [DOI] [PubMed] [Google Scholar]
- Mervis J.S., Borda L.J., Miteva M. 'Post-finasteride syndrome': what to tell our female patients? Br. J. Dermatol. 2018;179:785–786. doi: 10.1111/bjd.16658. [DOI] [PubMed] [Google Scholar]
- Mitchell J.B., Stewart J. Effects of castration, steroid replacement, and sexual experience on mesolimbic dopamine and sexual behaviors in the male rat. Brain Res. 1989;491:116–127. doi: 10.1016/0006-8993(89)90093-0. [DOI] [PubMed] [Google Scholar]
- Mondaini N., Gontero P., Giubilei G., Lombardi G., Cai T., Gavazzi A., Bartoletti R. Finasteride 5 mg and sexual side effects: how many of these are related to a nocebo phenomenon? J. Sex. Med. 2007;4:1708–1712. doi: 10.1111/j.1743-6109.2007.00563.x. [DOI] [PubMed] [Google Scholar]
- Moreno-Indias I., Sanchez-Alcoholado L., Sanchez-Garrido M.A., Martin-Nunez G.M., Perez-Jimenez F., Tena-Sempere M., Tinahones F.J., Queipo-Ortuno M.I. Neonatal androgen exposure causes persistent gut microbiota dysbiosis related to metabolic disease in adult female rats. Endocrinology. 2016;157:4888–4898. doi: 10.1210/en.2016-1317. [DOI] [PubMed] [Google Scholar]
- Motofei I.G., Rowland D.L., Georgescu S.R., Baconi D.L., Dimcevici N.P., Paunica S., Constantin V.D., Balalau C. A pilot study on the sexual side effects of finasteride as related to hand preference for men undergoing treatment of male pattern baldness. BJU Int. 2013;111:E221–226. doi: 10.1111/j.1464-410X.2012.11580.x. [DOI] [PubMed] [Google Scholar]
- Motofei I.G., Rowland D.L., Georgescu S.R., Tampa M., Baconi D., Stefanescu E., Baleanu B.C., Balalau C., Constantin V., Paunica S. Finasteride adverse effects in subjects with androgenic alopecia: a possible therapeutic approach according to the lateralization process of the brain. J. Dermatol. Treat. 2016:1–3. doi: 10.3109/09546634.2016.1161155. [DOI] [PubMed] [Google Scholar]
- Motofei I.G., Rowland D.L., Manea M., Georgescu S.R., Paunica I., Sinescu I. Safety profile of finasteride: distribution of adverse effects according to structural and informational dichotomies of the mind/brain. Clin. Drug Investig. 2017;37:511–517. doi: 10.3109/09546634.2016.1161155. [DOI] [PubMed] [Google Scholar]
- Narasimhalu C.R. Randomized questionnaire based case-control research study on evaluation of sexual function in Indian patients taking oral finasteride for androgenetic alopecia. Dermatol. Ther. 2015;5:231–234. doi: 10.1007/s13555-015-0084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseribafrouei A., Hestad K., Avershina E., Sekelja M., Linlokken A., Wilson R., Rudi K. Correlation between the human fecal microbiota and depression. Neuro Gastroenterol. Motil. 2014;26:1155–1162. doi: 10.1111/nmo.12378. [DOI] [PubMed] [Google Scholar]
- Nickel J.C., Fradet Y., Boake R.C., Pommerville P.J., Perreault J.P., Afridi S.K., Elhilali M.M. Efficacy and safety of finasteride therapy for benign prostatic hyperplasia: results of a 2-year randomized controlled trial (the PROSPECT study). PROscar Safety Plus Efficacy Canadian Two year Study. CMAJ (Can. Med. Assoc. J.) 1996;155:1251–1259. [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Soares R., JM E.S., Correia M.P., Andre M.C. Finasteride 5 mg/day treatment of patterned hair loss in normo-androgenetic postmenopausal women. Int. J. Trichol. 2013;5:22–25. doi: 10.4103/0974-7753.114709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Org E., Mehrabian M., Parks B.W., Shipkova P., Liu X., Drake T.A., Lusis A.J. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microb. 2016;7:313–322. doi: 10.1080/19490976.2016.1203502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K.H., Kim S.W., Kim K.D., Paick J.S. Effects of androgens on the expression of nitric oxide synthase mRNAs in rat corpus cavernosum. BJU Int. 1999;83:327–333. doi: 10.1046/j.1464-410x.1999.00913.x. [DOI] [PubMed] [Google Scholar]
- Peeters M., Giuliano F. Central neurophysiology and dopaminergic control of ejaculation. Neurosci. Biobehav. Rev. 2008;32:438–453. doi: 10.1016/j.neubiorev.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Pinsky M.R., Gur S., Tracey A.J., Harbin A., Hellstrom W.J. The effects of chronic 5-alpha-reductase inhibitor (dutasteride) treatment on rat erectile function. J. Sex. Med. 2011;8:3066–3074. doi: 10.1111/j.1743-6109.2011.02425.x. [DOI] [PubMed] [Google Scholar]
- Price V.H., Roberts J.L., Hordinsky M., Olsen E.A., Savin R., Bergfeld W., Fiedler V., Lucky A., Whiting D.A., Pappas F., Culbertson J., Kotey P., Meehan A., Waldstreicher J. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J. Am. Acad. Dermatol. 2000;43:768–776. doi: 10.1067/mjd.2000.107953. [DOI] [PubMed] [Google Scholar]
- Ridlon J.M., Ikegawa S., Alves J.M., Zhou B., Kobayashi A., Iida T., Mitamura K., Tanabe G., Serrano M., De Guzman A., Cooper P., Buck G.A., Hylemon P.B. Clostridium scindens: a human gut microbe with a high potential to convert glucocorticoids into androgens. J. Lipid Res. 2013;54:2437–2449. doi: 10.1194/jlr.M038869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrborn C.G., Boyle P., Nickel J.C., Hoefner K., Andriole G., Aria A., Investigators A.S. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology. 2002;60:434–441. doi: 10.1016/s0090-4295(02)01905-2. [DOI] [PubMed] [Google Scholar]
- Romer B., Gass P. Finasteride-induced depression: new insights into possible pathomechanisms. J. Cosmet. Dermatol. 2010;9:331–332. doi: 10.1111/j.1473-2165.2010.00533.x. [DOI] [PubMed] [Google Scholar]
- Romer B., Pfeiffer N., Lewicka S., Ben-Abdallah N., Vogt M.A., Deuschle M., Vollmayr B., Gass P. Finasteride treatment inhibits adult hippocampal neurogenesis in male mice. Pharmacopsychiatry. 2010;43:174–178. doi: 10.1055/s-0030-1249095. [DOI] [PubMed] [Google Scholar]
- Santarelli L., Saxe M., Gross C., Surget A., Battaglia F., Dulawa S., Weisstaub N., Lee J., Duman R., Arancio O., Belzung C., Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sasibhushana R.B., Shankaranarayana Rao B.S., Srikumar B.N. Repeated finasteride administration induces depression-like behavior in adult male rats. Behav. Brain Res. 2019;365:185–189. doi: 10.1016/j.bbr.2019.03.006. [DOI] [PubMed] [Google Scholar]
- Seale L.R., Eglini A.N., McMichael A.J. Side effects related to 5 alpha-reductase inhibitor treatment of hair loss in women: a review. J. Drugs Dermatol. JDD. 2016;15:414–419. [PubMed] [Google Scholar]
- Sharon G., Sampson T.R., Geschwind D.H., Mazmanian S.K. The central nervous system and the gut microbiome. Cell. 2016;167:915–932. doi: 10.1016/j.cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum K.W., Cullen D.R., Messenger A.G. Hair loss in women with hyperandrogenism: four cases responding to finasteride. J. Am. Acad. Dermatol. 2002;47:733–739. doi: 10.1067/mjd.2002.124608. [DOI] [PubMed] [Google Scholar]
- Snyder J.S., Soumier A., Brewer M., Pickel J., Cameron H.A. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmeier C.A., Mahajan G.J., Konick L.C., Overholser J.C., Jurjus G.J., Meltzer H.Y., Uylings H.B., Friedman L., Rajkowska G. Cellular changes in the postmortem hippocampus in major depression. Biol. Psychiatry. 2004;56:640–650. doi: 10.1016/j.biopsych.2004.08.022. S0006-3223(04)00936-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H.H., Yu J., Kang S.J., Chae M.R., So I., Park J.K., Lee S.W. Persistent erectile dysfunction after discontinuation of 5-alpha reductase inhibitor therapy in rats depending on the duration of treatment. World J Mens Health. 2019;37:240–248. doi: 10.5534/wjmh.180082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetel M.J., de Vries G.J., Melcangi R.C., Panzica G., O'Mahony S.M. Steroids, stress and the gut microbiome-brain axis. J. Neuroendocrinol. 2018;30 doi: 10.1111/jne.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai K.E., Sinclair R.D. Finasteride for female androgenetic alopecia. Br. J. Dermatol. 2002;147:812–813. doi: 10.1046/j.1365-2133.2002.49084.x. [DOI] [PubMed] [Google Scholar]
- Todorovic N., Filipovic D. The antidepressant- and anxiolytic-like effects of fluoxetine and clozapine in chronically isolated rats involve inhibition of hippocampal TNF-alpha. Pharmacol. Biochem. Behav. 2017;163:57–65. doi: 10.1016/j.pbb.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Tosti A., Pazzaglia M., Soli M., Rossi A., Rebora A., Atzori L., Barbareschi M., Benci M., Voudouris S., Vena G.A. Evaluation of sexual function with an international index of erectile function in subjects taking finasteride for androgenetic alopecia. Arch. Dermatol. 2004;140:857–858. doi: 10.1001/archderm.140.7.857. [DOI] [PubMed] [Google Scholar]
- Tosti A., Piraccini B.M., Soli M. Evaluation of sexual function in subjects taking finasteride for the treatment of androgenetic alopecia. J. Eur. Acad. Dermatol. Venereol. 2001;15:418–421. doi: 10.1046/j.1468-3083.2001.00315.x. [DOI] [PubMed] [Google Scholar]
- Traish A.M., Melcangi R.C., Bortolato M., Garcia-Segura L.M., Zitzmann M. Adverse effects of 5alpha-reductase inhibitors: what do we know, don't know, and need to know? Rev. Endocr. Metab. Disord. 2015;16:177–198. doi: 10.1007/s11154-015-9319-y. [DOI] [PubMed] [Google Scholar]
- Tsunemi Y., Irisawa R., Yoshiie H., Brotherton B., Ito H., Tsuboi R., Kawashima M., Manyak M., Group A.R.I.S. Long-term safety and efficacy of dutasteride in the treatment of male patients with androgenetic alopecia. J. Dermatol. 2016;43:1051–1058. doi: 10.1111/1346-8138.13310. [DOI] [PubMed] [Google Scholar]
- Valsecchi R., Leghissa P., Riva M. Female androgenetic alopecia treated by finasteride: a case forward. Acta Derm. Venereol. 2004;84:488–489. [PubMed] [Google Scholar]
- van Zuuren E.J., Fedorowicz Z., Schoones J. Interventions for female pattern hair loss. Cochrane Database Syst. Rev. 2016:CD007628. doi: 10.1002/14651858.CD007628.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varothai S., Bergfeld W.F. Androgenetic alopecia: an evidence-based treatment update. Am. J. Clin. Dermatol. 2014;15:217–230. doi: 10.1007/s40257-014-0077-5. [DOI] [PubMed] [Google Scholar]
- Walf A.A., Kaurejo S., Frye C.A. Research brief: self-reports of a constellation of persistent antiandrogenic, estrogenic, physical, and psychological effects of finasteride usage among men. Am. J. Men's Health. 2018;12:900–906. doi: 10.1177/1557988317750989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.L., Han Q.Q., Gong W.Q., Pan D.H., Wang L.Z., Hu W., Yang M., Li B., Yu J., Liu Q. Microglial activation mediates chronic mild stress-induced depressive- and anxiety-like behavior in adult rats. J. Neuroinflammation. 2018;15:21. doi: 10.1186/s12974-018-1054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will R.G., Hull E.M., Dominguez J.M. Influences of dopamine and glutamate in the medial preoptic area on male sexual behavior. Pharmacol. Biochem. Behav. 2014;121:115–123. doi: 10.1016/j.pbb.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Wilton L., Pearce G., Edet E., Freemantle S., Stephens M.D., Mann R.D. The safety of finasteride used in benign prostatic hypertrophy: a non-interventional observational cohort study in 14,772 patients. Br. J. Urol. 1996;78:379–384. doi: 10.1046/j.1464-410x.1996.00091.x. [DOI] [PubMed] [Google Scholar]
- Won Y.Y., Lew B.L., Sim W.Y. Clinical efficacy of oral administration of finasteride at a dose of 2.5 mg/day in women with female pattern hair loss. Dermatol. Ther. 2018;31 doi: 10.1111/dth.12588. [DOI] [PubMed] [Google Scholar]
- Wu M., Yu Q., Li Q. Differences in reproductive toxicology between alopecia drugs: an analysis on adverse events among female and male cases. Oncotarget. 2016;7:82074–82084. doi: 10.18632/oncotarget.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeon J.H., Jung J.Y., Choi J.W., Kim B.J., Youn S.W., Park K.C., Huh C.H. 5 mg/day finasteride treatment for normoandrogenic Asian women with female pattern hair loss. J. Eur. Acad. Dermatol. Venereol. 2011;25:211–214. doi: 10.1111/j.1468-3083.2010.03758.x. [DOI] [PubMed] [Google Scholar]
- Yirmiya R., Rimmerman N., Reshef R. Depression as a microglial disease. Trends Neurosci. 2015;38:637–658. doi: 10.1016/j.tins.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Yu M., Jia H., Zhou C., Yang Y., Zhao Y., Yang M., Zou Z. Variations in gut microbiota and fecal metabolic phenotype associated with depression by 16S rRNA gene sequencing and LC/MS-based metabolomics. J. Pharm. Biomed. Anal. 2017;138:231–239. doi: 10.1016/j.jpba.2017.02.008. [DOI] [PubMed] [Google Scholar]
- Yurkovetskiy L., Burrows M., Khan A.A., Graham L., Volchkov P., Becker L., Antonopoulos D., Umesaki Y., Chervonsky A.V. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39:400–412. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M.G., Wu W., Zhang C.M., Wang X.J., Gao P.J., Lu Y.L., Shen Z.J. Effects of oral finasteride on erectile function in a rat model. J. Sex. Med. 2012;9:1328–1336. doi: 10.1111/j.1743-6109.2012.02661.x. [DOI] [PubMed] [Google Scholar]
- Zorumski C.F., Paul S.M., Izumi Y., Covey D.F., Mennerick S. Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci. Biobehav. Rev. 2013;37:109–122. doi: 10.1016/j.neubiorev.2012.10.005. S0149-7634(12)00172-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]