Abstract
Avoidance behavior is a hallmark in pathological anxiety disorders and results in impairment of daily activities. Individual differences in avoidance responses are critical in determining vulnerability or resistance to anxiety disorders. Dopaminergic activation is implicated in the processing of avoidance responses; however, the mechanisms underlying these responses are unknown. In this sense, we used a preclinical model of avoidance behavior to investigate the possibility of an intrinsic differential dopaminergic pattern between good and poor performers. The specific goal was to assess the participation of dopamine (DA) through pharmacological manipulation, and we further evaluated the effects of systemic injections of the dopaminergic receptor type 1 (D1 antagonist - SCH23390) and dopaminergic receptor type 2 (D2 antagonist - sulpiride) antagonists in the good performers. Additionally, we evaluated the effects of intra-amygdala microinjection of a D1 antagonist (SCH23390) and a D2 antagonist (sulpiride) in good performers as well as intra-amygdala microinjection of a D1 agonist (SKF38393) and D2 agonist (quinpirole) in poor performers. Furthermore, we quantified the contents of dopamine and metabolites (3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA)) in the amygdala, evaluated the basal levels of tyrosine hydroxylase expression (catecholamine synthesis enzyme) and measured the volume of the substantia nigra, ventral tegmental area and locus coeruleus. Our results showed that it could be possible to convert animals from good to poor performers, and vice versa, by intra-amygdala (basolateral and central nucleus) injections of D1 receptor antagonists in good performers or D2 receptor agonists in poor performers. Additionally, the good performers had lower levels of DOPAC and HVA in the amygdala, an increase in the total volume of the amygdala (AMG), substantia nigra (SN), ventral tegmental area (VTA) and locus coeruleus (LC), and an increase in the number of tyrosine hydroxylase-positive cells in SN, VTA and LC, which positively correlates with the avoidance behavior. Taken together, our data show evidence for a dopaminergic signature of avoidance performers, emphasizing the role of distinct dopaminergic receptors in individual differences in avoidance behavior based on pharmacological, immunohistochemical, neurochemical and volumetric analyses. Our findings provide a better understanding of the role of the dopaminergic system in the execution of avoidance behavior.
Keywords: Anxiety disorders, Avoidance, Amygdala, Prefrontal cortex, Dopamine, Aversive learning
Highlights
-
•
The role of dopamine in individual differences in avoidance behavior.
-
•
Dopamine modulates avoidance behavior.
-
•
Dopaminergic evidence of individual difference in avoidance behavior.
-
•
Good and poor avoiders distinction based on dopaminergic signature.
-
•
Dopaminergic signature of avoidance performers: poor versus good avoiders.
1. Introduction
Avoidance behavior consists of the transition from fear reactions to motor actions to avoid a harmful or unpleasant stimulus, increasing the animal's chance of survival in the face of potential damage (Skinner, 1969; Lang et al., 1998). It has been observed in different species, including humans, primates and rodents (LeDoux, 1996; Sheynin et al., 2014). Conversely, the manifestation of avoidance responses in the absence of real potential danger is the central characteristic of several mental disorders (e.g., panic disorder, avoidant personality disorder) (World Health Organization, 2004; Lampe, 2016) and is related to anxiety disorders (e.g., social anxiety disorder and social phobia) (Moitra et al., 2008; American Psychiatric Association, 2013; Bardeen et al., 2014).
Preclinical models of avoidance behavior are fundamental to better understand the course and etiology of the behavior and to provide insights for new human pharmacological treatments (Krypotos et al., 2015). The two-way active avoidance task is performed in a shuttle box that is divided into two compartments by a door, and the rats are trained to exhibit the avoidance behavior by moving from one compartment to another in order to avoid the delivery of the footshocks (Martinez et al., 2013). This is a particularly interesting model because two different subpopulations are distinguished based on the avoidance response, the good (high avoiders) and poor (low avoiders) performers, with higher levels of anxiety displayed by the poor performers (Martinez et al., 2013). Therefore, in the context of post-traumatic stress disorder (PTSD) and other anxiety disorders, the distinction between good and poor performers could be helpful to study the persistent and maladaptive threat responses (Mahan and Ressler, 2012; Galatzer-Levy et al., 2014).
It is suggested that a fronto-limbic-striatal network controls this behavior and that maladaptive responses could be due to a reduction in frontal and limbic activity, especially related to the amygdala and prefrontal cortex (Schlund et al., 2011; Martinez et al., 2013; Ramirez et al., 2015). The central (Ce), basolateral (BLA) and basomedial (BMA) amygdala nuclei are critical for the modulation of avoidance behavior (Choi et al., 2010; Martinez et al., 2013; Ramirez et al., 2015; Jiao et al., 2015). In addition, the close involvement of the ventral tegmental area, nucleus accumbens and habenula to this neurocircuitry points to a possible dopaminergic modulation of avoidance behavior (Carlezon and Thomas, 2009; Darvas et al., 2011; Stamatakis and Stuber, 2012; Fernando et al., 2014; Sanna et al., 2014; 2017). In fact, dopamine (DA)-deficient mice have impaired avoidance behavior, and the restoration of the DA system in the amygdala and striatum is sufficient to restore this behavior (Darvas et al., 2011). In this sense, dopaminergic activation in the ventral tegmental area and substantial nigra has been implicated in processing avoidance response (Rigoli et al., 2016); however, the mechanisms underlying these responses are unknown.
Considering the key role of avoidance in normal and psychiatric status, it is critical to improve our understanding of the mechanisms responsible for modulating avoidance response. To investigate the participation of DA through pharmacological manipulation, we evaluated the effects of systemic injections of a D1 antagonist (SCH23390) and a D2 antagonist (sulpiride) in the good performers. Additionally, we evaluated the effects of intra-amygdala microinjection of dopaminergic receptor type 1 (D1) antagonist (SCH23390) and a dopaminergic receptor type 2 (D2) antagonist (sulpiride) in the good performers and intra-amygdala microinjection of a D1 agonist (SKF38393) and a D2 agonist (quinpirole) in poor performers. Furthermore, to assess the possibility of an intrinsic differential DA pattern between good and poor performers, we quantified DA and its metabolites (3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) in the amygdala, evaluated the basal levels of tyrosine hydroxylase (TH) expression (catecholamine synthesis enzyme) and measured the volumes of the substantia nigra, ventral tegmental area and locus coeruleus.
2. Materials and methods
2.1. Subjects
Male Wistar rats (N = 246) from the animal facility of the University of Sao Paulo were used as subjects. The animals weighed 200–300 g and were housed in polypropylene cages (40 × 34 × 17 cm) in groups of three under a 12:12 dark/light cycle (lights on at 07:00 h), room temperature maintained at 24 °C ± 1 °C, wood shavings and free access to food and water throughout the experiment. Animals were maintained in the animal facility for 7 days before experiments for habituation. The experiments reported in this paper were performed in compliance with the recommendations of the Brazilian Society of Neuroscience and Behavior, which, in turn, are based on the US National Institutes of Health Guide for Care and Use of Laboratory Animals. The study was approved by the Ethics Committee on the Use of Animals at Hospital Sirio-Libanes (CEUA #2013/12) and the Medical School of the University of Sao Paulo (CEP #083/11).
2.2. Apparatus/procedure
2.2.1. Sidman active avoidance
Behavioral training/testing was conducted in two-way shuttle boxes (Insight Equipments, Brazil). Animals were randomly assigned for experimental or box control; experimental animals were submitted to seven 25-min daily training sessions in the two-way shuttle box and tested on the eighth day; control animals received equivalent exposure to the box without the footshock delivered. Shuttling between compartments delayed the delivery of scrambled footshock unconditioned stimulus - US (0.6 mA; 0.5 s) by 30 s (R–S interval). In the absence of shuttling, US delivery occurred every 5 s. The response to stimulus (R–S) interval shuttles comprised avoidance responses and stimulus to stimulus (S–S) interval shuttles comprised escape responses. All shuttles produced 0.3 s feedback stimuli (house light blink). Animals were divided into good and poor performance groups according to the number of avoidance responses exhibited in the last two training sessions. The animals that performed more than 20 avoidance responses were considered good performers, while animals that did not achieve this number were considered poor performers (Lázaro-Muñoz et al., 2010; Martinez et al., 2013; de Oliveira et al., 2016). Freezing was defined as the absence of movement except that required for breathing (Blanchard and Blanchard, 1969) and it was assessed during the first 2 min of the tests based on a previous study (Lázaro-Muñoz et al., 2010). The evaluation was performed by an observer blinded to the group specification.
2.2.2. Open field test
After the test at day 8 on the two-way shuttle boxes, animals were evaluated in the open field for general motor activity. The apparatus consists of a 0.6 m square dark gray Formica surrounded by 50-cm high Formica walls. Each rat was placed in the center of the open field and allowed to explore it freely for 10 min. Sessions were recorded with a video camera for future analysis. After each animal completed the test, the open field was cleaned with 5% ethanol and then dried with a dry cloth.
2.2.3. Dopaminergic drugs
For the pharmacological manipulation of avoidance behavior, the following drugs were used: (see Table 1)
Table 1.
Drugs and doses used for pharmacological DA manipulation.
| Type of Drug | Intraperitoneal Injection (Name/Dose) | Intra-amygdala Injection (Name/Dose) |
|---|---|---|
| D1 agonist | NA | (SKF38393, Tocris) 0.4 μL |
| D1 antagonist | (SCH23390, Sigma-Aldrich) 0.025 mg/kg and 0.05 mg/kg |
(SCH23390, Sigma-Aldrich) 0.3 μL (0.1 μg/0.1 μL) |
| D2 agonist | NA | (Quinpirole, Tocris) 0.1 μL |
| D2 antagonist | (Sulpiride, Sigma-Aldrich) 20 mg/kg and 40 mg/kg |
(Sulpiride, Tocris) 0.2 μL |
| Saline | Sterile Saline Solution (0.9%) 10 mL/kg |
Sterile Saline Solution (0.9%) 0.1–0.4 μL |
Abbreviations: DA: dopaminergic; NA: not applicable; D1: dopamine receptor 1; D2: dopamine receptor 2. Intra-amygdala drug dilution 1:1.
2.2.4. Cannula implantation
Animals were anesthetized with isoflurane (4% induction; 2% maintenance in 100% oxygen) through a face-mask attached to the stereotaxic instrument (David Kopf, Germany). For optimizing analgesia, immediately after hair trichotomy, animals had received local anesthetic (0.1 mL of Lidocaine chlorhydrate with norepinephrine, Cristalia, Brazil) and were injected intraperitoneally with 0.1 mL of morphine sulfate (10 mg/mL, Cristalia, Brazil). The animal's scalp was cleaned with 10% iodopovidone, the skull was exposed and the periosteum was removed. Using a dental drill (LB-100; Beltec - Brazil), bilateral holes were made in the skull for the implantation of the cannula directed to the basolateral (AP: 3.0 mm; ML: 5.1 mm; DV: 7.0 mm) or central (AP: 2.4 mm; ML: 4.0 mm; DV: 7.0 mm) nuclei of the amygdala, following the coordinates described at Paxinos and Watson Atlas (Paxinos and Watson, 2005). The cannulas were fixed with acrylic resin (JET, Brazil) and two screws previously fixed and sealed with a stainless steel wire to protect it against obstruction, which was removed at the time of the microinjection.
After surgery, animals were injected subcutaneously with 0.3 mL broad-spectrum veterinarian Pentabiotic Reinforced (Wyeth, Brazil) and received sodium dipyrone (50 mg/mL, Medley) diluted in sterile injection water (final concentration: 2.5 mg/mL) for three consecutive days. Animals were allowed to recover from surgery for seven days.
2.2.5. Amygdala drugs microinjection
On the test day (8th day), the cannula seal was removed from the animal's head, and an infusion needle was introduced (1 mm below the guide cannula) for solution injection. An infusion pump (Harvard Apparatus) with a flow rate of 0.25 μL/min was used for drugs and saline microinjection. The infusion needle was kept in place for one extra minute after the end of the microinjection to maximize the diffusion process (Macedo et al., 2005). After a 10-min interval, animals were tested on the two-way shuttle box for avoidance and freezing behavior evaluation. Poor performers received microinjections of DA agonists (quinpirole; SKF38393) or saline in the basolateral or central nuclei of the amygdala, while good performers received DA antagonist microinjections (SCH23390; sulpiride) or saline in the same targets.
2.2.6. Systemic drug administration
On the test day (8th day), good performers were intraperitoneally injected with dopaminergic receptor antagonist (SCH23390 30-min interval; sulpiride 10-min interval) or saline and tested in the two-way shuttle box for avoidance and freezing behaviors evaluation (de Souza Caetano et al., 2013).
2.2.7. Brain extraction
2.2.7.1. Perfusion
Ninety minutes after testing, the animals were deeply anesthetized with thiopental (40 mg/kg) and morphine sulfate (10 mg/mL) and perfused transcardially with a solution of 4.0% paraformaldehyde in 0.1 M phosphate buffer, using a peristaltic pump (Cole Parmer). Brains were removed, placed in paraformaldehyde for 3 h, and then transferred to a 30% sucrose/0.1 M phosphate buffer at 4 °C. Frozen whole brain coronal sections (40 mm thick) were sliced with a sliding microtome (Leica Biosystems).
2.2.7.2. Decapitation
Animals were deeply anesthetized with thiopental (40 mg/kg) and morphine sulfate (10 mg/mL), and heads were removed using a rodent guillotine (Insight Equipments, Brazil). After removal from the skull, brains were placed on an iced brain matrix and cut (1 mm), according to bregma reference (Paxinos and Watson, 2005). Samples were weighed and kept in a deep freezer (−80 °C) until analysis.
2.2.8. C-fos and TH immunohistochemistry
Brain sections were processed with anti-c-Fos antiserum raised in rabbit (Ab-5, Calbiochem, lot-D07099; dilution 1:20,000) or anti-TH raised in mouse (Millipore; Lot-LV1679333; dilution 1:1000). Primary antiserum was localized using an avidin–biotin complex system (ABC; Vector Laboratories). Briefly, sections were incubated in biotinylated goat anti-rabbit IgG solution (Vector Laboratories), then placed in the mixed avidin–biotin horseradish peroxidase complex solution (ABC Elite Kit; Vector Laboratories) (90 min/step at 22 °C). The peroxidase complex was incubated in a chromogen solution containing 0.02% 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma) with 0.3% nickel-ammonium sulfate in 0.05 M Tris-buffer (pH 7.6), followed by incubation in this chromogen solution with hydrogen peroxide (1:3000) (10 min/step) (Introini-Collison et al., 1979). Extensive washing in PBS buffer (pH 7.4) halted the DAB reaction. Sections were mounted on gelatin-coated slides, dehydrated, and coverslipped with DPX (Sigma). An adjacent reference series was Nissl stained for cytoarchitectonic purposes.
2.2.9. Quantification
For c-Fos-immunoreactive neuron quantification, brain slides were scanned using Pannoramic and Case Viewer (Biogen), and images were processed using ImageJ (News Version 1.44b). c-Fos positive neuron density was determined by dividing the resulting number by the area of the region of interest (delimited based on the Paxinos and Watson atlas) (Paxinos and Watson, 2005). The structures evaluated along the anteroposterior axis were prefrontal cortex (prelimbic and infralimbic) in bregma: 3.00 mm, 3.75 mm and 3.72 mm and amygdaloid nuclei (basolateral, lateral, medial and central) in bregma: 2.04 mm, −2.28 mm, −2.76 mm and −3.00 mm. For the TH quantification, photomicrographs of brain slides were taken using a 10x objective of a microscope equipped with Camera Lucida (NIKON ECLIPSE E600). The count was performed using ImageJ (News Version 1.44b) following the same protocol described above. The structures evaluated along the anteroposterior axis were the SN, VTA and LC in bregma: 6.72 mm; −10.08 mm; −9.96 mm. All the quantifications were performed by an observer blinded to the behavioral results.
2.2.10. High-performance liquid chromatography (HPLC)
The HPLC system was equipped with a reversed-phase column (Hypurity Elite C18, 250 mm × 4.6 mm, 5 μm and 100 Å pore-diameter particle size; Hypersil, Cheshire, United Kingdom) together with electrochemical detection. The samples were homogenized in 0.2 M perchloric acid containing dihydroxybenzylamine (DHBA) and centrifuged at 15,000 rpm for 20 min at 6 °C. A 50 μL sample dilution was injected into the HPLC system along with a proper buffer (150 mM chloroacetic acid, 120 mM NaOH, 0.67 mM EDTA, 0.86 mM sodium octylsulfate, 3.5% acetonitrile, 2.6% tetrahydrofuran; adjusted to pH 3.0) at a flow rate of 1.2 mL/min. The maximum sensitivity of the electrochemical detector was set at 2 nA, and the electrode oxidation potential was set at 850 mV versus a reference electrode. The ratios of DOPAC/DA and HVA/DA were used as indices of dopaminergic activity (Carvalho et al., 2005).
2.2.11. Volumetric analysis
Brain sections were mounted on gelatin-coated slides and Nissl stained according to the protocol previously described (Paul et al., 2008). Corresponding bregma slices were selected for evaluation, based on The Rat Brain Atlas (Paxinos and Watson, 2005), as follows: 1. Amygdala: bregma −1.20 to −4.08; 2. SN: bregma −4.36 to −6.72; 3. VTA: bregma −4.68 to −6.84; 4. LC: bregma −9.48 to −10.32. Images were acquired using a Digital Microscope Zeiss AxioCam Plus (exposure: 28 ms; length: 2.5 × 1300 zvs) and analyzed with the software AxioVision 3.0.6.1©1998–2000 (Carl Zeiss Vision Microimaging GmbH, Germany). The final volume estimation was determined as the sum of all bregma measures for that specific structure, considering the interval between slices (150 μm), as described in a previous work (Keeley et al., 2015).
2.2.12. Statistical analyses
Data are presented as the mean ± standard error of the mean (SEM). Freezing time, number of avoidance responses and administration of drugs were analyzed with two-way repeated measure analysis of variance ANOVA (factors: Group and Session). Data obtained in the open field, dopamine and metabolites quantification, volume and TH staining and c-Fos pattern were evaluated with the one-way ANOVA test. Correlations were set using the Pearson correlation test. When necessary, the Newman-Keuls post hoc test was applied. Significance was set at p < 0.05.
2.3. Study design
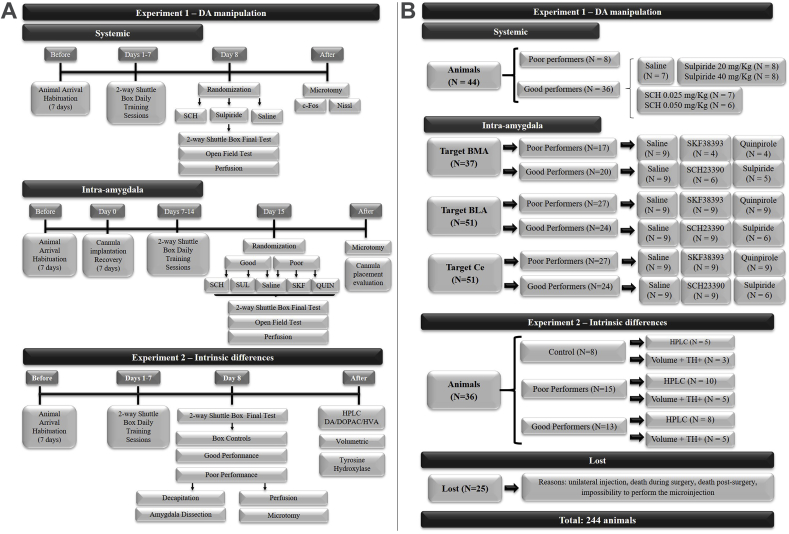
The study design is shown in Fig. 1A.
Fig. 1.
(A). Experimental design of the study divided in Experiment 1 that consisted of dopaminergic manipulation that was performed systemic and intra-amygdala manipulation in good and poor performers; and Experiment 2 that evaluated intrinsic differences. (B). Flowchart of the animals used in the study illustrating the number of the animals used per experiment in each experimental condition. QUIN: quinpirole. SCH: SCH23390; SKF: SKF38393.
2.4. Experiment 1 – dopaminergic manipulation
2.4.1. Systemic pharmacological DA manipulation
As shown in Fig. 1A, after the habituation period in our animal facility, animals were submitted to seven daily training sessions in the two-way shuttle box and subsequently divided in good and poor performance according to the behavioral response exhibited. On the eighth day, good performers were randomly assigned to the control or experimental group and systemic administration of saline or dopaminergic antagonists, respectively. After a standard period of time, animals were evaluated in the two-way shuttle box and in the open field test. Ninety minutes after testing, animals were perfused; brains were removed, sliced in a freezing microtome and processed for c-Fos immunohistochemistry and Nissl staining.
2.4.2. Intra-amygdala pharmacological DA manipulation
After the habituation period in our animal facility, animals were submitted to a surgical procedure for the implantation of the infusion cannula. Consecutively to the surgical recovery period, animals were submitted to seven daily training sessions in the two-way shuttle box and divided in good and poor performance according to the number of avoidance responses exhibited in the last two sessions. On the eighth day, animals were randomly assigned to the control or experimental group and administered saline or pharmacological agents. Good performers assigned for the experimental group received dopaminergic antagonists and poor performers assigned for the experimental group received dopaminergic agonists. After a standard period of time, animals were evaluated in the two-way shuttle box and in the open field test. Immediately after testing, animals were perfused; brains were removed, sliced in freezing microtome and processed for Nissl staining for validation of cannula placement.
2.5. Experiment 2 –basal DA levels
As shown in Fig. 1A, after a habituation period in the animal facility, the animals were randomly divided into experimental or control groups. Animals in the experimental group were submitted to eight daily training sessions in the two-way shuttle box and subsequently divided in good and poor performance groups according to the behavioral response exhibited in the last two sessions. Control animals were equally exposed to the shuttle box but never received any footshock.
On the last day, immediately after testing, a portion of the animals was decapitated, the brain was removed, and the amygdala was dissected for the quantification of dopamine and dopamine metabolites by HPLC. Another portion of the animals was perfused; the brains were removed, sliced in a freezing microtome and processed for TH immunohistochemistry and volumetric analysis.
3. Results
The number of animals used in each Experiment is shown in Fig. 1B.
3.1. Experiment 1 – DA manipulation
3.1.1. Behavior assessment
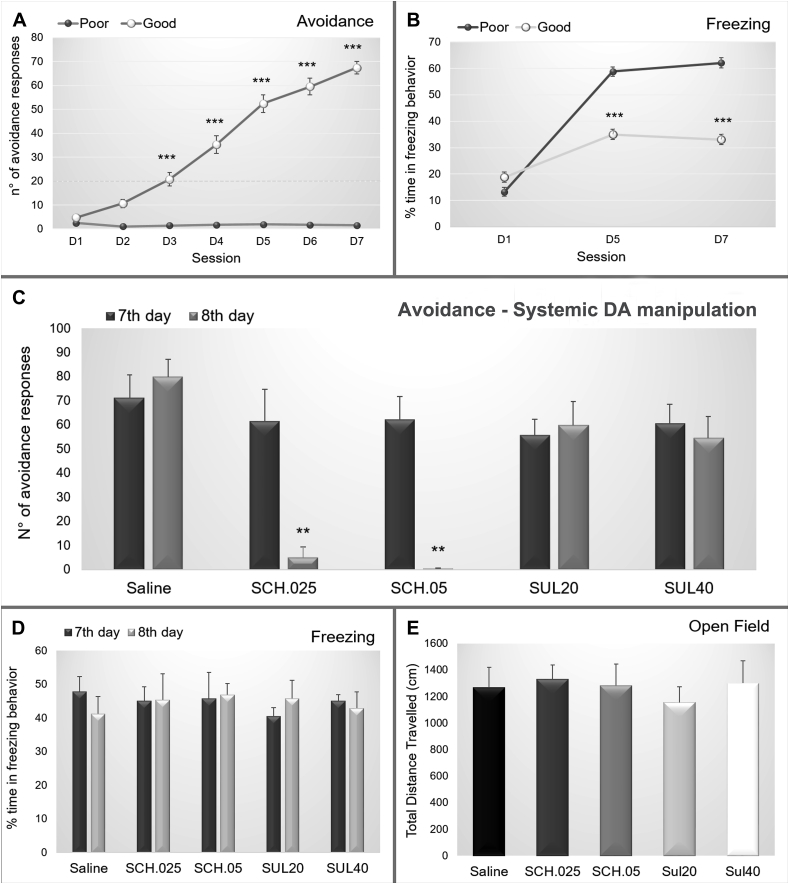
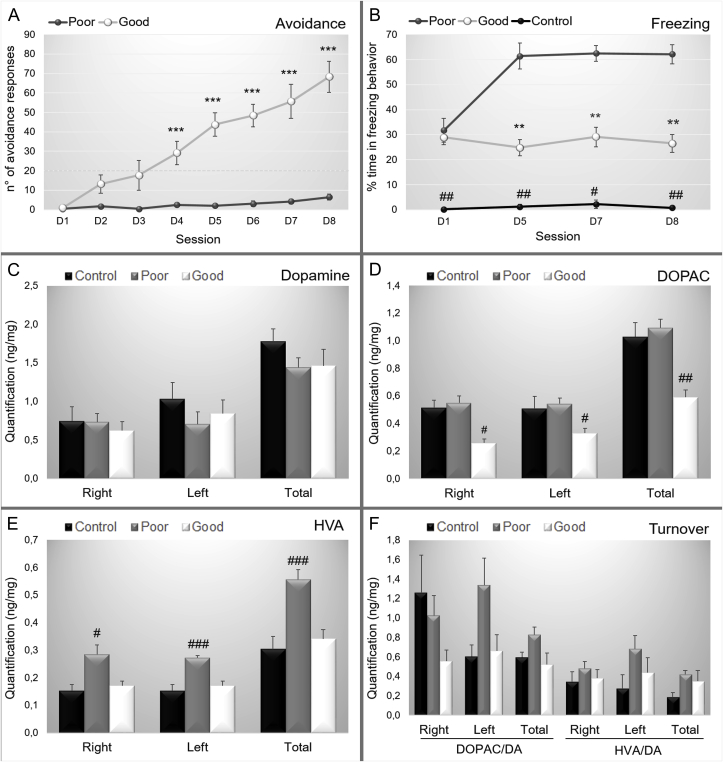
After the habituation period in the animal facility, the animals were trained in the two-way shuttle box. Along the seven daily training sessions, the animals were divided into good and poor performance groups based on the number of avoidance responses. The good performance group presented a continuous increase in the number of avoidance responses (interaction factor F(12,1790) = 29.487; p = 0.000000; Fig. 2 A) and a reduction in the percentage of freezing (interaction factor F(4,766) = 19.501; p = 0.00000; Fig. 2 B).
Fig. 2.
(A) Number of avoidance and (B) Percentage of the time spent in freezing exhibited by the poor and good avoiders. (C) Number of avoidance performed and (D) Percentage of time spent in freezing behavior by the good performers on the 7th day of training and on the 8th day of training after the administration of saline (N = 7), SCH 0.025 mg/kg (N = 7), SCH 0.05 mg/kg (N = 6), sulpiride 20 mg/kg (N = 8) or sulpiride 40 mg/kg (N = 8). (E) Total travel distance showed by good performers after the administration saline (N = 7), SCH 0.25 mg/kg (N = 7), SCH 0.5 mg/kg (N = 6), sulpiride 20 mg/kg (N = 8) or sulpiride 40 mg/kg (N = 8). ***: p < 0.001 in comparison with poor performers.
3.1.2. Systemic pharmacological DA manipulation
On the 8th day, animals with good performance (N = 36) were randomly divided into five groups: 1) saline (N = 7), 2) dopamine D1 receptor antagonist SCH 0.025 mg/kg (N = 7), 3) dopamine D1 receptor antagonist SCH 0.05 mg/kg (N = 6), 4) dopamine D2 receptor antagonist sulpiride 20 mg/kg (N = 8), and 5) dopamine D2 receptor antagonist sulpiride 40 mg/kg (N = 8). After systemic drug administration, the behavior was evaluated in the two-way shuttle box and open field. Groups SCH 0.025 mg/kg and SCH 0.05 mg/kg showed a decreased number of avoidance responses after drug administration (F(8,122) = 3.4368; p = 0.00133; Fig. 2 C). No changes were observed in freezing behavior (F(8,122) = 0.20408; p = 0.98969; Fig. 2 D) or in general motor activity (F(4,31) = 0.23501; p = 0.91648; Fig. 2 E).
3.1.3. c-Fos immunohistochemistry
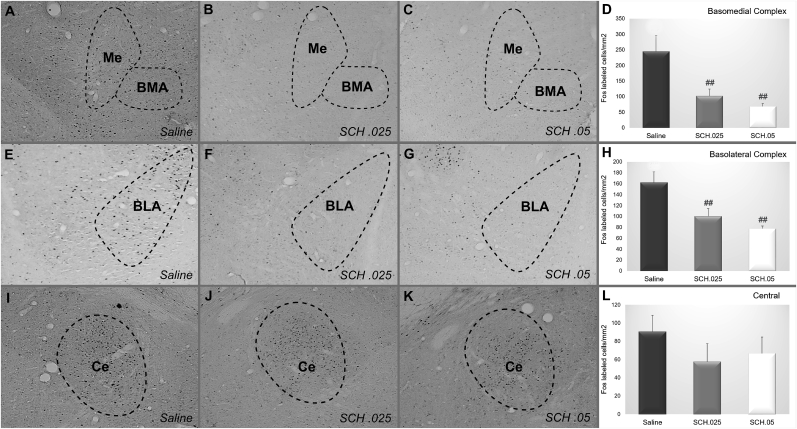
The saline (N = 6), SCH 0.025 mg/kg (N = 6) and SCH 0.05 mg/kg (N = 5) groups were evaluated for c-Fos-positive cell density in the basolateral complex (basolateral + lateral, BLA), basomedial complex (basomedial + medial, BMA) and central nucleus (Ce) of the amygdala. Both SCH groups presented smaller c-Fos positive cell densities in relation to controls in the BMA (F(2,14) = 7.0708; p = 0.00754; Fig. 3A–D) and BLA (F(2,14) = 8.5815; p = 0.00369; Fig. 3E–H), and no differences were observed in the Ce (F(2,14) = 0.82084; p = 0.46017; Fig. 3I–L). Additional data regarding c-Fos expression of other amygdala subnuclei and in SCH 0.025 mg/kg, SCH 0.05 mg/kg, sulpiride 20 mg/kg and sulpiride 40 mg/kg groups are shown in Supplementary Table 1.
Fig. 3.
Representative photomicrographs of c-Fos staining in the basomedial complex, basolateral complex and central amygdala after the administration of (A, E, I) saline, (B, F, J) SCH 0.25 mg/kg or (C, G, K) SCH 0.5 mg/kg in good performers. Mean density of c-Fos + cells in (D) Basomedial complex, (H) Basolateral complex and (L) Central amygdala for good avoiders after the systemic injection of saline (N = 6), SCH 0.25 mg/kg (N = 6) or SCH 0.5 mg/kg (N = 5). Scale bars represent 400 μm in all photographs. **: p < 0.01 in comparison with the other groups.
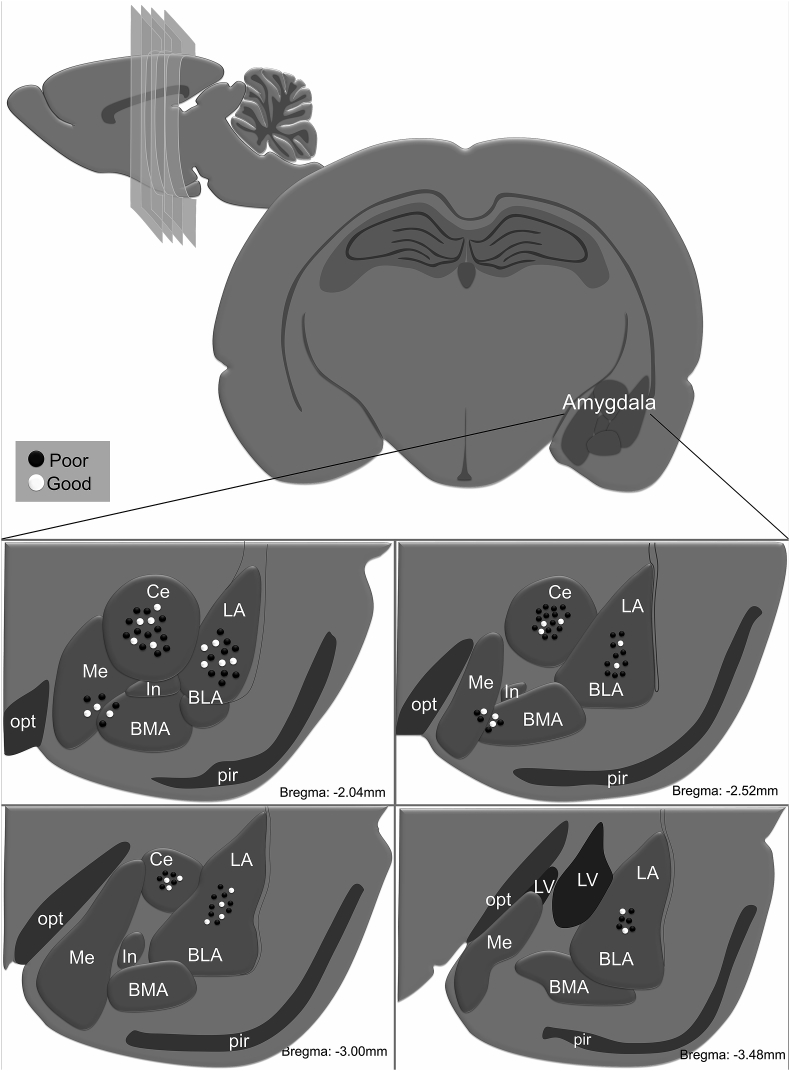
3.1.4. Intra-amygdala pharmacological DA manipulation
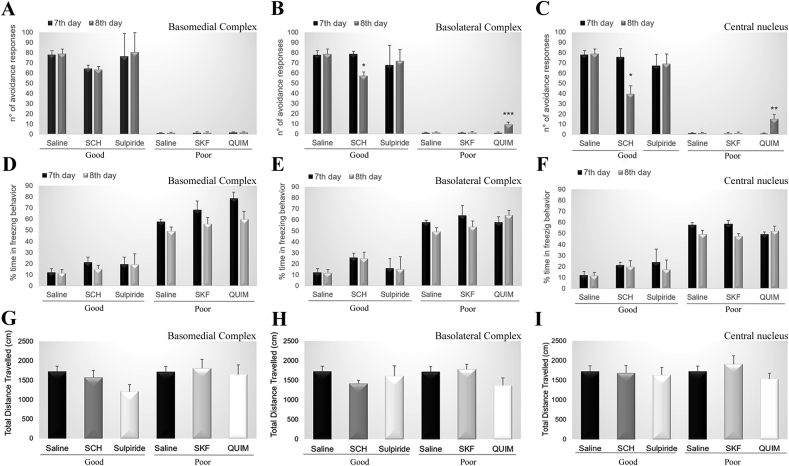
Prior to training in the two-way shuttle box, animals were submitted to stereotaxic surgery for bilateral cannula placement in the BMA, BLA or Ce. Fig. 4 illustrates the cannula placement. On the 8th day, animals with good performance were randomly divided into groups 1) Saline (N = 9), 2) Dopamine D1 receptor antagonist SCH (BMA N = 6, BLA N = 9, Ce N = 9), and 3) Dopamine D2 receptor antagonist sulpiride (BMA N = 5, BLA N = 6, Ce N = 6) and the poor performers were randomly divided into three groups 1) Saline (N = 9), 2) Dopamine D1 receptor agonist SKF (BMA N = 4, BLA N = 9, Ce N = 9), and 3) Dopamine D2 receptor agonist quinpirole (BMA N = 4, BLA N = 9, Ce N = 9). After drug administration, behavior was evaluated in the two-way shuttle box and open field. No differences were observed in avoidance behavior in animals with cannula targeting the BMA (good: F(2,25) = 0.2462; p = 0.97571, poor: F(4,54) = 0.01181; p = 0.99972; Fig. 5 A). For the BLA and Ce cannula groups, SCH animals presented decreased avoidance behavior (BLA: F(2,31) = 4.3462; p = 0.02168; Ce F(2,31) = 3.7541; p = 0.03468; Fig. 5B and C), and quinpirole animals showed increased avoidance behavior (BLA: F(4,94) = 5.3927; p = 0.00059; Ce F(4,94) = 4.5334; p = 0.00216; Fig. 5B and C). No changes were observed in freezing behavior (BMA: good F(2,25) = 0.35890; p = 0.70198, poor: F(4,54) = 0.51430; p = 0.72548; BLA: good F(2,31) = 0.00009; p = 0.99991, poor: F(4,94) = 0.73201; p = 0.57235; Ce: good F(2,31) = 0.00962; p = 0.99043, poor: F(4,94) = 1.4678; p = 0.21821; Fig. 5D–F) or in general motor activity (BMA: F(4,27) = 0.21867; p = 0.92570; BLA: F(4,40) = 1.8856; p = 0.13183; Ce: F(4,40) = 0.63915; p = 0.63765; Fig. 5G–I).
Fig. 4.
Representative microinjection sites for basomedial complex, basolateral complex and central nucleus of amygdala along bregma −2.04, −2.52, −3.00 and −3.48 mm. Each dot indicates the site of injection corresponding to poor avoiders (black dots) and good avoiders (white dots). BLA: basolateral nucleus of amygdala, BMA: basomedial nucleus of the amygdala; Ce: central nucleus of amygdala, In: intercalated nucleus of the amygdala, LA: lateral nucleus of amygdala, LV: lateral ventricle, Me: medial nucleus of amygdala; pir: piriform nucleus, opt: optic tract.
Fig. 5.
Number of avoidance performed by good performers on the 7th day of training (basal) and on the 8th day of training after the administration of saline, SCH 0.3 μL, sulpiride 0.2 μL and by poor avoiders on the 7th day of training (basal) and on the 8th day of training after the administration of saline, SKF 0.4 μL, or quinpirole 0.1 μL into the (A) basomedial complex, (B) basolateral complex or (C) central nucleus of amygdala. Percentage of time spent in freezing behavior performed by good performers on the 7th day of training (basal) and on the 8th day of training after the administration of saline, SCH 0.3 μL, sulpiride 0.2 μL and by poor avoiders on the 7th day of training (basal) and on the 8th day of training after the administration of saline, SKF 0.4 μL, or quinpirole 0.1 μL into the (D) basomedial complex, (E) basolateral complex or (F) central nucleus of the amygdala. Total travel distance showed by good performers after the administration of saline, SCH 0.3 μL, sulpiride 0.2 μL and by the poor avoiders on the 7th day of training (basal) and on the 8th day of training after the administration of saline, SKF 0.4 μL, or quinpirole 0.1 μL into the (G) basomedial complex, (H) basolateral complex or (I) central nucleus of amygdala. Group good avoiders: saline (N = 9), SCH 0.3 μL (basomedial N = 6, basolateral N = 9, central N = 9), sulpiride 0.2 μL (basomedial N = 5, basolateral N = 6, central N = 6). Group poor avoiders: saline (N = 9), SKF 0.4 μL (basomedial N = 4, basolateral N = 9, central N = 9) or quinpirole 0.1 μL (basomedial N = 4, basolateral N = 9, central N = 9). *: p < 0.05 in comparison with the corresponding 7th day.
3.2. Experiment 2 – basal DA levels
3.2.1. Behavior assessment
After a habituation period in the animal facility, the animals were randomly divided into experimental or control groups (see Fig. 1B – Experiment 2). During the training sessions, the experimental group was divided into good performance (N = 13) and poor performance (N = 15) groups based on the avoidance response presented in the two-way shuttle box. The good performance group presented a continuous increase in the number of avoidance responses (interaction factor F(14,414) = 4.9798; p = 0.0000; Fig. 6 A) and a reduction in the percentage of freezing (interaction factor F(12,288) = 2.3964; p = 0.00610; Fig. 1 B). Control animals were exposed to the shuttle box but received no footshock; thus, control animals presented a low percentage of freezing behavior and no avoidance responses (Fig. 6 B).
Fig. 6.
Data are reported as the means ± SEM. (A). Number of active avoidance responses in good and poor performers across training sessions. (B). Percentage of time spent in freezing behavior along sessions. (C). Quantification of dopamine (ng/mg of tissue) in the right, left and both hemispheres considering control, poor and good performers. (D). Quantification of 3,4-dihydroxyphenylacetic acid - DOPAC (ng/mg of tissue) in the right, left and both hemispheres considering control, poor and good performers. (E). Quantification of homovanillic acid - HVA (ng/mg of tissue) in the right, left and both hemispheres considering control, poor and good performers. (F). Quantification of dopamine turnover of DOPAC and HVA in the right, left and both hemispheres considering control, poor and good performers. Poor: poor performers, Good: good performers, Control: control group. DOPAC/DA: turnover rate for DOPAC; HVA/DA: turnover rate for HVA, right: right hemisphere, left: left hemisphere, total: right and left hemisphere. For the behavioral data: control (N = 5), good (N = 13) and poor (N = 15). For the HPLC data: control (N = 5), good (N = 8) and poor (N = 10) and **: p < 0.01 in comparison with poor performers, ***: p < 0.001 in comparison with poor performers, #: p < 0.05 in comparison with good and poor performers, ##: p < 0.01 in comparison with good and poor performers; ###: p < 0.001 in comparison with good and poor performers.
3.2.2. Dopamine and metabolite quantification
The concentration of dopamine and metabolites (DOPAC and HVA) was evaluated in the total amygdala of controls (N = 5), good (N = 8) and poor (N = 10) performers. There were no differences in dopamine levels between groups in the right (F(2,20) = 0.17934; p = 0.83715), left (F(2,20) = 0.59812; p = 0.55938) or total amygdala (F(2,20) = 0.67928; p = 0.51830; Fig. 6 C). However, the metabolite quantification showed lower levels of DOPAC in good performers (Right: F(2,20) = 3.5959 p = 0.04634; Left: F(2,20) = 4.4693 p = 0.02486; Total: F(2,20) = 7.3101 p = 0.00414; Fig. 6 D) and higher levels of HVA in poor performers (Right: F(2,20) = 5.0862 p = 0.01638; Left: F(2,20) = 15.663 p = 0.00008; Total: F(2,20) = 10.482 p = 0.00077; Fig. 6 E). No differences were observed in dopamine turnover in the right (DOPAC/DA: F(2,20) = 1.6276; p = 0.22136; HVA/DA: F(2,20) = 0.24807; p = 0.78191), left (DOPAC/DA: F(2,20) = 2.4942; p = 0.10787; HVA/DA: F(2,20) = 1.9352; p = 0.17049) or total amygdala (DOPAC/DA: F(2,20) = 2.6346; p = 0.09647; HVA/DA: F(2,20) = 1.9714; p = 0.16540; Fig. 6 F).
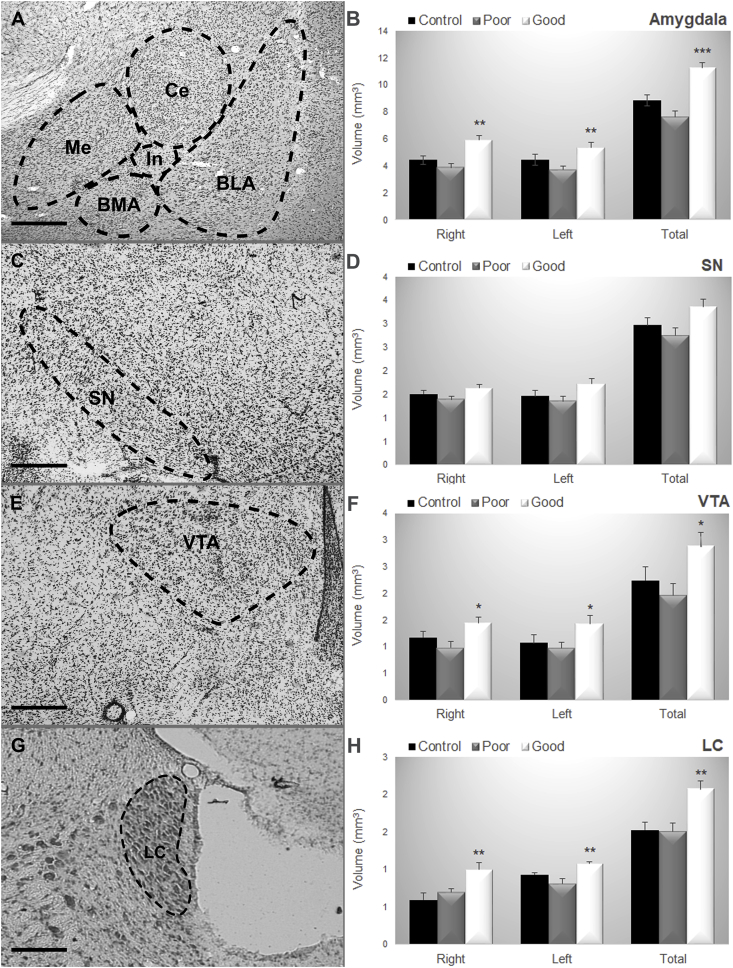
3.2.3. Volumetric analysis
The volume of the amygdala, substantia nigra (SN), ventral tegmental area (VTA) and locus coeruleus (LC) was estimated in control (N = 3) good (N = 5) and poor (N = 5) performers. The Newman-Keuls test showed that the good performance group presented greater volume in 1) Amygdala (Right: F(2,10) = 6.60; p = 0.01; Left: F(2,10) = 5.96; p = 0.02, Total: F(2,10) = 9.49; p = 0.005; Fig. 2, Fig. 7) VTA (Right: F(2,10) = 5.00; p = 0.03; Left: F(2,10) = 4.33; p = 0.04; Total: F(2,10) = 4.71; p = 0.036; Fig. 3, Fig. 7) LC (Right: F(2,10) = 8.38; p = 0.007; Left: F(2,10) = 5.71; p = 0.02; Total: F(2,10) = 9.04; p = 0.006; Fig. 7G and H). The Newman-Keuls test did not show difference in the volume of the SN (Right: F(2,10) = 1.49; p = 0.27; Left: F(2,10) = 2.11; p = 0.17; Total: F(2,10) = 2.40; p = 0.14; Fig. 7C and D).
Fig. 7.
Representative photomicrographs of (A) the Amygdala nuclei, (C) Substantia Nigra, (E) Ventral Tegmental Area, (G) Locus Coeruleus. Data are reported as the means ± SEM. Volumetric estimates in (B) the Amygdala nuclei, (D) Substantia nigra, (F) Ventral Tegmental Area, (H) Locus Coeruleus considering the right and left hemisphere and the total volume considering control, poor and good performers. BLA: basolateral nucleus of amygdala; BMA: basomedial nucleus of amygdala; Ce: central nucleus of amygdala, LC: locus coeruleus; Me: medial nucleus of amygdala; SN: substantia nigra; VTA: ventral tegmental area. Control (N = 3), Good (N = 5), Poor (N = 5). Scale bars represent 400 μm in all photographs. *: p < 0.05 in comparison with poor performers; **: p < 0.01 in comparison with poor performers, ***: p < 0.001 in comparison with poor performers.
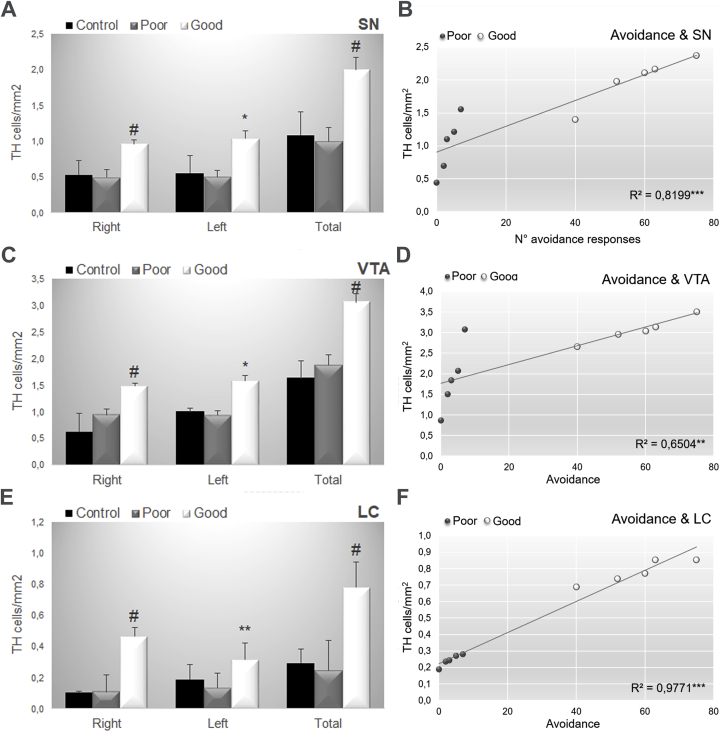
3.2.4. TH immunohistochemistry
TH+ neurons were evaluated in the SN, VTA and LC of control (N = 3) good (N = 5) and poor (N = 5) performers. The Newman-Keuls test showed that the good performance group showed greater staining in all structures 1) SN (Right: F(2,10) = 5.61; p = 0.02; Left: F(2,10) = 5.59; p = 0.00.023; Total: F(2,10) = 7.53; p = 0.011; Fig. 2, Fig. 8) VTA (Right: F(2,10) = 4.59; p = 0.04; Left: F(2,10) = 4.67; p = 0.00.037; Total: F(2,10) = 7.97; p = 0.008; Fig. 3, Fig. 8) LC (Right: F(2,10) = 138.56; p = 0.0001; Left: F(2,10) = 6.77; p = 0.014 Total: F(2,10) = 155.78; p = 0.0001; Fig. 8E). Furthermore, there was a significant correlation between volume and the number of avoidance responses in all structures (SN: R2 = 0.8199; p < 0.00031, VTA: R2 = 0.6504; p < 0.00482; LC: R2 = 0.9771; p < 0.00000; Fig. 8B, C, D).
Fig. 8.
Data are reported as the means ± SEM. TH positive cells/mm2 in (A) Substantia nigra, (C) Ventral Tegmental Area, (E) Locus Coeruleus considering the right and left hemisphere and the total volume considering control, poor and good performers. Correlation data between the number of avoidance and tyrosine hydroxylase-positive cells/mm2 in the (B) Substantia Nigra, (D) Ventral Tegmental Area and (E) Locus Coeruleus considering poor and good performers. Control (N = 3), Good (N = 5), Poor (N = 5). LC: locus coeruleus; SN: substantia nigra; VTA: ventral tegmental area. Scale bars represent 400 μm in all photographs. *: p < 0.05 in comparison with poor performers; **: p < 0.01 in comparison with poor performers, ***: p < 0.001 in comparison with poor performers, #: P < 0.05 in comparison with all groups.
4. Discussion
The present work showed that there is a distinct dopaminergic pattern for good and poor performers that is critical for the expression of avoidance behavior. Moreover, it could be possible to switch from good to poor performer, and vice versa, by intra-amygdala (BLA and Ce) injections of D1 receptor antagonist drugs in good performers or D2 receptor agonist drugs in poor performers. Furthermore, in comparison with poor performers, the good performers had lower levels of DOPAC in the amygdala, an increase in the total volume of the AMG, SN, VTA and LC, and an increase in the number of TH + cells in the SN, VTA and LC, which positively correlated with the avoidance behavior. Taken together, our results suggest that the dopaminergic system could modulate avoidance behavior.
The behavioral distinction between good and poor avoiders is well established in the literature (Choi et al., 2010; Lázaro-Muñoz et al., 2010; Martinez et al., 2013; Galatzer-Levy et al., 2014). The poor performers failed in the transition from freezing to avoidance behavior even after training (Campese et al., 2016; LeDoux et al., 2017; Boeke et al., 2017).
Considering dopaminergic modulation, our data showed that systemic injection of SCH23390, a D1 antagonist drug, could disrupt the avoidance response in good performers without affecting motor activity. These data have been supported by previous published papers that showed that subtype-dopaminergic receptors modulate avoidance (Beninger et al., 1989; Aguilar et al., 2000; Reis et al., 2004; Boschen et al., 2011; Wietzikoski et al., 2012). According to our data, the substrate for this systemic modulation is a reduction in the c-Fos activation of the basomedial and basolateral nucleus of the amygdala. A possible reason for this result could be the main localization of D1 receptors in both nuclei (Weiner et al., 1991; Boyson et al., 1986). Specifically, the anatomical pathway that regulates approach-avoidance conflict behavior could be from the medial nucleus projecting to the ventromedial hypothalamus and bed nucleus of the stria terminalis through the D1 receptor neuron population (Miller et al., 2019).
Regarding D2 receptors, the systemic injection of sulpiride, a D2 antagonist, did not affect the avoidance responses or motor activity exhibited by the good performers. Previous papers (Reis et al., 2004; Boschen et al., 2011) showed a reduction in avoidance response after systemic administration of sulpiride. However, a possible reason for these discrepant results could be the training protocol: unsignaled avoidance in our experiment versus signaled protocols (Reis et al., 2004; Boschen et al., 2011). It has been shown that unsignaled and signaled protocols have different learning pattern (Ulrich et al., 1964; Powell, 1976; Mertens et al., 2018; Hurtado-Parrado et al., 2019) and different neuroanatomical pathways (Troncoso et al., 1998; Cohen and Castro-Alamancos, 2007, 2010; Hormigo et al., 2016, 2019). For instance, in signaled protocols there is the involvement of the sensory thalamus, which fires during signaled avoidance responses (Cohen and Castro-Alamancos, 2010). Additionally, there is the projection of the substantia nigra pars reticulata, a main GABAergic output, to the superior colliculus, which is responsible for the expression of signaled avoidance responses (Hormigo et al., 2016). An interesting hypothesis concerns the possible role of dopaminergic inputs to the superior colliculus, which could contribute to the avoidance response (Essig and Felsen, 2016). Another explanation could be the possibility that the signaled protocol modulates the anxiety levels considering that there is predictability and control over the harmful outcome (Sheynin et al., 2014; Ng and Lovibond, 2017; Hormigo et al., 2019). In this line with this thinking, it has been proposed that different mechanisms could underlie the aversive response depending on the experimental protocol (Louilot et al., 1986; Campese et al., 2016, 2013).
Considering our microinjection target, the importance of basolateral and central nuclei for avoidance behavior has been reported in several papers (Werka et al., 1978; Wilensky et al., 2000; Rorick-Kehn and Steinmetz, 2005; Lázaro-Muñoz et al., 2010; Moscarello and LeDoux, 2013; Jiao et al., 2015). Specifically, the role of the central amygdala in avoidance has been investigated in active and passive avoidance protocol with different results. In passive avoidance CeA lesions impaired avoidance behavior (Grossman et al., 1975; Jellestad and Bakke, 1985). In active avoidance, Grossman et al. (1975) showed that lesions in CeA consistently produced facilitatory effects on active avoidance, which is supported by more recent studies (Lázaro-Muñoz et al., 2010; Choi et al., 2010). The reason for this apparently contradictory effect could be the technical method for performing the lesion, i.e., older studies lesions were often large and/or not particularly specific to the amygdala subnuclei; and different avoidance protocol.
The microinjection of the D1 antagonist into the basolateral and central nuclei of the amygdala decreased the number of avoidances in the good performers. The basolateral complex receives dopaminergic innervation from the ventral tegmental area (Swanson, 1982; Nader and LeDoux, 1999; de la Mora et al., 2010) and has D1 and D2 dopaminergic type receptors (Weiner et al., 1991; Scibilia et al., 1992; Levey et al., 1993; Rouillard and Freeman, 1995; de la Mora et al., 2010), while the central nucleus receives innervation from the SN (Rouillard and Freeman, 1995) and has D1 and D2 type receptors (Weiner et al., 1991; Scibilia et al., 1992). It has been shown that microinjection of D2 (sulpiride) or D1 (SCH23390) dopaminergic antagonist in the nucleus accumbens reduced the number of avoidance responses (Wadenberg et al., 1990; Wietzikoski et al., 2012). However, the administration of sulpiride into the prefrontal cortex and dorsal striatum and SCH233990 in the dorsal striatum did not affect avoidance responses (Wadenberg et al., 1990; Wietzikoski et al., 2012), suggesting that the microinjection site is the crucial factor. Supporting our data, de la Mora et al. (2010) proposed that dopaminergic modulation in the central nucleus is responsible for modulating avoidance.
The role of dopamine in acquisition is more consistent than in the expression of the avoidance behavior. It has been shown that impairment in the dopaminergic system elicits severe deficit in avoidance acquisition (Fibiger et al., 1974; Beninger, 1983; Koob et al., 1984; Wadenberg and Hicks, 1999; Darvas et al., 2011), while for avoidance expression, some studies have shown that DA is involved (Wadenberg et al., 1990; Takamatsu et al., 2015), while others have shown that it is not (Dunn et al., 1986; Nasello and Felicio, 1990). A possible reason for the discrepant results could be the variety of avoidance protocols used in the literature (Nasello and Felicio, 1990; Johnson et al., 2001; Declercq and De Houwer, 2008; Maia, 2010). Supporting this suggestion, a phasic dopamine release pattern has been shown depending on the experimental protocol (Oleson et al., 2012; Wenzel et al., 2018; Pultorak et al., 2018). Specifically, warning signal protocols release dopamine in comparison with unavoidable footshock which suppress dopamine release (Overmier and Seligman, 1967; Oleson et al., 2012), supporting the hypothesis that the protocol is an essential aspect for explaining the variety of results concerning dopaminergic modulation. Another confounder could be the dopaminergic role in encoding value for the signals. It has also been shown a phasic dopaminergic pattern depending on the value signal (Bromberg-Martin et al., 2010; Fiorillo, 2013; Gentry et al., 2016; Pultorak et al., 2018). Another possibility of bias is the testing condition. When an animal is paired with a conspecific that is shocked, the non-shocked rat exhibits an increase in the DA release, reflecting the importance of the test environment in the results (Lichtenberg et al., 2018). An acute dopaminergic reduction can also have different results on avoidance learning varying with the age of the animals that were used (Kelm & Boettiger, 2015). A last interesting possibility is that dopamine has different roles in avoidance behavior considering the concepts of safety, opponency and controllability (Huys and Dayan, 2009; Lloyd and Dayan, 2016).
A possible explanation for the differential role of D1 and D2 in poor and good performers could be due to the bidirectional modulatory role of those subtypes of receptors. The opposite modulations of D1 and D2 receptor signaling have already been shown in previous publication (Moustafa et al., 2008; Doll et al., 2011; Cox et al., 2015; Nguyen et al., 2019). There are bidirectional differences in D1 and D2 concerning transduction mechanisms. In the central nervous system, the activation of D2 receptors is through the mediation of G (βγ) proteins by inhibiting adenylate cyclase and reducing the intracellular cyclic adenosine monophosphate (cAMP) concentration while D1-receptor activation is linked to an increase in cyclic adenosine monophosphate and the activation of phospholipase C (Onali et al., 1985; Undie and Friedman, 1990; Gingrich and Caron, 1993, Jin et al., 2001; Neve et al., 2004; de la Mora et al., 2010). A bidirectional modulatory role for dopamine in avoidance through segregated D1 and D2 cortico-striatal pathways has also been proposed (Cox et al., 2014).
Dopaminergic neurons are phasically activated by alerting signals (Bromberg-Martin et al., 2010), and dysfunction in this system could contribute to an abnormal processing of environment cues in the basolateral and central nucleus of the amygdala, leading to an impairment in the avoidance response. In this sense, our data support the hypothesis (LeDoux et al., 2017) that the dopaminergic system could be considered a potential target for pharmacological therapy.
Some lines of data point to a competition between the production of the freezing response that directly opposes avoidance (Wilensky et al., 2006; Ciocchi et al., 2010; Haubensak et al., 2010; Choi et al., 2010; Lázaro-Muñoz et al., 2010). Our data regarding pharmacological intervention did not show this pattern which could be due to the variability in freezing assessments, such that in our protocol was evaluated at the beginning of the test, but in other paradigms, was evaluated throughout the session (Choi et al., 2010; Ciocchi et al., 2010). Our data is supported by the suggestion that fear levels are not crucial for affecting avoidance (Grossman et al., 1975).
Focusing on the intrinsic difference between groups, we consider that the maladaptive behavior shown by the poor performers could be attributed to the dopaminergic systems. Our data showed that good performers have a decreased DOPAC content in the amygdala without affecting DA levels and dopaminergic turnover. These data are supported by Csillag (1999), who compared brain samples of control and trained animals and showed that there is no difference in the avoidance task considering dopamine and dopamine turnover rate. Although quantitatively DA levels are the same, the mechanism of the adaptive compensatory response may be changed in these animals, specifically those related to the availability of dopaminergic receptors and the pattern of phosphorylation of the D1 and D2 receptors (Carvalho et al., 2005; Frederick et al., 2015; Yapo et al., 2017). Considering the HVA and DOPAC results, our data suggest that timing for metabolization could be responsible for the intrinsic differences between poor and good performers. To support this suggestion, Heffner et al. (1984) showed that dopaminergic metabolites are quickly eliminated during the learning process.
Other data supporting intrinsic differences could be observed in the increase in the volume and quantification of TH + cells of the SN, ventral tegmental area and locus coeruleus, as shown by the good performers that were positively correlated with the avoidance behavior. The VTA and SN contain DA neurons (Watabe-Uchida et al., 2012; Beier et al., 2015; Lerner et al., 2015; Menegas et al., 2015). The reduction in the pattern of TH neurons in the SN can lead to impairments in memory and, learning (Moreira et al., 2012) and in avoidance tasks (Díaz-Véliz et al., 2002). Additionally, monitoring the substantia nigra and VTA volume could be an index of cognitive status, considering that their volume has been considered a parameter of the progression of neurodegenerative diseases (Chen et al., 2014; De Marco and Venneri, 2018; D'Amelio et al., 2018). Additionally, the dopaminergic system controls motivation, and electrical stimulation of the VTA has been suggested as an effective target for depressive disorders (Yadid and Friedman, 2008; Friedman et al., 2009; 2012; Gazit et al., 2015).
The LC is the main noradrenergic nucleus (Schwarz and Luo, 2015; Feinstein et al., 2016) that has been suggested to be responsible for the elaboration of the avoidance response (Shelkar et al., 2016; Clewett et al., 2018) probably due to the locus coeruleus-amygdala circuitry (Sabban et al., 2018). Clarifying the elaboration of the avoidance, intra-LC injections of the putative neurotransmitter agmatine increases the inhibitory avoidance mediated by alfa2-adrenoreceptors and nitric oxide (Shelkar et al., 2016). Further, approximately a month after the inhibitory avoidance test, there is an increase in noradrenergic receptors, neuropeptide Y receptors, corticotropin-releasing hormone receptors and the endocannabinoid system in the LC and amygdala (Clewett et al., 2018). Good performers present a higher number of TH neurons than poor performers in the LC, which could be supported by a study that showed that injury of the noradrenergic system impairs instrumental avoidance probably through the loss of cells in the LC (Radwanska et al., 2010). Another possible interpretation could be that TH + cells in the LC may reflect signaling of a different catecholamine instead of only noradrenergic regulation. Supporting this hypothesis, there is close interaction between the dopaminergic and noradrenergic systems (Antelman and Caggiula, 1977; Lisieski et al., 2019; Zerbi et al., 2019), as LC stimulation induces a concomitant release of DA and NA (Devoto et al., 2001; 2003a, 2003b, 2004, 2005) and there is an anatomically specific connectomic fingerprint of LC with dopamine receptors (Zerbi et al., 2019).
In the same line of thinking, previous works from other paradigms such as reversal learning, lever press, the decision-making task and, instrumental learning support the assumption that the dopamine system is crucial for modulating individual differences (Tomie et al., 2000; Cheng and Feenstra, 2006; Randall et al., 2012; Vollbrecht et al., 2015; Klanker et al., 2015; Groman et al., 2019).
Although volumetric changes in the LC could not be directly correlated with function (Mounton et al., 1984), it could represent a cognitive status (Theofilas et al., 2017) because there is a decrease in LC volume in neurodegenerative diseases (Chen et al., 2014; Theofilas et al., 2017).
The amygdala is involved in the modulation of avoidance responses (LeDoux, 2000; Schlund and Cataldo, 2010; Lázaro-Muñoz et al., 2010; Martinez et al., 2013; Ramirez et al., 2015; Ardeshiri et al., 2017; Korn et al., 2017). Our data are in contrast with a previous published paper (Rio-Álamos et al., 2017) that showed that low avoider rats had greater amygdala volume in comparison with high avoider rats. A possible reason for this difference could be the use of high- and low-avoidance animals inbred strains, which were originally selected and bred in accordance with their performance in the avoidance task, as previously suggested by our research group (de Oliveira et al., 2016). In this same line of thinking, Mozhui et al. (2007) quantified the basolateral amygdala across 35 inbred lines, showing huge variability in volume and cell populations between strains. Additionally, Sultana et al. (2019) showed that inbred strains exhibited contrasting characteristic behaviors and differences in genetic background.
Taken together, our data have provided evidence for a dopaminergic signature of avoidance performers, emphasizing the role of distinct dopaminergic receptors in individual differences in avoidance behavior based on pharmacological, immunohistochemical, neurochemical and volumetric analyses.
Regarding technical limitations, the invasiveness of the guide cannula and the microinjection procedure, and the impossibility to perform a drug-free test on the day 9 should be considered. Additionally, the pattern of drug spread was not evaluated and there were no offsite controls; however, the drug and dose used was based on previous papers that targeted the same structure. Further, we did not infuse the agonist in the good performers or the antagonist in the poor performers as counterproof and to minimize the drug effects.
5. Conclusion
The results of our study provide a better understanding of the role of the dopaminergic system in the execution of avoidance behavior. Specifically, good performers have DA patterns in the amygdala, SN, VTA and LC that are intrinsically different from those of poor performers, and it could be possible to convert animals from good to poor performers through manipulation of D1 and D2 receptors. In the future, we expect that our results will provide insights into the treatment of psychiatric disorders.
Authors’ contributions
GFA, FVA, and FSR: performed the experiments, data curation, and formal analyses, and reviewed and edited the paper; MDJS, MCC, CCO, LCTS, MCC, and MAK: performed the experiments and formal analyses, and edited the paper; MJT, JPO, MB, and ETF: conceptualized the experiments and reviewed and edited the paper; RCRM: conceptualized the experiments, obtained the funding, administered and supervised the project, and wrote the paper.
CRediT authorship contribution statement
Geiza Fernanda Antunes: Formal analysis, Data curation, Writing - review & editing. Flavia Venetucci Gouveia: Formal analysis, Data curation, Writing - review & editing. Fabiana Strambio Rezende: Formal analysis, Data curation, Writing - review & editing. Midiã Dias de Jesus Seno: Formal analysis, Writing - review & editing. Milene Cristina de Carvalho: Formal analysis, Writing - review & editing. Caroline Cruz de Oliveira: Formal analysis, Writing - review & editing. Lennon Cardoso Tosati dos Santos: Formal analysis, Writing - review & editing. Marina Correia de Castro: Formal analysis, Writing - review & editing. Mayra Akemi Kuroki: Formal analysis, Writing - review & editing. Manoel Jacobsen Teixeira: Writing - review & editing, Conceptualization. José Pinhata Otoch: Writing - review & editing, Conceptualization. Marcus Lira Brandao: Writing - review & editing, Conceptualization, Writing - review & editing, Conceptualization. Erich Talamoni Fonoff: Conceptualization, Supervision, Validation, Writing - review & editing.
Declaration of competing interest
The authors report no conflicts of interest.
Acknowledgments
The authors wish to thank Mr. Bruno Gregnanin Petron, Professor Vera Demarchi Aiello, and Mr. Ernande Xavier dos Santos for their help during the development of this project. The authors also wish to thank all the research assistants and staff of Hospital Sirio-Libanes and to the Laboratório de Anatomia Patológica from the Instituto do Coração InCor/FMUSP. The following author are the recipients of grants from the government of Brazil: R.C.R.M. FAPESP #11/08575–7; G.F.A. CAPES# 88882.366209/2019–01; F.V.G. FAPESP #13/20602–5 and FAPESP #17/10466–8; C.C.O. FAPESP#13/03039–5; M.C. C FAPESP #13/03040–3; L.C.T.S. FAPESP #14/12999–5; M.D.J.S. CAPES#33160015002P8.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2020.100219.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Aguilar M.A., Marí-Sanmillán M.I., Morant-Deusa J.J., Miñarro J. Different inhibition of conditioned avoidance response by clozapine and DA D1 and D2 antagonists in male mice. Behav. Neurosci. 2000 Apr;114(2):389–400. [PubMed] [Google Scholar]
- American Psychiatric Association . American Psychiatric Pub; 2013. Diagnostic and Statistical Manual of Mental Disorders (DSM-5R) [Google Scholar]
- Antelman S.M., Caggiula A.R. Norepinephrine-dopamine interactions and behavior. Science. 1977 Feb 18;195(4279):646–653. doi: 10.1126/science.841304. [DOI] [PubMed] [Google Scholar]
- Ardeshiri M.R., Hosseinmardi N., Akbari E. The effect of orexin 1 and orexin 2 receptors antagonisms in the basolateral amygdala on memory processing in a passive avoidance task. Physiol. Behav. 2017 May 15;174:42–48. doi: 10.1016/j.physbeh.2017.03.004. [DOI] [PubMed] [Google Scholar]
- Bardeen J.R., Tull M.T., Stevens E.N., Gratz K.L. Exploring the relationship between positive and negative emotional avoidance and anxiety symptom severity: the moderating role of attentional control. J. Behav. Ther. Exp. Psychiatr. 2014 Sep;45(3):415–420. doi: 10.1016/j.jbtep.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Beier K.T., Steinberg E.E., DeLoach K.E., Xie S., Miyamichi K., Schwarz L. Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell. 2015 Jul 30;162(3):622–634. doi: 10.1016/j.cell.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beninger R.J. The role of dopamine in locomotor activity and learning. Brain Res. 1983 Oct;287(2):173–196. doi: 10.1016/0165-0173(83)90038-3. [DOI] [PubMed] [Google Scholar]
- Beninger R.J., Hoffman D.C., Mazurski E.J. Receptor subtype-specific dopaminergic agents and conditioned behavior. Neurosci. Biobehav. Rev. 1989;13(2–3):113–122. doi: 10.1016/s0149-7634(89)80019-3. Summer-Fall. [DOI] [PubMed] [Google Scholar]
- Blanchard R.J., Blanchard D.C. Crouching as an index of fear. J. Comp. Physiol. Psychol. 1969 Mar;67(3):370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Boeke E.A., Moscarello J.M., LeDoux J.E., Phelps E.A., Hartley C.A. Active avoidance: neural mechanisms and attenuation of pavlovian conditioned responding. J. Neurosci. 2017 May 3;37(18):4808–4818. doi: 10.1523/JNEUROSCI.3261-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschen S.L., Wietzikoski E.C., Winn P., Da Cunha C. The role of nucleus accumbens and dorsolateral striatal D2 receptors in active avoidance conditioning. Neurobiol. Learn. Mem. 2011;96:254–262. doi: 10.1016/j.nlm.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Boyson S.J., McGonigle P., Molinoff P.B. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J. Neurosci. November. 1986;6:3177–3188. doi: 10.1523/JNEUROSCI.06-11-03177.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin E.S., Matsumoto M., Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010 Dec 9;68(5):815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campese V., McCue M., Lázaro-Muñoz G., Ledoux J.E., Cain C.K. Development of an aversive Pavlovian-to-instrumental transfer task in rat. Front. Behav. Neurosci. 2013 Nov 26;7:176. doi: 10.3389/fnbeh.2013.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campese V.D., Sears R.M., Moscarello J.M., Diaz-Mataix L., Cain C.K., LeDoux J.E. The neural foundations of reaction and action in aversive motivation. Curr. Top. Behav. Neurosci. 2016;27:171–195. doi: 10.1007/7854_2015_401. [DOI] [PubMed] [Google Scholar]
- Carlezon W.A., Jr., Thomas M.J. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl. 1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho M.C., Albrechet-Souza L., Masson S., Brandão M.L. Changes in the biogenic amine content of the prefrontal cortex, amygdala, dorsal hippocampus, and nucleusaccumbens of rats submitted to single and repeated sessions of the elevatedplus-maze test. Braz. J. Med. Biol. Res. 2005 Dec;38(12):1857–1866. doi: 10.1590/s0100-879x2005001200014. [DOI] [PubMed] [Google Scholar]
- Chen X., Huddleston D.E., Langley J., Ahn S., Barnum C.J., Factor S.A., Levey A.I., Hu X. Simultaneous imaging of locus coeruleus and substantia nigra with a quantitative neuromelanin MRI approach. Magn. Reson. Imaging. 2014 Dec;32(10):1301–1306. doi: 10.1016/j.mri.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Cheng J., Feenstra M.G. Individual differences in dopamine efflux in nucleus accumbens shell and core during instrumental learning. Learn. Mem. 2006;13(2):168–177. doi: 10.1101/lm.1806. Mar-Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.S., Cain C.K., LeDoux J.E. The role of amygdala nuclei in the expression of auditory signaled two-way active avoidance in rats. Learn. Mem. 2010 Feb 26;17(3):139–147. doi: 10.1101/lm.1676610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S., Herry C., Grenier F., Wolff S.B., Letzkus J.J., Vlachos I. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010 Nov 11;468(7321):277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Clewett D.V., Huang R., Velasco R., Lee T.H., Mather M. Locus coeruleus activity strengthens prioritized memories under arousal. J. Neurosci. 2018 Feb 7;38(6):1558–1574. doi: 10.1523/JNEUROSCI.2097-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.D., Castro-Alamancos M.A. Early sensory pathways for detection of fearful conditioned stimuli: tectal and thalamic relays. J. Neurosci. 2007 Jul 18;27(29):7762–7776. doi: 10.1523/JNEUROSCI.1124-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.D., Castro-Alamancos M.A. Neural correlates of active avoidance behavior in superior colliculus. J. Neurosci. 2010 Jun 23;30(25):8502–8511. doi: 10.1523/JNEUROSCI.1497-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox S.M., Frank M.J., Larcher K., Fellows L.K., Clark C.A., Leyton M., Dagher A. Striatal D1 and D2 signaling differentially predict learning from positive and negative outcomes. Neuroimage. 2015 Apr 1;109:95–101. doi: 10.1016/j.neuroimage.2014.12.070. [DOI] [PubMed] [Google Scholar]
- Csillag A. Striato-telencephalic and striato-tegmental circuits: relevance to learning in domestic chicks. Behav. Brain Res. 1999 Feb 1;98(2):227–236. doi: 10.1016/s0166-4328(98)00088-6. [DOI] [PubMed] [Google Scholar]
- D'Amelio M., Serra L., Bozzali M. Ventral tegmental area in prodromal alzheimer's disease: bridging the gap between mice and humans. J. Alzheim. Dis. 2018;63(1):181–183. doi: 10.3233/JAD-180094. [DOI] [PubMed] [Google Scholar]
- Darvas M., Fadok J.P., Palmiter R.D. Requirement of dopamine signaling in the amygdala and striatum for learning and maintenance of a conditioned avoidance response. Learn. Mem. 2011 Feb 16;18(3):136–143. doi: 10.1101/lm.2041211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Mora M.P., Gallegos-Cari A., Arizmendi-García Y., Marcellino D., Fuxe K. Role of dopamine receptor mechanisms in the amygdaloid modulation of fear and anxiety: structural and functional analysis. Prog. Neurobiol. 2010 Feb 9;90(2):198–216. doi: 10.1016/j.pneurobio.2009.10.010. [DOI] [PubMed] [Google Scholar]
- De Marco M., Venneri A. Volume and connectivity of the ventral tegmental area are linked to neurocognitive signatures of alzheimer's disease in humans. J. Alzheim. Dis. 2018;63(1):167–180. doi: 10.3233/JAD-171018. [DOI] [PubMed] [Google Scholar]
- de Oliveira C.C., Gouveia F.V., de Castro M.C., Kuroki M.A., Dos Santos L.C., Fonoff E.T. A window on the study of aversive instrumental learning: strains, performance, neuroendocrine, and immunologic systems. Front. Behav. Neurosci. 2016 Aug 24;10:162. doi: 10.3389/fnbeh.2016.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza Caetano K.A., de Oliveira A.R., Brandão M.L. Dopamine D2 receptors modulate the expression of contextual conditioned fear: role of the ventral tegmental area and the basolateral amygdala. Behav. Pharmacol. 2013 Aug;24(4):264–274. doi: 10.1097/FBP.0b013e32836356c4. [DOI] [PubMed] [Google Scholar]
- Declercq M., De Houwer J. On the role of US expectancies in avoidance behavior. Psychon. Bull. Rev. 2008 Feb;15(1):99–102. doi: 10.3758/pbr.15.1.99. [DOI] [PubMed] [Google Scholar]
- Devoto P., Flore G., Pani L., Gessa G.L. Evidence for co-release of noradrenaline and dopamine from noradrenergic neurons in the cerebral cortex. Mol. Psychiatr. 2001 Nov;6(6):657–664. doi: 10.1038/sj.mp.4000904. [DOI] [PubMed] [Google Scholar]
- Devoto P., Flore G., Vacca G., Pira L., Arca A., Casu M.A. Co-release of noradrenaline and dopamine from noradrenergic neurons in the cerebral cortex induced by clozapine, the prototype atypical antipsychotic. Psychopharmacology (Berl.) 2003 Apr;167(1):79–84. doi: 10.1007/s00213-002-1381-y. [DOI] [PubMed] [Google Scholar]
- Devoto P., Flore G., Longu G., Pira L., Gessa G.L. Origin of extracellular dopamine from dopamine and noradrenaline neurons in the medial prefrontal and occipital cortex. Synapse. 2003 Dec 1;50(3):200–205. doi: 10.1002/syn.10264. [DOI] [PubMed] [Google Scholar]
- Devoto P., Flore G., Pira L., Longu G., Gessa G.L. Alpha2-adrenoceptor mediated co-release of dopamine and noradrenaline from noradrenergic neurons in the cerebral cortex. J. Neurochem. 2004 Feb;88(4):1003–1009. doi: 10.1046/j.1471-4159.2003.02239.x. [DOI] [PubMed] [Google Scholar]
- Devoto P., Flore G., Saba P., Fà M., Gessa G.L. Stimulation of the locus coeruleus elicits noradrenaline and dopamine release in the medial prefrontal and parietal cortex. J. Neurochem. 2005 Jan;92(2):368–374. doi: 10.1111/j.1471-4159.2004.02866.x. [DOI] [PubMed] [Google Scholar]
- Díaz-Véliz G., Mora S., Dossi M.T., Gómez P., Arriagada C., Montiel J. Behavioral effects of aminochrome and dopachrome injected in the rat substantia nigra. Pharmacol. Biochem. Behav. 2002 Nov;73(4):843–850. doi: 10.1016/s0091-3057(02)00923-1. [DOI] [PubMed] [Google Scholar]
- Doll B.B., Hutchison K.E., Frank M.J. Dopaminergic genes predict individual differences in susceptibility to confirmation bias. J. Neurosci. 2011 Apr 20;31(16):6188–6198. doi: 10.1523/JNEUROSCI.6486-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn A.J., Elfvin K.L., Berridge C.W. Changes in plasma corticosterone and cerebral biogenic amines and their catabolites during training and testing of mice in passive avoidance behavior. Behav. Neural. Biol. 1986 Nov;46(3):410–423. doi: 10.1016/s0163-1047(86)90422-x. [DOI] [PubMed] [Google Scholar]
- Essig J., Felsen G. Warning! Dopaminergic modulation of the superior colliculus. Trends Neurosci. 2016 Jan;39(1):2–4. doi: 10.1016/j.tins.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein D.L., Kalinin S., Braun D. Causes, consequences, and cures for neuroinflammation mediated via the locus coeruleus: noradrenergic signaling system. J. Neurochem. 2016 Oct;139(Suppl. 2):154–178. doi: 10.1111/jnc.13447. [DOI] [PubMed] [Google Scholar]
- Fernando A.B., Urcelay G.P., Mar A.C., Dickinson T.A., Robbins T.W. The role of the nucleus accumbens shell in the mediation of the reinforcing properties of a safety signal in free-operant avoidance: dopamine-dependent inhibitory effects of d-amphetamine. Neuropsychopharmacology. 2014 May;39(6):1420–1430. doi: 10.1038/npp.2013.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibiger H.C., Phillips A.G., Zis A.P. Deficits in instrumental responding after 6-hydroxydopamine lesions of the nigro-neostriatal dopaminergic projection. Pharmacol. Biochem. Behav. 1974;2(1):87–96. doi: 10.1016/0091-3057(74)90139-7. Jan-Feb. [DOI] [PubMed] [Google Scholar]
- Fiorillo C.D. Two dimensions of value: dopamine neurons represent reward but not aversiveness. Science. 2013 Aug 2;341(6145):546–549. doi: 10.1126/science.1238699. [DOI] [PubMed] [Google Scholar]
- Frederick A.L., Yano H., Trifilieff P., Vishwasrao H.D., Biezonski D., Mészáros J. Evidence against dopamine D1/D2 receptor heteromers. Mol. Psychiatr. 2015 Nov;20(11):1373–1385. doi: 10.1038/mp.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A., Frankel M., Flaumenhaft Y., Merenlender A., Pinhasov A., Feder Y. Programmed acute electrical stimulation of ventral tegmental area alleviates depressive-like behavior. Neuropsychopharmacology. 2009 Mar;34(4):1057–1066. doi: 10.1038/npp.2008.177. [DOI] [PubMed] [Google Scholar]
- Friedman A., Lax E., Abraham L., Tischler H., Yadid G. Abnormality of VTA local field potential in an animal model of depression was restored by patterned DBS treatment. Eur. Neuropsychopharmacol. 2012 Jan;22(1):64–71. doi: 10.1016/j.euroneuro.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Galatzer-Levy I.R., Moscarello J., Blessing E.M., Klein J., Cain C.K., LeDoux J.E. Heterogeneity in signaled active avoidance learning: substantive and methodological relevance of diversity in instrumental defensive responses to threat cues. Front. Syst. Neurosci. 2014 Sep 24;8:179. doi: 10.3389/fnsys.2014.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazit T., Friedman A., Lax E., Samuel M., Zahut R., Katz M., Abraham L., Tischler H., Teicher M., Yadid G. Programmed deep brain stimulation synchronizes VTA gamma band field potential and alleviates depressive-like behavior in rats. Neuropharmacology. 2015 Apr;91:135–141. doi: 10.1016/j.neuropharm.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Gentry R.N., Lee B., Roesch M.R. Phasic dopamine release in the rat nucleus accumbens predicts approach and avoidance performance. Nat. Commun. 2016 Oct 27;7:13154. doi: 10.1038/ncomms13154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich J.A., Caron M.G. Recent advances in the molecular biology of dopamine receptors. Annu. Rev. Neurosci. 1993;16:299–321. doi: 10.1146/annurev.ne.16.030193.001503. [DOI] [PubMed] [Google Scholar]
- Groman S.M., Massi B., Mathias S.R., Lee D., Taylor J.R. Model-free and model-based influences in addiction-related behaviors. Biol. Psychiatr. 2019 Jun 1;85(11):936–945. doi: 10.1016/j.biopsych.2018.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman S.P., Grossman L., Walsh L. Functional organization of the rat amygdala with respect to avoidance behavior. J. Comp. Physiol. Psychol. 1975 Feb;88(2):829–850. doi: 10.1037/h0076396. [DOI] [PubMed] [Google Scholar]
- Haubensak W., Kunwar P.S., Cai H., Ciocchi S., Wall N.R., Ponnusamy R. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010 Nov 11;468(7321):270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner T.G., Vosmer G., Seiden L.S. Increased transport of 3,4-dihydroxyphenylacetic acid from brain during performance of operant behavior in the rat. Brain Res. 1984 Feb 13;293(1):85–91. doi: 10.1016/0006-8993(84)91455-0. [DOI] [PubMed] [Google Scholar]
- Hormigo S., Vega-Flores G., Castro-Alamancos M.A. Basal ganglia output controls active avoidance behavior. J. Neurosci. 2016 Oct 5;36(40):10274–10284. doi: 10.1523/JNEUROSCI.1842-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormigo S., Vega-Flores G., Rovira V., Castro-Alamancos M.A. Circuits that mediate expression of signaled active avoidance converge in the pedunculopontine tegmentum. J. Neurosci. 2019 Jun 5;39(23):4576–4594. doi: 10.1523/JNEUROSCI.0049-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado-Parrado C., Acevedo-Triana C., Pear J. Swimming against the current": behavioral data of Betta splendens during an escape and avoidance task with water flows as the aversive stimulus. Data Brief. 2019 Jul 15;25:104260. doi: 10.1016/j.dib.2019.104260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huys Q.J.M., Dayan P. A Bayesian formulation of behavioral control. Cognition. 2009 Dec;113(3):314–328. doi: 10.1016/j.cognition.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Introini-Collison I.B., Miyazaki B., McGaugh J.L. Involvement of the amygdala in the memory-enhancing Itoh K, Konishi A, Nomura S, Mizuno N, Nakamura Y, Sugimoto T. Application of coupled oxidation reaction to electron microscopic demonstration of horseradish peroxidase: cobalt-glucose oxidase method. Brain Res. 1979 Oct 19;175(2):341–346. doi: 10.1016/0006-8993(79)91013-8. [DOI] [PubMed] [Google Scholar]
- Jellestad F.K., Bakke H.K. Passive avoidance after ibotenic acid and radio frequency lesions in the rat amygdala. Physiol. Behav. 1985 Feb;34(2):299–305. doi: 10.1016/0031-9384(85)90119-2. [DOI] [PubMed] [Google Scholar]
- Jiao X., Beck K.D., Myers C.E., Servatius R.J., Pang K.C. Altered activity of the medial prefrontal cortex and amygdala during acquisition and extinction of an active avoidance task. Front. Behav. Neurosci. 2015 Sep 15;9:249. doi: 10.3389/fnbeh.2015.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L.Q., Wang H.Y., Friedman E. Stimulated D(1) dopamine receptors couple to multiple Galpha proteins in different brain regions. J. Neurochem. 2001 Sep;78(5):981–990. doi: 10.1046/j.1471-4159.2001.00470.x. [DOI] [PubMed] [Google Scholar]
- Johnson J.D., Li W., Li J., Klopf A.H. A computational model of learned avoidance behavior in a one-way avoidance experiment. Adapt. Behav. 2001;9(2):91–104. [Google Scholar]
- Keeley R.J., Bye C., Trow J., McDonald R.J. Strain and sex differences in brain and behaviour of adult rats: learning and memory, anxiety and volumetric estimates. Behav. Brain Res. 2015 Jul 15;288:118–131. doi: 10.1016/j.bbr.2014.10.039. [DOI] [PubMed] [Google Scholar]
- Klanker M., Sandberg T., Joosten R., Willuhn I., Feenstra M., Denys D. Phasic dopamine release induced by positive feedback predicts individual differences in reversal learning. Neurobiol. Learn. Mem. 2015 Nov;125:135–145. doi: 10.1016/j.nlm.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F., Simon H., Herman J.P., Le Moal M. Neuroleptic-like disruption of the conditioned avoidance response requires destruction of both the mesolimbic and nigrostriatal dopamine systems. Brain Res. 1984 Jun 15;303(2):319–329. doi: 10.1016/0006-8993(84)91218-6. [DOI] [PubMed] [Google Scholar]
- Korn C.W., Vunder J., Miró J., Fuentemilla L., Hurlemann R., Bach D.R. Amygdala lesions reduce anxiety-like behavior in a human benzodiazepine-sensitive approach-avoidance conflit test. Biol. Psychiatr. 2017 Oct 1;82(7):522–531. doi: 10.1016/j.biopsych.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krypotos A.M., Effting M., Kindt M., Beckers T. Avoidance learning: a review of theoretical models and recent developments. Front. Behav. Neurosci. 2015 Jul 21;9:189. doi: 10.3389/fnbeh.2015.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe L. Avoidant personality disorder as a social anxiety phenotype: risk factors, associations and treatment. Curr. Opin. Psychiatr. 2016 Jan;29(1):64–69. doi: 10.1097/YCO.0000000000000211. [DOI] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. Emotion, motivation, and anxiety: brain mechanisms and psychophysiology. Biol. Psychiatr. 1998 Dec 15;44(12):1248–1263. doi: 10.1016/s0006-3223(98)00275-3. [DOI] [PubMed] [Google Scholar]
- Lázaro-Muñoz G., LeDoux J.E., Cain C.K. Sidman instrumental avoidance initially depends on lateral and basal amygdala and is constrained by central amygdala-mediated Pavlovian processes. Biol. Psychiatr. 2010 Jun 15;67(12):1120–1127. doi: 10.1016/j.biopsych.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. vol. 107. Elsevier; 1996. Chapter 26 Emotional networks and motor control: a fearful view; pp. 437–446. (The Emotional Motor System). [DOI] [PubMed] [Google Scholar]
- LeDoux J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E., Moscarello J., Sears R., Campese V. The birth, death and resurrection of avoidance: a reconceptualization of a troubled paradigm. Mol. Psychiatr. 2017 Jan;22(1):24–36. doi: 10.1038/mp.2016.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner T.N., Shilyansky C., Davidson T.J., Evans K.E., Beier K.T., Zalocusky K.A. Intact-brain analyses reveal distinct information carried by SNc dopamine subcircuits. Cell. 2015 Jul 30;162(3):635–647. doi: 10.1016/j.cell.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey A.I., Hersch S.M., Rye D.B., Sunahara R.K., Niznik H.B., Kitt C.A. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc. Natl. Acad. Sci. U. S. A. 1993 Oct 1;90(19):8861–8865. doi: 10.1073/pnas.90.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenberg N.T., Lee B., Kashtelyan V., Chappa B.S., Girma H.T., Green E.A. Rat behavior and dopamine release are modulated by conspecific distress. Elife. 2018 Nov 28;7 doi: 10.7554/eLife.38090. pii: e38090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisieski M.J., Karavidha K., Gheidi A., Garibyan R.L., Conti A.C., Morrow J.D. Divergent effects of repeated cocaine and novel environment exposure on locus coeruleus c-fos expression and brain catecholamine concentrations in rats. Brain Behav. 2019 Mar;9(3) doi: 10.1002/brb3.1222. Epub 2019 Feb 20. PubMed PMID: 30790470; PubMed Central PMCID: PMC6422811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd K., Dayan P. Safety out of control: dopamine and defence. Behav. Brain Funct. 2016 May 23;12(1):15. doi: 10.1186/s12993-016-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louilot A., Le Moal M., Simon H. Differential reactivity of dopaminergic neurons in the nucleus accumbens in response to different behavioral situations. An in vivo voltammetric study in free moving rats. Brain Res. 1986 Nov 12;397(2):395–400. doi: 10.1016/0006-8993(86)90646-3. [DOI] [PubMed] [Google Scholar]
- Macedo C.E., Martinez R.C., de Souza Silva M.A., Brandão M.L. Increases in extracellular levels of 5-HT and dopamine in the basolateral, but not in the central, nucleus of amygdala induced by aversive stimulation of the inferior colliculus. Eur. J. Neurosci. 2005 Feb;21(4):1131–1138. doi: 10.1111/j.1460-9568.2005.03939.x. [DOI] [PubMed] [Google Scholar]
- Mahan A.L., Ressler K.J. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2012 Jan;35(1):24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia T.V. Two-factor theory, the actor-critic model, and conditioned avoidance. Learn. Behav. 2010 Feb;38(1):50–67. doi: 10.3758/LB.38.1.50. [DOI] [PubMed] [Google Scholar]
- Martinez R.C., Gupta N., Lázaro-Muñoz G., Sears R.M., Kim S., Moscarello J.M., LeDoux J.E., Cain C.K. Active vs. reactive threat responding is associated with differential c-Fos expression in specific regions of amygdala and prefrontal cortex. Learn. Mem. 2013 Jul 18;20(8):446–452. doi: 10.1101/lm.031047.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegas W., Bergan J.F., Ogawa S.K., Isogai Y., Umadevi Venkataraju K., Osten P. Dopamine neurons projecting to the posterior striatum form an anatomically distinct subclass. Elife. 2015 Aug 31;4 doi: 10.7554/eLife.10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens G., Van Dessel P., De Houwer J. The contextual malleability of approach-avoidance training effects: approaching or avoiding fear conditioned stimuli modulates effects of approach-avoidance training. Cognit. Emot. 2018 Mar;32(2):341–349. doi: 10.1080/02699931.2017.1308315. [DOI] [PubMed] [Google Scholar]
- Miller S.M., Marcotulli D., Shen A., Zweifel L.S. Divergent medial amygdala projections regulate approach-avoidance conflict behavior. Nat. Neurosci. 2019 Apr;22(4):565–575. doi: 10.1038/s41593-019-0337-z. Epub 2019 Feb 25. PubMed PMID: 30804529; PubMed Central PMCID: PMC6446555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moitra E., Herbert J.D., Forman E.M. Behavioral avoidance mediates the relationship between anxiety and depressive symptoms among social anxiety disorder patients. J. Anxiety Disord. 2008 Oct;22(7):1205–1213. doi: 10.1016/j.janxdis.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Moreira C.G., Barbiero J.K., Ariza D., Dombrowski P.A., Sabioni P., Bortolanza M. Behavioral, neurochemical and histological alterations promoted by bilateral intranigral rotenone administration: a new approach for an old neurotoxin. Neurotox. Res. 2012 Apr;21(3):291–301. doi: 10.1007/s12640-011-9278-3. [DOI] [PubMed] [Google Scholar]
- Moscarello J.M., LeDoux J.E. Active avoidance learning requires prefrontal suppression of amygdala-mediated defensive reactions. J. Neurosci. 2013 Feb 27;33(9):3815–3823. doi: 10.1523/JNEUROSCI.2596-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa A.A., Cohen M.X., Sherman S.J., Frank M.J. A role for dopamine in temporal decision making and reward maximization in parkinsonism. J. Neurosci. 2008 Nov 19;28(47):12294–12304. doi: 10.1523/JNEUROSCI.3116-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozhui K., Hamre K.M., Holmes A., Lu L., Williams R.W. Genetic and structural analysis of the basolateral amygdala complex in BXD recombinant inbred mice. Behav. Genet. 2007 Jan;37(1):223–243. doi: 10.1007/s10519-006-9122-3. [DOI] [PubMed] [Google Scholar]
- Nader K., LeDoux J.E. Inhibition of the mesoamygdala dopaminergic pathway impairs the retrieval of conditioned fear associations. Behav. Neurosci. 1999 Oct;113(5):891–901. doi: 10.1037//0735-7044.113.5.891. [DOI] [PubMed] [Google Scholar]
- Nasello A.G., Felicio L.F. Avoidance behavior, prolactin, HVA and DOPAC in offspring of bromopride-treated rats. Pharmacol. Biochem. Behav. 1990 Nov;37(3):571–575. doi: 10.1016/0091-3057(90)90030-l. [DOI] [PubMed] [Google Scholar]
- Neve K.A., Seamans J.K., Trantham-Davidson H. Dopamine receptor signaling. J. Recept. Signal Transduct. Res. 2004 Aug;24(3):165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- Ng A., Lovibond P.F. Intentions matter: avoidance intentions regulate anxiety via outcome expectancy. Behav. Res. Ther. 2017 Sep;96:57–65. doi: 10.1016/j.brat.2017.03.007. [DOI] [PubMed] [Google Scholar]
- Nguyen D., Alushaj E., Erb S., Ito R. Dissociative effects of dorsomedial striatum D1 and D2 receptor antagonism in the regulation of anxiety and learned approach-avoidance conflict decision-making. Neuropharmacology. 2019 Mar 1;146:222–230. doi: 10.1016/j.neuropharm.2018.11.040. [DOI] [PubMed] [Google Scholar]
- Oleson E.B., Gentry R.N., Chioma V.C., Cheer J.F. Subsecond dopamine release in the nucleus accumbens predicts conditioned punishment and its successful avoidance. J. Neurosci. 2012;32(42):14804–14808. doi: 10.1523/JNEUROSCI.3087-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onali P., Olianas M.C., Gessa G.L. Characterization of dopamine receptors mediating inhibition of adenylate cyclase activity in rat striatum. Mol. Pharmacol. 1985 Aug;28(2):138–145. [PubMed] [Google Scholar]
- Overmier J.B., Seligman M.E. Effects of inescapable shock upon subsequent escape and avoidance responding. J. Comp. Physiol. Psychol. 1967;63(1):28–33. doi: 10.1037/h0024166. [DOI] [PubMed] [Google Scholar]
- Paul C.A., Beltz B., Berger-Sweeney J. The nissl stain: a stain for cell bodies in brain sections. Cold Spring Harb. Protoc. 2008 Sep 1 doi: 10.1101/pdb.prot4805. 2008:pdb.prot4805. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. Academic Press; 2005. The Rat Brain in Stereotaxic Coordinates - the New Coronal Set. [Google Scholar]
- Powell R.W. A comparison of signaled vs. unsignaled free-operant avoidance in wild and domesticated rats. Anim. Learn. Behav. 1976;4:279–286. doi: 10.3758/BF03214050. [DOI] [Google Scholar]
- Pultorak K.J., Schelp S.A., Isaacs D.P., Krzystyniak G., Oleson E.B. A transient dopamine signal represents avoidance value and causally influences the demand to avoid. eNeuro. 2018 May 15;5(2) doi: 10.1523/ENEURO.0058-18.2018. pii: ENEURO.0058-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwanska K., Nikolaev E., Kaczmarek L. Central noradrenergic lesion induced by DSP-4 impairs the acquisition of avoidance reactions and prevents molecular changes in the amygdala. Neurobiol. Learn. Mem. 2010 Oct;94(3):303–311. doi: 10.1016/j.nlm.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Ramirez F., Moscarello J.M., LeDoux J.E., Sears R.M. Active avoidance requires a serial basal amygdala to nucleus accumbens shell circuit. J. Neurosci. 2015 Feb 25;35(8):3470–3477. doi: 10.1523/JNEUROSCI.1331-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall P.A., Pardo M., Nunes E.J., López Cruz L., Vemuri V.K., Makriyannis A. Dopaminergic modulation of effort-related choice behavior as assessed by a progressive ratio chow feeding choice task: pharmacological studies and the role of individual differences. PloS One. 2012;7(10) doi: 10.1371/journal.pone.0047934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis F.L., Masson S., de Oliveira A.R., Brandão M.L. Dopaminergic mechanisms in the conditioned and unconditioned fear as assessed by the two-way avoidance and light switch-off tests. Pharmacol. Biochem. Behav. 2004 Oct;79(2):359–365. doi: 10.1016/j.pbb.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Rigoli F., Chew B., Dayan P., Dolan R.J. Multiple value signals in dopaminergic midbrain and their role in avoidance contexts. Neuroimage. 2016 Jul 15;135:197–203. doi: 10.1016/j.neuroimage.2016.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Río-Álamos C., Oliveras I., Piludu M.A., Gerbolés C., Cañete T., Blázquez G. Neonatal handling enduringly decreases anxiety and stress responses and reduces hippocampus and amygdala volume in a genetic model of differential anxiety: behavioral-volumetric associations in the Roman rat strains. Eur. Neuropsychopharmacol. 2017 Feb;27(2):146–158. doi: 10.1016/j.euroneuro.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Rorick-Kehn L.M., Steinmetz J.E. Amygdalar unit activity during three learning tasks: eyeblink classical conditioning, Pavlovian fear conditioning, and signaled avoidance conditioning. Behav. Neurosci. 2005 Oct;119(5):1254–1276. doi: 10.1037/0735-7044.119.5.1254. [DOI] [PubMed] [Google Scholar]
- Rouillard C., Freeman A.S. Effects of electrical stimulation of the central nucleus of the amygdala on the in vivo electrophysiological activity of rat nigral dopaminergic neurons. Synapse. 1995 Dec;21(4):348–356. doi: 10.1002/syn.890210410. [DOI] [PubMed] [Google Scholar]
- Sabban E.L., Serova L.I., Newman E., Aisenberg N., Akirav I. Changes in gene expression in the locus coeruleus-amygdala circuitry in inhibitory avoidance PTSD model. Cell. Mol. Neurobiol. 2018 Jan;38(1):273–280. doi: 10.1007/s10571-017-0548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna F., Piludu M.A., Corda M.G., Argiolas A., Giorgi O., Melis M.R. Dopamine is involved in the different patterns of copulatory behaviour of Roman high and low avoidance rats: studies with apomorphine and haloperidol. Pharmacol. Biochem. Behav. 2014 Sep;124:211–219. doi: 10.1016/j.pbb.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Sanna F., Bratzu J., Piludu M.A., Corda M.G., Melis M.R., Giorgi O. Dopamine, noradrenaline and differences in sexual behavior between roman high and low avoidance male rats: a microdialysis study in the medial prefrontal cortex. Front. Behav. Neurosci. 2017 Jun 7;11:108. doi: 10.3389/fnbeh.2017.00108.eCollection2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlund M.W., Cataldo M.F. Amygdala involvement in human avoidance, escape and approach behavior. Neuroimage. 2010 Nov 1;53(2):769–776. doi: 10.1016/j.neuroimage.2010.06.058. Epub 2010 Jun 30. PubMed PMID: 20600966; PubMed Central PMCID: PMC2930061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlund M.W., Magee S., Hudgins C.D. Human avoidance and approach learning: evidence for overlapping neural systems and experiential avoidance modulation of avoidance neurocircuitry. Behav. Brain Res. 2011 Dec 1;225(2):437–448. doi: 10.1016/j.bbr.2011.07.054. [DOI] [PubMed] [Google Scholar]
- Schwarz L.A., Luo L. Organization of the locus coeruleus-norepinephrine system. Curr. Biol. 2015 Nov 2;25(21):R1051–R1056. doi: 10.1016/j.cub.2015.09.039. [DOI] [PubMed] [Google Scholar]
- Scibilia R.J., Lachowicz J.E., Kilts C.D. Topographic nonoverlapping distribution of D1 and D2 dopamine receptors in the amygdaloid nuclear complex of the rat brain. Synapse. 1992 Jun;11(2):146–154. doi: 10.1002/syn.890110208. [DOI] [PubMed] [Google Scholar]
- Shelkar G.P., Gakare S.G., Chakraborty S., Dravid S.M., Ugale R.R. Interactions of nitric oxide with α2 -adrenoceptors within the locus coeruleus underlie the facilitation of inhibitory avoidance memory by agmatine. Br. J. Pharmacol. 2016 Sep;173(17):2589–2599. doi: 10.1111/bph.13531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheynin J., Beck K.D., Servatius R.J., Myers C.E. Acquisition and extinction of human avoidance behavior: attenuating effect of safety signals and associations with anxiety vulnerabilities. Front. Behav. Neurosci. 2014 Sep 15;8:323. doi: 10.3389/fnbeh.2014.00323.eCollection2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner B.F. Appleton-Century-Crofts, Educational Division, Meredith Corporation; 1969. Contingencies of Reinforcement: A Theoretical Analysis. [Google Scholar]
- Stamatakis A.M., Stuber G.D. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat. Neurosci. 2012 Jun 24;15(8):1105–1107. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana R., Ogundele O.M., Lee C.C. Contrasting characteristic behaviours among common laboratory mouse strains. R. Soc. Open Sci. 2019 Jun 12;6(6):190574. doi: 10.1098/rsos.190574.eCollection2019Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson L.W. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res. Bull. 1982 Jul-Dec;9(1–6):321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Takamatsu Y., Hagino Y., Sato A., Takahashi T., Nagasawa S.Y. Improvement of learning and increase in dopamine level in the frontal cortex by methylphenidate in mice lacking dopamine transporter. Curr. Mol. Med. 2015;15(3):245–252. doi: 10.2174/1566524015666150330144018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilas P., Ehrenberg A.J., Dunlop S., Di Lorenzo Alho A.T., Nguy A., Leite R.E.P. Locus coeruleus volume and cell population changes during Alzheimer's disease progression: a stereological study in human postmortem brains with potential implication for early-stage biomarker discovery. Alzheimer's Dementia. 2017 Mar;13(3):236–246. doi: 10.1016/j.jalz.2016.06.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A., Aguado A.S., Pohorecky L.A., Benjamin D. Individual differences in pavlovian autoshaping of lever pressing in rats predict stress-induced corticosterone release and mesolimbic levels of monoamines. Pharmacol. Biochem. Behav. 2000 Mar;65(3):509–517. doi: 10.1016/s0091-3057(99)00241-5. [DOI] [PubMed] [Google Scholar]
- Troncoso A.C., Cirilo-Júnior G., Sandner G., Brandão M.L. Signaled two-way avoidance learning using electrical stimulation of the inferior colliculus as negative reinforcement: effects of visual and auditory cues as warning stimuli. Braz. J. Med. Biol. Res. 1998 Mar;31(3):391–398. doi: 10.1590/s0100-879x1998000300011. [DOI] [PubMed] [Google Scholar]
- Ulrich R.E., Holz W.C., Azrin N.H. Stimulus control of avoidance behavior. J. Exp. Anal. Behav. 1964 Mar;7:129–133. doi: 10.1901/jeab.1964.7-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undie A.S., Friedman E. Stimulation of a dopamine D1 receptor enhances inositol phosphates formation in rat brain. J. Pharmacol. Exp. Therapeut. 1990 Jun;253(3):987–992. [PubMed] [Google Scholar]
- Vollbrecht P.J., Nobile C.W., Chadderdon A.M., Jutkiewicz E.M., Ferrario C.R. Pre-existing differences in motivation for food and sensitivity to cocaine-induced locomotion in obesity-prone rats. Physiol. Behav. 2015 Dec 1;152(Pt A):151–160. doi: 10.1016/j.physbeh.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadenberg M.L., Hicks P.B. The conditioned avoidance response test re-evaluated: is it a sensitive test for the detection of potentially atypical antipsychotics? Neurosci. Biobehav. Rev. 1999;23(6):851–862. doi: 10.1016/s0149-7634(99)00037-8. [DOI] [PubMed] [Google Scholar]
- Wadenberg M.L., Ericson E., Magnusson O., Ahlenius S. Suppression of conditioned avoidance behavior by the local application of (-)sulpiride into the ventral, but not the dorsal, striatum of the rat. Biol. Psychiatr. 1990 Aug 15;28(4):297–307. doi: 10.1016/0006-3223(90)90657-n. [DOI] [PubMed] [Google Scholar]
- Watabe-Uchida M., Zhu L., Ogawa S.K., Vamanrao A., Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012 Jun 7;74(5):858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Weiner D.M., Levey A.I., Sunahara R.K., Niznik H.B., O'Dowd B.F., Seeman P., Brann M.R. D1 and D2 dopamine receptor mRNA in rat brain. Proc. Natl. Acad. Sci. U. S. A. 1991 Mar 1;88(5):1859–1863. doi: 10.1073/pnas.88.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel J.M., Oleson E.B., Gove W.N., Cole A.B., Gyawali U., Dantrassy H.M. Phasic dopamine signals in the nucleus accumbens that cause active avoidance require endocannabinoid mobilization in the midbrain. Curr. Biol. 2018 May 7;28(9):1392–1404. doi: 10.1016/j.cub.2018.03.037. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werka T., Skår J., Ursin H. Exploration and avoidance in rats with lesions in amygdala and piriform cortex. J. Comp. Physiol. Psychol. 1978 Aug;92(4):672–681. doi: 10.1037/h0077505. [DOI] [PubMed] [Google Scholar]
- Wietzikoski E.C., Boschen S.L., Miyoshi E., Bortolanza M., Dos Santos L.M., Frank M. Roles of D1-like dopamine receptors in the nucleus accumbens and dorsolateral striatum in conditioned avoidance responses. Psychopharmacology (Berl.) 2012 Jan;219(1):159–169. doi: 10.1007/s00213-011-2384-3. [DOI] [PubMed] [Google Scholar]
- Wilensky A.E., Schafe G.E., LeDoux J.E. The amygdala modulates memory consolidation of fear-motivated inhibitory avoidance learning but not classical fear conditioning. J. Neurosci. 2000 Sep 15;20(18):7059–7066. doi: 10.1523/JNEUROSCI.20-18-07059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky A.E., Schafe G.E., Kristensen M.P., LeDoux J.E. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J. Neurosci. 2006 Nov 29;26(48):12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2004. International Statistical Classification of Diseases and Related Health Problems. [Google Scholar]
- Yadid G., Friedman A. Dynamics of the dopaminergic system as a key component to the understanding of depression. Prog. Brain Res. 2008;172:265–286. doi: 10.1016/S0079-6123(08)00913-8. [DOI] [PubMed] [Google Scholar]
- Yapo C., Nair A.G., Clement L., Castro L.R., Hellgren Kotaleski J., Vincent P. Detection of phasic dopamine by D1 and D2 striatal medium spiny neurons. J. Physiol. 2017 Dec 15;595(24):7451–7475. doi: 10.1113/JP274475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbi V., Floriou-Servou A., Markicevic M., Vermeiren Y., Sturman O., Privitera M. Rapid reconfiguration of the functional connectome after chemogenetic locus coeruleus activation. Neuron. 2019 Aug 21;103(4):702–718. doi: 10.1016/j.neuron.2019.05.034. e5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.