Summary
Chronic spontaneous urticaria (CSU) pathogenesis shows a complex and still unclear interplay between immunoglobulin (Ig)G‐ and IgE‐mediated autoimmunity, leading to mast cell and basophil degranulation and wheal formation. The objective of this study was to evaluate at the same time IgE‐ and IgG‐reactivity to well recognized and recently reported autoantigens in CSU patients, and to assess the effects of such reactivity on response to the anti‐IgE monoclonal antibody omalizumab. Twenty CSU patients underwent omalizumab treatment. Urticaria activity score 7 (UAS7) was recorded at baseline and at different drug administration time‐points for categorizing early‐, late‐ or non‐responders. At baseline, sera from the 20 patients and from 20 controls were tested for IgE and IgG autoantibodies to high‐ and low‐affinity IgE receptors (FcεRI and FcεRII), tissue factor (TF) and thyroglobulin (TG) by immunoenzymatic methods. Antibody levels were compared with those of controls and analysed according to response. Eighteen patients were omalizumab responders (11 early and seven late), while two were non‐responders. More than 50% of patients had contemporary IgE and IgG to at least to one of the four different autoantigens. Late responders showed higher levels of both anti‐TF IgE and IgG than early responders (P = 0·011 and P = 0·035, respectively). Twenty‐five per cent of patients had levels of anti‐FcεRI IgE, exceeding the upper normal limit, suggesting that it could be a novel auto‐allergen in CSU. In CSU, there is an autoimmune milieu characterized by the co‐existence of IgE and IgG autoantibodies to the same antigen/allergen, particularly in late responders to omalizumab, possibly explaining the slower response.
Keywords: autoimmunity, IgE, IgG, omalizumab, urticaria
Chronic spontaneous urticaria (CSU) has a complex and still unclear pathogenesis mainly involving autoimmunity and/or autoallergy, eventually leading to mast cell/basophil degranulation and wheal formation. The humanized monoclonal anti‐IgE antibody omalizumab is very effective in the treatment of CSU resistant to antihistamines; however, several patients show a late response to this drug. In CSU patients, our study demonstrates the coexistence of IgE‐ and IgG‐mediated autoimmunity to the same autoantigens, particularly in late omalizumab responders, explaining the slower clinical response to the drug when the high affinity IgE receptor (FcεRI) is involved.

Introduction
Chronic spontaneous urticaria (CSU), defined as the recurrent occurrence of wheals sometimes associated with angioedema for more than 6 weeks, is a common and potentially disabling disease. The long‐term uncertainty that has surrounded its pathogenesis is gradually clearing. The finding, some 20–30 years ago, of functionally active immunoglobulin (Ig)G autoantibodies to the high‐affinity IgE receptor (FcεRI) or to IgE but only in a minority of patients with CSU 1, 2, 3, 4 has, in more recent years, been reached by the detection of IgE class autoantibodies, first to thyroid peroxidase 5 and subsequently to a number of autoallergens 6, including thyroglobulin (TG) and tissue factor (TF) 7, in a much larger proportion of cases. This latter finding has provided a solid rationale for the very rapid response to omalizumab, a humanized anti‐IgE monoclonal antibody (mAb), which is observed in a large proportion of patients with severe CSU. The co‐existence of both IgG‐mediated (type IIb after Gell and Coombs) autoimmunity and of IgE‐mediated (type I) autoimmunity in patients with CSU and its effect on omalizumab response has not been investigated so far. In the present study, we measured IgE and IgG reactivity to four autoantigens in patients with CSU: two well‐recognized, such as high‐ and low‐affinity IgE receptors (FcεRI and FcεRII) and two recently reported, such as TF and TG.
Patients and methods
Patients
Twenty patients (three male, 17 female; median age = 53·5 years, min 24–max 80 years) with severe CSU (UAS7 > 25) underwent subcutaneous omalizumab treatment at a dose of 300 g/month following the indications of the European Academy of Allergy and Clinical Immunology (EAACI) guidelines 8. Patients were evaluated at baseline and 1, 3 and 4 months after omalizumab treatment initiation and UAS7 was recorded at each time‐point in order to categorize the patients into early‐, late‐ or non‐responders. Clinical response was considered as early if it occurred within 4 weeks after the first administration with reduction of UAS7 to at least < 6, late if the response was observed within 3 months with a reduction to at least < 6, or absent (symptoms remaining unchanged 1 month after the third administration) 9.
The clinical response to omalizumab is depicted in Table 1. Eighteen (90%) patients were considered omalizumab responders; 11 were early responders and seven were late responders. Two patients did not show any response to the drug.
Table 1.
Urticaria activity score values over 7 days (UAS7) at different time‐points based on omalizumab response in 20 patients with chronic spontaneous urticaria
| UAS7 at baseline Median (min–max) | UAS7 1 month after starting omalizumab Median (min–max) | UAS7 3 months after starting omalizumab Median (min–max) | |
|---|---|---|---|
| Early responders, n = 11 | 31 (16–42) | 0 (0–6) | 0 (0–5) |
| Late responders, n = 7 | 33 (22–37) | 16 (8–30) | 3 (0–6) |
| Non‐responders, n = 2 | 31 (27–35) | 29 (27–30) | 18 (14–22) |
At baseline, sera from the 20 CSU patients and from 20 sex‐ and age‐matched healthy controls were studied for IgE and IgG autoantibodies specific for TF, TG, high‐affinity IgE receptor (FcεRI) and low‐affinity IgE receptor (FcεRII). The antigens to be tested were chosen: (i) in view of the well‐known association of CSU with IgG‐mediated thyroid autoimmunity 10 together with the more recently described IgE thyroid autoreactivity 5, 7; (ii) in order to detect whether the high‐ and the low‐affinity IgE receptors might represent a direct target for IgE‐mediated autoimmune responses, as is the case with the well‐known IgG autoimmunity 3, 11; and (iii) in consideration of all the recent work showing the activation of the coagulation cascade in patients with severe CSU, with the aim of detecting whether IgE autoallergy to TF (the main initiator of coagulation) may occur in CSU 12. In both patients and controls, endocrine and coagulation diseases were ruled out; thyroid hormone levels as well as anti‐nuclear, anti‐extractable nuclear antigen and anti‐dsDNA antibodies were normal or negative. In both groups, atopic status was excluded based on clinical history (absence of atopic symptoms). Moreover, in CSU patients, an accurate diagnostic work‐up was performed, including skin‐prick tests with commercial extracts of the most common airborne allergens and foods. Total IgE, measured by an immunoenzymatic method, were 186 kUA/l (19‐1027 kUA/l) in patients and 40 kUA/l (4‐102 kUA/l) in controls.
The study was conducted following the ethical principles of the Declaration of Helsinki, regulatory requirements and the code of Good Clinical Practice. Patients and controls gave an informed written consent to the use of their sera and relative data. The local review board approved the study.
Detection of specific IgE and IgG
Anti‐TF, anti‐TG, anti‐ FcεRI and anti‐ FcεRII IgE and IgG autoantibodies were measured by sandwich enzyme‐linked immunosorbent assay (ELISA). Each antigen [TF (recombinant TF; Haematologic Technologies Inc., Essex, VT, USA); TG (human TG; Sigma, St Louis, MO, USA); FcεRI (human FcERI; Sino Biological, Wayne, PA, USA) and FcεRII (recombinant human CD23; Abcam, Cambridge, UK)] was coated overnight onto microtitration plates at a concentration of 10 μg/ml and, after washing, the residual binding sites were blocked with bovine serum albumin 1%. After further washes, plasma dilutions (1 : 10, 1 : 100 and 1 : 1000) from patients and controls were added and incubated for 45 min at room temperature. After washing, goat anti‐human IgE (ε chain‐specific) (Sigma) was added for the determination of specific IgE and, after further washing, peroxidase‐conjugated donkey polyclonal anti‐goat IgG (Santa Cruz Biotechnology Inc, Dallas, TX, USA) was added. For the determination of specific total IgG, we added a goat anti‐human IgG (γ‐chain‐specific) horseradish peroxidase (HRP) conjugate (Sigma); after further washing, rabbit polyclonal anti‐mouse IgG peroxidase‐conjugated was added. All the reactions were revealed by ortho‐phenylenediamine (Sigma). As cut‐off levels, we adopted the highest value recorded among normal controls for each assay; levels exceeding that value among patients were regarded as positive.
Statistical analysis
The results are expressed as median and ranges (min–max). The non‐parametric Mann–Whitney U‐test for unpaired values was used to assess statistical significance of differences between groups. Significance level was set at P < 0·05. The sample size was calculated in order to obtain a statistical power of 80%, with an alpha error of 5%, based on two previous studies. The first, by Ulambayar et al. 13, showed 54% higher levels of IgG anti‐FcεRI in patients with CSU than in normal controls, while the second, by Cugno et al. 7, showed more than 111% higher levels of IgE anti‐TF and anti‐TG in CSU patients than in normal controls.
Data were analysed using the spss PC statistical package version 25.0 (IBM, Armonk, NY, USA).
Results
IgE and IgG autoantibodies to TF
TF IgE and IgG autoreactivity is depicted in Fig. 1. Median TF IgE levels did not differ between patients [median = 498 optical density (OD) (min–max = 73–1597 OD)] and controls [363 OD (min–max = 50–475 OD)] (P = not significant (n.s.). However, within the patient group, late responders showed significantly higher levels of anti‐TF IgE [815 OD (min–max = 168–1597 OD)] than early responders [415 OD (min–max = 87–806 OD)] (P = 0·011). The two non‐responders showed very low levels of TF IgE.
Fig. 1.
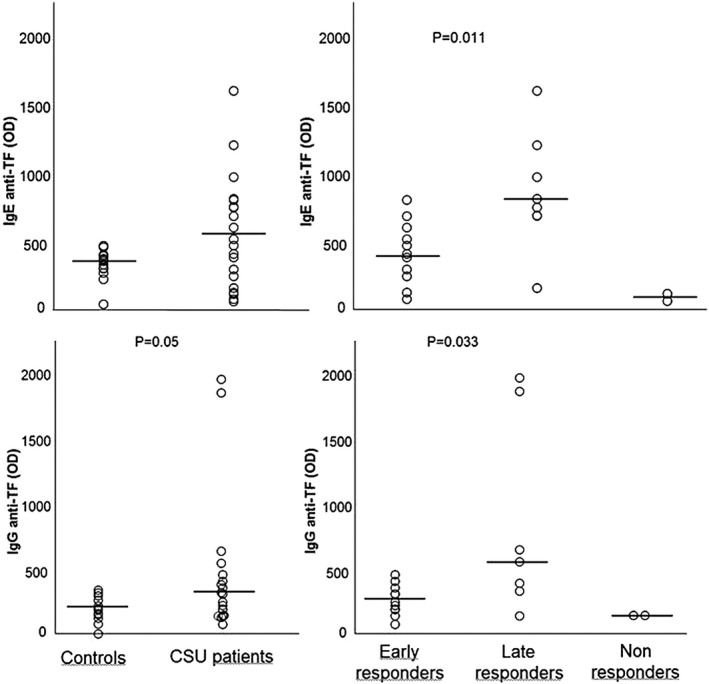
Immunoglobulin (Ig)E and IgG autoimmune response to tissue factor (TF) in healthy controls and patient subgroups divided based on clinical response to omalizumab. Horizontal lines represent medians.
Median TF IgG levels were higher in patients [314 OD (min–max = 84–1956 OD)] than in controls [197 OD (min–max = 11–343 OD)], although probably due to small numbers the difference did not reach statistical significance (P = 0·051). Again, marked differences were found within the patient group, as in late omalizumab responders TF IgG levels were significantly higher [559 OD (min–max = 148–1956 OD)] than in early responders [253 OD (min–max = 84–161 OD)] (P = 0·035). Again, the two non‐responders did not show any TF IgG autoreactivity.
IgE and IgG autoantibodies to TG
TG IgE and IgG autoreactivity is depicted in Fig. 2. Median TG IgE levels did not differ significantly between patients [median 324 OD (min–max = 38–2034 OD)] and controls [236 OD (min–max = 129–330 OD)] (P = n.s.). Late responders showed slightly higher levels of TF IgE [408 OD (min–max = 172–800 OD)] than early responders [261 OD (min–max = 163–2034 OD)], although without reaching statistical significance due to the presence of two outliers in the group of early responders. Non‐responders did not show any IgE reactivity to TG.
Fig. 2.
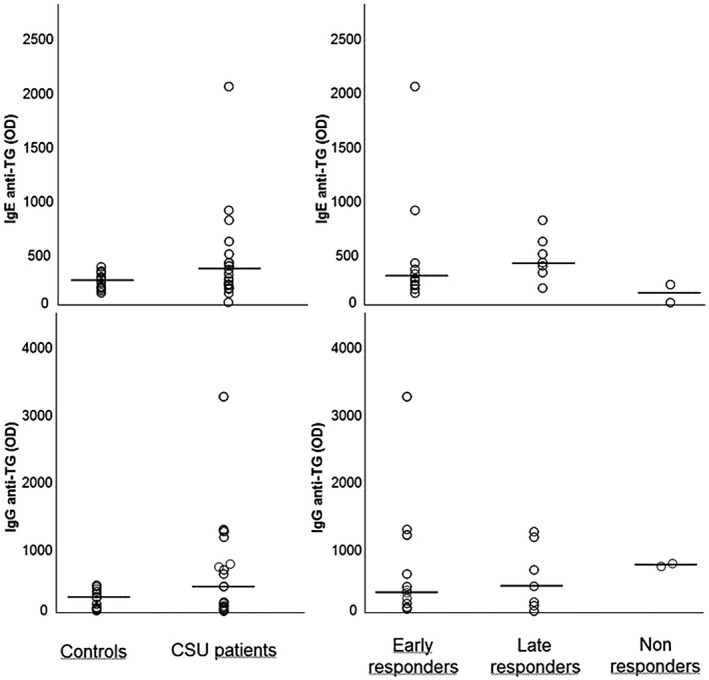
Immunoglobulin (Ig)E and IgG autoimmune response to thyroglobulin (TG) in healthy controls and patient subgroups divided based on clinical response to omalizumab. Horizontal lines represent medians.
Median TG IgG was higher in patients [509 OD (min–max = 50–3231 OD)] than in controls [252 OD (min–max = 55–412 OD)], although the difference did not reach statistical significance (P = 0·054). Late responders [418 OD (min–max = 50–1227 OD)] and early responders [413 OD (min–max = 61–3231 OD)] showed similar median levels of TG IgG. Interestingly, both patients not responding to omalizumab showed elevated levels of IgG to TG.
IgE and IgG autoantibodies to FcεRI
FcεRI IgE and IgG autoreactivity is shown in Fig. 3. Median FcεRI IgE levels did not differ between patients [190 OD (min–max = 22–839 OD)] and controls [197 OD (min–max = 4–290 OD)], although six patients (30%) showed levels exceeding the upper limit of controls. FcεRI IgE levels did not differ in the three subgroups of patients.
Fig. 3.
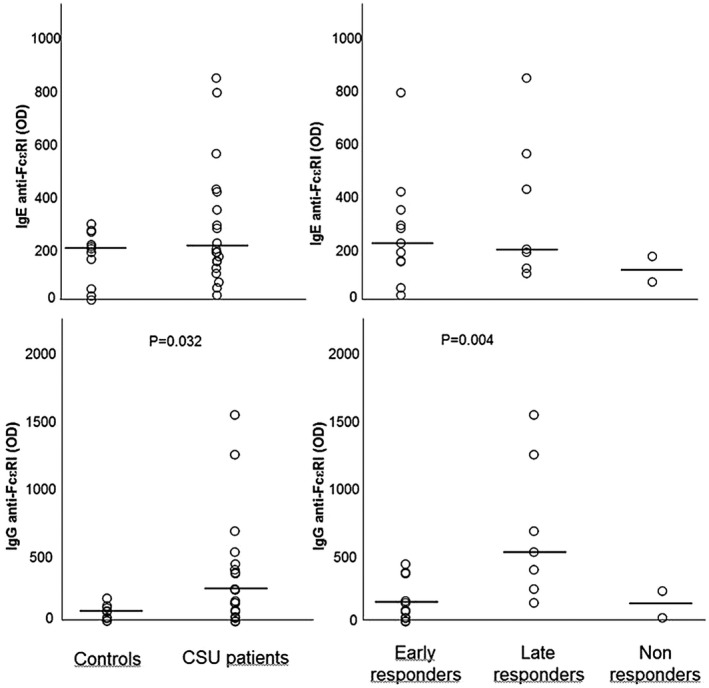
Immunoglobulin (Ig)E and IgG autoimmune response to the high‐affinity IgE receptor (FcεRI) in healthy controls and patient subgroups divided based on clinical response to omalizumab. Horizontal lines represent medians.
Median FcεRI IgG levels were significantly higher in patients [190 OD (min–max = 1–1526 OD)] than in controls [77 OD (min–max = 6–173 OD)] (P = 0·032). Late responders showed significantly higher levels of anti‐ FcεRI IgG [669 OD (min–max = 140–1526 OD)] than early responders [85 OD (min–max = 26–426 OD)] (P = 0·004) and non‐responders.
IgE and IgG autoantibodies to FcεRII
FcεRII IgE and IgG autoreactivity is depicted in Fig. 4. Median FcεRII IgE levels were similar in patients [284 OD (min–max = 183–406 OD)] and controls [272 OD (min–max = 199–397 OD)] (P = n.s.). Further, FcεRII IgE levels did not differ between late [286 OD (min–max = 205–406 OD)], early [304 OD (min–max = 183–361 OD)] and non‐responders.
Fig. 4.
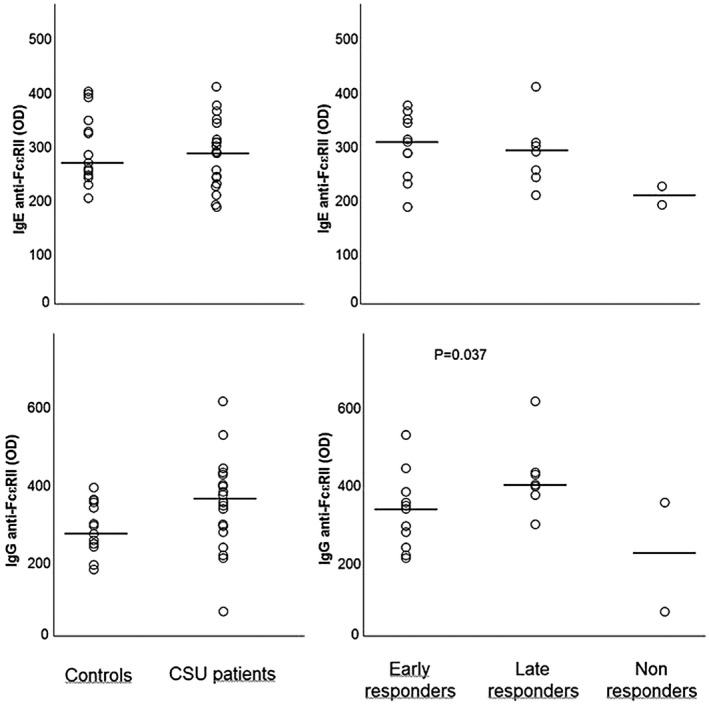
Immunoglobulin (Ig)E and IgG autoimmune response to the low‐affinity IgE receptor (FcεRII) in healthy controls and patient subgroups divided based on clinical response to omalizumab. Horizontal lines represent medians.
Median FcεRII IgG levels were higher in patients [348 OD (min–max = 67–609 OD)] than in controls [235 OD (min–max = 176–387 OD)] although, probably due to the small number of patients, the difference did not reach the statistical significance (P = 0·074). Again, within the patient group, late omalizumab responders showed higher FcεRII IgG levels [394 OD (min–max = 292–609 OD)] than early responders [272 OD (min–max = 206–522 OD)] (P = 0·05).
Overview of the results in individual patients
The results of the serological investigation in individual patients are shown in Table 2. Only two of 20 (10%) patients did not show specific IgE or IgG to any of the four autoantigens studied. Notably, both responded promptly to omalizumab therapy. Ten of 20 (50%) patients showed the contemporary presence of IgE and IgG reactivity to different autoantigens.
Table 2.
Detection of IgE and IgG autoantibodies in 20 patients with severe CSU
| Patient | TF IgE | TF IgG | TG IgE | TG IgG | FcεRI IgE | FcεRI IgG | FcεRII IgE | FcεRII IgG | Omalizumab response | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | L | |||||||||||
| 2 | L | |||||||||||
| 3 | L | |||||||||||
| 4 | E | |||||||||||
| 5 | E | |||||||||||
| 6 | L | |||||||||||
| 7 | E | |||||||||||
| 8 | E | |||||||||||
| 9 | L | |||||||||||
| 10 | E | |||||||||||
| 11 | E | |||||||||||
| 12 | N | |||||||||||
| 13 | E | |||||||||||
| 14 | L | |||||||||||
| 15 | E | |||||||||||
| 16 | E | |||||||||||
| 17 | E | |||||||||||
| 18 | E | |||||||||||
| 19 | L | |||||||||||
| 20 | N |
CSU = chronic spontaneous urticaria; TG = thyroglobulin; TF = tissue factor; Ig = immunoglobulin.
Gray color represents positivity.
Sixteen of 20 (80%) patients showed signs of IgG‐mediated autoimmunity to at least one of the four antigens tested; seven, seven and two of these showed an early, late or non‐response to omalizumab, respectively.
Thirteen of 20 (65%) patients showed IgE‐mediated autoimmunity to at least one of the antigens studied; seven and six of these showed an early or late response to omalizumab, respectively. None of the two non‐responders showed autoreactive IgE.
Of 10 patients showing IgG to the high affinity IgE receptor, six and one were late‐ and non‐responders to omalizumab, respectively.
Five patients showed IgG reactivity against the low affinity IgE receptor FcεRII (CD23).
Discussion
The existence of IgG‐mediated autoimmune processes in a proportion of patients with CSU has been known for 30 years. Much more recent is the observation that chronic urticaria patients may show auto‐allergic, IgE‐mediated events 5, 6, 7. In this study, we looked for both IgE and IgG directed against four autoantigens possibly involved in the pathogenesis of CSU. One potential drawback of our study is the limited number of antigens studied, as Schmetzer et al. recently showed IgE autoreactivity to hundreds of auto‐allergens in patients with CSU 6. Thus, we cannot exclude that the autoimmune response, which was already detected in 90% of our cases (18 of 20; Table 2), was possibly directed against a much larger array of autoantigens and was present in virtually all the population. Another limit of this study is the low number of patients enrolled, but due to cost limitations we were unable to investigate a broader population.
Our main finding is that approximately half of our CSU patients (11 of 20) showed the contemporaneous presence of both IgE and IgG autoantibodies directed against the same antigen/allergen or against distinct targets. This finding may potentially increase our understanding of the immunological events occurring in CSU, but at the same time clearly shows the complexity of the pathogenesis of this disease.
In CSU patients a prompt omalizumab response has been associated with elevated total IgE levels 14, 15, 16, 17 and with an elevated expression of high‐affinity IgE receptor on effector cells 18, 19. Conversely, IgG autoimmunity has been associated with a slow or absent response to the drug 9, 20. Thus, it is clearly possible that the contemporary presence of both IgE and IgG class autoantibodies renders the response to the drug unpredictable in the single patient and probably dependent on a fine balance between the two types of autoimmune response, or on the specific clinical effect of these autoantibodies. For instance, in our population 70% of patients showing IgG autoantibodies to the high‐affinity IgE receptor, FcεRI, responded slowly or did not respond at all to omalizumab. This is in keeping with the recent observation that omalizumab is able to bind not only circulating IgE, but also IgE fixed to the high‐affinity receptor and to detach them from the receptor 21, which leads (in a long term) to a down‐regulation of the receptor and a probable later response to the drug. In fact, a lower number of high‐affinity IgE receptors is available for activation by specific IgG autoantibodies. Interestingly, both patients who did not show IgE or IgG to any of the studied allergens responded promptly to omalizumab; this might suggest the presence of an autoimmune IgE response to an allergen other than those studied here 6.
The finding of IgE‐ and IgG‐mediated responses to TF, the main activator of the extrinsic pathway of the coagulation cascade, represents a link (and possibly the sign of an interplay) between the activation of the coagulation/fibrinolysis system and the auto‐allergic/autoimmune pathways of the disease 7. Although the effective clinical relevance of these autoantibodies has to be more clearly defined in future studies, it is nonetheless of interest that late responders to omalizumab showed significantly higher levels of both IgG and IgE to TF than early responders, suggesting that autoreactive autoantibodies levels might influence the pattern of response to omalizumab. TF is expressed mainly by eosinophils and endothelial cells 22, 23, and eosinophils may be activated upon stimulation of the low‐affinity IgE receptor. A quarter of our patients showed IgG against FcεRII, confirming previous findings by Puccetti et al 24. This mechanism may provide an alternative pathway leading to histamine release from mast cells and basophils.
We detected IgE specific for the high‐affinity IgE receptor in six cases, indicating that FcεRI could be a novel auto‐allergen in patients with CSU 6, 7. The in‐vivo relevance of these autoantibodies remains unclear, and deserves further investigation. However, they could be directly functionally active, causing the direct cross‐link of high‐affinity IgE receptors with their antigen binding fragment (as is the case for FcεRI IgG), or might induce mast cell degranulation when fixed to the receptor by the Fc domain after binding of free FcεRI, or both events may occur at the same time.
In conclusion, we have detected autoimmune responses sustained by both IgE and IgG antibody classes in more than half of patients with CSU. Such autoimmune responses may co‐exist and possibly influence the clinical response to anti‐IgE therapy. Further studies are needed to assess the clinical relevance of single autoantibodies in the disease of single CSU patients.
Disclosures
The authors have no conflicts of interest to declare.
Author contributions
R. A., A. V. M. and M. C. designed the study. R. A. and S. F. followed the patients and collected the clinical specimens. M. L. and V. C. performed the laboratory tests. R. A., A. V. M. and M. analyzed all the data and drafted the manuscript. S. F, M. L. and V. C. revised the manuscript.
Acknowledgements
The authors declare no sources of funding.
References
- 1. Gruber BL, Baeza ML, Marchese MJ, Agnello V, Kaplan AP. Prevalence and functional role of anti‐IgE autoantibodies in urticarial syndromes. J Invest Dermatol 1988; 90:213–7. [DOI] [PubMed] [Google Scholar]
- 2. Grattan CEH, Francis DM, Hide M, Greaves MW. Detection of circulating histamine releasing autoantibodies with functional properties of anti IgE in chronic urticaria. Clin Exp Allergy 1991; 21:695–704. [DOI] [PubMed] [Google Scholar]
- 3. Hide M, Francis DM, Grattan CEH, Hakimi J, Kochan JP, Greaves MW. Autoantibodies against the high‐affinity IgE receptor as a cause of histamine release in chronic urticaria. N Engl J Med 1993; 328:1599–604. [DOI] [PubMed] [Google Scholar]
- 4. Fiebiger E, Maurer D, Holub H et al Serum IgG autoantibodies directed against the alpha chain of FcεRI: a selective marker and pathogenetic factor for a distinct subset of chronic urticaria patients? J Clin Invest 1995; 96:2606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Altrichter S, Peter HJ, Pisarevskaja D, Metz M, Martus P, Maurer M. IgE mediated autoallergy against thyroid peroxidase–a novel pathomechanism of chronic spontaneous urticaria? PLOS ONE 2011; 6:e14794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmetzer O, Lakin E, Topal FA et al IL‐24 is a common and specific autoantigen of IgE in patients with chronic spontaneous urticaria. J Allergy Clin Immunol 2017; 142:876–82. [DOI] [PubMed] [Google Scholar]
- 7. Cugno M, Asero R, Ferrucci S et al Elevated IgE to tissue factor and thyroglobulin are abated by omalizumab in chronic spontaneous urticaria. Allergy 2018; 73:2408–10. [DOI] [PubMed] [Google Scholar]
- 8. Zuberbier T, Aberer W, Asero R et al The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy 2018; 73:1393–414. [DOI] [PubMed] [Google Scholar]
- 9. Gericke J, Metz M, Ohanyan T et al Serum autoreactivity predicts time to response to omalizumab therapy in chronic spontaneous urticaria. J Allergy Clin Immunol 2017; 139:1059–61. [DOI] [PubMed] [Google Scholar]
- 10. Leznoff A, Sussman GL. Syndrome of idiopathic chronic urticaria and angioedema with thyroid autoimmunity: a study of 90 patients. J Allergy Clin Immunol 1989; 84:66–71. [DOI] [PubMed] [Google Scholar]
- 11. Puccetti A, Bason C, Simeoni S et al In chronic idiopathic urticaria autoantibodies against Fc epsilonRII/CD23 induce histamine release via eosinophil activation. Clin Exp Allergy 2005; 35:1599–607. [DOI] [PubMed] [Google Scholar]
- 12. Asero R, Tedeschi A, Marzano AV, Cugno M. Chronic spontaneous urticaria: immune system, blood coagulation, and more. Expert Rev Clin Immunol 2016; 12:229–31. [DOI] [PubMed] [Google Scholar]
- 13. Ulambayar B, Chen YH, Ban GY et al Detection of circulating IgG autoantibody to FcεRIα in sera from chronic spontaneous urticaria patients. J Microbiol Immunol Infect 2020; 53: 141–7. [DOI] [PubMed] [Google Scholar]
- 14. Marzano AV, Genovese G, Casazza G et al Predictors of response to omalizumab and relapse in chronic spontaneous urticaria: a study of 470 patients. J Eur Acad Dermatol Venereol 2019; 33:918–24. [DOI] [PubMed] [Google Scholar]
- 15. Ertas R, Ozyurt K, Atasoy M, Hawro T, Maurer M. The clinical response to omalizumab in chronic spontaneous urticaria patients is linked to and predicted by IgE levels and their change. Allergy 2018; 73:705–12. [DOI] [PubMed] [Google Scholar]
- 16. Weller K, Ohanyan T, Hawro T et al Total IgE levels are linked to the response of chronic spontaneous urticaria patients to omalizumab. Allergy 2018; 73:2406–8. [DOI] [PubMed] [Google Scholar]
- 17. Cugno M, Genovese G, Ferrucci S, Casazza G, Asero R, Marzano AV. IgE and D‐dimer baseline levels are higher in responders than nonresponders to omalizumab in chronic spontaneous urticaria. Br J Dermatol 2018; 179:776–7. [DOI] [PubMed] [Google Scholar]
- 18. Deza G, Bertolín‐Colilla M, Pujol RM et al Basophil FcεRI expression in chronic spontaneous urticaria: a potential immunological predictor of response to omalizumab therapy. Acta Derm Venereol 2017; 97:698–704. [DOI] [PubMed] [Google Scholar]
- 19. Deza G, Bertolín‐Colilla M, Sánchez S et al Basophil FcɛRI expression is linked to time to omalizumab response in chronic spontaneous urticaria. J Allergy Clin Immunol 2018; 141:2313–6. [DOI] [PubMed] [Google Scholar]
- 20. Palacios T, Stillman L, Borish L, Lawrence M. Lack of basophil CD203c‐upregulating activity as an immunological marker to predict response to treatment with omalizumab in patients with symptomatic chronic urticaria. J Allergy Clin Immunol Pract 2016; 4:529–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maggi L, Rossettini B, Montaini G et al Omalizumab dampens type 2 inflammation in a group of long‐term treated asthma patients and detaches IgE from FcεRI. Eur J Immunol 2018; 48:2005–14. [DOI] [PubMed] [Google Scholar]
- 22. Cugno M, Marzano AV, Tedeschi A, Fanoni D, Venegoni L, Asero R. Expression of tissue factor by eosinophils in patients with chronic urticaria. Int Arch Allergy Immunol 2009; 148:170–4. [DOI] [PubMed] [Google Scholar]
- 23. Yanase Y, Takahagi S, Hide M. Chronic spontaneous urticaria and the extrinsic coagulation system. Allergol Int 2018; 67:191–4. [DOI] [PubMed] [Google Scholar]
- 24. Puccetti A, Bason C, Simeoni S et al In chronic idiopathic urticaria autoantibodies against Fc epsilonRII/CD23 induce histamine release via eosinophil activation. Clin Exp Allergy 2005; 35:1599–607. [DOI] [PubMed] [Google Scholar]


