Abstract
Cavitary lung lesions are quite common findings on chest imaging and often pose a diagnostic challenge to the clinicians. We describe a case of a 75-year-old male who presented to the emergency room with hemoptysis. Computed tomography of the chest demonstrated multiple cavitary pulmonary nodules with peripheral groundglass opacities. Bronchoscopy did not reveal any active bleeding source, and washings were negative for malignancy and infectious cause. Computed Tomography guided biopsy of the left lung nodule showed metastatic carcinoma consistent with papillary renal cell carcinoma. This case highlights the unusual presentation of metastatic renal cell carcinoma.
Key words: Renal cell carcinoma, cavitation
Introduction
A pulmonary cavitary lesion is defined pathologically as a vacuolated lesion with gas/air. It will appear radiologically as an area of low-attenuation or lucency within an area of consolidation, mass or nodule.1 Based on the etiology, it can be distinguished into either infectious or non-infectious. The infectious causes can be bacterial, fungal, mycobacterial, or parasitic, while non-infectious reasons are usually noted to be malignancies or rheumatologic diseases.
The most likely cause of a thick-walled cavitary lesion is malignancy.2 Cavitation within a nodule is more often than not associated with a worse prognostic factor. Metastatic diseases are rarer causes of cavitary pulmonary lesions, which can cause multiple cavitations as compared to primary lung cancers which typically present as solitary cavitations. Most common metastatic cavitary lesions are seen due to head, neck, esophageal, and uterine carcinomas. Renal, bladder, and synovial sarcomas are far rarer causes for cavitation of metastasis to the lung.3 We present a patient with an unusual presentation of hemoptysis with cavitary pulmonary lesions secondary to renal cell carcinoma metastasis.
Case Report
A 75-year-old male with a history of hypertension, diabetes mellitus, chronic kidney disease due to focal segmental glomerular sclerosis, and paroxysmal atrial fibrillation on anticoagulation, who presented to the emergency room with hemoptysis for three days in duration. He also endorsed long-standing hematuria, for which he had been evaluated 18 months ago via a percutaneous biopsy of a 2.7 cm right renal mass. It showed focal segmental glomerulosclerosis with no malignant component. Unfortunately, he was unable to follow up with nephrology and urology after this episode. He also missed his follow up imaging appointments and did not receive any glucocorticoid therapy. He reported no significant worsening of his hematuria. He denied fever, chills, night sweats, and weight loss. He has a smoking history of 24 pack-years and quit twenty-five years earlier. He was born and lived in the United States with no prior exposure to tuberculosis (TB). His home medications included apixaban, atorvastatin, and sacubitril-valsartan. On physical exam, the patient was in no apparent distress. Oropharynx was moist and had good oral dentition and hygiene. Lungs were clear to auscultation bilaterally with good movement of air, and no wheezing or rales were noted. No inflammation or edema noted on extremities. The skin was warm and dry, with no rashes. Upon presentation, his initial hemoglobin was 7.3 g/dL, and hematocrit was 23.7 g/dL. His baseline hemoglobin and hematocrit was 14 g/dL and 43 g/dL, respectively. A few hours later, the patient’s hemoglobin and hematocrit dropped to 6.6 g/dL and 21.1 g/dL, respectively. His apixaban was held, and he had been transfused two units of packed red blood cells with appropriate improvement. He also had large blood in his urine. Computed tomography of the chest demonstrated multiple cavitary pulmonary nodules with peripheral ground-glass opacities along with subcarinal and mediastinal lymphadenopathy (Figure 1).
He then underwent bronchoscopy, which revealed healthy and clear airways bilaterally, with a minimal amount of red blood in the medial basal, anterior basal and posterior basal segment of the right lower lobe with no active bleeding. Sequential bronchoalveolar lavages were not obtained due to a low suspicion of diffuse alveolar hemorrhage or vasculitis. He had a mildly enlarged subcarinal lymph node (1.3 cm) in an otherwise non-pathologic mediastinum therefore, endobronchial ultrasound and transbronchial needle biopsy was not performed. The washings were negative for malignancy and infectious causes which further prompted a Computed Tomography guided biopsy of the left lung nodule, which showed metastatic carcinoma consistent with papillary renal cell carcinoma (RCC). This prompted a computed tomography of abdomen and pelvis which showed a 2.8 cm hypodense right upper lobe renal mass, subcentimeter retroperitoneal and mesenteric lymph nodes without any evidence of retroperitoneal hemorrhage. He had no further episodes of hemoptysis and required no further transfusions. He was discharged with oncology follow up, where he was recommended to initiate a dual immune checkpoint inhibitor therapy with nivolumab and ipilimumab with regular follow-ups.
Discussion
Cysts are very similar to cavities and are classified on a spectrum in the transition from one to the other. In theory, cysts are usually thin-walled (<4 mm), and cavities have thicker walls which are further classified as benign or malignant with a direct correlation to the increasing thickness. However, it is difficult to make this differentiation due to the overlap of the two.2
The differentials for a pulmonary cavitary lesion are extensive and differentiated as non-infectious and infectious. Infectious causes can be bacterial, fungal, mycobacterial, or parasitic. Bacterial infections cause cavitary lesions by bypassing the host’s defenses and colonizing or creating necrotizing pneumonia or abscess within the lung tissue. This process is more commonly seen in cases of infection with S. aureus or K. pneumonia and occasionally with S. pneumonia or H. influenza. It can also be seen less frequently with Actinomyces, Nocardia, or Rhodococcus species.4 Common fungal causes include aspergillosis, zygomycosis, histoplasmosis, blastomycosis, and more. Mycobacterium is a renowned cause with tuberculosis having a high prevalence for causing cavitary lesions in people with pulmonary infection, due to its caseating necrosis.4 Cavitary lesions can also be a complication of septic emboli as a result of the thrombosis of pulmonary vessels by microorganisms.5
Although cavitary lung lesions are most commonly due to an infectious process, the possibility of a metastatic sequela in the setting of a pre-existing malignancy cannot be undermined. In a case of lung metastasis in the context of squamous cell carcinoma (SCC) of the uterine cervix, which presented as a thin-walled cavitary lesion in a Moroccan woman. The etiology was assumed to be tuberculosis due to endemic prevalence which led to initial treatment with anti bacillary treatment. Without any clinical improvement, the patient underwent bronchoscopy with transbronchial biopsy, which proved the lesion to be metastatic SCC in nature. Despite starting systemic chemotherapy, the patient died nine months later.6 This case portrays the importance of not undermining the malignant potential of a newly found cavitary lesion. Although the most common causes might be related to an infectious cause, it is essential also procedurally to rule out malignant process, since missing such a diagnosis can be fatal for the patient.
Non-infectious causes are primarily malignancies or rheumatologic diseases such as Wegner’s granulomatosis or sarcoidosis. Cavitation in lung cancer is associated with worse prognosis but is more common amongst the squamous cell carcinomas. 7 Other primary causes of cavitary malignancies are seen in Kaposi’s sarcoma and lymphomas in the setting of an immunocompromised patient. Lymphamatoid granulomatosis is a rare malignancy associated with Epstein Barr virus, which can result in multiple pulmonary cavitary lesions.8 Given the known association that the majority of cavitary pulmonary lesions are a rare finding in metastatic diseases, they are still known to occur in some malignancies such as head, neck, esophageal, and uterine. Cavitary lesions are an atypical presentation for renal cell carcinoma metastasis, however, among most cases with renal cell carcinoma, the most common anatomic site of dissemination is the lungs, which accounts for 45% of all metastasis.
Pulmonary metastases are usually asymptomatic (90% of cases). On CT images, they typically manifest as multiple nodules or solitary nodules and in subpleural locations. Renal cell carcinoma is also well known for its cannonball metastases.9 Some cases of lung metastases may show necrosis and cavitation generally after antiangiogenic therapy. The exact mechanism of cavitation is unknown, but the cause is presumed to be either tumor necrosis or a check-valve mechanism that develops through tumor infiltration into the bronchial structure. The wall of a cavitated mass is generally irregular and thick.10
The distinction of a new pulmonary malignancy in the setting of metastatic pulmonary malignancy is a growing area of interest. A study was done by Bowman et al., which looked at three different individuals who have pulmonary metastasis from primary renal cell carcinoma. 11 This study looked at 3 cases who developed new primary lung malignancies with pre-existing pulmonary metastasis. In all of these cases, patients would be responding well to treatment targeting the RCC metastasis. Over time they would develop an isolated nodule, which might be seen incidentally on imaging which remains stable or continues to grow despite the treatment. It is only upon biopsy that these were found to be primary malignancies and therapy was initiated, focusing that specific malignancy as well. If caught early as it was in 2 of the cases, the patients were able to achieve resolution via surgical resection or stereotactic radiation. 11 However, in one case, it had already metastasized to the brain, and despite whole brain radiation and systemic chemo, the patient passed away.11 These cases go to exemplify the importance of continually monitoring for the progression of new nodules or cavitary lesions during routine imaging of these patients and being overtly dismissive. It also ties into the importance of having regularly scheduled follow-ups with the patient and ensuring that they are compliant with follow-ups to screen for these possible outcomes.
The standard first-line treatment for metastatic renal cell carcinoma was Sunitinib, which is an EGFR tyrosine kinase inhibitor. Motzer et al., phase 3 trial compared nivolumab plus ipilimumab with Sunitinib for advanced renal-cell carcinoma. The study proved that when used in combination, the two drugs displayed a response rate of 40% and a 2-year survival rate of over 67-70% in both untreated and previously treated patients with metastatic renal cell carcinoma as compared to Sunitinib.12
Conclusions
Although common etiologies of cavitary lung disease have been extensively classified, rare types of infrequent associations of cavitary lung lesions with renal cell carcinoma should also be considered. Prompt recognition and appropriate management are essential as they can be lifesaving in these patient populations.
Figure 1.
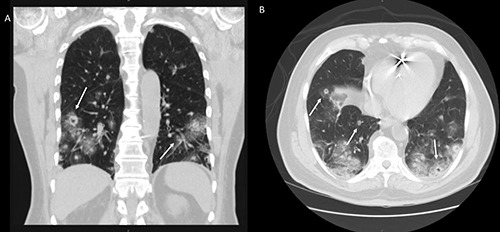
Computed tomography of the chest demonstrated multiple metastases in both lungs. Few of these lesions (arrow) show cavitation. (A) Coronal view (B) Axial view.
References
- 1.Hansell DM, Bankier AA, Macmahon H, et al. Fleischner Society: Glossary of Terms for Thoracic Imaging. Radiology 2008;246:697-722. [DOI] [PubMed] [Google Scholar]
- 2.Ryu JH, Swensen SJ. Cystic and Cavitary Lung Diseases: Focal and Diffuse. Mayo Clin Proc 2003;78:744-52. [DOI] [PubMed] [Google Scholar]
- 3.Song J, Yu J, Ma Z, Lu S. Rare occurrence of cavitation of lung metastases following ef-fective targeted therapy: A case report. Oncol Lett 2016;11:1589-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gadkowski LB, Stout JE. Cavitary Pulmonary Disease. Clin Microbiol Rev 2008;21:305-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parkar AP, Kandiah P. Differential Diagnosis of Cavitary Lung Lesions. J Belg Soc Radiol 2016;100:1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raissouni S, Ghizlane R, Mouzount H., et al. Unusual case of cavitary lung metastasis from squamous cell carcinoma of the uterine cervix. Pan African Med J 2013;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duda K, Kolodziejski L, Góralczyk J, et al. Cavitated tumor as a clinical subentity in squa-mous cell lung cancer patients. Neoplasma 2003;50:66-73. [PubMed] [Google Scholar]
- 8.Mccloskey M, Catherwood M, McManus D, et al. A case of lymphomatoid granulomato-sis masquerading as a lung abscess. Thorax 2004;59:818-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brufau BP, Cerqueda CS, Villalba LB, et al. Metastatic Renal Cell Carcinoma: Radiologic Findings and Assessment of Response to Targeted Antiangiogenic Therapy by Using Mul-tidetector CT. RadioGraphics 2013;33:1691-716. [DOI] [PubMed] [Google Scholar]
- 10.Seo JB, Im J-G, Goo JM, et al. Atypical Pulmonary Metastases: Spectrum of Radiologic Findings. RadioGraphics 2001;21:403-17. [DOI] [PubMed] [Google Scholar]
- 11.Bowman IA, Pedrosa I, Kapur P, Brugarolas J. Renal Cell Carcinoma With Pulmonary Metastasis and Metachronous Non-Small Cell Lung Cancer. Clin Genitour Cancer 2017;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Motzer RJ, Rini BI, Mcdermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol 2019;20:1370-85. [DOI] [PMC free article] [PubMed] [Google Scholar]


