Abstract
Background
Exhaled nitric oxide (NO), especially fractional concentration of exhaled NO (FENO) has been used to predict the responsiveness to inhaled corticosteroid (ICS) in children with asthma. However, the use of exhaled NO for predicting asthma control in children is still controversial.
Methods
This was a perspective observational study. Asthmatic children who were naïve to inhaled corticosteroid (ICS) were included in the present study. The measurements of FENO and CANO (concentration of NO in the gas phase of the alveolar), spirometry, blood eosinophil counts (BEC), and total IgE levels were done for each asthmatic child. All study subjects started proper asthma treatment after the enrollment.
Results
Ninety three asthmatic children (9±3 years) with moderate (63.4%) to severe (36.6%) asthma were included and finished the 3-month study. The levels of FENO and CANO at inclusion were 37±11 ppb and 5.8±1.4 ppb, respectively; the mean of BEC was 617±258 cells/μL; the level of total IgE was 1563±576 UI/mL; 89% of subjects were positive for at least one respiratory allergen. The percentage of severe asthma was reduced significantly after 3 months (P<0.001). Well controlled asthma subjects at 3 months had higher levels of FENO and lower levels of CANO at inclusion (P<0.05 and P<0.05). FENO<20 ppb or CANO>5ppb had a risk of uncontrolled asthma at 3 months (OR: 1.7, CI 95% [(0.8) - (3.3)], P<0.05; OR: 1.9, CI 95% [(0.9) - (2.7)], P<0.05; respectively). FENO>35 ppb at inclusion had a positive predictive value for asthma control at 3 months (OR: 3.5, CI 95% [2.2-5.9], P<0.01).
Conclusions
Exhaled NO is a biomarker of asthma which may have a potential role to predict the control of asthma in short-term follow up in asthmatic children.
Key words: Asthma, asthma control, exhaled NO, FENO, CANO
Introduction
Asthma is a very common chronic respiratory disease in children [1]. It remains a leading cause of emergency department visits, hospitalization, and unscheduled school absences due to asthma exacerbations [2,3]. In Vietnam, the prevalence of school-age children with asthma has been varied from 5.1% up to 12.1% [4,5]. Although many advancements have been discovered in the last decades over asthma management, obtaining well-controlled asthma in children is still a big challenge for physicians in practice. As with adults, the control of asthma in children is dependent mainly on their treatment responsiveness and adherence [1]. Moreover, it is challenging for pediatric physicians to predict the different levels of asthma control in short-term follow up of their asthmatic patients. This problem is due to the heterogeneity of asthma symptoms and phenotypes, especially in young children. Currently, the control of asthma is measured by symptoms during the day and at night, during physical activities, and associated with the use of rescue treatment to relieve symptoms with short acting beta 2 agonist (SABA). However, in asthmatic children with moderate to severe asthma, the choice of predictive factors for asthma control remains an important issue for asthma management.
Currently, the use of GINA (Global Initiative for Asthma) recommendations for asthma control is often used in practice with three different control levels, including controlled (or totally controlled), partially controlled, and uncontrolled asthma [1]. In addition to GINA recommendations, the use of ACT (Asthma Control Test) and especially Childhood Asthma Control Test (cACT), useful for youngest children, have been equally available to evaluate the control of asthma [1,6]. In Vietnam, the local version of ACT for asthmatic children is also available for pediatric physicians and it has been considered as a useful additional tool for childhood asthma control [7,8]. According to that, different biomarkers have been studied and used to evaluate the level of asthma control in short-term follow up such as blood or sputum eosinophil count, periostin, and exhaled nitric oxide (NO) [9-12]. In asthmatic adults, previous studies suggest that FENO (fractional concentration of exhaled NO) might be used to predict the persistence of asthma control in asymptomatic patients and can be used in asthma management [13-15]. Actually, the association between FENO and current asthma control in adults is much more complex, with a clear correlation in patients who are naïve to ICS (inhaled corticosteroids) and very weak correlation when patients are properly treated; while FENO seems to be useful in predicting future risk of losing asthma control [16,17]. FENO can be measured by portable devices for evaluating the airway inflammation in asthmatic patients [18]. The use of exhaled NO for predicting asthma control in children is still controversial. However, NICE guidelines recommend to consider FENO test in children and young people in case of uncertainty in the diagnosis (with a cut-off 35 ppb) [19].
In addition, the use of NO concentration in the gas phase of the alveolar (CANO) as an inflammatory biomarker of small airways in patients with asthma has been demonstrated [20-22]. It has been suggested that the increase of CANO level might reflect a severe or persistent asthma and the increase of CANO might reflect the allergic inflammation (Type 2 asthma) in distal airways [23,24]. Therefore, the present study aimed to clarify a predictive role of exhaled NO (FENO and CANO) in the control of asthma in children in short-term follow up.
Methods
Patients
Asthmatic children who presented to the Clinical Research Center of Lamdong Medical College (Dalat, Vietnam) from June 2018 to June 2019 were eligible for the present study after their parents/guardians signed an Institutional Review Board-Approved consent form on the patient’s behalf. This study was approved by the Ethic Council of Lamdong Medical College (04.18/LMCTTYSH- YD, approved in March 2018). The parents/guardians and patients also had been informed that they could withdraw of the study without the impact on asthma management.
Inclusion criteria
Asthmatic children over 6 years old who were newly diagnosed with asthma according to GINA [1] beginning of the study or who were previously diagnosed with asthma and did not take any daily treatment over one month were included in the present study. The included subjects were able to perform spirometry, exhaled NO measurements (FENO, CANO and FnNO [fractional concentration of nasally aspirated NO]), blood tests, and skin prick test (SPT). Every subject had been followed and finished the study after three months.
Exclusion criteria
Patients who had one of the following criteria were excluded from the present study: being unable to perform spirometry or exhaled NO measurements; having other acute or chronic diseases such as congenital heart disease, hepatobiliary disorders, nephritic syndrome or chronic glomerulonephritis, acute respiratory infection, or psychological problems; being unable to obtain consent or absent during follow up; having acute asthma exacerbations (AAE) at the beginning.
Study design
This was a prospective observational study. Patients with uncontrolled asthma who met the inclusion criteria were included and treated as recommended by GINA [1] and followed up for three months. All the data concerning medical history, exposure to tobacco smoke, disease severity, laboratory tests including peripheral blood eosinophil counts (BEC), total IgE, skin prick test (SPT) with standard respiratory allergens, exhaled NO measurement (FENO, CANO, and nNO), and lung function testing were recorded for analysis.
The severity of asthma was defined by daytime symptoms, symptoms at night, asthma crisis, and airflow limitation measured by FEV1. Fluticasone propionate was used as ICS with moderate or high doses depending on asthma severity and combined with long acting beta agonist (LABA; salmeterol); short acting beta 2 agonist (SABA; albuterol) was used as a rescue treatment. All subjects had clinic visits after one month and three months for the assessment of asthma severity, asthma control by GINA and ACT (Asthma Control Test) in Vietnamese version for children of 4-11 years and >11 years, frequency of SABA use, lung function testing, and exhaled NO measurement (FENO, CANO, and FnNO). The adherence of asthmatic children was evaluated by the percentage of patients who did regularly their daily treatment during follow up.
Laboratory techniques
Blood Eosinophil Count (BEC) and total IgE concentration quantifying. Blood samples of all study patients were collected through venipuncture and used for counting eosinophils and measuring total IgE. BEC in peripheral blood was analyzed by automatic machine. IgE concentration in peripheral blood was quantified by chemical luminescence technique (COBASC 501; Hitachi, Japan). The increases of eosinophil and total IgE in peripheral blood were defined by local Biology Lab (eosinophilils 6%; total IgE >214 UI/mL) and as described previously [8].
Skin prick test (SPT). SPT was done for all study patients with standardized respiratory allergens (Stallergenes; London, UK) including Dermatophagoides Pteronyssius (Dp), Dermatophagoides Farinae (Df), Blomia tropicalis (Blo), Phoenix dactylisera, Alternaria spp, mixed pollens (Dactylus glomerata, Phleum pratense, Lolium perenne), dog hairs, cat hairs, and cockroaches. Negative control with 0.9% saline solution and positive control with 1 mg/mL of histamine was done for each SPT. The test was positive when the wheal size exceeded the negative control ≥3 mm.
Lung Function Testing (LFT). LFT (spirometry) was done by Blue Spiro (Medisoft; Sorinnes, Belgium). The reversibility of airway obstruction was measured by forced expiratory volume in one second (FEV1) after 15 min using 200 mcg albuterol. The reversibility test was defined as positive when there was an increase of FEV1 ≥12% and >200 mL as described previously [8,24]. The reference values and used equations were defined by integrated Expair Software (Medisoft; Sorinnes, Belgium) with race correction for Asian people. The level of obstruction was defined as mild obstruction (FEV1 ≥ 80% predicted, moderate obstruction (FEV1 of 60-79% predicted), and severe obstruction (FEV1 < 59% predicted). LFT was done routinely after the measurement of exhaled nitric oxide.
Exhaled nitric oxide (NO) measurements. FENO, CANO and FnNO were done by Hypair FENO+ Device (Medisoft; Sorinnes, Belgium) according to manufacturer’s instructions as described previously with expiratory air flows of 50 mL/sec for FENO and with multiple flows for CANO [8]. CANO was done by machine’s integrated Expair Software using a linear equation of y = ax + b (x: flow rate [4 flow rates of 50/100/150/350 mL/s]; b: JawNO 115 [maximal bronchial production of NO in the airways]; a: CANO). CANO and JawNO have been corrected according to the Condorelli equation [25]. The mean value of two correct measurements was used for analysis. FENO, CANO and FnNO levels were classified as recommended by the ATS (American Thoracic Society) / ERS (European Respiratory Society) and previous publications [16,24,26].
Statistical analysis
The statistical analysis was performed with SPSS software 22.0 (Chicago, IL, USA) for all recorded data. Categorical variables were presented absolute and relative frequencies (n and %) and continuous variables were described by mean and standard deviation (mean ± SD). Normal distribution was evaluated by using Skewness-Kurtosis test. The pair-comparison of mean was done by Mann-Whitney U test or by Kruskal-Wallis test for more than two groups. Odds ratios (OR) with 95% confidence interval (CI) was used to measure the association between clinical and functional parameters at inclusion with controlled or uncontrolled asthma after 3 months. P<0.05 was considered as statistically significant.
Results
Clinical and functional characteristic of study patients at inclusion
From June 2018 to June 2019, 93 asthmatic children more than 6 years of age who met the inclusion criteria and followed up during 3 months were included in the study (Figure 1). The mean age of patients was 9 ± 3 years; 62.4% male; and asthma onset age of 3.5 ± 2.5 years (Table 1). There were 54.8% passive smokers (exposed to second-hand smoke), 87.1% personal allergic history, and 62.3% had family history of allergies (Table 1). The percentage of moderate and severe asthma was respectively 63.4% and 36.6%. 8.6%, 59.1% and 32.3% of subjects were never treated, discontinued treatment or unregularly treated with an asthma preventive drug, respectively (Table 1).
The result of spirometry showed study subjects had a moderate to severe reduction of FEV1 and PEFR with mean values of 64±18% and 58±15%, respectively (Table 1). The levels of FENO, CANO, JawNO, and FnNO were 37±11 ppb, 5.8±1.4 ppb, 77±22 nL/min, and 1,826±379 ppb, respectively. The mean of BEC was 617±258 cells/μL or 6.3±3.5% of total white blood cells; the level of total IgE was 1,563±576 UI/mL (Table 1). 89% of study subjects were positive with at least one respiratory allergen confirmed by SPT.
Table 1.
Clinical and functional characteristics of patients at the beginning of study.
| Characteristics (N=93) | Mean ± SD or Percentage % (N) |
|---|---|
| Age, years | 9 ± 3 |
| Gender | |
| Male | 62.4 (58 |
| Female | 47.6(35) |
| Age of asthma onset, years | 3.5 ± 2.5 |
| BMI, kg/m2 | 17.5 ± 1.5 |
| Passive smokers, % | 54.8(51) |
| Atopy | |
| Personal allergic history | 87.1(81) |
| Familiar allergic history | 62.3(58) |
| Asthma severity, % | |
| Moderate asthma | 63.4(59) |
| Severe asthma | 36.6(34) |
| AAE with hospitalization, times/year | 2.4 ± 1.6 |
| Asthma preventive treatment | |
| Never treated with preventive drugs | 8.6(8) |
| Discontinued treatment | 59.1(55) |
| Unregularly treated | 32.3(22) |
| ACT, scores | 8 ± 4 |
| Comorbidity | |
| Allergic rhinitis | 83.8(78) |
| Eczema | 31.2(29) |
| Spirometry | |
| FEV1, % of predicted | 64 ± 18 |
| FVC, % of predicted | 73 ± 12 |
| FEV1/FVC, % of predicted | 67 ± 11 |
| FEF25-75, % of predicted | 48 ± 19 |
| PEFR, % of predicted | 58 ± 15 |
| Reversibility*, % (N) | 76.3(71) |
| Exhaled NO | |
| FENO, ppb | 37 ± 11 |
| CANO, ppb | 5.8 ± 1.4 |
| JawNO, nL/min | 77 ± 22 |
| FnNO, ppb | 1826 ± 379 |
| Total IgE, UI/mL | 1563 ± 576 |
| BEC, % (cells/μL) | 6.3 ± 3.5 (617 ± 258) |
| SPT (+)§ | 89 |
BMI, body mass index; AAE, acute asthma exacerbation; ACT, asthma control test; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; FEF25-75, forced expiratory flow at 25-75% of time; PEFR, peak expiratory flow rate; NO, nitric oxide; FENO, fractional concentration of exhaled nitric oxide; CANO, concentration of nitric oxide in the gas phase of the alveolar; JawNO, total flux of NO in the conducting airway compartment; FnNO, fractional concentration of nasally aspirated nitric oxide; ppb: part per billion; BEC, blood eosinophil count; SPT, skin prick test
*defined by increase of FEV1>12% and 200 mL
§positive at least with one allergen on skin prick test.
Clinical and functional characteristics of study patients after asthma management
The results after one month of asthma treatment showed the percentage of passive smokers, moderate or severe asthma, and asthma treatment with high or moderate dose of ICS plus LABA were not significant different in comparison to those at the beginning of study in study subjects (47.3%, 72.1%, 27.9%, 27.9%, and 72.1% vs 54.8%, 63.4%, 36.6%, 36.6%, and 63.4%; P>0.05; Table 2, Figure 2). The daily use of SABA and ACT scores were significantly improved after one month compared to that at inclusion in study patients (3.5±1.7 and 15±6 vs 6.2±3.4 and 8±4; P<0.05 and P<0.01, respectively; Table 2). The percentage of uncontrolled asthma was significantly reduced after one month in comparison to that at inclusion (49.5% vs 100%; P<0.001; Table 2, Figure 2). After one month of asthma treatment, there was significant improvement of spirometric parameters (Table 2). The levels of FENO, CANO, JawNO, and FnNO were significant reduced after one month in compared to those at inclusion (25±12 ppb, 4.9±2.1 ppb, 41±16 nL/min, and 1432±561 ppb vs 37±11 ppb, 5.8±1.4 ppb and 1826±379 ppb; P<0.01, P<0.05 and P<0.05, respectively; Table 2).
The results after 3 months of asthma treatment showed there were significant reductions of severe asthma, weekly use of SABA, high dose of ICS + LABA, LTRA treatment, and uncontrolled asthma in comparison to those at inclusion and after one month of treatment (13.9%, 2.6±1.5 times, 13.9%, and 66.5% vs 36.6% and 27.9%, 6.2±3.4 and 3.5±1.7 times, 36.6% and 27.9%, and 100% and 100%; P<0.001 and P<0.01, P<0.01 and P<0.05, P<0.001 and P<0.01, and P<0.001 and P<0.001, respectively; Table 2, Figure 2). The ACT scores and percentage of controlled asthma were also significantly increased after 3 months of treatment in comparison to those at inclusion and after one month of treatment (19±5 vs 8±4 and 15±6; 50.5% vs 0% and 4.3%; P<0.001 and P<0.05; P<0.001, respectively; Table 2, Figure 2). There was the significant improvement of FEV1 and PEFR after 3 months of treatment compared to the values at inclusion (P<0.01 and P<0.01, respectively; Table 2). The levels of FENO, CANO, and FnNO were also significantly reduced after 3 months of treatment in comparison to those at inclusion and after one month of treatment (16±9 ppb vs 37±11 and 25±12 ppb, 3.7±1.8 ppb vs 5.8±1.4 and 4.9±2.1 ppb, and 978±425 vs 1,826±379 and 1,432±561 ppb; P<0.001 and P<0.01, P<0.01 and P<0.05, and P<0.001 and P<0.01, respectively; Table 2).
Figure 1.

Flow chart of study with asthmatic children after three months followed-up. ACT, Asthma Control Test; LFT, lung function test; NO, nitric oxide; SPT, skin prick test.
Figure 2.

Significant modification of clinical and functional characteristics of study patients after 1 month and 3 months. FEV1, forced expiratory volume in 1 second; NS, not significant difference.
Comparison of clinical and functional characteristics at inclusion between uncontrolled and controlled asthmatic patients at 3 months
The results of the present study showed there was no significant difference between controlled and uncontrolled asthmatic patients classified at 3 months for age, gender, BMI, and atopy measured at inclusion (9±2 vs 8±3, 29% vs 20%, 17.3±1.4 vs 17.9±1.6 kg/m2, 87.2% vs 80.6%; P>0.05, P>0.05, P>0.05, and P>0.05, respectively; Table 3). Controlled asthmatic patients were older and had moderate asthma severity at inclusion than uncontrolled asthmatic patients (5.5±2.0 vs 2.5±1.5 and 80.8% vs 25.8%; P<0.01 and P<0.001, respectively; Table 3, Figure 3).
In comparison to uncontrolled asthmatic patients, study patients with controlled asthma at 3 months had higher FEV1, PEFR, and FENO levels at inclusion than those with uncontrolled asthma (78±19% vs 52±16%, 69±17% vs 46±12%, and 45±14 ppb vs 29±8 ppb; P<0.05, P<0.05, and P<0.05, respectively; Table 3, Figure 3). Controlled asthma also had a higher reversibility rate than uncontrolled asthma (91.4% vs 54.8%, P<0.01; Table 3). Uncontrolled asthmatic patients at 3 months had a higher level of CANO, nNO, total IgE, and BEC at inclusion than controlled asthmatic patients (6.9±1.9 ppb vs 4.7±0.8 ppb, 2,341±487 ppb vs 1,322±256 ppb, 2,154±785 UI/mL vs 1,143±437 UI/mL, and 823±367 cells/μL vs 451±184 cells/μL; P<0.05, P<0.01, P<0.01, and P<0.05, respectively; Table 3).
Odds ratios and 95% confidence intervals for the predictive factors at inclusion on controlled and uncontrolled asthma at 3 months
The severity of asthma evaluated at inclusion had a significant odds ratio (OR) for the control of asthma defined by GINA or ACT scores >20 (Table 4, Figure 4). The medical history of hospitalization due to asthma exacerbation in previous year had significant and negative OR for uncontrolled asthma defined by GINA at 3 months (OR = 1.9 and P<0.05; Table 4, Figure 4). Asthmatic patients who had allergic rhinitis also had significant OR for asthma control defined by ACT scores (OR = 1.4 and P<0.05). FEV1 <60% of predicted value at inclusion had a risk of uncontrolled asthma in study subjects (OR = 1.8 and P<0.05). FEV1 ≥80% of predicted value at inclusion had a good agreement of OR for asthma control defined by both criteria of GINA and ACT scores (OR = 1.6 and P<0.05; OR = 1.5 and P<0.05, respectively; Table 4, Figure 4). FENO <20 ppb had a risk of uncontrolled asthma at 3 months in study patients (OR = 1.7 and 1.5; P<0.05 and P<0.05; Table 4, Figure 4). However, FENO >35 ppb had a good agreement for asthma control defined by GINA (OR = 3.5 and P<0.01) whereas CANO >5 ppb had a risk of asthma control defined by GINA and ACT scores at 3 months (Table 4, Figure 4).
Table 2.
Comparison of clinical and functional characteristics at the beginning of study vs after 1 month and 3 months.
| Characteristics | At inclusion (N=93) |
1 month (N=93) |
3 months (N=93) |
P |
|---|---|---|---|---|
| Passive smokers, % (N) | 54.8(51) | 47.3(44) | 44.1(41) | NS#,§,° |
| Asthma severity, % | ||||
| Mild asthma | 0 (0) | 0 | 23.5(31) | n/a#,§,° |
| Moderate asthma | 63.4(59) | 72.1(67) | 52.6(49) | NS#,§; <0.05 |
| Severe asthma | 36.6(34) | 27.9(26) | 13.9(13) | NS#; <0.001§; <0.01° |
| Use of SABA, times/week | 6.2 ±3.4 | 3.5 ± 1.7 | 2.6 ± 1.5 | <0.05#; <0.01§; <0.05° |
| Asthma treatment* | ||||
| High dose of ICS+LABA | 36.6(34) | 27.9(26) | 13.9(13) | NS#; <0.001§; <0.01° |
| Moderate dose of ICS+LABA | 63.4(59) | 72.1(67) | 52.6(49) | NS#,§; <0.01° |
| LTRA | 100(93) | 100(93) | 66.5(62) | NS#; <0.001§; <0.001° |
| Moderate dose of ICS | 0 (0) | 0 (0) | 23.5(31) | n/a#,§,° |
| ACT, scores | 8 ± 4 | 15 ± 6 | 19 ± 5 | <0.01#; <0.001§; <0.05° |
| Control of asthma | ||||
| Uncontrolled, % | 100(93) | 49.5(46) | 33.3(31) | <0.001#; <0.001§; <0.01° |
| Partially controlled, % | 0 (0) | 46.2(43) | 16.1(15) | n/a#,§; <0.001° |
| Total controlled, % | 0 (0) | 4.3(4) | 50.5(47) | n/a#,§; <0.001° |
| Treatment adherence | ||||
| Good | 0 (0) | 87.1(81) | 100(93) | n/a#,§; <0.001° |
| Poor | 100(93) | 12.9(12) | 0 (0) | <0.001#; n/a§,° |
| Spirometry, % of predicted | ||||
| FEV1 | 64 ± 18 | 75 ± 12 | 84 ± 14 | <0.05#; <0.01§; <0.05° |
| FVC | 73 ± 12 | 81 ± 14 | 88 ± 11 | <0.05#; <0.05§; NS° |
| FEV1/FVC | 67 ± 11 | 78 ± 8 | 81 ± 12 | <0.05#; <0.01§; NS° |
| FEF25-75 | 48 ± 19 | 59 ± 15 | 64 ± 12 | <0.05#; <0.05§; NS° |
| PEFR | 58 ± 15 | 77 ± 12 | 81 ± 11 | <0.01#; <0.01§; NS° |
| Exhaled NO | ||||
| FENO, ppb | 37 ± 11 | 25 ± 12 | 16 ± 9 | <0.01#; <0.001§; 0.01° |
| CANO, ppb | 5.8 ± 1.4 | 4.9 ± 2.1 | 3.7 ± 1.8 | <0.05#; <0.01§; 0.05° |
| JawNO, nL/min | 77 ± 22 | 41 ± 16 | 38 ± 13 | <0.01#; <0.001§; NS° |
| FnNO, ppb | 1826 ± 379 | 1432 ± 561 | 978 ± 425 | <0.05#; <0.001§; 0.01° |
SABA, short acting beta 2 agonist; ICS, inhaled corticosteroid; LABA, long acting beta 2 agonist; LTRA, leukotriene receptor antagonist; ACT, asthma control test; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FEF25-75, forced expiratory flow at 25-75% of time; PEFR, peak expiratory flow rate; NO, nitric oxide; FENO, fractional exhaled nitric oxide; CANO, concentration of nitric oxide in the gas phase of the alveolar; JawNO, total flux of NO in the conducting airway compartment; FnNO, fractional concentration of nasally aspirated nitric oxide; ppb: part per billion
*treatment started at the beginning of study
#at the beginning vs 1 month
§at the beginning vs 3 months
°3 months vs 1 month.
Discussion
The results of this study showed that i) the cut-off of FENO <20 ppb or CANO >5 ppb demonstrated a risk of uncontrolled asthma at 3 months in study patients; ii) the level of FENO and CANO, percentage of uncontrolled asthma, daily use of SABA were significantly reduced after one and three months of asthmatic treatment; 3) the controlled asthmatic subjects evaluated at three months had higher levels of FENO and CANO and lower percentages of severe asthma and hospitalization frequency in previous year measured at inclusion than uncontrolled asthmatic subjects.
The asthmatic children in this study had the general characteristics of asthma in childhood with early asthma onset (<5 yearsold) and medical history of allergy and atopy (Table 1). The percentage of second-hand smoke exposure in asthmatic subjects was very high (54.8%; Table 1); however, there was no significant difference of FENO level in subjects with or without passive smoking (36±10 ppb vs 38±11 ppb; data not shown). Bobrowska- Korzeniowska et al. have suggested that FENO measurement could be interpreted in the context of environmental tobacco smoke exposure in asthmatic children [27]. Second-hand smoke exposure is a significant and complicating factor for asthma management in children in emerging countries such as Vietnam where the prevalence of adult smokers is high [28]. Asthmatic children exposed to tobacco smoke (passive smoking / second-hand smokers) are at higher risk for uncontrolled asthma with more severe asthma symptoms and exacerbations [29,30]. Therefore, cigarette smoking avoidance in childhood asthma education should be highly emphasized.
The present study showed that at inclusion, the majority of subjects had moderate asthma and their asthma treatment was discontinued or irregular; and especially, they had high levels of FENO, CANO, and FnNO (Table 1, Figure 3). Although the use of FENO in diagnosis and treatment of Type 2 asthma has been recommended previously [1,7,31], its role in predicting the control of asthma in children in short-term follow up has not been well demonstrated. In asthmatic patients, the level of airway inflammation measured by FENO may be used to step-up (with increased FENO) or step-down (with decreased FENO) the dose of ICS treatment [8,18,26,32]. A recent study of ours showed that the use of FENO in combination with GINA recommendations may help to reduce the dose of ICS vs using GINA alone in asthmatic children [8]. In the present study, the level of FENO and CANO has been reduced after asthma treatment at one month and three months (Table 2). Interestingly, our study found that a reduction of FENO and CANO was associated with an increased percentage of subjects with controlled asthma (Table 2). Therefore, the reduction of FENO and CANO level might predict the responsiveness of asthma treatment in patients who are naïve to ICS. However, the results of our study showed that JawNO level was significantly reduced after one month but not after three months and especially there was no significant difference of JawNO level between controlled asthma vs uncontrolled asthma at inclusion (Tables 2 and 3) Although the use of FENO in patients with asthma, especially in severe asthma, has been considered currently as biomarker of ICS and biologic therapy [33], the use of CANO in asthma is still controversial. The results of recent studies suggest CANO might be used as a biomarker of distal airways in asthmatic patients [33-35]. Therefore, CANO has been considered as an additional tool to categorize asthmatic children who have atopy and are sensitive to ICS [33]. A previous study demonstrated the level of CANO was higher in asthmatic children than that in healthy subjects and it was related to asthma control and depended on alveolar inflammation [35]. It has been suggested that an increase of CANO level could reflect a severe or persistent asthma [21,23]. Until now, the phenotype of asthmatic children with high level of CANO has not been well clarified. Increased CANO in exhaled breath might reflect the allergic inflammation in small airways in patients with asthma, whereas it has been reduced in patients with lung fibrosis or pulmonary hypertension [36]. In adults, the increase of CANO level could reflect a severe or persistent asthma during asthma follow up [7,30]. Thus, the reduction of CANO may be used as a predictive biomarker of the responsiveness of ICS treatment in asthmatic children with increased exhaled NO.
Table 3.
Comparison of clinical and functional characteristics at the beginning of study between controlled and uncontrolled asthma patients classified at 3 months.
| Characteristics | Uncontrolled asthma (N=31) |
Total controlled asthma (N=47) |
P |
|---|---|---|---|
| Age, years | 8 ± 3 | 9 ± 2 | NS |
| Gender | |||
| Male | 20 | 29 | NS |
| Female | 11 | 18 | NS |
| Age of asthma onset, years | 2.5 ± 1.5 | 5.5 ± 2.0 | <0.01 |
| BMI, kg/m2 | 17.9 ± 1.6 | 17.3 ± 1.4 | NS |
| Passive smokers, % | 61.3(19) | 38.2(18) | <0.05 |
| Atopy | |||
| Personal allergic history | 80.6(25) | 87.2(41) | NS |
| Familiar allergic history | 58.1(18) | 61.7(29) | NS |
| Asthma severity, % | |||
| Moderate asthma | 25.8(8) | 80.8(38) | <0.001 |
| Severe asthma | 74.2(23) | 19.2(9) | <0.001 |
| AAE with hospitalization, times/ | 3.2±1.8 | 1.8±0.9 | <0.05 |
| Asthma treatment* | |||
| High dose of ICS+LABA | 74.2(23) | 19.2(9) | <0.001 |
| Moderate dose of ICS+LABA | 25.8(8) | 80.8(38) | <0.001 |
| LTRA | 100(31) | 100(47) | NS |
| Moderate dose of ICS | 0 (0) | 0 (0) | n/a |
| Use of SABA, times/week | 8.5 ± 2.6 | 2.4 ± 1.3 | <0.001 |
| Comorbidity | |||
| Allergic rhinitis | 90.3(28) | 65.9(31) | <0.01 |
| Eczema | 29.0(9) | 31.9(15) | NS |
| Spirometry | |||
| FEV1, % of predicted | 52 ± 16 | 78 ± 19 | <0.05 |
| FVC, % of predicted | 69 ± 11 | 79 ± 14 | NS |
| FEV1/FVC, % of predicted | 66 ± 9 | 69 ± 12 | NS |
| FEF25-75, % of predicted | 46 ± 17 | 51 ± 21 | NS |
| PEFR, % of predicted | 46 ± 12 | 69 ± 17 | <0.05 |
| Reversibility§, %(N) | 54.8(17) | 91.4(43) | <0.01 |
| Exhaled NO, ppb | |||
| FENO, ppb | 29 ± 8 | 45 ± 14 | <0.05 |
| CANO, ppb | 6.9 ± 1.9 | 4.7 ± 0.8 | <0.05 |
| JawNO, nL/min | 79 ± 23 | 75 ± 21 | NS |
| FnNO, ppb | 2341 ± 487 | 1322 ± 256 | <0.01 |
| Total IgE, UI/mL | 2154 ± 785 | 1143 ± 437 | <0.01 |
| Blood eosinophil count, cells/μL | 823 ± 367 | 451 ± 184 | <0.05 |
| Skin prick test (+)° | 83.9(26) | 80.8(38) | NS |
BMI, body mass index; AAE, acute asthma exacerbation; SABA, short acting beta 2 agonist; ICS: inhaled corticosteroid; LABA, long acting beta 2 agonist; LTRA, leukotriene receptor antagonist; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; FEF25-75, forced expiratory flow at 25-75% of time; PEFR, peak expiratory flow rate; NO, nitric oxide; FENO, fractional exhaled nitric oxide; CANO, concentration of nitric oxide in the gas phase of the alveolar; JawNO, total flux of NO in the conducting airway compartment; FnNO, fractional concentration of nasally aspirated nitric oxide; ppb: part per billion; BEC, blood eosinophil count; SPT, skin prick test
*treatment started at inclusion
§defined by increase of FEV1>12% and 200mL
°positive at least with one allergen on skin prick test; NS, not significant difference.
Figure 3.

Comparison of clinical and functional characteristics measured at inclusion of controlled and uncontrolled asthma at 3 months. BMI, body mass index; FENO, fractional exhaled nitric oxide; CANO, concentration of alveolar nitric oxide; NS, not significant difference.
Figure 4.
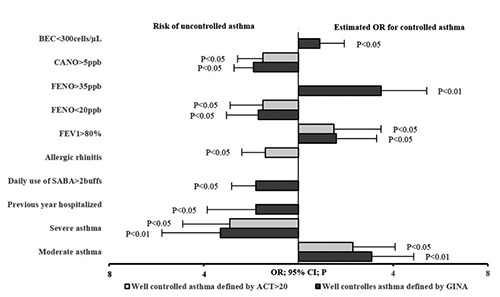
Odds ratios (OR) and 95% confidence intervals (CI) for the predictive factors at inclusion on controlled and uncontrolled asthma at 3 months. BEC, blood eosinophil count; FENO, fractional exhaled nitric oxide; CANO, concentration of alveolar nitric oxide; FEV1, forced expiratory volume in one second; SABA, short acting beta 2 agonist.
Table 4.
Odds ratios (OR) and 95% confidence intervals for the predictive factors at inclusion on controlled and uncontrolled asthma at 3 months.
| Parameters | Well controlled asthma | ||||||
|---|---|---|---|---|---|---|---|
| Defined by GINA | Defined by ACT scores ≥ 20 | ||||||
| OR (min/max) | CI 95% | P | OR | CI 95% | P | ||
| Clinical characteristics* | |||||||
| Asthma onset >5 years (+) | 1.3 | 0.5 / 1.8 | NS | 1.5 | 0.8 / 1.9 | NS | |
| Passive smoker (-) | 1.4 | 0.7 / 2.1 | NS | 1.3 | 0.7 / 1.8 | NS | |
| Asthma severity | Moderate (+) | 3.1 | 1.8 / 5.3 | <0.01 | 2.3 | 1.6 / 4.1 | <0.05 |
| Severe (-) | 3.3 | 1.7 / 5.9 | <0.01 | 2.9 | 1.4 / 4.8 | <0.05 | |
| AAE hospitalized in previous year ≥2 times (-) | 1.9 | 0.9 / 2.9 | <0.05 | 1.7 | 0.6 / 3.1 | NS | |
| Daily used SABA ≥2 buffs§ (-) | 1.8 | 0.8 / 2.7 | <0.05 | 1.5 | 0.7 / 2.7 | NS | |
| Allergic rhinitis (-) | 1.5 | 0.7 / 2.9 | NS | 1.4 | 0.7 / 2.4 | <0.05 | |
| Functional characteristics* | |||||||
| FEV1, % | <60 (-) | 1.8 | 0.1 / 4.1 | <0.05 | 1.4 | 0.9 / 3.9 | NS |
| 60 – 80 (+) | 0.9 | 0.5 / 2.1 | NS | 0.7 | 0.2 / 1.9 | NS | |
| ≥ 80 (+) | 1.6 | 0.9 / 3.2 | <0.05 | 1.5 | 0.7 / 3.8 | <0.05 | |
| nNO, ppb | >500 (-) | 1.4 | 0.7 / 3.5 | NS | 1.3 | 0.6 / 3.1 | NS |
| 500-1000 (-) | 1.5 | 0.5 / 3.1 | NS | 1.4 | 0.8 / 2.4 | NS | |
| >1000 (-) | 1.3 | 0.7 / 3.1 | NS | 0.9 | 0.4 / 2.7 | NS | |
| FENO, ppb | <20 (-) | 1.7 | 0.8 / 3.3 | <0.05 | 1.5 | 0.9 / 3.1 | <0.05 |
| 20 – 35 (+) | 1.3 | 0.3 / 3.4 | NS | 0.9 | 0.4 / 2.9 | NS | |
| >35 (+) | 3.5 | 2.2 / 5.9 | <0.01 | 2.9 | 1.4 / 4.9 | NS | |
| CANO, ppb | >5 (-) | 1.9 | 0.9 / 2.7 | <0.05 | 1.5 | 0.8 / 2.6 | <0.05 |
| ≤5 (+) | 0.8 | 0.2 / 1.7 | NS | 0.7 | 0.3 / 1.9 | NS | |
| <300 (+) | 0.9 | 0.3 / 2.1 | <0.05 | 0.7 | 0.2 / 1.8 | NS | |
| BEC, cells/μL | 300-600 (+) | 1.6 | 0.8 / 3.5 | NS | 1.4 | 0.7 / 2.9 | NS |
| ≥600 (-) | 1.3 | 0.6 / 2.3 | NS | 1.2 | 0.5 / 2.1 | NS | |
AAE, acute asthma exacerbation; SABA, short acting beta 2 agonist; FEV1, forced expiratory volume in 1 s; FENO, fractional exhaled nitric oxide; CANO, concentration of nitric oxide in the gas phase of the alveolar; FnNO, fractional concentration of nasally aspirated nitric oxide; ppb, part per billion; BEC, blood eosinophil count; NS, not significant difference
*measured at inclusion
§measured at 1 month; (-), risk of uncontrolled asthma; (+), positive agreement of controlled asthma.
In the management of asthma, especially in asthmatic children, the other important issue is how to predict the success of asthma management in order to recommend the appropriate asthma action plan. The results of this study showed that some clinical characteristics of subjects at inclusion had the significant predictive factors of asthma control at 3 months, evaluated by OR. These predictive factors included age of asthma onset, frequency of hospitalization in previous year, and asthma severity with airflow limitation (Table 4, Figure 4). For asthmatic children with low level of FENO at inclusion, there was a risk of uncontrolled asthma at 3 months, whereas for whom with high level of FENO at inclusion there was a good agreement for asthma control (Table 4, Figure 4). Interestingly, high level of CANO had a risk of uncontrolled asthma defined by GINA and ACT scores at 3 months (Table 4, Figure 4). These results suggest that the measurement of exhaled NO (FENO and CANO) might be used to predict the control of asthma in children who are naïve with ICS.
Until now, the studies on the role of CANO in asthmatic children are limited. The present study suggests a new approach in the control of asthma by measuring CANO concomitant with FENO in asthmatic children. However, the present study still has some limitations relating to the small number of study subjects and lack of long-term follow up. In addition, the measurement of CANO needs the multiple flow device which is costlier and has not been extensively equipped in all asthma care centers. Thus, more studies on the role of exhaled NO as predictive biomarkers of asthma control in children should be undertaken to clarify its benefits and costeffectiveness.
Conclusions
Children with asthma who are naïve with ICS usually have high levels of exhaled NO. High levels of FENO in asthmatic children might have a predictive value for controlled asthma. However, low levels of FENO and high levels of CANO can predict uncontrolled asthma in children. The measurement of exhaled NO, especially CANO, is still a new area in the field of asthma management; therefore, more studies in this field should be done in the future.
Acknowledgements
The authors would like to thank all the Members of the Clinical Research Centers of Lam Dong Medical College for their contribution to this work.
List of abbreviations
- AAE
acute asthma exacerbation;
- ACT
asthma control test;
- BEC
blood eosinophil count;
- BMI
body mass index;
- CANO
concentration of alveolar nitric oxide;
- FEF25-75
forced expiratory flow at 25-75% of time;
- FEV1
forced expiratory volume in 1 second;
- FENO
fractional exhaled nitric oxide;
- FnNO
nasal nitric oxide;
- FVC
forced vital capacity;
- ICS
inhaled corticosteroid;
- LABA
long acting beta 2 agonist;
- LTRA
leukotriene receptor antagonist;
- NO
nitric oxide;
- PEFR
peak expiratory flow rate;
- Ppb
part per billion;
- SABA
short acting beta 2 agonist;
- SPT
skin prick test.
Funding Statement
Funding: The study was supported by the grant from Lam Dong Medical College (LDMC-SR.03.2018), Dalat city, Vietnam.
References
- 1.Global Initiative for Asthma. [Internet]. Archived reports. Accessed on: 29 April 2020. Available from: http://ginasthma.org/archived-reports/ [Google Scholar]
- 2.Centers for Disease Control and Prevention. [Internet]. Asthma in the US vital signs. 2011. Accessed on: 29 April 2020. Available from: https://www.cdc.gov/vitalsigns/asthma/index.html [Google Scholar]
- 3.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief 2012;1–8. [PubMed] [Google Scholar]
- 4.Toizumi M, Hashizume M, Nguyen HAT, Yasunami M, Kitamura N, Iwasaki C, et al. Asthma, rhinoconjunctivitis, eczema, and the association with perinatal anthropometric factors in Vietnamese children. Sci Rep 2019;9:2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nga NN, Chai SK, Bihn TT, Redding G, Takaro T, Checkoway H, et al. ISAAC-based asthma and atopic symptoms among Ha Noi school children. Pediatr Allergy Immunol 2003;14:272-9. [DOI] [PubMed] [Google Scholar]
- 6.Cloutier MM, Schatz M, Castro M, Clark N, Kelly HW, Mangione-Smith R, et al. Asthma outcomes: composite scores of asthma control. J Allergy Clin Immunol 2012;129:S24-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen-Thi-Bich H, Duong-Thi-Ly H, Thom VT, Pham-Thi-Hong N, Dinh LD, Le-Thi-Minh H, et al. Study of the correlations between fractional exhaled 359 nitric oxide in exhaled breath and atopic status, blood eosinophils, FCER2 mutation, and asthma control in Vietnamese children. J Asthma Allergy 2016;9:163-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinh-Thi-Dieu H, Vo-Thi-Kim A, Tran-Van H, Tang-Thi-Thao T, Duong-Quy S. Study of the beneficial role of exhaled nitric oxide in combination with GINA guidelines for titration of inhaled corticosteroids in children with asthma. J Breath Res 2020. doi: 10.1088/1752-7163/ab6809. [DOI] [PubMed] [Google Scholar]
- 9.Eguiluz-Gracia I, Tay TR, Hew M, Escribese MM, Barber D, O’Hehir RE, et al. Recent developments and highlights in biomarkers in allergic diseases and asthma. Allergy 2018;73: 2290-305. [DOI] [PubMed] [Google Scholar]
- 10.Fitzpatrick AM. Biomarkers of asthma and allergic airway diseases. Ann Allergy Asthma Immunol 2015;115:335-40. [DOI] [PubMed] [Google Scholar]
- 11.Hancox RJ, Pavord ID, Sears MR. Associations between blood eosinophils and decline in lung function among adults with and without asthma. Eur Respir J 2018;51:1702536. [DOI] [PubMed] [Google Scholar]
- 12.Konradsen JR, Skantz E, Nordlund B, Lidegran M, James A, Ono J, et al. Predicting asthma morbidity in children using proposed markers of Th2-type inflammation. Pediatr Allergy Immunol 2015;26:772-9. [DOI] [PubMed] [Google Scholar]
- 13.Sippel JM, Holden WE, Tilles SA, O'Hollaren M, Cook J, Thukkani N, et al. Exhaled nitric oxide levels correlate with measures of disease control in asthma. J Allergy Clin Immunol 2000;106:645-50. [DOI] [PubMed] [Google Scholar]
- 14.Ozier A, Girodet PO, Bara I, Tunon de Lara JM, Marthan R, Berger P. Control maintenance can be predicted by exhaled NO monitoring in asthmatic patients. Respir Med 2011;105:989-96. [DOI] [PubMed] [Google Scholar]
- 15.Michils A, Baldassarre S, Van Muylem A. Exhaled nitric oxide and asthma control: a longitudinal study in unselected patients. Eur Respir J 2008;31:539-46. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Pianosi P, Keogh K, Zaiem F, Alsawas M, Alahdab F, et al. The clinical utility of fractional exhaled nitric oxide (FENO) in asthma management. Report No. 17(18)-EHC030- EF. AHRQ Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality (US); 2017. [PubMed] [Google Scholar]
- 17.Malinovschi A, Van Muylem A, Michiels S, Michils A. FENO as a predictor of asthma control improvement after starting inhaled steroid treatment. Nitric Oxide 2014;40:110-6. [DOI] [PubMed] [Google Scholar]
- 18.Duong-Quy S. Clinical utility of the exhaled nitric oxide (NO) measurement with portable devices in the management of allergic airway inflammation and asthma. J Asthma Allergy 2019;12:331-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institute for Health and Care Excellence (NICE). [Internet]. Asthma: diagnosis, monitoring and chronic asthma management. Accessed on: 28 April 2020. Available from: https://www.nice.org.uk/guidance/ng80/chapter/Recommendations#diagnosing-asthma-in-young-children [PubMed] [Google Scholar]
- 20.Scichilone N, Battaglia S, Taormina S, Modica V, Pozzecco E, Bellia V. Alveolar nitric oxide and asthma 352 control in mild untreated asthma. J Allergy Clin Immunol 2013;131:1513-7. [DOI] [PubMed] [Google Scholar]
- 21.Berry M, Hargadon B, Morgan A, Shelley M, Richter J, Shaw D, et al. Alveolar nitric oxide in adults with asthma: Evidence of distal lung inflammation in 355 refractory asthma. Eur Respir J 2005;25:986-91. [DOI] [PubMed] [Google Scholar]
- 22.Sardón O, Corcuera P, Aldasoro A, Korta J, Mintegui J, Emparanza JI, et al. Alveolar nitric oxide and its role in pediatric asthma control assessment. BMC Pulm Med 2014; 14:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen-Thi-Bich H, Duong-Thi-Ly H, Thom VT, Pham-Thi-Hong N, Doan Dinh L, Le-Thi-Minh H, et al. Study of the correlations between fractional exhaled 359 nitric oxide in exhaled breath and atopic status, blood eosinophils, FCER2 mutation, and asthma control in Vietnamese children. J Asthma Allergy 2016;9:163-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Condorelli P, Shin HW, Aledia AS, Silkoff PE, George SC. A simple technique to characterize proximal and peripheral nitric oxide exchange using constant flow exhalations and an axial diffusion model. J Appl Physiol 2007;102:417-25. [DOI] [PubMed] [Google Scholar]
- 25.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 2011;184:602-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bobrowska-Korzeniowska M, Stelmach I, Brzozowska A, Jerzyńska J, Mitał M, Stelmach W. The effect of passive smoking on exhaled nitric oxide in asthmatic children. Nitric Oxide 2019;86:48-53. [DOI] [PubMed] [Google Scholar]
- 27.Duong-Quy S, Hua-Huy T, Mai-Huu-Thanh B, Doan-Thi-Quynh N, Le-Quang K, Nguyen-Van H, et al. Early detection of smoking related chronic obstructive pulmonary disease in Vietnam. Rev Mal Respir 2009;26:267-74. [DOI] [PubMed] [Google Scholar]
- 28.Akinbami LJ, Kit BK, Simon AE. Impact of environmental tobacco smoke on children with asthma, United States, 2003-2010. Acad Pediatr 2013;13:508-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, May SM, Charoenlap S, Pyle R, Ott NL, Mohammed K, et al. Effects of secondhand smoke exposure on asthma morbidity and health care utilization in children: a systematic review and metaanalysis. Ann Allergy Asthma Immunol 2015;115:396-401. [DOI] [PubMed] [Google Scholar]
- 30.Beck-Ripp J, Griese M, Arenz S, Köring C, Pasqualoni B, Bufler P. Changes of exhaled nitric oxide during steroid treatment of childhood asthma. Eur Respir J 2002;19:1015-9. [DOI] [PubMed] [Google Scholar]
- 31.Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med 2005;352:2163-73. [DOI] [PubMed] [Google Scholar]
- 32.Puckett JL, Taylor RW, Leu SY, Guijon OL, Aledia AS, Galant SP, George SC. Clinical patterns in asthma based on proximal and distal airway nitric oxide categories. Respir Res 2010; 11:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zinellu E, Piras B, Ruzittu GGM, Fois SS, Fois AG, Pirina P. Recent advances in inflammation and treatment of small airways in asthma. Int J Mol Sci 2019;20:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paredi P, Kharitonov SA, Meah S, Barnes PJ, Usmani OS. A novel approach to partition central and peripheral airway nitric oxide. Chest 2014;145:113-9. [DOI] [PubMed] [Google Scholar]
- 35.Dinh-Xuan AT, Annesi-Maesano I, Berger P, Chambellan A, Chanez P, Chinet T, et al. Contribution of exhaled nitric oxide measurement in airway inflammation assessment in asthma. A position paper from the French Speaking Respiratory Society. Rev Mal Respir 2015;32:193-215. [DOI] [PubMed] [Google Scholar]


