Abstract
Introduction
Mesenchymal stem cells (MSCs) have always been the center of the experimental exploration of regenerative therapy together with other stem cells. Among with, peripheral blood-derived mesenchymal stem cells (PBMSCs) have been regarded as promising in clinical applications for its convenience of acquisition from peripheral blood. However, few reported experiments so far to elucidate the exact mechanisms of how PBMSC influence regeneration. As the ability of immunomodulatory is one of the crucial features that influence MSC to reconstruct impaired tissue, we decided to focus on the immunomodulatory abilities of PBMSCs and conducted experiments associated with macrophages and T lymphocytes, which are two main cell types that dominate the innate and acquired immunity. Therefore, a basis can be made from these experiments for applications of PBMSCs in regenerative therapy in the future.
Methods
A Transwell system was used for the coculturing of PBMSCs with macrophages. T lymphocytes were cultured directly with PBMSCs. Flow cytometry and immunochemistry were conducted for identifying the phenotypes. Immunomagnetic microspheres, ELISA and RT-qPCR were used to detect the expressions of relevant molecules or mRNAs.
Results
After coculturing PBMSCs with M0, the anti-inflammatory IL-10 was increased whereas the proinflammatory TNF-α decreased; the expression of CD11b, CD68, CD206, Arg-1, IL-10 and CCL-22 was up-regulated whereas IL-1β down-regulated. The expression of TGF-β, RORγt, Foxp3 and IL-10 was increased in the cocultured lymphocytes whereas IL-17 and IL-6 decreased; the ratio of CD4+IL-17+ Th17/CD25+Foxp3+ Treg was reduced.
Conclusion
The findings demonstrated that PBMSCs promoted the anti-inflammatory features of macrophages and the Th17/Treg system. PBMSCs are able to inhibit inflammation associated with these two immune cell systems, and thus provide insight into how PBMSCs achieve their immunomodulatory ability.
Keywords: Stem cells, Mesenchymal stem cells, Inflammation, Macrophages, T lymphocytes
Highlights
-
•
Anti-inflammatory effect of peripheral blood-derived mesenchymal stem cells.
-
•
Co-culture promotes the polarization of M2 macrophages.
-
•
Co-culture alters the balance of Th17/Tregs.
1. Introduction
Mesenchymal stem cells (MSCs) have received increasing attention due to their multipotentiality, which may enable them to promote tissue regeneration and provide a novel strategy for the clinical treatment of many intractable diseases [[1], [2], [3]]. Nevertheless, several studies on inflammatory/immunological diseases, especially spinal cord injuries (SCI) have demonstrated that the survival rates of MSCs after implantation are extremely low (~0.1% in the spinal cord), yet they still improve prognosis in animal models [[1], [2], [3]]. Such therapeutic effects may be largely due to the immunomodulatory ability of MSCs; however, the mechanism of their effects remains unclear.
MSCs exhibit plastic adherence, multilineage differentiation capacity, and expression of MSC markers and pluripotent genes [4]. Postnatal organs and tissues serve as primary MSC sources; however, different sources of MSCs have varying differentiation potentialities, as well as variation in the expression stem cell-related markers and other important features, including proliferation, immunomodulation and xenotransplantation abilities [4]. Therefore, MSCs should be carefully validated and studied prior to their application.
Compared to the invasiveness of the harvesting procedure of other tissue-derived MSCs, peripheral blood-derived MSCs (PBMSCs) can be easily obtained from blood samples in a sterile and less invasive manner, and thus provide an efficient method of collecting autologous MSCs for regenerative therapies [4,5]. Additionally, recent studies have identified unique biological features of PBMSCs that are different from other MSCs, and have demonstrated that PBMSCs have great potentiality for the treatment of wounds and diseases in animal models, which supports our previous work in which PBMSCs were transplanted into SCI Sprague Dawley (SD) rats [[6], [7], [8], [9], [10], [11]]; however, the specific mechanisms of such modulatory abilities remain unclear.
Originating from the concept of Th1/Th2 polarization, it is established that macrophages can be polarized to a proinflammatory state, termed M1, or an anti-inflammatory state, termed M2 [12,13]. It has been reported that MSCs have the ability to promoting macrophage polarization to an M2 phenotype in vivo, and thus suppress inflammation resulting in remodeling and disease-curing effects [[14], [15], [16], [17]]; however, the secretion patterns of MSCs are complex and needed to be clarified.
CD4+ T cells can differentiate into distinct subtypes, including T helper 1 (Th1), T helper 2 (Th2), T helper 17 (Th17) and regulatory T (Treg) cells [18]. Tregs are regarded as immunosuppressive and have the ability to inhibit inflammation by secreting anti-inflammatory cytokines, interleukin-10 (IL-10) and transforming growth factor-β (TGF-β) [[19], [20], [21]]. By contrast, Th17 cells are characterized by the production of the key proinflammatory cytokine, IL-17, and are considered to be proinflammatory cells [22,23]. Recent studies have demonstrated that the ratio of Th17/Treg cells is critical in regulating immunity [[24], [25], [26]]. Thus, Tregs and Th17 need to reach a balance to maintain homeostasis, otherwise, there may be excessive and invasive inflammatory reactions. Several studies have focused on using bone marrow-derived MSCs to treat autoimmune diseases; however, an exploration of how PBMSCs influence the Th17/Treg balance has never been conducted, to the best of our knowledge.
Taken together, it is necessary to further elucidate the mechanisms of the therapeutic effects of PBMSCs, especially regarding inflammatory/immunological diseases. The current study focused on the two cell systems mentioned above, with the cells co-cultured with PBMSCs in order to determine how PBMSCs influence inflammation by regulating these two important cell types. It was noted that the populations of M2 macrophages and Tregs increased, while M1 macrophages and Th17 cells were decreased by co-culture with PBMSCs. The results further confirmed the effectiveness of PBMSCs in regulating M2 polarization and the differentiation of Tregs; thus, indicating that PBMSCs could be used within the clinical treatment of inflammatory diseases in the future.
2. Materials and methods
2.1. Animals
Specific pathogen-free SD rats were purchased from the Animal Center of Third Military Medical University (production license no. SCXK Yu 2012-0005). A total of 40 SD rats (age, 6–8 weeks; weight, 80–120 g; both sexes) were used in this experiment conforming to the 3Rs (Reduction, Replacement, and Refinement) principle. The animal studies were performed after receiving approval from the Institutional Animal Care and Use Committee of Zunyi Medical University.
2.2. Cells
2.2.1. Isolation, culture, and identification of PBMSCs
PBMSCs were processed following our previously described methods [11]. A total of 25 SD rats were subcutaneously injected with granulocyte-colony stimulating factor (G-CSF; 100 μg/kg/day) for 6 days. Peripheral blood of 5–10 ml was harvested from the left ventricles. Immediately, the sample was diluted with an equal volume of PBS and carefully layered onto the rat Ficoll solution (1.083 g/l; Chuanye Biochemicals). After centrifugation at 400×g for 30 min, the layer at the interface was collected and rinsed with PBS. The pellet was pipetted and resuspended in 5 ml complete α-Eagle's Minimum Essential Medium (α-MEM; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 2 mmol/l glutamine (Hyclone GE Healthcare Life Sciences) and 0.01 μg/ml basic fibroblast growth factor (Peprotech, Inc.) after centrifugation. Subsequently, the cell suspensions were transferred into a disposable plastic 25-cm2 culture flask, and placed into an incubator (37 °C, 5% CO2 and saturated humidity). The medium was changed when required. The cells were subcultured once 80–90% confluent. Passage 3 (P3) cells were analyzed by flow cytometry and immunocytochemical staining for identification, and then used in the subsequent experiments. Mesoderm multi-lineage differentiation capacities were analyzed according to our previous research [27].
2.2.2. Isolation and culture of M0 macrophages
L929 conditioned medium (LCM) was collected according to previous protocols by culturing L929 cells [28]. SD rats (n = 15; age, 6–8 weeks; weight, 80–120 g) were sacrificed by cervical dislocation, and then femurs were collected under sterile conditions. The bone marrow cavities were flushed into a clean 50 ml tube using RPMI-1640 medium. After centrifugation, the pellet was resuspended with differentiation medium (10% FBS, 20% LCM and 70% RPMI-1640) then seeded into 6-well plates at a density of 2 × 106 cells/ml. The medium was first changed on the 2nd day. The macrophages obtained from the plates 6 days after culturing were regarded as M0 macrophages and were used for further experiments.
2.2.3. Isolation and culture of rat spleen lymphocytes
The spleens of the same 15 SD rats were obtained under aseptic conditions as soon as the bone marrow collection described above was completed and were ground gently in 1 ml RPMI-1640 medium in a glass grinder. The liquid was filtered through a 300-mesh nylon filter. Gently, the filtration solution was added onto the top of Ficoll solutions. The lymphocyte layer was then collected after centrifugation at 400×g for 30 min. The pellet was resuspended with RPMI-1640 conditioned medium (10% FBS, 2 mmol/l glutamine) and centrifuged at 400×g for 10 min. Again, the pellet was resuspended in conditioned medium. Following seeding into a 6-well plate, the lymphocytes were cultured in an incubator (37 °C, 5% CO2 and saturated humidity).
2.2.4. Indirect co-culture of PBMSCs and M0 macrophages
Purified bone marrow-derived macrophages were seeded in a 0.4-μm Transwell culture system at a density of 1 × 105 cells/cm2. Cells were divided into three groups: PBMSCs (1 × 105 cells/cm2) group, macrophage (M0) group, PBMSCs (1 × 105 cells/cm2) and macrophage co-culture group (PBMSCs + M0). In the co-culture group, PBMSCs were seeded in the upper Transwell chamber and co-cultured with M0 macrophages for 3 days. On the 4th day, the medium was changed to serum-free medium and supernatant was collected after 24 h. The supernatant was stored at −80 °C for cytokine detection using immunomagnetic microspheres (Bio-Rad Laboratories, Inc.). Briefly, the standards and samples were diluted according to the Bio-Plex Cytokine Reagent Kit (Bio-Rad Laboratories, Inc.) and incubated on ice for 30 min; the immunomagnetic microspheres were gently shaken for ~30 s then were diluted in assay buffer (Bio-Rad Laboratories, Inc.) and 50 μl was added to plates. After adding the antibodies to standards and samples accordingly, the plates were incubated for 30 min and centrifuged at 850×g avoiding light. After washing, the plates were detected using the Bio-Plex 200 system (Bio-Rad Laboratories, Inc.).
2.2.5. Direct co-culture of PBMSCs and lymphocytes
The lymphocyte co-culture was performed using two groups: Direct co-culture group (PBMSCs + lymphocytes) and the lymphocytes group. Specifically, for the direct co-culture group, 1 × 106 PBMSCs were added into a T-75 flask and cultured for ~8 h in 10 ml complete α-MEM until they became adherent. Then, mitomycin (40 μg/ml) was added to the flask. After 3 h, the medium was replaced with RPMI-1640 supplemented with 10% FBS and 2 mmol/l glutamine. The lymphocytes (1 × 107 cells) were added to reach a ratio of 10:1 with PBMSCs [29]. The supernatants were collected after 5 and 10 days of co-culturing for further experiments.
2.3. Flow cytometry
PBMSC identification. P3 PBMSCs were obtained and resuspended in PBS solutions (1% BSA) then added into flow tubes (1 × 106 cells/ml in 100 μl). FITC-conjugated mouse antibodies against CD90 (1:100; eBioscience; Thermo Fisher Scientific, Inc.), CD29 (1:100; eBioscience; Thermo Fisher Scientific, Inc.), CD45 (1:100; eBioscience; Thermo Fisher Scientific, Inc.), and the PE-conjugated mouse antibodies against rat CD11b (1:100; eBioscience; Thermo Fisher Scientific, Inc.), CD79a (1:100; eBioscience; Thermo Fisher Scientific, Inc.) were added into the tubes. After fixation with 4% paraformaldehyde, the samples were examined using a FACS Calibur flow cytometer (BD Biosciences; Becton, Dickinson and Company) and analyzed using CELL Quest software. FITC-conjugated and PE-conjugated mouse anti-rat IgG (Invitrogen; Thermo Fisher Scientific, Inc.) were used as controls.
2.3.1. Macrophages
Macrophages of the M0 group and PBMSCs + M0 group were collected and resuspended in PBS solutions (1% BSA), and then added into flow tubes (1 × 106 cells/ml in 100 μl). Antibodies against mouse anti-rat CD206 (1:100; Santa Cruz Biotechnology, Inc.), CD68 (1:100; Santa Cruz Biotechnology, Inc.), CD11b (1:100; Santa Cruz Biotechnology, Inc.) were added into the tubes for subsequent detection. For the inducible nitric oxide synthase (iNOS), arginase-1 (Arg-1) and tumor necrosis factor-α (TNF-α) detection, macrophages were first treated with Protein Transport Inhibitor Cocktail (2 μM; eBioscience; Thermo Fisher Scientific, Inc.) 6 h before collection, then were obtained and resuspended. After fixation with 4% paraformaldehyde, permeabilization buffer (eBioscience; Thermo Fisher Scientific, Inc.) was added. Samples were incubated with the antibody for 30 min and then examined using a FACS Calibur flow cytometer (BD Biosciences; Becton, Dickinson and Company). The data acquired were analyzed using CELL Quest software.
2.3.2. Th17/Treg
As described previously (11 Fu et al., 2017), the lymphocytes were obtained and resuspended in RPMI-1640 (10% FBS) after culturing for 5 and 10 days. After transfer to flow tubes, the cells were pretreated with PMA/lonomycin mixture (250X, 12.5 mg/ml and 0.25 mg/ml; Multi Sciences) for 4 h. The CD4-FITC (1:100; eBioscience; Thermo Fisher Scientific, Inc.) and CD25-PE (1:100; eBioscience; Thermo Fisher Scientific, Inc.) antibodies were added to the tubes and incubated for 1 h avoiding light. Fixation/permeabilization buffer (eBioscience; Thermo Fisher Scientific, Inc.) was added in the following procedure. Then, the forkhead box p3 (Foxp3)-APC (1:100; eBioscience; Thermo Fisher Scientific, Inc.) and IL-17-PE (eBioscience; Thermo Fisher Scientific, Inc.) antibodies were added to the tubes to detect CD4+CD25+Foxp3+ Treg cells, and CD4+IL17+ Th17 cells. The cell samples were resuspended and examined using a FACS Calibur flow cytometer (BD Biosciences; Becton, Dickinson and Company) and analyzed with CELL Quest Software.
2.4. Immunocytochemistry
P3 PBMSCs were obtained and seeded into 6-well plates at a density of 1 × 105 cells/cm2. Once 80% confluent, mouse anti-rat CD34 (Santa Cruz Biotechnology, Inc.), CD73 (Santa Cruz Biotechnology, Inc.) and CD105 (Santa Cruz Biotechnology, Inc.) antibodies were added, and then incubated with the cells overnight. Then, the samples were washed with PBS, followed by incubation with horseradish peroxidase-conjugated goat anti-mouse secondary antibodies (Invitrogen; Thermo Fisher Scientific, Inc.) for 35 min. After reaction with 3,3′-diaminobenzidine (DAB; Sigma–Aldrich; Merck KGaA) and staining with hematoxylin (Solarbio Science & Technology), the samples were examined using an inverted microscope (Olympus Corporation).
2.5. Enzyme-linked immunosorbent assay (ELISA)
The concentrations of IL-17 and TGF-β in the supernatants of the lymphocyte group and PBMSCs + lymphocytes group were detected using ELISA kits (eBioscience; Thermo Fisher Scientific, Inc.). The 96-well plates were measured using the Bio-Rad Plate Reader model 680 (Bio-Rad Laboratories, Inc.) and the following data were analyzed using the ELISA Calc software.
2.6. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
RT-qPCR techniques were used to examine the mRNA levels of IL-6, Foxp3, RAR-related orphan receptor γT (RORγt), TGF-β, IL-17 and IL-10 in T cells, and IL-6, IL-1β, C–C motif chemokine ligand 22 (CCL22) and IL-10 in macrophages. Total mRNA was extracted using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) and reverse transcribed with the GoScript™ RT System (Promega Corporation), according to the manufacturer's protocol. GoTaq® Probe RT-qPCR System (Promega Corporation) was used in the subsequent amplification. The thermocycling conditions were as follows: Initial denaturation at 95 °C for 5 min, followed by 40 cycles of 95 °C for 10 s, 60 °C annealing for 30 s and 72 °C extension for 10 s. The relative mRNA expressions were calculated using the ΔΔCq method. The primers used in the procedures were designed using Primer 3 software and synthesized by Sangon Biotech Company (Table 1).
Table 1.
Primer sets for RT-qPCR.
| Gene | Sequence (5′–3′), upstream | Sequence (5′–3′), downstream | Bp |
|---|---|---|---|
| IL-6 | TTCACAGAGGATACCACCCACA | AATCAFAATTFCCATTGCAC | 124 |
| IL-10 | GAAAAATTGAACCACCCGGCA | TTCCAAGGAGTTGCTCCCGT | 99 |
| TGF-β | CCGCAACAACGCAATCTATG | AGCCCTGTATTCCGTCTCCTT | 180 |
| IL-17A | CAAACGCCGAGGCCAATAAC | AGAGTCCAGGGTGAAGTGGA | 130 |
| FoxP3 | AGCTTGTTTGCTGTGCGGAG | GTGGCATAGGTGAAAGGGGG | 122 |
| RORγT | AGGCTACGAGGGTCAGGATT | AGACCGTAGCACCATTCCAC | 167 |
| IL-1β | AATGACCTGTTCTTTGAGGCTGAC | CGAGATGCTGCTGTGAGATTTGAAG | 115 |
| CCL22 | GGCAGGAAGGACCATACAAA | TCCAGAGGAGCAAGCAGATT | 200 |
IL, interleukin; TGF, transforming growth factor; FoxP3, forkhead box P3; ROR, retinoic acid receptor-related orphan receptor.
2.7. Statistical analysis
All data were processed and presented as the mean ± SD, or as the percentage of controls ± SD. Means were compared using the Student's t-test or one-way ANOVA (LSD was chosen for post-hoc multiple comparisons). P < 0.05 was considered to indicate a statistically significant difference.
3. Results
3.1. PBMSCs have MSC characteristics
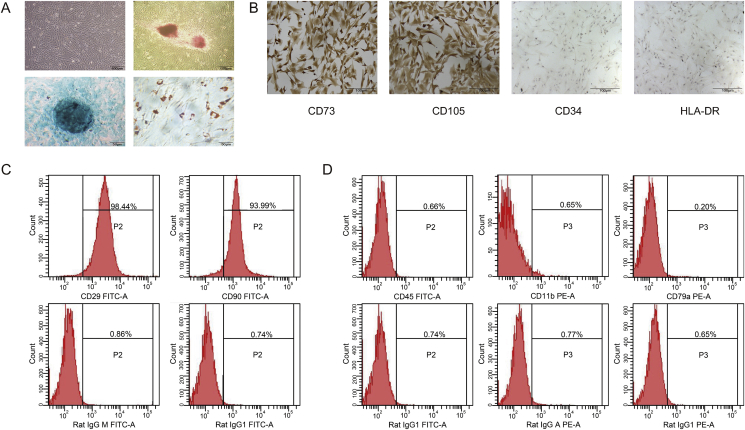
MSCs have a characteristic fibroblast-like shape and grow in a radial manner. Morphological observations showed that the cells cultured using established procedures in the present study exhibited the typical fibroblast-like shape after primary culture for 20 days (Fig. 1A). The subcultured cells had the same spindle shape and growth pattern. Immunocytochemistry and flow cytometry revealed that CD90, CD29, CD73 and CD105 were highly expressed in the MSCs, whereas these cells were negative for CD45, CD11b, CD79a, CD34 and HLA-DR (Fig. 1B–D), which is consistent with previous research on MSCs [30].
Fig. 1.
Identification of cultured rat PBMSCs microscopy, immunocytochemical staining and flow cytometry techniques (A) Morphology of P3 PBMSCs; Osteogenesis, chondrogenesis, adipogenesis-committed differentiation of PBMSCs were determined by alizarin red, alcian blue and oil red staining, respectively (B) CD73, CD105, CD34 and HLA-DR of P3 PBMSCs treated with DAB and colored with hematoxylin, then observed under an inverted microscope (x100). Phenotypic analysis of PBMSCs analyzed by flow cytometry (C) CD29 FITC-A and CD90 FITC-A of P3 PBMSCs, together with the control isotypes Rat IgGM FITC-A and Rat IgG1 FITC-A detection (D) CD45 FITC-A, CD11b PE-A and CD79a PE-A of P3 PBMSCs, together with the control isotypes Rat IgG1 FITC-A, Rat IgG APE-A and Rat IgG1 PE-A detection. PBMSCs, peripheral blood-derived mesenchymal stem cells; P3, passage 3.
3.2. The co-culture of M0 and PBMSCs promotes the secretion of anti-inflammatory cytokines
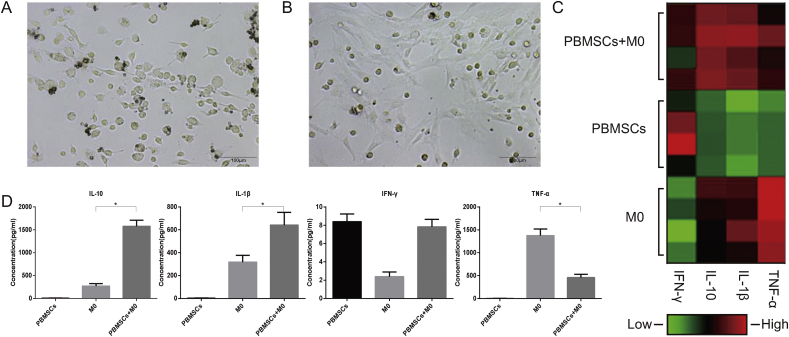
Primarily, cultured M0 macrophages grew adherently with extended rod-like pseudopodia and were highly heterogeneous in morphology (Fig. 2A). After co-culture with PBMSCs for 5 days, M0 macrophages become more spherical and uniform in shape (Fig. 2B). The refraction rate increased, indicating enhanced cellular viability (Fig. 2B).
Fig. 2.
Morphology of rat macrophages under different culture conditions and cytokine levels in the supernatant of different culture groups (A) Morphology of M0 macrophages under an inverted microscope (x200) (B) Morphology of M0 macrophages co-cultured with PBMSCs (x200) (C) The heatmap of cytokines IFN-γ, IL-10, IL-1β and TNF-α in PBMSCs + M0, PBMSCs and M0 groups (n = 4) after conducting immune-microsphere detection (D) Quantitative analysis of the concentration changes of IFN-γ, IL-10, IL-1β and TNF-α in the supernatant of PBMSCs + M0, PBMSCs and M0 groups (n = 4; ∗P < 0.05). PBMSCs, peripheral blood-derived mesenchymal stem cells; IFN-γ, interferon-γ; IL, interleukin; TNF-α, tumor necrosis factor-α.
The supernatants of the M0, PBMSCs and M0 + PBMSCs groups were analyzed using the Bio-Plex 200 System. As a typical anti-inflammatory cytokine, IL-10 was highly expressed in the co-culture group compared to the other groups (P < 0.05; Fig. 2C and D). By contrast, TNF-α, a well-known proinflammatory cytokine, was highly expressed in the M0 group, but after co-culture with PBMSCs, the amount of TNF-α in the supernatant was significantly reduced (P < 0.05; Fig. 2C and D). Other cytokines, such as IL-1β and interferon-γ (IFN-γ), which are involved in inflammatory reactions, were also examined. The IL-1 family consists of 11 members; among them, IL-1β has been demonstrated to have broad proinflammatory activity. The amount of IL-1β was increased after the co-culture of M0 cells with PBMSCs. There was no difference in IFN-γ secretion among the three groups.
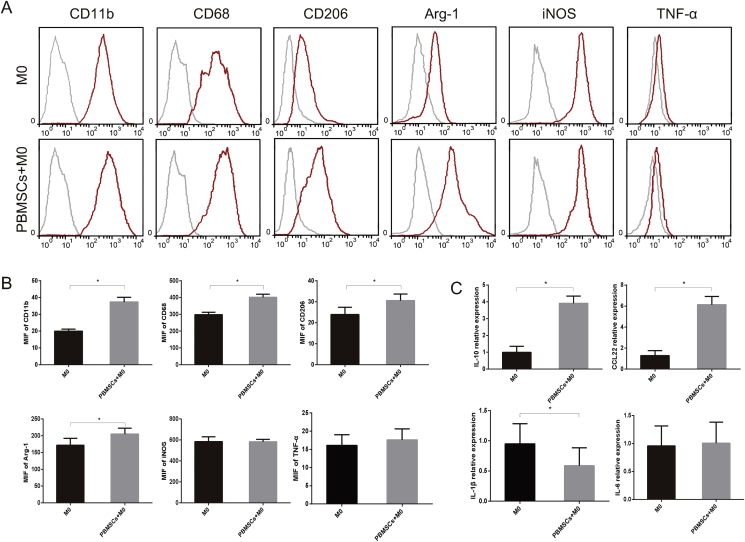
3.3. The Co-culture of M0 with PBMSCs promotes the expressions of M2 surface markers and cytokines
For furthering exploration, flow cytometry and RT-qPCR assays were performed (Fig. 3A–C). Flow cytometry analysis demonstrated that the expression of CD11b, CD68 and CD206 was upregulated when M0 macrophages were co-cultured with PBMSCs (Fig. 3A and B). Arg-1, as a key factor that facilitates the function of M2 macrophages, was increased after co-culturing. iNOS and TNF-α levels were not significantly different between the two groups (Fig. 3A and B). RT-qPCR demonstrated that the M2-related cytokine, IL-10, and chemokine, CCL22, were increased by co-culture with PBMSCs (Fig. 3C). In contrast, M1-related cytokine, IL-1β, was decreased by co-culture, while there was no significant change in IL-6 levels (Fig. 3C).
Fig. 3.
Expressions of surface markers and relevant cytokines in macrophages. Detection of surface markers and cytokines of macrophages from M0 and PBMSCs + M0 groups by flow cytometry (A) Expression of CD11b, CD68, CD206, Arg-1, iNOS and TNF-α (B) quantitative analysis of the concentration changes of CD11b, CD68, CD206, Arg-1, iNOS and TNF-α (n = 4; ∗P < 0.05) (C) Quantitative analysis of the expressions of IL-10, CCL22, IL-1β and IL-6 of macrophages by reverse transcription-quantitative PCR. PBMSCs, peripheral blood-derived mesenchymal stem cells; Arg-1, arginase-1; iNOS, inducible nitric oxide synthase; TNF-α, tumor necrosis factor-α; IL, interleukin; CCL22, C–C motif chemokine ligand 22.
3.4. The co-culture of T cells with PBMSCs altered the balance of Th17/Treg
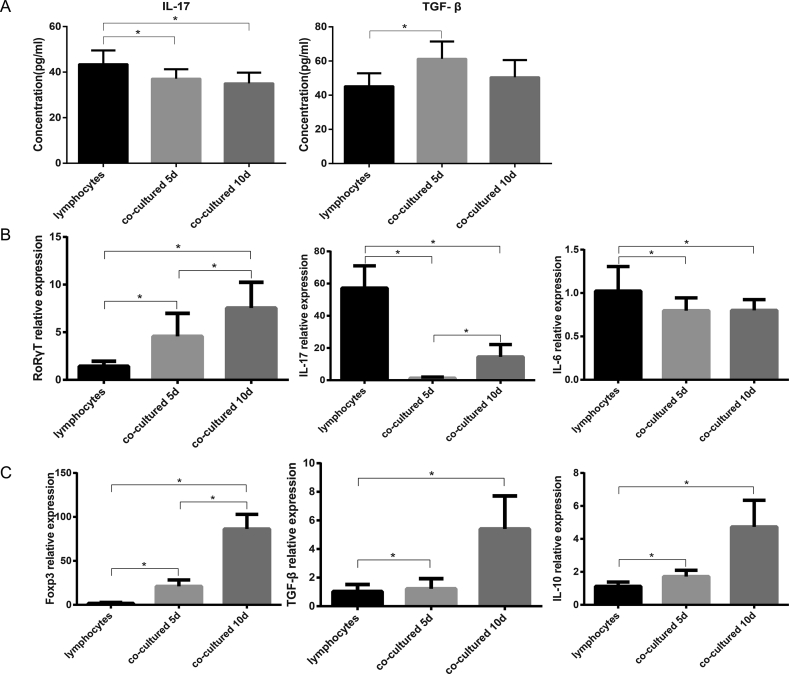
Given that TGF-β and IL-17 are associated with the balance of Th17/Treg cells, ELISA was used to measure the concentration of these two cytokines in the supernatant. The expression of Treg-associated cytokine TGF-β was higher in the co-culture group after 5 days of direct co-culturing compared with the lymphocyte only group (P < 0.05); whereas, the Th17-associated cytokine, IL-17, exhibited the opposite trend (P < 0.05; Fig. 4A). However, there was no significant difference in expression between the samples taken at the two time points (P>0.05; Fig. 4A).
Fig. 4.
Expression of relevant cytokines and genes associated with the balance of Th17/Treg under different culture conditions. The relevant cytokines detected by ELISA (A) Concentrations of TGF-β and IL-17 of the supernatant in lymphocytes and co-culturing group (mean ± SD, n = 6; ∗P < 0.05) (B) The Th17 associated (proinflammatory) genes, RORγt, IL-17 and IL-6 expressions of the lymphocyte as well as co-culturing groups were detected using reverse transcription-quantitative PCR assays (mean ± SD, n = 6; ∗P < 0.05) (C) The Treg associated (anti-inflammatory) genes, Foxp3, TGF-β and IL-10 expressions of the lymphocyte as well as co-culturing groups were detected using reverse transcription-quantitative PCR assays (mean ± SD, n = 6; ∗P < 0.05). TGF-β, transforming growth factor-β; IL, interleukin; RORγt, RAR-related orphan receptor γT; Foxp3, forkhead box p3.
Further analysis of the expression of associated genes was performed by RT-qPCR. The mRNA expression of genes associated with Th17 cells, such as IL-17, which is considered to be proinflammatory, was decreased after co-culturing (P < 0.05; Fig. 4B). There was no difference in the mRNA level of IL-6 in both co-culturing groups; whereas, IL-17 was increased at day 10 compared with day 5 (P < 0.05; Fig. 4B). Interestingly, RORγt, which is considered to be critical for the differentiation of Th17 cells, was increased by co-culture with PBMSCs (P < 0.05; Fig. 4B). and expression was higher at day 10 than that at day 5 (P < 0.05; Fig. 4B). As for Tregs, crucial genes associated with differentiation or secretion, including TGF-β, IL-10 and Foxp3, were highly expressed at day 5, and even higher at day 10 (P < 0.05; Fig. 4C).
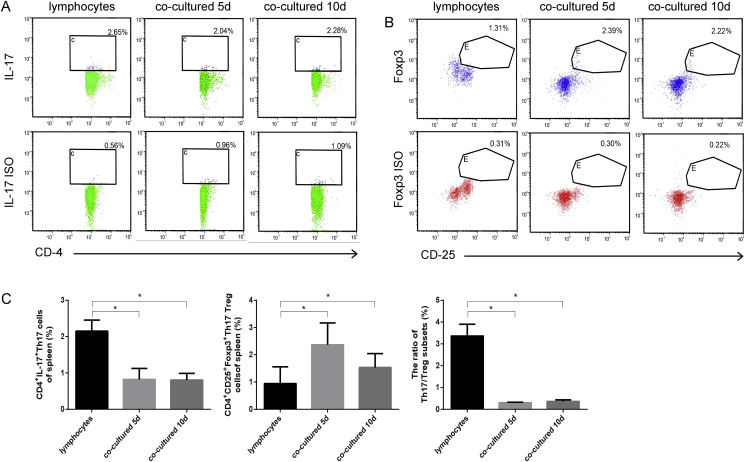
Furthermore, flow cytometry was performed to detect the Th17 and Treg ratio. The proportion of CD4+IL-17+ Th17 cells was reduced after co-culturing with PBMSCs, whereas CD25+Foxp3+ Tregs were increased (P < 0.05; Fig. 5A–C). Thus, the ratio of Th17/Tregs was significantly reduced (P < 0.05; Fig. 5C). The difference between the co-cultured groups at the two time points was not significant (P > 0.05; Fig. 5A–C).
Fig. 5.
Flow cytometry analysis of Th17 and Treg cells under different culturing conditions. Markers of Th17 and Tregs were analyzed using flow cytometry (A) CD4, IL-17 together with the isotype of IL-17 in the lymphocyte and co-culturing groups (n = 6) (B) CD25 and Foxp3, together with the isotype of Foxp3 (n = 6) (C) The percentage of CD4+IL-17+ Th17 cells and CD4+CD25+Foxp3+ Treg cells in the lymphocyte and co-culturing groups, the ratios of Th17/Treg in each group were also calculated (n = 6; ∗P < 0.05). IL, interleukin; Foxp3, forkhead box p3.
4. Discussion
MSCs have the advantages of multi-differentiation potentiality and hypoimmunogenicity, and have been applied in various animal models designed to replace cells or tissues that have been damaged by various diseases and injuries. Prior to their branding as MSCs, marrow-derived fibroblasts were first used in the Dexter assay as feeder cells to sustain the survival of primitive murine hematopoietic stem cells in vitro. Regardless of their limited properties in primitive applications and mesodermal structural capacity, the profound effects of MSCs on innate and adaptive immune cells were identified [31]. Thus, given the fact that the survival rates of injected MSCs (intravenous or localized) are extremely low, the focus of research has switched from tissue regeneration to the immunomodulatory ability of MSCs [32,33].
At present, MSCs have been demonstrated to be efficient in numerous models of inflammation-related diseases [[34], [35], [36]]. In the current study, PBMSCs were selected as seed cells because of the ease of obtaining samples, which would drastically reduce the invasiveness compared to the harvesting of MSC from traditional sources (such as bone marrow-derived MSCs). Previous studies had already established the method of collecting PBMSCs using G-CSF, and confirmed that the collected cells were MSCs [27]. Flow cytometry and immunocytochemistry confirmed that the cells acquired from peripheral blood in the current study were CD90+CD29+CD73+CD105+HLA-DR- MSCs.
Macrophages have a pivotal role in innate immunity and participate in the entire inflammatory process throughout the body [37]. During inflammation, macrophages can polarize into different subtypes according to the environment that they are in. At the early stage of inflammation, M1 macrophages have the predominant role in destroying invading organisms and damaged cells by releasing reactive oxygen species or proinflammatory cytokines (nitric oxide, IL-1, IL-6, TNF-α and others); however, excessive inflammatory reactions may cause unrestrained expression of proinflammatory chemokines, which may hinder recovery [13,[38], [39], [40], [41]]. By contrast, at the later stages, M1 macrophages are stimulated by cytokines, such as IL-4, and are reprogrammed to become M2 macrophages, which are characterized by high expression of anti-inflammatory cytokines, such as IL-10, which stops the acute phase of tissue inflammation, and thus promotes tissue self-reconstruction and repair [13,38,39].
In the current study, macrophages co-cultured with PBMSCs had a cytokine secretion pattern similar to M2 macrophages, which express high levels of anti-inflammatory cytokines (IL-10) and reduced levels of proinflammatory cytokines (TNF-α). Although, IL-1β was unexpectedly increased after co-culture with PBMSCs, which conflicted with the RT-qPCR results of IL-1β mRNA expression in macrophages within the co-culture system; we assumed that this may result from the time point we choose to evaluate cytokines happened to be the very time when PBMSCs were just skewing the macrophages from pro-inflammatory M1 towards the anti-inflammatory M2, similar to the circumstances of the resolving stage of inflammation when organisms’ immune systems encounter stimuli. Still, more specific researches are needed for further elucidation.
Arg-1 has a critical role in regulating the function of macrophages. Arg-1 reduces nitric oxide synthesis by consuming l-arginine, the substrate of iNOS [42,43]. Thus, the Arg-1/iNOS balance can provide a reflection of the M1/M2 ratio. Furthermore, the chemokine CCL22 is predominantly secreted by macrophages [44]. IL-4 is a well-known cytokine that promotes the polarization of M2 macrophages and induces the expression of CCL22 [44,45]. By contrast, IFN-γ promotes the M1 macrophage phenotype and decreases the production of CCL22. Therefore, the expression of CCL22 may also indicate the polarization state of macrophages [46].
Taken together, the findings of the current study indicate that PBMSCs change the ratio of M1/M2 macrophages and skew the balance towards the M2 phenotype, which provides a novel direction for further exploration of the use of PBMSCs. Additionally, the experiments were conducted using indirect co-culturing, considering the complexity of the effects of direct contact between the phagocytic macrophages and PBMSCs. If in direct contact, the effects could be caused by activation of surface receptors, such as Toll-like receptors, or through efferocytosis. Thus, the paracrine mechanisms were examined using indirect culture to provide preliminary data for further experiments.
T lymphocytes provide a crucial link between innate and adaptive immunity. CD4+/helper T cells form the majority of T lymphocytes. Among all subtypes differentiated from CD4+ T cells, Tregs are the only subtype that has immunosuppressive effects on T cell activation or proliferation, and function through direct cell–cell contact and the secretion of cytokines [47]. The anti-inflammatory mediators, IL-10 and TGF-β, and the transcription factor Foxp3 are regarded as markers of activated Tregs [48]. Th17 cells secrete IL-17, which is a key factor that acts synergistically with other proinflammatory cytokines, such as TNF-α and IL-1β, in most circumstances [49]. IL-17 recruits neutrophils, promotes chemokine synthesis and intermediates the interactions between lymphocytes and macrophages; thus, indicating that the IL-17-producing Th17 cells promote inflammatory reactions [49]. The helper T cell family can undergo transformation between subtypes depending on the specific circumstances encountered in vivo, and together with the fact that Tregs and Th17 cells interact antagonistically with each other to maintain homeostasis, it is clear that skewing the balance of Th17/Treg cells will have a marked effect on immunity, which has already been validated in various previous studies [[24], [25], [26]].
In the present study, the expression levels of relevant genes and cytokines in the co-culture system were examined using ELISA, RT-qPCR and flow cytometry to determine the effect of PBMSCs on the Th17/Treg balance. The results demonstrated that IL-10, TGF-β and Foxp3 levels, which are the typical genes expressed by Tregs, were increased in the co-culture system, whereas the expression of IL-6 and IL-17 was decreased. Together with ELISA and flow cytometry analysis, the results indicated that the relative quantity of Th17 cells was reduced compared with Tregs when co-cultured with PBMSCs. Notably, the expression of RORγt is commonly associated with Th17 cells, but RORγt is also present in activated Treg cells [50]. As Th17s may partway through their transformation to Tregs, even RORγt+ cells should be considered Tregs transformed from Th17 (according to our interpretation of the increase in RORγt expression in the co-culture system); or the increase in RORγt might be the result of negative feedback created by the decline of Th17 cells.
It is clear that exaggerated, excessive synthesis of IL-6 leads to an acute, severe inflammatory response known as a ‘cytokine storm’; thus IL-6 has always been regarded as a proinflammatory cytokine. However, research on macrophages and other cells has recently revealed that IL-6 promotes the activation of STAT3, and STAT3 can stimulate target cells to have an anti-inflammatory phenotype; whether the same effect occurs in lymphocytes is still unknown. Therefore, evaluating the precise effects of IL-6 expression changes is complex and requires further investigation.
MSCs are known to produce TGF-β and IL-10 [51,52]. Despite the fact that TGF-β has effects on both Tregs and Th17 cells, it has been reported that TGF-β can only promote the differentiation of Th17 in cooperation with proinflammatory cytokines (IL-1 and IL-6), and promotes the differentiation of Tregs when combined with IL-10 [53,54]. This suggests that PBMSCs promoted Treg differentiation by secreting TGF-β and IL-10. However, it has been reported that direct contact between MSCs and T cells is more important for differentiation than the paracrine mechanisms [55]. Thus, it is necessary to design further experiments to block the expression of TGF-β or/and IL-10 in PBMSCs in order to understand the mechanisms involved and resolve this controversial issue. Interestingly, it has been previously reported that the pre-stimulation of MSCs with proinflammatory cytokines enhances their ability to modulate Treg differentiation. In summary, the regulatory effects of PBMSCs on Th17/Treg cells are likely to depend on paracrine mechanisms, direct contact with T cells and the stimulation state.
5. Conclusions
In conclusion, the present study demonstrated that PBMSCs promote the anti-inflammatory M2 macrophage phenotype and differentiation of Tregs, thereby skewing the M1/M2 ratio and the balance of Th17/Treg cells toward an anti-inflammatory state. Although the environment is far more complex PBMSCs are transplanted into subjects, the two main immune cell types have been confirmed to be responsive to the immunomodulatory effects of PBMSCs in vitro and may be able to reduce inflammation. These findings provide preliminary evidence and novel directions for further exploring the immunomodulatory ability of PBMSCs, and thereby promoting the potential application of PBMSCs as a clinical treatment for immune or inflammatory diseases in the future.
Declaration of competing interest
There is no conflict of interest exist in this article.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No.81960299);Project for Academic Seedling Cultivation and Innovation Exploration of Zunyi Medical University, (No. [2017] 5733-029).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Qian Zhang, Email: zmczq@163.com.
Tao Zhang, Email: oceanzt@163.com.
References
- 1.Kumagai G., Tsoulfas P., Toh S., McNiece I., Bramlett H.M., Dietrich W.D. Genetically modified mesenchymal stem cells (MSCs) promote axonal regeneration and prevent hypersensitivity after spinal cord injury. Exp Neurol. 2013;248:369–380. doi: 10.1016/j.expneurol.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 2.Monsanto M.M., Wang B.J., Sussman M.A. Synthetic MSC? Nothing beats the real thing. Circ Res. 2017;120(11):1694–1695. doi: 10.1161/CIRCRESAHA.117.310986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park D.H., Lee J.H., Borlongan C.V., Sanberg P.R., Chung Y.G., Cho T.H. Transplantation of umbilical cord blood stem cells for treating spinal cord injury. Stem Cell Rev Rep. 2011;7(1):181–194. doi: 10.1007/s12015-010-9163-0. [DOI] [PubMed] [Google Scholar]
- 4.Calle A., Barrajón-Masa C., Gómez-Fidalgo E., Martín-Lluch M., Cruz-Vigo P., Sánchez-Sánchez R. Iberian pig mesenchymal stem/stromal cells from dermal skin, abdominal and subcutaneous adipose tissues, and peripheral blood: in vitro characterization and migratory properties in inflammation. Stem Cell Res Ther. 2018;9(1):178. doi: 10.1186/s13287-018-0933-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chong P.P., Selvaratnam L., Abbas A.A., Kamarul T. Human peripheral blood derived mesenchymal stem cells demonstrate similar characteristics and chondrogenic differentiation potential to bone marrow derived mesenchymal stem cells. J Orthop Res. 2012;30(4):634–642. doi: 10.1002/jor.21556. [DOI] [PubMed] [Google Scholar]
- 6.Li S., Huang K.J., Wu J.C., Hu M.S., Sanyal M., Hu M. Peripheral blood-derived mesenchymal stem cells: candidate cells responsible for healing critical-sized calvarial bone defects. Stem Cells Transl Med. 2015;4(4):359–368. doi: 10.5966/sctm.2014-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu W.L., Zhou C.Y., Yu J.K. A new source of mesenchymal stem cells for articular cartilage repair: MSCs derived from mobilized peripheral blood share similar biological characteristics in vitro and chondrogenesis in vivo as MSCs from bone marrow in a rabbit model. Am J Sports Med. 2014;42(3):592–601. doi: 10.1177/0363546513512778. [DOI] [PubMed] [Google Scholar]
- 8.Bussche L., Van de Walle G.R. Peripheral blood-derived mesenchymal stromal cells promote angiogenesis via paracrine stimulation of vascular endothelial growth factor secretion in the equine model. Stem Cells Transl Med. 2014;3(12):1514–1525. doi: 10.5966/sctm.2014-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen T., Chen D., Li F., Tan Z. Netrin-1 with stem cells promote angiogenesis in limb ischemic rats. J Surg Res. 2014;192(2):664–669. doi: 10.1016/j.jss.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Nichols J.E., Niles J.A., DeWitt D., Prough D., Parsley M., Vega S. Neurogenic and neuro-protective potential of a novel subpopulation of peripheral blood-derived CD133+ ABCG2+ CXCR4+ mesenchymal stem cells: development of autologous cell-based therapeutics for traumatic brain injury. Stem Cell Res Ther. 2013;4(1):3. doi: 10.1186/scrt151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu Q., Liu Y., Liu X., Zhang Q., Chen L., Peng J. Engrafted peripheral blood-derived mesenchymal stem cells promote locomotive recovery in adult rats after spinal cord injury. Am J Transl Res. 2017;9(9):3950–3966. [PMC free article] [PubMed] [Google Scholar]
- 12.Romagnani S. T-cell subsets (Th1 versus Th2) Ann Allergy Asthma Immunol. 2000;85(1):9–18. doi: 10.1016/S1081-1206(10)62426-X. [DOI] [PubMed] [Google Scholar]
- 13.Murray P.J. Macrophage polarization. Annu Rev Physiol. 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 14.Selleri S., Bifsha P., Civini S., Pacelli C., Dieng M.M., Lemieux W. Human mesenchymal stromal cell-secreted lactate induces M2-macrophage differentiation by metabolic reprogramming. Oncotarget. 2016;7(21):30193–30210. doi: 10.18632/oncotarget.8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrison T.J., Jackson M.V., Cunningham E.K., Kissenpfennig A., McAuley D.F., O'Kane C.M. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196(10):1275–1286. doi: 10.1164/rccm.201701-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geng Y., Zhang L., Fu B., Zhang J., Hong Q., Hu J. Mesenchymal stem cells ameliorate rhabdomyolysis-induced acute kidney injury via the activation of M2 macrophages. Stem Cell Res Ther. 2014;5(3):80. doi: 10.1186/scrt469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song J.Y., Kang H.J., Hong J.S., Kim C.J., Shim J.Y., Lee C.W. Umbilical cord-derived mesenchymal stem cell extracts reduce colitis in mice by re-polarizing intestinal macrophages. Sci Rep. 2017;7(1):9412. doi: 10.1038/s41598-017-09827-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iborra S., Gonzalezgranado J.M. In Vitro differentiation of naive CD4 (+) T cells: a tool for understanding the development of atherosclerosis. Methods Mol Biol. 2015;1339:177–189. doi: 10.1007/978-1-4939-2929-0_12. [DOI] [PubMed] [Google Scholar]
- 19.Lewkowicz N., Mycko M.P., Przygodzka P., Cwiklinska H., Cichalewska M., Matysiak M. Induction of human IL-10-producing neutrophils by LPS-stimulated Treg cells and IL-10. Mucosal Immunol. 2015;9(2):364–378. doi: 10.1038/mi.2015.66. [DOI] [PubMed] [Google Scholar]
- 20.Laidlaw B.J., Cui W., Amezquita R.A., Gray S.M., Guan T., Lu Y. Production of IL-10 by CD4+ regulatory T cells during the resolution of infection promotes the maturation of memory CD8+ T cells. Nat Immunol. 2015;16(8):871–879. doi: 10.1038/ni.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanjabi S., Oh S.A., Li M.O. Regulation of the immune response by TGF-β: from conception to autoimmunity and infection. Cold Spring Harb Perspect Biol. 2017;16(9):236. doi: 10.1101/cshperspect.a022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owaga E., Hsieh R.H., Mugendi B., Masuku S., Shih C.K., Chang J.S. Th17 cells as potential probiotic therapeutic targets in, inflammatory bowel diseases. Int J Mol Sci. 2015;16(9):20841–20858. doi: 10.3390/ijms160920841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace K.L., Zheng L.B., Kanazawa Y., Shih D.Q. Immunopathology of inflammatory bowel disease. World J Gastroenterol. 2014;20(1):6–21. doi: 10.3748/wjg.v20.i1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng T.T., Zou T., Wang X., Zhao W.F., Qin A.L. Clinical significance of changes in the Th17/Treg ratio in autoimmune liver disease. World J Gastroenterol. 2017;23(21):3832–3838. doi: 10.3748/wjg.v23.i21.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L., Wang X., Tong L., Wang J., Dou M., Ji S. Recovery from acute lung injury can be regulated via modulation of regulatory T cells and Th17 cells. Scand J Immunol. 2018;88(5) doi: 10.1111/sji.12715. [DOI] [PubMed] [Google Scholar]
- 26.Fasching P., Stradner M., Graninger W., Dejaco C., Fessler J. Therapeutic potential of targeting the Th17/treg Axis in autoimmune disorders. Molecules. 2017;22(1):134. doi: 10.3390/molecules22010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu Q., Zhang Q., Jia L.Y., Fang N., Chen L., Yu L.M. Isolation and characterization of rat mesenchymal stem cells derived from granulocyte colony-stimulating factor-mobilized peripheral blood. Cells Tissues Organs. 2016;201(6):412–422. doi: 10.1159/000445855. [DOI] [PubMed] [Google Scholar]
- 28.Pinedatorra I., Gage M., de J.A., Pello O.M. Isolation, culture, and polarization of murine bone marrow-derived and peritoneal macrophages. Methods Mol Biol. 2015;1339:101–109. doi: 10.1007/978-1-4939-2929-0_6. [DOI] [PubMed] [Google Scholar]
- 29.Le Blanc K., Tammik L., Sundberg B., Haynesworth S.E., Ringdén O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57(1):11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 30.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F.C., Krause D.S. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 31.Bernardo M.E., Fibbe W.E. Mesenchymal stromal cells. sensors and switchers of inflammation. Cell Stem Cell. 2013;13(4):392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Barui A., Chowdhury F., Pandit A., Datta P. Rerouting mesenchymal stem cell trajectory towards epithelial lineage by engineering cellular niche. Biomaterials. 2018;156:28–44. doi: 10.1016/j.biomaterials.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 33.Galipeau J., Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeda K., Webb T.L., Ning F., Shiraishi Y., Regan D.P., Chow L. Mesenchymal stem cells recruit CCR2+ monocytes to suppress allergic airway inflammation. J Immunol. 2018;200(4):1261–1269. doi: 10.4049/jimmunol.1700562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun X., Hao H., Han Q., Song X., Liu J., Dong L. Human umbilical cord-derived mesenchymal stem cells ameliorate insulin resistance by suppressing NLRP3 inflammasome-mediated inflammation in type 2 diabetes rats. Stem Cell Res Ther. 2017;8(1):241. doi: 10.1186/s13287-017-0668-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Najar M., Krayem M., Merimi M., Burny A., Meuleman N., Bron D. Insights into inflammatory priming of mesenchymal stromal cells: functional biological impacts. Inflamm Res. 2018;67(6):467–477. doi: 10.1007/s00011-018-1131-1. [DOI] [PubMed] [Google Scholar]
- 37.Mantovani A. Wandering pathways in the regulation of innate immunity and inflammation. J Autoimmun. 2017;85:1–5. doi: 10.1016/j.jaut.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sica A., Erreni M., Allavena P., Porta C. Macrophage polarization in pathology. Cell Mol Life Sci. 2015;72(21):4111–4126. doi: 10.1007/s00018-015-1995-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y.C., Zou X.B., Chai Y.F., Yao Y.M. Macrophage polarization in inflammatory diseases. Int J Biol Sci. 2014;10(5):520–529. doi: 10.7150/ijbs.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kong X., Gao J. Macrophage polarization: a key event in the secondary phase of acute spinal cord injury. J Cell Mol Med. 2017;21(5):941–954. doi: 10.1111/jcmm.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Isidro R.A., Appleyard C.B. Colonic macrophage polarization in homeostasis, inflammation, and cancer. Am J Physiol Gastrointest Liver Physiol. 2016;311(1):G59–G73. doi: 10.1152/ajpgi.00123.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faro M.L., Fox B., Whatmore J.L., Winyard P.G., Whiteman M. Hydrogen sulfide and nitric oxide interactions in inflammation. Nitric Oxide. 2014;41:38–47. doi: 10.1016/j.niox.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 43.Voronova I.P., Khramova G.M., Kulikova E.A., Petrovskii D.V., Bazovkina D.V., Kulikov A.V. 5-HT2A receptors control body temperature in mice during LPS-induced inflammation via regulation of NO production. Pharmacol Res. 2016;103:123–131. doi: 10.1016/j.phrs.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 44.Yoshie O., Matsushima K. CCR4 and its ligands: from bench to bedside. Int Immunol. 2015;27(1):11–20. doi: 10.1093/intimm/dxu079. [DOI] [PubMed] [Google Scholar]
- 45.Hao J., Hu Y., Li Y., Zhou Q., Lv X. Involvement of JNK signaling in IL4-induced M2 macrophage polarization. Exp Cell Res. 2017;357(2):155–162. doi: 10.1016/j.yexcr.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 46.Ivashkiv L.B. IFNγ: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat Rev Immunol. 2018;18(9):545–558. doi: 10.1038/s41577-018-0029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barbi J., Pardoll D., Pan F. Treg functional stability and its responsiveness to the microenvironment. Immunol Rev. 2014;259(1):115–139. doi: 10.1111/imr.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morikawa H., Sakaguchi S. Genetic and epigenetic basis of Treg cell development and function: from a FoxP3-centered view to an epigenome-defined view of natural Treg cells. Immunol Rev. 2014;259(1):192–205. doi: 10.1111/imr.12174. [DOI] [PubMed] [Google Scholar]
- 49.Veldhoen M. Interleukin 17 is a chief orchestrator of immunity. Nat Immunol. 2017;18(6):612–621. doi: 10.1038/ni.3742. [DOI] [PubMed] [Google Scholar]
- 50.Kim B.S., Lu H., Ichiyama K., Chen X., Zhang Y.B., Mistry N.A. Generation of RORγt +, antigen-specific T regulatory 17 cells from Foxp3 + precursors in autoimmunity. Cell Rep. 2017;21(1):195–207. doi: 10.1016/j.celrep.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mehler V.J., Burns C., Moore M.L. Concise review: exploring immunomodulatory features of mesenchymal stromal cells in humanized mouse models. Stem Cell. 2018;37(3):298–305. doi: 10.1002/stem.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferreira J.R., Teixeira G.Q., Santos S.G., Barbosa M.A., Almeida-Porada G., Gonçalves R.M. Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Front Immunol. 2018;9:2837. doi: 10.3389/fimmu.2018.02837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Veldhoen M., Hocking R.J., Atkins C.J., Locksley R.M., Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 54.Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 55.Meesuk L., Tantrawatpan C., Kheolamai P., Manochantr S. The immunosuppressive capacity of human mesenchymal stromal cells derived from amnion and bone marrow. Biochem Biophys Rep. 2016;8:34–40. doi: 10.1016/j.bbrep.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]