Abstract
Introduction
Researchers have investigated the use of platelet-rich plasma (PRP) therapy. However, the mechanisms through which PRP affects tissue repair remain unclear. We hypothesize that PRP promotes tissue repair through not only via direct manner on the local cells but also via indirect manner that encourage the recruitment of reparative cells such as macrophages (MPs), and it depends on the quality of PRP including the concentration of leukocytes. The aim of this study is to elucidate the actions of the MPs in the mechanisms of PRP on tissue repair processes.
Methods
Leukocyte-rich (LR) PRP and leukocyte-poor (LP) PRP were prepared from 12-week-old C57BL6 mice. Full-thickness defects were created in central third of patellar tendons of 12-week-old C57BL/6 mice for histologic analysis (n = 36) and 12-week-old B6.129P-Cx3cr1tm1Litt/J mice for flow cytometry analysis (n = 108). B6.129P-Cx3cr1tm1Litt/J mouse is GFP-positive only in the MP-linage cells thus MPs recruited to the repair tissue can be distinguished whether it had originated from administrated PRP or recruited from host mouse. Mice were treated either with LR-PRP, LP-PRP, or without PRP (control group). Histological analyses were performed to evaluate the tendon healing using Bonar score as semi-quantitative histological scoring system. Flow cytometric analyses were performed to count the number of GFP-positive cells around repaired patellar tendon. In addition, the ratio of pro-inflammatory MPs (M1)/anti-inflammatory MPs (M2) were analyzed in those GFP-positive cells. The statistical analysis was performed using GraphPad Prism ver6. P values < 0.05 were considered statistically significant.
Results
In LR-PRP and LP-PRP groups, all variables in Bonar score such as cell morphology, cellularity, vascularity, and collagen arrangement were significantly improved in comparison with control group, indicating that both PRPs promote tendon hearing. LP-PRP promoted the tendon healing significantly faster than that of LR-PRP on postoperative day 28 (P < 0.001). LR-PRP enhanced angiogenesis (vascularity: P < 0.001), while LP-PRP improved the collagen arrangement on postoperative day 28 (collagen arrangement: P < 0.01). In other variables such as cell morphology and cellularity score, there were no significant differences between LR-PRP and LP-PRP groups in any time points. Flow cytometric findings showed that recruitment of GFP-positive MPs in the LR and LP-PRP groups were significantly increased from postoperative day 4 compared with control group without PRP treatment (P < 0.001). The majority of GFP-positive MPs were M1 at the initiation of tendon healing phase, and M2 were gradually increased from postoperative day 4. The number of M1 was significantly high both in the LP- and LR-PRP groups (day 4 and 7, p < 0.001), but the number of M2 was high only in the LP-PRP group (day 7 and 14, P < 0.05) when it compared with control group. The M1/M2 ratio on postoperative day 7 was significantly lower in the LP-PRP group than those in the control group (P < 0.05).
Conclusions
This study demonstrated that PRP enhanced the tendon healing and promoted the recruitment of MPs to the injured tissue. The subtypes of MPs were different depends on the types of PRPs, suggesting that leukocytes in PRP influence the effect of PRP therapy.
Keywords: Platelet-rich plasma, Macrophage, Immune response, Inflammatory cell balance, Tissue repair
Abbreviations: PRP, platelet rich plasma; MPs, macrophages; PPP, platelet poor plasma
Highlights
-
•
Both leukocyte poor- and rich-PRP promoted the recruitment of MPs to the injured tissue.
-
•
Leukocyte poor PRP recruited more anti-inflammatory macrophages (M2 phenotype) which encourage tissue repair.
-
•
PRP acts not only to localized cells (paracrine) but also to the circulating reparative cells (chemotaxis).
1. Introduction
Platelet-rich plasma (PRP) is an autologous blood that concentrates platelets and contains diverse growth factors and cytokines [1,2]. PRP therapy is a promising as that is simple, safe, low cost, and minimally invasive and could be used to promote the tissue repair process [[2], [3], [4]]. PRP has recently been used as a therapeutic material in many fields. As the use of PRP has increased in the clinic, the number of clinical and basic studies supporting the efficacy of PRP therapy has also increased [[3], [4], [5], [6], [7]]. However, some studies have shown less favorable results [8,9], and evidence supporting the use of PRP in the clinical setting remains insufficient. Moreover, researchers have proposed several problems related to the use of PRP therapy.
First, although some studies have supported the efficacy of PRP in the clinical setting during the tissue repair process [[10], [11], [12], [13]], the molecular mechanisms through which PRP exerts these effects are still unclear. In a previous study, administration of PRP significantly increased angiogenesis during the early phase of the tendon repair process [14]. However, the cells participating in the early phase of the PRP-dependent tissue repair process have not been identified. Second, although some classification systems based on leukocyte numbers, leukocyte activation status, and platelet concentration have been proposed [15,16], few studies have been conducted to evaluate the effects of the quality of PRP on the tissue repair process. Therefore, the effects of differences in the quality of PRP remain unclear.
Generally, macrophages (MPs) are thought to play important roles in the early phase of the tissue repair process. MPs are unique effector cells in innate immunity and play critical roles in tissue repair [[17], [18], [19]]. In addition, MPs comprise two phenotypically distinct subtypes, i.e., pro-inflammatory MPs (M1), which promote the inflammation phase, and anti-inflammatory MPs (M2), which promote the tissue regeneration phase. The timely shift from M1 to M2 is thought to be crucial for tissue repair [[19], [20], [21], [22], [23], [24], [25], [26], [27]].
In addition, there has been some discussion about whether the efficacy of the PRP is affected by the concentration and composition of leukocytes in the PRP [28,29], particularly regarding leukocyte-rich PRP (LR-PRP) versus leukocyte-poor PRP (LP-PRP). Indeed, our previous work showed that the leukocyte concentration and composition in the PRP influenced the expression of growth factors and cytokines [30]. Additionally, these leukocytes may have positive effects, such as antimicrobial effects, or negative effects, such as excessive inflammation, via the release of catabolic cytokines [31,32]. Some studies have also suggested that LR-PRP possesses both catabolic and anabolic effects [30,33,34], whereas LP-PRP exerts anabolic effects rather than catabolic effects in injured tissues [30,35,36]. In contrast, some studies have suggested that LR-PRP and LP-PRP have similar safety profiles, and adverse reactions to PRP may not be directly related to leukocyte concentrations [37,38]. Thus, further studies are needed to standardize the concentrations of leukocytes needed in optimal PRP, which may vary according to pathophysiology, and to elucidate the effects of molecular mechanisms on the quality of PRP.
In this study, we hypothesized that the tissue repair mechanism of PRP may involve both direct and indirect effects, e.g., recruitment of reparative cells through blood flow, particularly MPs, depending on the quality of PRP. Accordingly, we aimed to elucidate the effects of MPs on the tissue repair process and to evaluate the influence of PRP quality on the activity of MPs.
2. Methods
2.1. Ethics statement
All experimental procedures and protocols in this study were approved by the Institutional Animal Care and Use Ethics Committee of Juntendo University (approval number 300200).
2.2. Animals
This study was a controlled laboratory study. In total, 58 female C57BL/6 mice (12 weeks old) and 108 female B6.129P-Cx3cr1tm1Litt/J mice expressing green fluorescent protein (GFP) in MPs obtained from The Jackson Laboratory (Bar Harbor, ME, USA) were used in this study. The effects of MPs on injured patella tendons were investigated by flow cytometry analysis.
2.3. Blood collection and PRP preparation
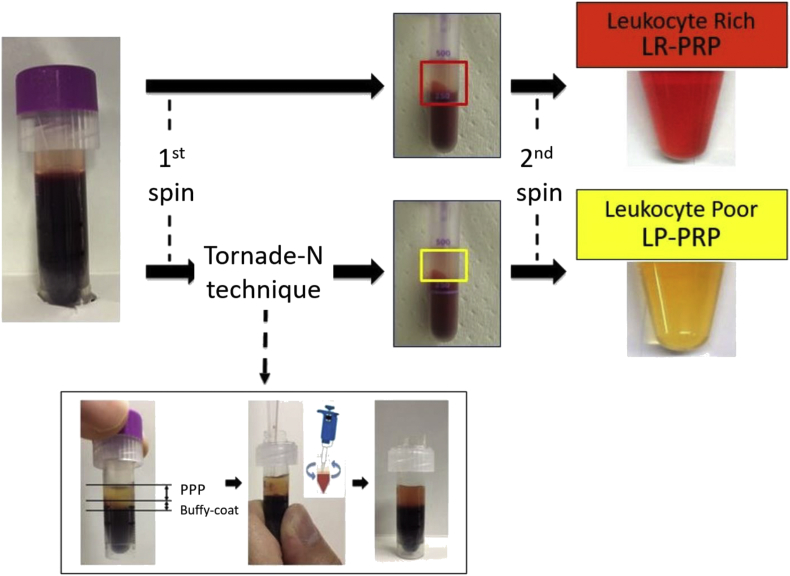
LR-PRP and LP-PRP were prepared from whole blood (WB) of C57BL/6 donor mice (n = 40) using the double spin technique. Approximately 1 mL WB was drawn via cardiac puncture into a micro tube containing EDTA-2Na as an anticoagulant (Microtainer; BD Biosciences, Bedford, MA, USA). After the first spin (220×g, 10 min, 25 °C), the upper layer, buffy coat, and the layer below the buffy coat were transferred to another tube for LR-PRP, and the upper layer and buffy coat were transferred to another tube using the original Tornado-N technique for LP-PRP (Fig. 1). After the second spin (2400 g, 10 min, 25 °C), the supernatant (platelet-poor plasma [PPP]) was removed, and approximately 100 μL PPP remained. The pellet from the bottom of each tube was resuspended in the remaining PPP to prepare LR-PRP and LP-PRP. LR-PRP and LP-PRP (50 μL each) was divided into another tube and cryopreserved at −80 °C until application (Fig. 1).
Fig. 1.
The Tornado-N technique for LR-PRP and LP-PRP preparation. The first spin was carried out at 220×g for 10 min at 25 °C, and the second spin was carried out at 2400×g for 10 min at 25 °C. After the first spin, the layer between the red layer (including neutrophils and erythrocytes) and the buffy coat (including platelets and a few lymphocytes) was shaken up carefully using a pipette.
2.4. Hematological analysis
The platelet, leukocyte, and erythrocyte concentrations and leukocyte compositions of whole-blood, LR-PRP, and LP-PRP samples were determined using an automated hematology analyzer (Poch-100iV Diff; Sysmex, Kobe, Japan) immediately after preparation.
2.5. Surgical procedure and PRP application
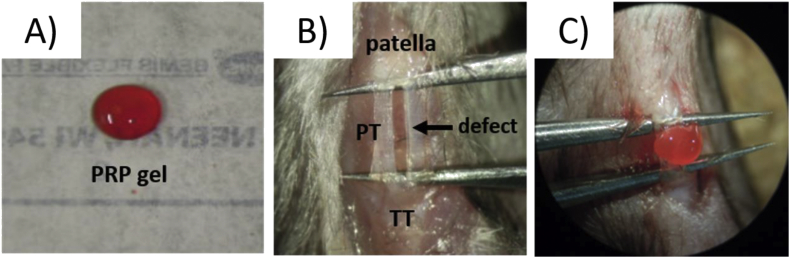
Twelve-week-old C57BL/6 mice and B6.129P-Cx3cr1tm1Litt/J mice were anesthetized with 4% isoflurane, a longitudinal skin incision was made over the patellar tendon. Then, full-thickness defects were created in the central third of the patellar tendon using a microsurgery technique described by Dyment et al. [39,40]. Microtweezers were slid under the tendon and spread to tension the tendon. The central third of the patellar tendon was cut away with micro scissors (Fig. 2B). The cryopreserved PRP prepared from C57BL/6 mice was thawed, 0.5 mM calcium chloride (Sigma Aldrich, St. Louis, MO, USA) was added, and the samples were incubated for 1 h at 37 °C in a water bath to activate the PRP and form a gel (Fig. 2A,C).
Fig. 2.
Surgical procedure and PRP application. A) PRP gel. B) A full-thickness defect was created in the central third of the patellar tendon. C) PRP gel was applied to the patellar tendon defect. PT = patellar tendon, TT = tibia tuberosity.
For histological analysis, C57BL/6 mice treated with LP-PRP (n = 12) or LR-PRP (n = 12) on the patellar tendon defect were defined as the PRP groups, and without application of PRP were defined as the control group (n = 12). For flow cytometry analysis, B6.129P-Cx3cr1tm1Litt/J mice treated with PRP on the patellar tendon defect were defined as the LR-PRP (n = 36) and LP-PRP groups (n = 36), and B6.129P-Cx3cr1tm1Litt/J mice without application of PRP were defined as the control group (n = 36). After the end of the procedure, the skin was closed with a 5–0 nylon suture. Mice were allowed to be fully active after the operation.
2.6. Histological analysis
Histological analysis were performed on postoperative day 7, 14, 28, or 42 (n = 3/time point/group) according to the method described by Kawamoto et al. [41]. After euthanasia, hind limb were harvested an the femur and tibia were cut at midshaft. The samples were fixated with 4% paraformaldehyde (Nakarai Tesque, Kyoto, Japan) for 1h at 4 °C. After fixation, the samples were cryoprotected with 30% sucrose (ATAGO, Tokyo, Japan) overnight at 4 °C. After three times wash with 1x PBS, the samples were frozen in a cooled hexane (Nakarai Tesque, Kyoto, Japan), and then freeze embedded with carboxymethyl cellulose (CMC) gel (Leica microsystems, Wetzlar, Germany). Then, sagittal frozen sections (5 μm thickness) were made within the defect using a tungsten carbide blade. Each sample were stained with hematoxylin and eosin (H&E). Semi-quantitative histological evaluation of tendon healing process was performed in accordance with Bonar score [42,43]. The variables included in this scoring system were cell morphology, collagen arrangement, cellularity, and vascularity with higher grades indicating worse tendon structure (each factor; 0–3 points).
2.7. Flow cytometry analysis
B6.129P-Cx3cr1tm1Litt/J mice from the three groups were sacrificed on days 1, 2, 4, 7, 14, or 28 after operation (n = 6/time point/group). CX3CR1-GFP mice were used to distinguish MPs derived from administrated PRP (GFP-negative) or recruited by host mice (GFP-positive). After euthanasia, the patellar tendons were harvested and dissociated using collagenase for 2 h. The solution was moved to Falcon round-bottom polypropylene tubes (Corning, NY, USA) for flow cytometry using a 100-μm nylon strainer. Fifty microliters of count beads (CountBright Absolute Counting Beads; Thermo Fisher Scientific, USA) and Fc blocking reagents were added to each tube, and tubes were centrifuged (180×g, 5 min, 4 °C). The supernatants were removed, and 80 μL of phosphate-buffered saline (PBS)/10% fetal calf serum (FCS) was added to each tube. Next, rat anti-mouse F4/80 APC antibodies (BioLegend, San Diego, CA, USA), rat anti-mouse CD11b Alexa Fluor 700 antibodies (BioLegend, San Diego, CA, USA) and rat anti-mouse Ly6C PerCP/Cy5.5 antibodies (BioLegend, San Diego, CA, USA) were added to each tube (volume: 10 μL). Tubes were then incubated on ice for 30 min and centrifuged (180×g, 5 min, 4 °C). The supernatants were removed, and 500 μL PBS/10% FCS +0.005% Hoechst was added. Compensation was performed on the BD LSRFORTESSA flow cytometer using BD FACSDiva software (BD Bioscience) at the beginning of each experiment. Data were analyzed using FlowJo software (FlowJo LLC, USA). Gating strategy was performed following exclusion of debris and cellular aggregates and live/dead discrimination. The positive populations were identified using unstained controls to determine the negative populations. The cells were plotted F4/80 and CD11b. We consider the positive populations as MPs. In the positive populations, the GFP-positive MPs were then distinguished from GFP-negative MPs. We counted absolute cell number of the GFP-positive MPs on each time points. Based on previous studies, characterizing Ly6Chi MPs as M1 and Ly6Clo MPs as M2 via flow cytometry [20,25,44,45], CX3CR1hiLy6C + cells were considered as M1 and CX3CR1low/Ly6C- cells as M2 [20]. The GFP-positive MPs were then analyzed by plotting Ly6C against CX3CR1. We performed absolute cell counts of M1 and M2 to analyze M1/M2 ratio on each time points.
2.8. Statistical analysis
All data were presented as means ± standard deviations (SDs). All analyses were performed using GraphPad Prism ver6.0 (GraphPad Software, Inc., La Jolla, CA, USA). P values of less than 0.05 were considered statistically significant.
3. Results
3.1. PRP characterization
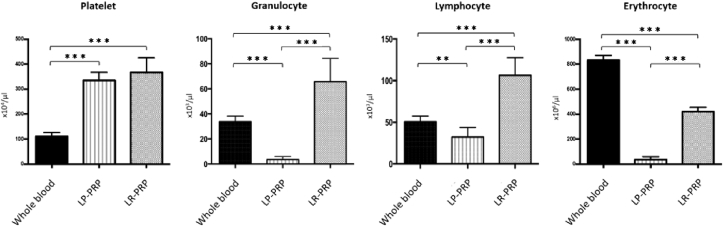
The platelet concentrations in LR-PRP and LP-PRP were three folds higher than that of WB and there was no difference between LR-PRP and LP- PRP (versus WB: LR = 3.24 ± 0.32 fold, LP = 3.13 ± 0.39 fold; LR versus LP: p = 0.560; Fig. 3). LR-PRP had abundant populations of granulocytes (65.8 × 103 ± 18.5 × 103/μL) and lymphocytes (106.6 × 103 ± 21.0 × 103/μL) (Fig. 3), whereas LP-PRP had very few granulocytes (3.5 × 103 ± 2.3 × 103/μL) and lower level of lymphocytes (32.3 × 103 ± 11.5 x103/μL). The erythrocyte concentrations in LR-PRP were approximately 0.5-fold lower than that of WB and those of LP-PRP were very low (versus WB: LR = 0.50 ± 0.05 fold, LP = 0.04 ± 0.03 fold; LR versus LP: p < 0.0001; Fig. 3).
Fig. 3.
PRP characterization. Platelet, granulocyte, lymphocyte and erythrocyte concentrations in whole blood, LP-PRP, and LR-PRP. Data are presented as means ± SDs (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001).
3.2. Histological findings
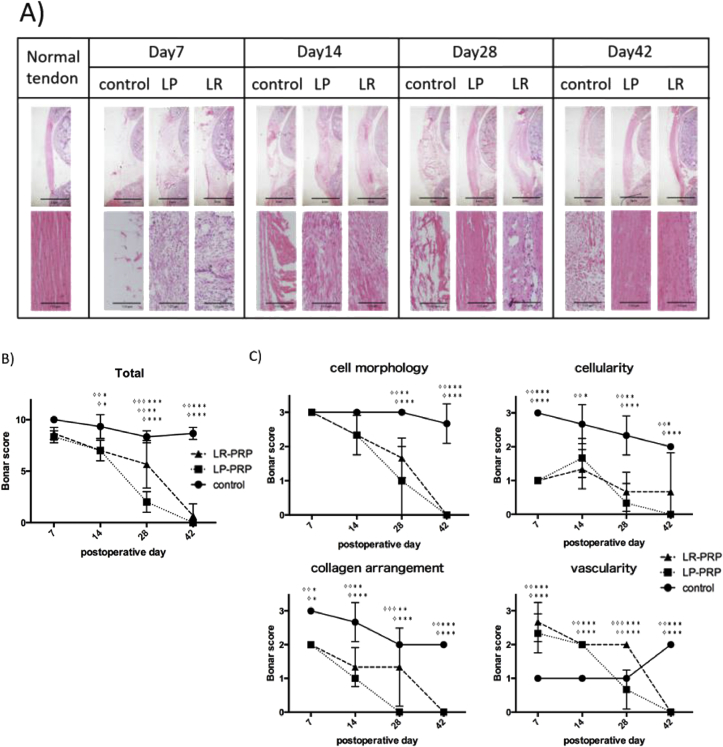
Histological analysis showed that the invasion of inflammatory cells occurred at initiation phase of tendon healing and gradually regenerated the tendon structure (Fig. 4A). On the day 7, inflammatory cells were abundantly observed in the LR-PRP and LP-PRP groups compared with control group. With regard to the Bonar score, total Bonar score was significantly improved both in LP and LR-PRP group in comparison with those of control group (on postoperative day 14: P < 0.05, day 28: P < 0.01, day42: P < 0.001, Fig. 4B, and almost all of the variables of Bonar score significantly improved both in LR-PRP and LP-PRP groups in comparison with control group as well (cell morphology on postoperative day 28, 42: P < 0.01, cellularity on postoperative day 7, 14, 28, 42: P < 0.05, vascularity on postoperative day 7, 14, 28,42: P < 0.001, collagen arrangement on postoperative day 7, 14, 28, 42: P < 0.05, Fig. 4C). The tendon healing was significantly earlier in LP-PRP group than those of LR-PRP group on postoperative day 28 (total Bonar score, P < 0.001), and collagen arrangement was improved earlier in LP-PRP than LR-PRP group on day 28 (P < 0.01, Fig. 4C). The vascularity was significantly high in LR-PRP group on day 28 (P < 0.001). In other variables such as cell morphology and cellularity score, there were no significant differences between LR-PRP and LP-PRP groups in any time points.
Fig. 4.
Histological analyses of patellar tendon. A) Hematoxylin and Eosin staining of sagittal section of patellar tendon. Magnifications are 20× (upper panels) and 200 x (lower panels). This figure showed the representative results of samples in each groups. B) The total Bonar score on each time points. C) The variables of Bonar score on each time points. Data are presented as means ± SDs (✧LP vs control, ✧ ✧ LR vs control, ✧ ✧ ✧ LP vs LR, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001).
3.3. Flow cytometry findings
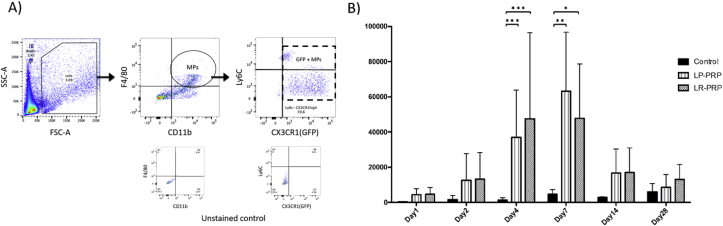
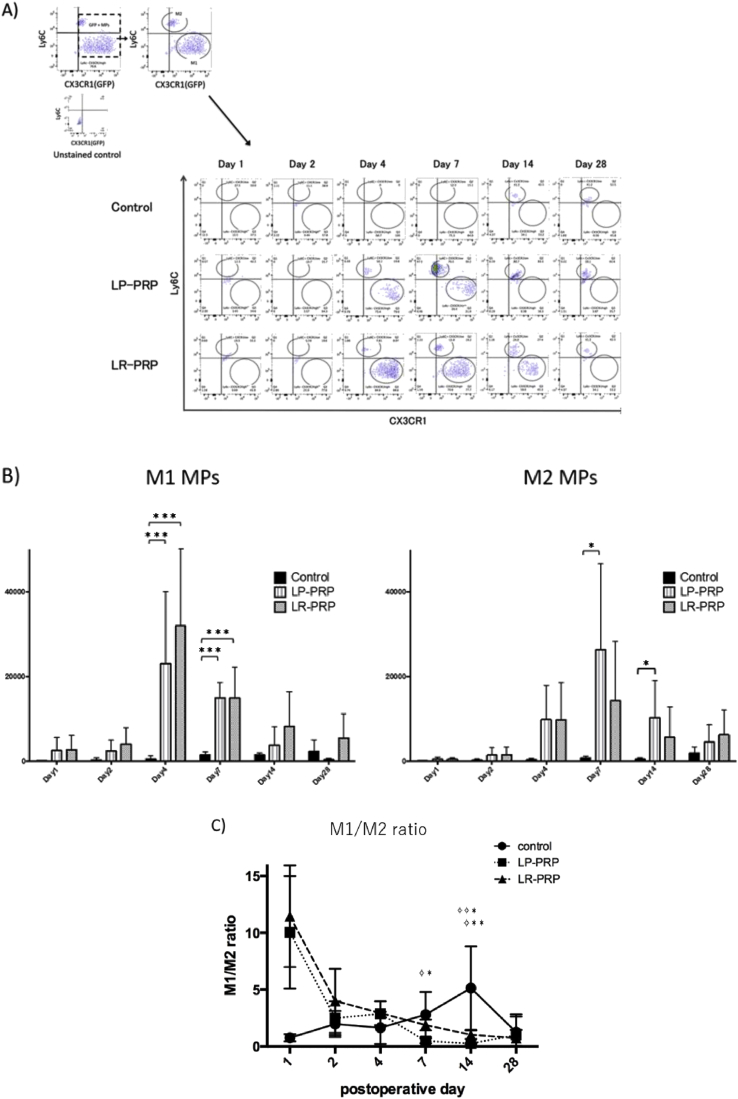
At first, the F4/80 and CD11b positive cells in the repaired tendon tissue were gathered, then GFP-positive cells were counted as MPs recruited from host mouse not from PRPs (Fig. 5A). Flow cytometry analysis showed that the number of GFP-positive MPs around the patellar tendon defects in the LR-PRP and LP-PRP groups were gradually increased from postoperative day 1 and significantly increased on postoperative day 4 and 7 in comparison with the control group (day 4: control = 1308 ± 550 cells, LR = 47,538 ± 19,948 cells, LP = 36,905 ± 10,961 cells; p values: control versus LR < 0.001, control versus LP < 0.001, LR versus LP = 0.9304/day 7: control = 4587 ± 1110 cells, LR = 47,179 ± 12,757 cells, LP = 63,177 ± 13,682 cells; p values: control versus LR = 0.031, control versus LP = 0.004, LR versus LP = 0.580) (Fig. 5B). Next, the GFP-positive MPs were sorted with Ly6C and CX3CR1 antibody to analyze the absolute cell counts of M1 and M2 MPs recruited from host mice (Fig. 6A). The number of M1 in control group was highest on postoperative day 28, while those in the LR-PRP and LP-PRP groups were highest at day 4 and decreased with time (Fig. 6B). The number of M1 were significantly higher than those of controls on postoperative day 4 and 7 (day 4: control = 485 ± 335 cells, LR = 31,964 ± 14,070 cells, LP = 23,030 ± 6953 cells; p values: control versus LR < 0.001, control versus LP < 0.001, LR versus LP = 0.7838/day 7: control = 1450 ± 313 cells, LR = 14,930 ± 2959 cells, LP = 14,918 ± 1488 cells; p values: control versus LR < 0.001, control versus LP < 0.001, LR versus LP = 0.999). Similarly, the number of M2 in control group was highest on postoperative day 28, while in the LR-PRP and LP-PRP groups were highest on postoperative day 7. The numbers of M2 were significantly higher in LP-PRP group than those of controls on postoperative day 7 and 14 (day 7: control = 724 ± 180 cells, LR = 14,254 ± 5715 cells, LP = 26,337 ± 8276 cells; p values: control versus LR = 0.257, control versus LP = 0.018, LR versus LP = 0.331/day 14: control = 437 ± 139 cells, LR = 5624 ± 2936 cells, LP = 10,270 ± 3590 cells; p values: control versus LR = 0.257, control versus LP = 0.018, LR versus LP = 0.331) (Fig. 6B).
Fig. 5.
The count of MPs after PRP therapy. A) Flow cytometry analysis. The MPs were analyzed by plotting F4/80 and CD11b. Then, the GFP-positive MPs were counted to distinguish MPs host origin from MPs contained in administrated PRP. This figure showed the representative results of a sample in LR-PRP group. B) The absolute cell counts of the GFP-positive MPs on each time points. Data are presented as means ± SDs (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001).
Fig. 6.
Calculation of M1/M2 ratio in each time point. A) The GFP-positive MPs were sorted with plotting Ly6C and CX3CR1 to count the absolute cell numbers of M1 and M2 MPs. This figure showed the representative results of each group. B) The absolute cell counts of the M1 MPs and M2 MPs on each time points. C) The M1/M2 ratio on each time points were performed. Data are presented as means ± SDs (✧LP vs control, ✧ ✧ LR vs control, ✧ ✧ ✧ LP vs LR, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001).
Finally, the M1/M2 ratio was analyzed. M1/M2 ratio >1 means that there are relatively more M1 than M2 MPs. In control group, M1/M2 ratio was highest on postoperative day 14, while those in LP- and LR-PRP was highest on postoperative day 1 (Fig. 6C) (day 1: control = 1.14 ± 0.39 folds, LR = 11.48 ± 5.42 folds, LP = 10.06 ± 4.73 folds; p values: control versus LR = 0.247, control versus LP = 0.340, LR versus LP = 0.970). The ratio of M1/M2 was below 1.0 only in LP-PRP groups at day 7 and 14 (day 7: control = 2.81 ± 0.81 folds, LR = 1.89 ± 0.19 folds, LP = 0.49 ± 0.08 folds; p values: control versus LR = 0.389, control versus LP = 0.010, LR versus LP = 0.134/day 14: control = 5.14 ± 1.50 folds, LR = 2.11 ± 1.09 folds, LP = 0.36 ± 0.10 folds; p values: control versus LR = 0.028, control versus LP = 0.009, LR versus LP = 0.857), therefore, both LP- and LR-PRP enhanced the recruitment of MPs but LP-PRP could lead more M2 phenotype that decrease inflammation and encourage tissue repair.
4. Discussion
The tissue repair process is traditionally divided into three sequential phases: inflammation, proliferation, and remodeling [46,47]. Platelets are the first cells that are accumulated at an injured site and initiate the repair process by secreting various cytokines and growth factors [48]. PRP has been used based on the hypothesis that cytokines and growth factors secreted by platelets will activate the tissue repair process by affecting the local cells at injured site [46]. In addition, PRP has been known to enhance angiogenesis during early tendon repair [14]. In this study, we revealed that PRP enhanced the recruitment of MPs from early phase of tendon repair and demonstrated that those cells were originated from not administrated PRP but from blood flow. This observation suggested that the cytokines and growth factors released from PRP would enhance the cell migration and invasion of MPs from immediately after the administration of PRP. This study demonstrated that this process is one of the mechanisms of PRP-enhanced tissue repair system.
MPs are essential for early-phase tissue repair. In addition, the balance between M1 and M2 is thought to be important for this process. M1 MPs are thought to promote the inflammation phase by producing cytokines/chemokines, such as C–C motif chemokine ligand 2, monocyte chemoattractant protein-1, inducible nitric oxide synthase, tumor necrosis factor-α, interleukin (IL)-12, IL-1β, and vascular endothelial growth factor. In contrast, M2 MPs promote the proliferation and remodeling phases by producing cytokines/chemokines, such as IL-4, IL-10, transforming growth factor-β, arginase-1, insulin-like growth factor-1, and platelet-derived growth factor-β [20,25,49]. In our data, both M1 and M2 in control group gradually increased with time and the peak of M1/M2 ratio was day 14 (Fig. 6). On the other hand, in the PRP groups, the peak was observed in early phase of tendon healing (M1: day 4, M2: day 7) and M1/M2 ratio was highest at the day after PRP administration (day1) both in LR- and LP-PRP. This observation suggests that PRP administration enhances the inflammation and initiate the tissue remodeling state. Therefore, PRPs would exert their effect especially in the degenerative tissue conditions with low or no blood supply as PRP enhances the recruitment of MPs via chemotaxis manner independent of blood flow.
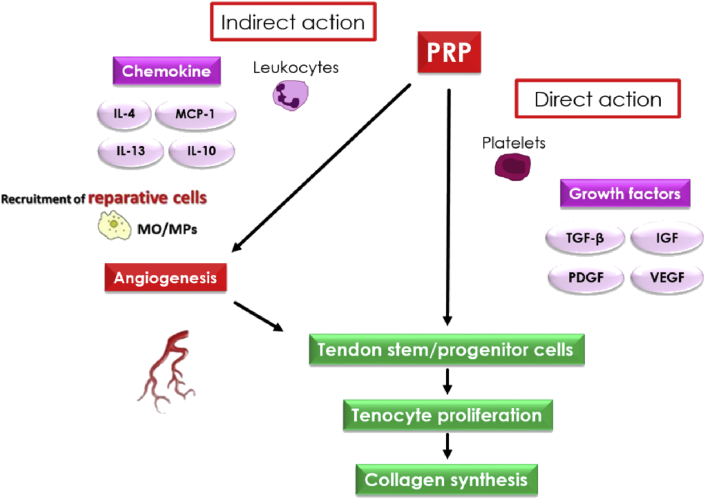
In this study, we demonstrated that local application of LR-PRP and LP-PRP to injured tissue promoted the recruitment of MPs derived from peripheral tissues and blood. Importantly, recruitment of reparative cells during the early phase is essential for inducing the normal cycle of the tissue repair process. Thus, local application of PRP could enhance the tissue repair process via not only direct mechanisms, such as inducing the proliferation of localized cells via growth factors within the PRP, but also indirect mechanisms, such as recruitment of reparative cells, particularly MPs, through peripheral tissue and blood flow (Fig. 7). Moreover, some reports have described the association between PRP and MPs subtypes. For example, Omar et al. suggested that PRP caused suppression of inflammation and induction of M2 MPs [50]. Accordingly, we hypothesized that the activity of MPs may be influenced by PRP quality, particularly the concentration and composition of leukocytes. We demonstrated that LR-PRP mainly enhanced the effects of M1 MPs, whereas LP-PRP more strongly induced the activity of M2 MPs. Commonly, M1 MPs can be generated by stimulation with bacterial lipopolysaccharides (LPS) in combination with IFN-γ. On the other hand, M2 MPs can be generated by various stimulation such as IL-4, IL-10 produced by Th2 lymphocyte [51]. The biological basis for this may be in the relative level of inflammatory versus anti-inflammatory mediators present in LR-PRP and LP-PRP. Inflammatory mediators such as TNF-α, IL-6, and IFN-ϒ are increased significantly in the presence of LR-PRP, whereas injection of LP-PRP increases anti-inflammatory mediators such as IL-4 and IL-10 [52,53]. This would be affected by the composition and concentration of leukocytes, platelets, and erythrocytes in PRP. It is known that granulocytes and erythrocytes abundantly contained in LR-PRP secrete a lot of inflammatory mediators, while lymphocytes abundantly contained in LP-PRP secrete a lot of anti-inflammatory mediators as represented by Th2 lymphocytes [54]. Also, These findings indicated that LR-PRP may cause a more pronounced inflammatory phase and that the acute infiltration and subsequent recruitment of inflammatory cells may stimulate the tissue repair process more rapidly. In contrast, LP-PRP may enhance the proliferation and remodeling phases more rapidly due to its strong potential to induce anabolic effects. Specifically, LR-PRP may need to be selected owing to its catabolic effects on the pathophysiology of degenerative changes in tissues, such as intractable tendinopathy.
Fig. 7.
Proposal of the tissue healing mechanism of PRP therapy. Local application of PRP could enhance the tissue repair process via not only direct mechanisms acting to the localized mesenchymal cells but also via indirect mechanisms through recruitment of tissue reparative cells.
A recent meta-analyses of PRP in tendinopathy showed that LR-PRP was associated with strongly positive outcomes [49,55]. In contrast, LP-PRP may have to be used in to exploit its anabolic effects, e.g., in osteoarthritis, tendon rupture, and pulled muscles. Accordingly, the optimal PRP may differ depending on the specific disease pathophysiology. In this study, we found that PRP promoted tendon repair through recruitment of MPs and that the leukocyte concentration and composition of PRP influenced the shift from M1 to M2 MPs. To the best of our knowledge, this is the first report describing these findings, and we expect that these results will contribute to elucidation of the mechanisms through which PRP therapy promotes the tissue repair process.
There are some limitations to this study. First, we used allogeneic PRP in this study; we could not use autologous PRP. However, the inbred animals used in this study are thought to be nearly identical to each other. Second, the murine patellar tendon defect model used in this study does not truly replicate human chronic degenerative changes. Third, we didn't administrate saline or platelet-poor plasma on the defect of patellar tendon in control group, therefore, there is possibility that the effect of inducing macrophages in LR-PRP and LP-PRP groups would be caused by foreign–body reaction. Forth, we have not confirmed whether tendon healing with PRP therapy is inhibited by suppression of the actions of MPs during the early phase of the tissue healing process. Further studies are needed to address these issues.
5. Conclusions
PRP therapy promoted the recruitment of macrophages in the process of tendon healing, and the leukocyte concentration and composition of PRP influenced the balance between M1 and M2 MPs. Thus, these results suggested that the tissue repair mechanism of PRP may involve both direct and indirect effects, with the latter being related to recruitment of reparative cells from the blood in a PRP quality-dependent manner. For further improvement of the efficacy of PRP therapy according to the specific pathophysiology of the disease, additional studies are needed to elucidate the mechanisms through which PRP quality affects tissue repair.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (grant no. 16K01832 and 19H04004). I am deeply grateful to Akemi Koyanagi and Tamami Sakanishi (Laboratory of Cell Biology, Research Support Center, Juntendo University Graduate School of Medicine)
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Alsousou J., Thompson M., Hulley P. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg Br. 2009;91:987–996. doi: 10.1302/0301-620X.91B8.22546. [DOI] [PubMed] [Google Scholar]
- 2.Foster T.E., Puskas B.L., Mandelbaum B.R. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez-Gonzalez D.J., Mendez-Bolaina E., Trejo-Bahena N.I. Platelet-rich plasma peptides: key for regeneration. Int J Pept. 2012:532519. doi: 10.1155/2012/532519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Werner S., Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 5.Kon E., Filardo G., Di Martino A. Platelet-rich plasma (PRP) to treat sports injuries: evidence to support its use. Knee Surg Sports Traumatol Arthrosc. 2011;19:516–527. doi: 10.1007/s00167-010-1306-y. [DOI] [PubMed] [Google Scholar]
- 6.Marx R.E., Carlson E.R., Eichstaedt R.M. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–646. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 7.Mishra A., Harmon K., Woodall J., Vieira A. Sports medicine applications of platelet rich plasma. Curr Pharmaceut Biotechnol. 2012;13:1185–1195. doi: 10.2174/138920112800624283. [DOI] [PubMed] [Google Scholar]
- 8.de Vos R.J., Weir A., van Schie H.T., Bierma-Zeinstra S.M., Verhaar J.A., Weinans H. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. J Am Med Assoc. 2010;303:144–149. doi: 10.1001/jama.2009.1986. [DOI] [PubMed] [Google Scholar]
- 9.Reurink G., Goudswaard G.J., Moen M.H., Weir A., Verhaar J.A., Bierma-Zeinstra S.M. Platelet-rich plasma injections in acute muscle injury. N Engl J Med. 2014;370:2546–2547. doi: 10.1056/NEJMc1402340. [DOI] [PubMed] [Google Scholar]
- 10.Chris J., Ji E.K., Kang S.Y., Sue S. Platelet-rich plasma stimulates cell proliferation and enhances matrix gene expression and synthesis in tenocytes from human rotator cuff tendons with degenerative tears. Am J Sports Med. 2012;40:1035–1045. doi: 10.1177/0363546512437525. [DOI] [PubMed] [Google Scholar]
- 11.de Mos M., van der Windt A.E., Jahr H., van Schie, HT., Weinans H., Verhaar J.A. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008;36:1171–1178. doi: 10.1177/0363546508314430. [DOI] [PubMed] [Google Scholar]
- 12.Dimitris N.L., Konstantinos K., George A. Temporal and spatial expression of TGF-b1 in the early phase of patellar tendon healing after application of platelet rich plasma. Arch Bone Jt Surg. 2016;4:156–160. [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J., Wang J. PRP treatment effects on degenerative tendinopathy - an in vitro model study. Muscles Ligaments Tendons J. 2014;4:10–17. [PMC free article] [PubMed] [Google Scholar]
- 14.Lyras D.N., Kazakos K., Verettas D. The influence of platelet-rich plasma on angiogenesis during the early phase of tendon healing. Foot Ankle Int. 2009;30:1101–1106. doi: 10.3113/FAI.2009.1101. [DOI] [PubMed] [Google Scholar]
- 15.DeLong J.M., Russell R.P., Mazzocca A.D. Platelet-rich plasma: the PAW classification system. Arthroscopy. 2012;28:998–1009. doi: 10.1016/j.arthro.2012.04.148. [DOI] [PubMed] [Google Scholar]
- 16.Dohan Ehrenfest D.M., Andia I., Zumstein M.A., Zhang C.Q., Pinto N.R., Bielecki T. Classification of platelet concentrates (platelet-rich plasma-PRP, platelet-rich fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 2014;4:3–9. [PMC free article] [PubMed] [Google Scholar]
- 17.Alberto M., Subhra K.B., Maria R.G., Antonio S., Locati M. Macrophage plasticity and polarization in tissue repair and remodeling. J Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 18.Gordon S., Martinez F. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Mantovani A., Sozzani S., Locati M. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 20.Arnold L., Henry A., Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into anti-inflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daley J.M., Brancato S.K., Thomay A.A., Reichner J.S., Albina J.E. The phenotype of murine wound macrophages. J Leukoc Biol. 2010;87:59–67. doi: 10.1189/jlb.0409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das A., Sinha M., Roy S. Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol. 2015;185:2596–2606. doi: 10.1016/j.ajpath.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barros M.H., Hauck F., Dreyer J.H., Kempkes B., Niedobitek G. Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PloS One. 2013;8 doi: 10.1371/journal.pone.0080908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novak M., Koh T. Macrophage phenotypes during tissue repair. J Leukoc Biol. 2013;93:875–881. doi: 10.1189/jlb.1012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramachandran P., Pellicoro A., Vernon M.A., Boulter L., Aucott R.L., Ali A. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:E3186–E3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor P.R., Gordon S. Monocyte heterogeneity and innate immunity. Immunity. 2003;19:2–4. doi: 10.1016/s1074-7613(03)00178-x. [DOI] [PubMed] [Google Scholar]
- 28.Andia I., Abate M. Platelet-rich plasma injections for tendinopathy and osteoarthritis. Int J Clin Rheumatol. 2012;7:397–412. [Google Scholar]
- 29.McCarrel T.M., Minas T., Fortier L.A. Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. J Bone Joint Surg Am. 2012;94:e143. doi: 10.2106/JBJS.L.00019. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi Y., Saita Y., Nishio H., Kaneko K. Leukocyte concentration and composition in platelet-rich plasma (PRP) influences the growth factor and protease concentrations. J Orthop Sci. 2016;21:683–689. doi: 10.1016/j.jos.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Moojen D.J., Everts P.A., Schure R.M. Antimicrobial activity of platelet-leukocyte gel against Staphylococcus aureus. J Orthop Res. 2008;26:404–410. doi: 10.1002/jor.20519. [DOI] [PubMed] [Google Scholar]
- 32.Oh J.H., Kim W., Park K.U., Roh Y.H. Comparison of the cellular composition and cytokine-release kinetics of various platelet-rich plasma preparations. Am J Sports Med. 2015;43:3062–3070. doi: 10.1177/0363546515608481. [DOI] [PubMed] [Google Scholar]
- 33.Dragoo J.L., Braun H.J., Durham J.L. Comparison of the acute inflammatory response of two commercial plateletrich plasma systems in healthy rabbit tendons. Am J Sports Med. 2012;40:1274–1281. doi: 10.1177/0363546512442334. [DOI] [PubMed] [Google Scholar]
- 34.Filardo G., Kon E., Pereira Ruiz M.T., Vaccaro F., Guitaldi R., Di Martino., A. Platelet-rich plasma intra-articular injections for cartilage degeneration and osteoarthritis: single- versus double- spinning approach. Knee Surg Sports Traumatol Arthrosc. 2012;20:2082–2091. doi: 10.1007/s00167-011-1837-x. [DOI] [PubMed] [Google Scholar]
- 35.Simental-Mendia M., Vilchez-Cavazos F., Martinez-Rodriguez H.G. The matrix synthesis and anti-inflammatory effects of autologous leukocyte-poor platelet rich plasma in human cartilage explants. Histol Histopathol. 2018;33:609–618. doi: 10.14670/HH-11-961. [DOI] [PubMed] [Google Scholar]
- 36.Simental-Mendia M., Vilchez-Cavazos F., Martinez-Rodriguez H.G. Leukocyte-poor platelet-rich plasma is more effective than the conventional therapy with acetaminophen for treatment of early knee osteoarthritis. Arch Orthop Trauma Surg. 2016;136:1723–1732. doi: 10.1007/s00402-016-2545-2. [DOI] [PubMed] [Google Scholar]
- 37.Cavallo C., Filardo G., Mariani E. Comparison of platelet rich plasma formulations for cartilage healing: an in vitro study. J Bone Joint Surg. 2014;96(5):5423–5429. doi: 10.2106/JBJS.M.00726. [DOI] [PubMed] [Google Scholar]
- 38.Riboh J.C., Saltzman B.M., Yanke A.B. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med. 2016;44:792–800. doi: 10.1177/0363546515580787. [DOI] [PubMed] [Google Scholar]
- 39.Dyment N.A., Kazemi N., Aschbacher-Smith L.E. The relationships among spatiotemporal collagen gene expression, histology, and biomechanics following full-length injury in the murine patellar tendon. J Orthop Res. 2012;30:28–36. doi: 10.1002/jor.21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dyment N.A., Liu C.F., Kazemi N. The paratenon contributes to scleraxis-expressing cells during patellar tendon healing. PloS One. 2013;8 doi: 10.1371/journal.pone.0059944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawamoto T., Kawamoto K. Preparation of thin frozen sections from nonfixed and undecalcified hard tissues using Kawamot's film method. Methods Mol Biol. 2014;1130:149–164. doi: 10.1007/978-1-62703-989-5_11. [DOI] [PubMed] [Google Scholar]
- 42.Cook J.L., Feller J.A., Bonar S.F. Abnormal tenocyte morphology is more prevalent than collagen disruption in asymptomatic athletes' patellar tendons. J Orthop Res. 2004;22:334–338. doi: 10.1016/j.orthres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 43.Maffulli N., Longo U.G., Franceschi F. Movin and Bonar scores assess the same characteristics of tendon histology. Clin Orthop Relat Res. 2008;466:1605–1611. doi: 10.1007/s11999-008-0261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nahrendorf M., Swirski F.K., Aikawa E., Stangenberg L., Wurdinger T., Figueiredo J.L. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swirski F., Nahrendorf M., Etzrodt M., Wildgruber M., Cortez-Retamozo V., Panizzi P. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nourissat G., Berenbaum F., Duprez D. Tendon injury: from biology to tendon repair. Nat Rev Rheumatol. 2015;11:223–233. doi: 10.1038/nrrheum.2015.26. [DOI] [PubMed] [Google Scholar]
- 47.Yang G., Rothrauff B.B., Tuan R.S. Tendon and ligament regeneration and repair: clinical relevance and developmental paradigm. Birth Defects Res C Embryo Today. 2013;99:203–222. doi: 10.1002/bdrc.21041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gawaz M., Vogel S. Platelets in tissue repair: control of apoptosis and interactions with regenerative cells. Blood. 2013;122:2550–2554. doi: 10.1182/blood-2013-05-468694. [DOI] [PubMed] [Google Scholar]
- 49.Ruffell D., Mourkioti F., Gambardella A., Kirstetter P., Lopez R.G., Rosenthal N. A CREB-C/EBPbeta cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc Natl Acad Sci U S A. 2009;106:17475–17480. doi: 10.1073/pnas.0908641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Omar N., El-Sabbagh Platelet-rich plasma-induced feedback inhibition of activin A/follistatin signaling: a mechanism for tumor-low risk skin rejuvenation in irradiated rats. J Photochem Photobiol, B. 2018;180:17–24. doi: 10.1016/j.jphotobiol.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 51.Thomas A., Kevin M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity, March. 2016;15 doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Braun Hillary J., Kim Hyeon Joo, Dragoo Jason L. The effect of platelet-rich plasma formulations and blood products on human synoviocytes. Am J Sports Med. 2014 May;42(5):1204–1210. doi: 10.1177/0363546514525593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sundman E.A., Cole B.J., Fortier L.A. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39:2135–2140. doi: 10.1177/0363546511417792. [DOI] [PubMed] [Google Scholar]
- 54.Mills Charles D., Kincaid Kristi, Annette M., Hill M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164(12):6166–6173. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 55.Fitzpatrick J., Bulsara M., Zheng M.H. The effectiveness of platelet-rich plasma in the treatment of tendinopathy: a meta-analysis of randomized controlled clinical trials. Am J Sports Med. 2017;45:226–233. doi: 10.1177/0363546516643716. [DOI] [PubMed] [Google Scholar]