Abstract
Immune thrombocytopenia (ITP) is a common hematological autoimmune disease, in which defective mesenchymal stem cells (MSCs) are potentially involved. Our previous study suggested that MSCs in ITP patients displayed enhanced apoptosis. MicroRNAs (miRNAs) play important roles in ITP by affecting megakaryopoiesis, platelet production and immunoregulation, whereas the roles of miRNAs in ITP-MSCs remain unknown. In a previous study, we performed microarray analysis to obtain mRNA and miRNA profiles of ITP-MSCs. In the present study, we reanalyze the data and identify miR-98-5p as a candidate miRNA contributing to MSC deficiency in ITP. miR-98-5p acts through targeting insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1), and the subsequent downregulation of insulin-like growth factor 2 (IGF-2) causes inhibition of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, which is involved in the process of MSC deficiency. Furthermore, miR-98-5p upregulates p53 by inhibiting β-transducin repeat-containing protein (β-TrCP)-dependent p53 ubiquitination. Moreover, miR-98-5p overexpression impairs the therapeutic effect of MSCs in ITP mice. All-trans retinoic acid (ATRA) protects MSCs from apoptosis by downregulating miR-98-5p, thus providing a potential therapeutic approach for ITP. Our findings demonstrate that miR-98-5p is a critical regulator of ITP-MSCs, which will help us thoroughly understand the pathogenesis of ITP.
Graphical Abstract
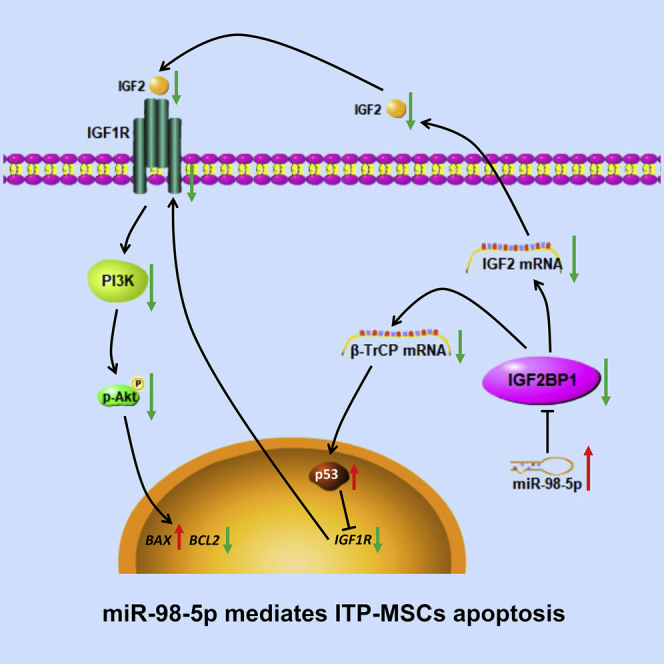
Immune thrombocytopenia (ITP) is a common hematological autoimmune disease, in which defective mesenchymal stem cells (MSCs) are potentially involved. Previous studies suggested that MSCs in ITP patients displayed enhanced apoptosis. Zhang and colleagues performed microarray analysis of ITP-MSCs and identified miR-98-5p as a candidate miRNA contributing to MSC deficiency in ITP. Further experiments were conducted, revealing that miR-98-5p exerts a pro-apoptotic effect through targeting IGF2BP1, and the resulting IGF-2/PI3K pathway inhibition and p53 accumulation are involved in ITP-MSC apoptosis.
Introduction
Thrombopoiesis occurs in the bone marrow microenvironment, and it begins with the commitment of hematopoietic stem cells to differentiate into megakaryocytic progenitors and eventually ends with maturation of megakaryocytes to produce platelets.1,2 As a main component of the hematopoietic niche, mesenchymal stem cells (MSCs) regulate megakaryocyte biogenesis and maturation and exhibit immune modulatory functions to maintain self-tolerance.3, 4, 5 MSCs are considered as the main regulators of megakaryocyte function, and MSC defects seem to play pivotal roles in the pathogenesis of immune thrombocytopenia (ITP). There is increasing evidence that MSCs in ITP exhibit impaired proliferative and functional capacities.6, 7, 8 Our previous study indicated that MSCs from ITP patients displayed increased apoptosis and showed an impaired immunosuppression function.9 We also demonstrated that the ability of ITP-MSCs to support megakaryocytic differentiation and thrombopoiesis was deficient,10 as was the ability to regulate dendritic cell differentiation.11 Based on the ability to modulate immune responses, MSCs have been used in the treatment of various inflammatory diseases, such as steroid-resistant acute graft-versus-host disease, cardiovascular disease, and autoimmune disorders.12, 13, 14 In particular, MSCs have been reported to be efficacious in improving platelet levels in ITP mice,15,16 and the intravenous infusion of umbilical cord-derived MSCs seems to be effective in refractory ITP patients.17 Given the promising therapeutic effects of MSCs in ITP and the key roles of MSCs during ITP development and progression, it is necessary to investigate the precise molecular signals that lead to MSC dysfunction in ITP. We have preliminarily explored the possible molecular regulations of MSC deficiency in ITP.9,10,18
MicroRNAs (miRNAs) are short (19–25 nt) evolutionarily conserved single-stranded RNA molecules that regulate gene expression. The effect of miRNA on mRNA is mediated through miRNA binding to the 3′ untranslated region (3′ UTR) of target mRNAs.19 miRNAs have been shown to play vital roles in immunoregulation, thereby participating in the pathogenesis of autoimmune diseases.20,21 The involvement of miRNAs in the pathogenicity of ITP remains unclear. miRNAs might be important regulatory molecules involved in the loss of tolerance in ITP.22 Several miRNAs have been shown to direct megakaryocyte proliferation, differentiation, and platelet production.23, 24, 25 Recently, miRNAs have been shown to play critical roles in regulating the proliferation, differentiation, and paracrine activity of MSCs.26 However, how miRNAs function in ITP-MSCs remains to be elucidated.
To address this issue, we profiled the expressions of both mRNAs and miRNAs by utilizing a microarray technique.18 In this study, we reanalyzed our previous miRNA profiling data from MSCs and identified miR-98-5p as a candidate miRNA that predisposes ITP-MSCs to be abnormal. We thus performed further experiments to determine whether miR-98-5p is involved in MSC deficiency in ITP, and the signaling mechanisms were also investigated.
Results
miRNA Profiling in MSCs Derived from ITP
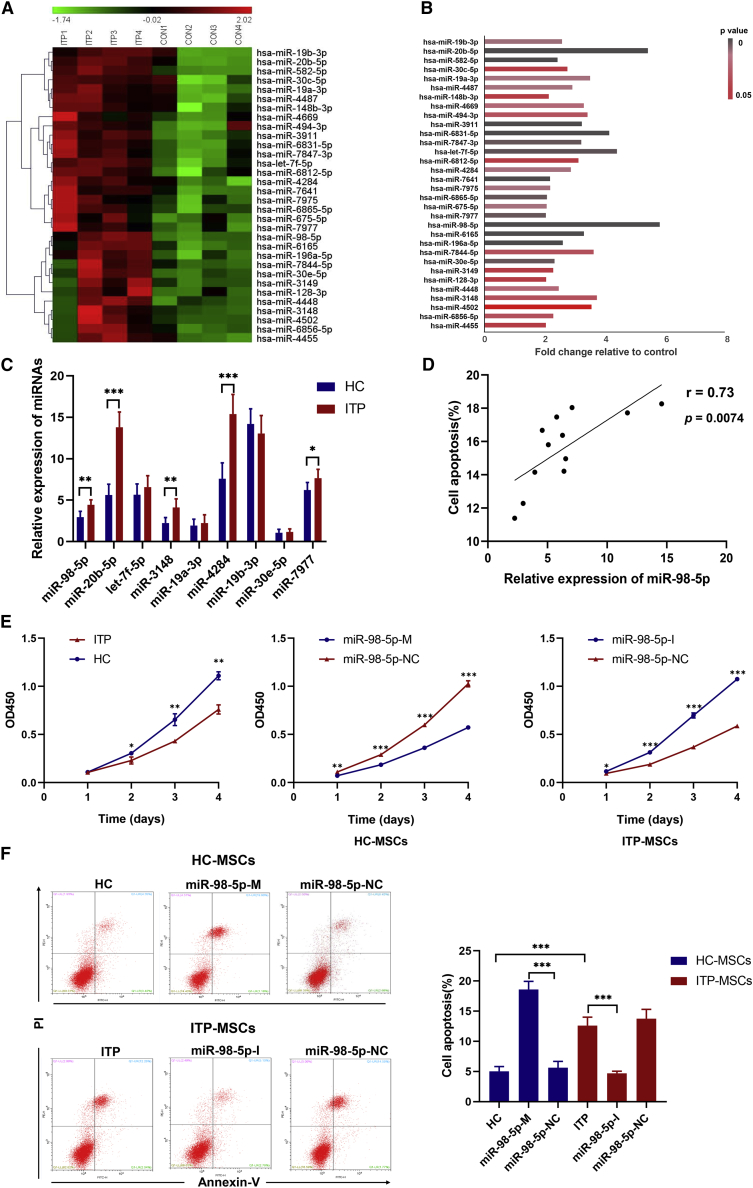
To better characterize the role of miRNAs in MSCs, we reanalyzed our previous microarray data from ITP-MSCs.18 Sixty-two miRNAs were detected to be significantly different between ITP and healthy controls. The TargetScan and Miranda algorithms were applied to evaluate whether these miRNAs were associated with changes in their target mRNA expression. Thirty-two miRNAs were found to be associated with mRNA expression in the database (Figure 1A), and the network of 32 differentially expressed miRNAs and their target mRNAs were also analyzed (Figure S1). Among the 32 miRNAs, miR-98-5p displayed the highest fold change (Figure 1B). Nine miRNAs (miR-98-5p, miR-20b-5p, let-7f-5p, miR-3148, miR-19a-3p, miR-4284, miR-19b-3p, miR-30e-5p, and miR-7977) among these 32 miRNAs were reported to be associated with autoimmune disorders or MSC functions.27, 28, 29, 30 Next, we performed quantitative real-time PCR experiments to validate the microarray data of these nine miRNAs using another set of MSCs from 12 ITP patients and 12 matched healthy controls. Of the nine miRNAs, five (miR-98-5p, miR-20b-5p, miR-3148, miR-4284, and miR-3977) remained increased in ITP-MSCs, and four (let-7f-5p, miR-19a-3p, miR-19b-3p, and miR-30e-5p) showed no difference between ITP-MSCs and healthy controls (Figure 1C). We further identified correlations between apoptosis rates and changes in miRNAs in ITP-MSCs. The results showed that miR-98-5p was positively correlated with apoptosis in ITP-MSCs (Figure 1D), whereas the other four miRNAs were not. miR-98-5p was previously reported to inhibit cell proliferation and induce apoptosis in hepatocellular carcinoma.31 In this study, we postulated that miR-98-5p is involved in the molecular regulation of ITP-MSCs apoptosis.
Figure 1.
MicroRNA Expression Profile in ITP-MSCs and the Correlation of miR-98-5p with MSC Apoptosis
(A) The expression profiling of 32 differential miRNAs (n = 4). (B) Fold change and p values of 32 miRNAs. (C) quantitative real-time PCR validation of miRNA expressions in MSCs from 12 ITP patients and 12 matched healthy controls (n = 12). (D) Association of miR-98-5p expression with apoptosis rate of ITP-MSCs (n = 12). (E) Cell proliferation of MSCs in each group was assessed by a CCK-8 assay (n = 6). (F) Flow cytometric analysis of apoptotic cells in each group (n = 6). All data are shown as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Increased miR-98-5p Expression in ITP-MSCs Correlates with Apoptosis
To investigate the roles of miR-98-5p in ITP-MSCs, expression vectors for miR-98-5p were constructed. We transfected healthy control MSCs (HC-MSCs) with primary (pri-)miR-98-5p vector (miR-98-5p-M) and miR-98-5p negative control (NC) vector (miR-98-5p-NC) and transfected ITP-MSCs with miR-98-5p-inhibitor vector (miR-98-5p-I) and miR-98-5p NC vector (miR-98-5p-NC), respectively (Figure S2B). miR-98-5p expression was significantly increased in miR-98-5p-M-transfected HC-MSCs and decreased in miR-98-5p-I-transfected ITP-MSCs (Figure S2C). MSC survival was evaluated by Cell Counting Kit-8 (CCK-8) assays, which revealed reduced cell survival of HC-MSCs when miR-98-5p was overexpressed, whereas survival of ITP-MSCs was improved when miR-98-5p was inhibited (Figure 1E). miR-98-5p-M-transfected HC-MSCs displayed abnormal morphology that was similar to ITP-MSCs, whereas miR-98-5p-I-transfected ITP-MSCs displayed improved morphology (Figure S2D). We further performed flow cytometric analysis of apoptotic cells, showing that apoptosis was increased in miR-98-5p-M-transfected HC-MSCs. Meanwhile, a decrease in apoptosis was observed in miR-98-5p-I-transfected ITP-MSCs (Figure 1F). These data indicated that increased apoptosis in ITP-MSCs was associated with overexpression of miR-98-5p.
IGF2BP1 Is a Direct Target of Posttranscriptional Repression by miR-98-5p
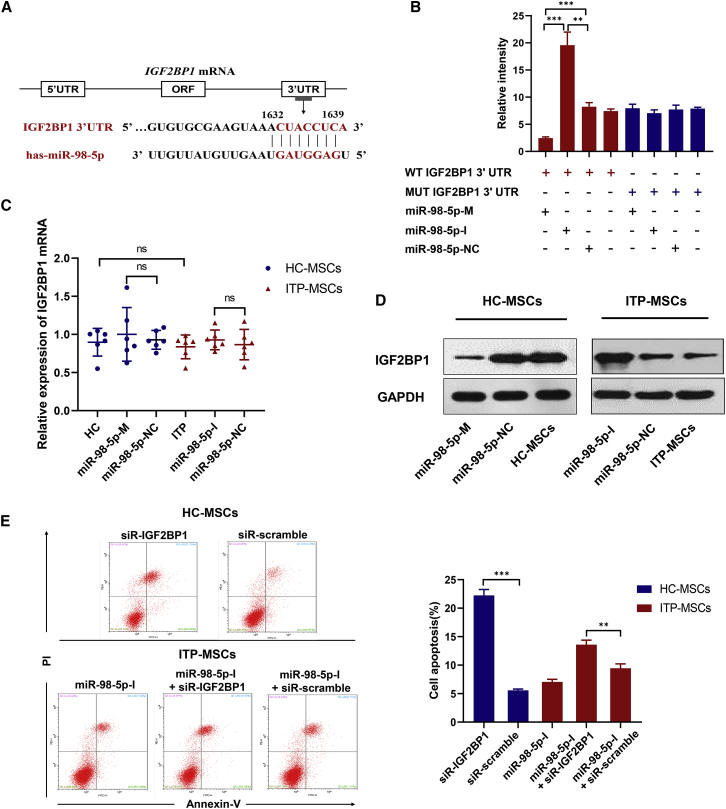
Microarray analysis indicated that insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1), an apoptosis-associated gene, was a direct target of miR-98-5p. In addition, by comparing the sequence of miR-98-5p and the 3′ UTR sequence of IGF2BP1 through miRDB, we predicted the most likely binding site for miR-98-5p in the IGF2BP1 3′ UTR (Figure 2A). A luciferase reporter containing the wild-type (WT) or the mutant (MUT) IGF2BP1 3′ UTR was constructed to confirm the targeting relationship between IGF2BP1 mRNA and miR-98-5p. Luciferase activity was markedly decreased in LM3 cells cotransfected with the WT IGF2BP1 3′ UTR plasmid and the pri-miR-98-5p plasmid (Figure 2B). However, cotransfection with the MUT IGF2BP1 3′ UTR plasmid and the pri-miR-98-5p plasmid in LM3 cells did not alter the luciferase activity (Figure 2B). We further detected IGF2BP1 expression in MSCs. Interestingly, IGF2BP1 mRNA levels were similar between ITP-MSCs and HC-MSCs (Figure 2C), but IGF2BP1 protein levels significantly decreased in ITP-MSCs (Figure 2D). Overexpression of miR-98-5p in HC-MSCs or inhibition of miR-98-5p in ITP-MSCs did not affect IGF2BP1 mRNA expression, but these treatments significantly inhibited or upregulated IGF2BP1 protein expression, respectively (Figures 2C and 2D). To investigate whether IGF2BP1 is involved in the process of ITP-MSC apoptosis, we transfected ITP-MSCs with a small interfering RNA (siRNA) to knock down IGF2BP1. As expected, the suppression of IGF2BP1 in HC-MSCs exacerbated apoptosis (Figure 2E). Furthermore, siRNA-IGF2BP1 transfection reversed the protective effect of miR-98-5p-I on ITP-MSCs (Figure 2E). These results suggest that miR-98-5p induces ITP-MSC apoptosis most likely by targeting IGF2BP1.
Figure 2.
IGF2BP1 Is a Direct Target of Posttranscriptional Repression by miR-98-5p
(A) The predicted binding site for miR-98-5p of the IGF2BP1 3′ UTR. (B) Luciferase reporter assay was conducted in MSCs. Data are presented as averages from three independent experiments. (C and D) qRT-PCR (C) and western blot (D) were used to detect the mRNA and protein levels of IGF2BP1 (n = 6). (E) Cell apoptosis was analyzed by flow cytometry in siRNA-IGF2BP1-transfected MSCs (n = 6). All data are shown as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. ns, not significant.
miR-98-5p Exerts Pro-apoptotic Effects by Inhibiting the IGF-2/PI3K/Akt Pathway
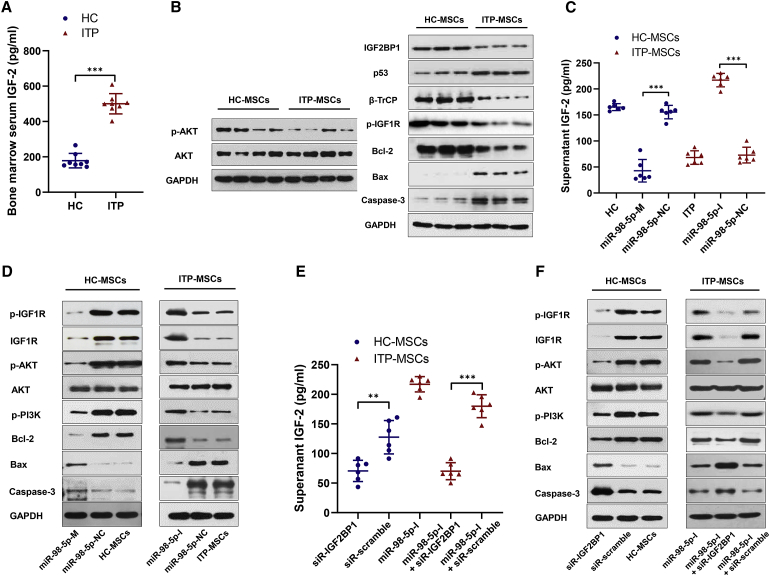
Insulin-like growth factors play important roles in the self-renewal and development of MSCs.32,33 IGF2BP1 mediates the stabilization of insulin-like growth factor 2 (IGF-2) mRNA, thus promoting the production of IGF-2,34 which is known to bind insulin-like growth factor 1 receptor (IGF1R), activating phosphatidylinositol 3-kinase (PI3K/Akt) signaling.32,33 We next addressed whether IGF-2/PI3K/Akt signaling was involved in the regulation of ITP-MSC apoptosis. Because IGF1R has the highest binding affinity for insulin-like growth factor 1 (IGF-1), followed by IGF-2, we first measured the concentrations of both IGF-1 and IGF-2 in bone marrow serum. Surprisingly, the soluble IGF-2 in bone marrow serum was significantly decreased in ITP patients (Figure 3A), whereas the IGF-1 concentration displayed no difference between ITP patients and healthy controls (Figure S3A). Further experiments indicated that ITP-MSCs showed compromised Akt signaling (Figure 3B). To identify whether the decreased IGF-2 and compromised Akt signaling in ITP-MSCs resulted from miR-98-5p upregulation and IGF2BP1 downregulation, we performed transfection experiments. Indeed, IGF-2 mRNA and soluble IGF-2 in culture medium were increased (Figure 3C; Figure S3B), and the IGF-2/PI3K/Akt pathway was simultaneously activated when miR-98-5p was inhibited in ITP-MSCs (Figure 3D). Accordingly, the IGF-2/PI3K/Akt pathway was attenuated when miR-98-5p was upregulated in miR-98-5p-M-transfected HC-MSCs (Figures 3C and 3D). Treatment of HC-MSCs with siRNA-IGF2BP1 resulted in the same effect on the IGF-2/PI3K/Akt pathway that was observed following the miR-98-5p-M treatment of HC-MSCs; treatment with siRNA-IGF2BP1 also reversed the activating IGF-2/PI3K/Akt effect of miR-98-5p-I on ITP-MSCs (Figure 3E; Figures S3C and S3F). Considering the crucial role of IGF-2 in MSCs, we preconditioned ITP-MSCs by treating them with exogenous IGF-2 in the culture medium. We found that IGF-2 supplementation markedly corrected ITP-MSCs apoptosis (Figure S3D), and it reversed the compromised IGF-2/PI3K/Akt signaling (Figure S3E). Collectively, these data suggest that miR-98-5p regulates ITP-MSC apoptosis by inhibiting the IGF-2/PI3K/Akt pathway.
Figure 3.
miR-98-5p Exerts Pro-apoptotic Effects by Inhibiting the IGF-2/PI3K/Akt Pathway
(A) The soluble IGF-2 in bone marrow serum from ITP patients and healthy controls was measured using an ELISA kit (n = 8). (B) Western blot was used to detect the activity of the IGF-2/PI3K/Akt pathway in ITP-MSCs and HC-MSCs (n = 6). (C) The supernatant IGF-2 was assessed in miR-98-5p-M-transfected HC-MSCs and miR-98-5p-I-transfected ITP-MSCs (n = 6). (D) The activity of the PI3K/Akt pathway was detected in miR-98-5p-M-transfected HC-MSCs and miR-98-5p-I-transfected ITP-MSCs (n = 6). (E) The supernatant IGF-2 was assessed in siRNA-IGF2BP1-transfected MSCs (n = 6). (F) The activity of PI3K/Akt pathway was detected in siRNA-IGF2BP1-transfected MSCs (n = 6). All data are shown as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
miR-98-5p Upregulates p53 through Inhibiting β-TrCP-Dependent Ubiquitination of p53
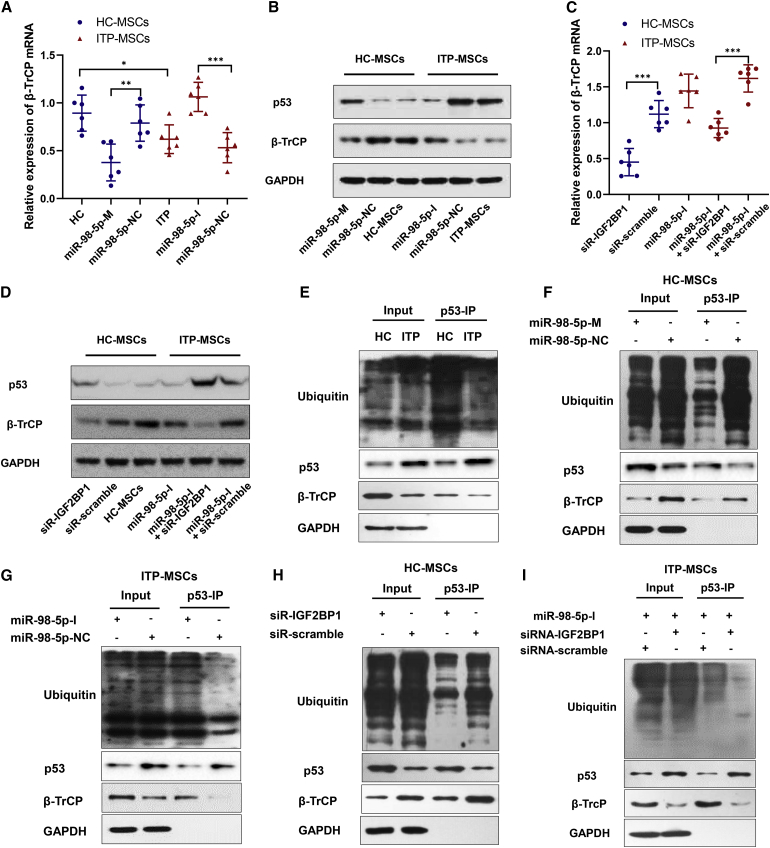
Our previous study demonstrated that the accumulation of p53 in ITP-MSCs is involved in ITP-MSC apoptosis.9 As a critical factor of the Skp1/Cul1/F-box (SCF) ubiquitin ligase complex, β-transducin repeat-containing proteins (β-TrCPs) mediate p53 ubiquitination and promote proteasome degradation of p53.35,36 Since IGF2BP1 could stabilize β-TrCP mRNA,37,38 we speculated that miR-98-5p targeting IGF2BP1 could contribute to the accumulation of p53 via inhibiting β-TrCP-dependent ubiquitination of p53. To confirm our speculation, we first measured β-TrCP and p53 expressions, revealing a marked decrease in β-TrCP at both the mRNA and protein levels in ITP-MSCs (Figures 4A and 4B); the observed upregulation of p53 (Figure 4B) was consistent with our previous study. Cell transfection experiments showed that β-TrCP was inhibited in miR-98-5p-M-transfected HC-MSCs and was upregulated in miR-98-5p-I-transfected ITP-MSCs (Figures 4A and 4B). In addition, siRNA-IGF2BP1 transfection downregulated β-TrCP in HC-MSCs and reversed the effect of miR-98-5p-I on ITP-MSCs (Figures 4C and 4D). These data indicated that miR-98-5p inhibited β-TrCP by targeting IGF2BP1 in ITP-MSCs. We next detected the ubiquitination of p53 mediated by β-TrCP. Interestingly, β-TrCP-mediated p53 ubiquitination was significantly inhibited in ITP-MSCs (Figure 4E). We performed more experiments to determine the role of miR-98-5p in p53 ubiquitination. The results showed that miR-98-5p-M transfection attenuated p53 ubiquitination in HC-MSCs, resulting in the accumulation of p53 (Figure 4F); however, inhibiting miR-98-5p markedly promoted p53 ubiquitination and downregulated p53 in ITP-MSCs (Figure 4G). Furthermore, β-TrCP-mediated p53 ubiquitination was inhibited in HC-MSCs when IGF2BP1 was knocked down by siRNA-IGF2BP1 transfection (Figure 4H), and siRNA-IGF2BP1 treatment reversed the promotion of p53 ubiquitination caused by miR-98-5p-I in ITP-MSCs (Figure 4I). Taken together, these data suggest that upregulation of miR-98-5p accounted for the accumulation of p53 in ITP-MSCs, most possibly by attenuating the p53 ubiquitinating effect mediated by β-TrCP.
Figure 4.
miR-98-5p Upregulates p53 through Inhibiting β-TrCP-Dependent Ubiquitination of p53
(A) qRT-PCR was used to detect the β-TrCP mRNA level in miR-98-5p-M transfected HC-MSCs and miR-98-5p-I transfected ITP-MSCs (n = 6). (B) β-TrCP and p53 were detected by western blot in miR-98-5p-M transfected HC-MSCs and miR-98-5p-I transfected ITP-MSCs (n = 6). (C) qRT-PCR was used to detect the β-TrCP mRNA level in siRNA-IGF2BP1 transfected MSCs (n = 6). (D) β-TrCP and p53 were detected by western blot in siRNA-IGF2BP1 transfected MSCs (n = 6). (E) β-TrCP-mediated p53 ubiquitination was detected in HC-MSCs and ITP-MSCs. Cells were treated with 20 μM proteasome inhibitor MG132 for 8 h, then p53 was immunoprecipitated and the ubiquitination of p53 was determined by western blot (n = 6). (F and G) β-TrCP-mediated p53 ubiquitination was detected in miR-98-5p-M-transfected HC-MSCs (n = 6) (F) and miR-98-5p-I-transfected ITP-MSCs (n = 6) (G). (H) The p53 ubiquitination was detected in siRNA-IGF2BP1-transfected HC-MSCs (n = 6). (I) The p53 ubiquitination was detected in miR-98-5p-I and siRNA-IGF2BP1 co-transfected ITP-MSCs (n = 6). All data are shown as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Since the IGF-2/PI3K/Akt pathway and the p53 pathway both participated in the process of ITP-MSC apoptosis, we wondered whether these two pathways had an essential connection to one another. Several studies have revealed that p53 suppresses IGF1R transcription, leading to abrogation of the IGF signaling network.39,40 To further assess whether p53 interacts with the IGF-2/PI3K/Akt pathway in ITP-MSCs, pifithrin-α (PFT-α) was used to inhibit p53, and then the activity of IGF-2/PI3K/Akt signaling was measured. The results showed that treatment with PFT-α markedly corrected ITP-MSC apoptosis (Figure S4A), which is consistent with our previous study. Meanwhile, PFT-α treatment upregulated IGF1R expression, and the phosphorylation of PI3K and Akt was also accordingly increased (Figure S4B). Taken together, these data reveal that miR-98-5p is involved in ITP-MSC apoptosis by interacting with p53.
miR-98-5p Overexpression Impairs the Treatment Effects of MSCs in ITP Mice
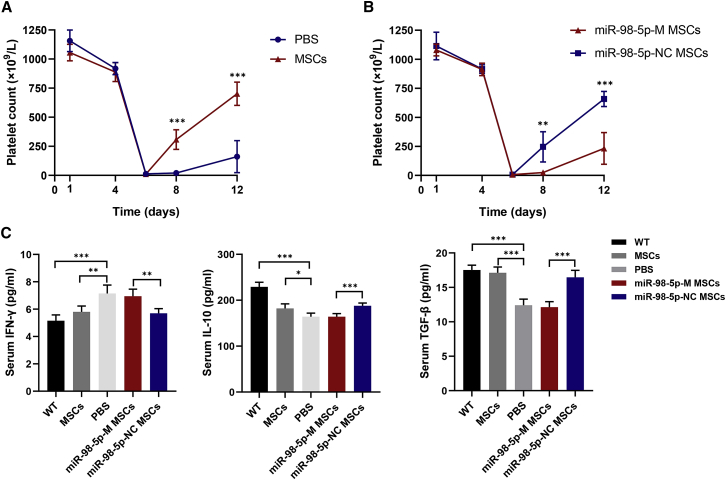
MSCs have been demonstrated to be beneficial for ITP mice.15 To better understand the role of miR-98-5p in MSCs during ITP, we established an active ITP model and injected ITP mice with miR-98-5p-modified MSCs to observe the resultant therapeutic effects. One week after transferring immunized splenocytes, platelet counts significantly decreased in ITP mice (Figure 5A), and there was obvious bleeding in subcutaneous tissues, abdomen, lungs, and intestines. Interestingly, we did not observe the upregulation of miR-98-5p in the bone marrow MSCs from ITP mice (data not shown). The normal MSC-treated group had a higher platelet count than did the untreated group at day 8 and day 12 (Figure 5A). However, miR-98-5p-M-transfected MSC-treated ITP mice showed no increase in platelets (Figure 5B). Alternatively, both miR-98-5p-M-transfected MSC- and miR-98-5p-NC-transfected MSC-treated mice displayed attenuated bleeding symptoms. Type 1 helper T cell-related cytokine interferon-γ (IFN-γ) was increased in ITP mice, and regulatory T cell-related cytokines transforming growth factor-β (TGF-β) and interleukin 10 (IL-10) were downregulated in ITP mice. Normal MSC treatment partly normalized the immune imbalance of ITP mice, whereas miR-98-5p-M MSCs treatment did not (Figure 5C). These results suggested that miR-98-5p overexpression in MSCs impaired the therapeutic effects of MSCs in ITP mice, which further demonstrated the critical role of miR-98-5p in ITP-MSCs.
Figure 5.
miR-98-5p Overexpression Impairs the Treatment Effects of MSCs in ITP Mice
(A) Platelet counts of MSC-treated ITP mice. Thrombocytopenia occurred in all mice on day 6. (B) Platelet counts of miR-98-5p-M-transfected MSC-treated ITP mice. (C) Cytokine levels in mice from five groups were detected using an ELISA kit. WT mice group, n = 6; PBS group, n = 4; MSC group, n = 5; miR-98-5p-M MSC group, n = 5; miR-98-5p-NC MSC group, n = 5. All data are shown as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
ATRA Protects ITP-MSCs from Apoptosis
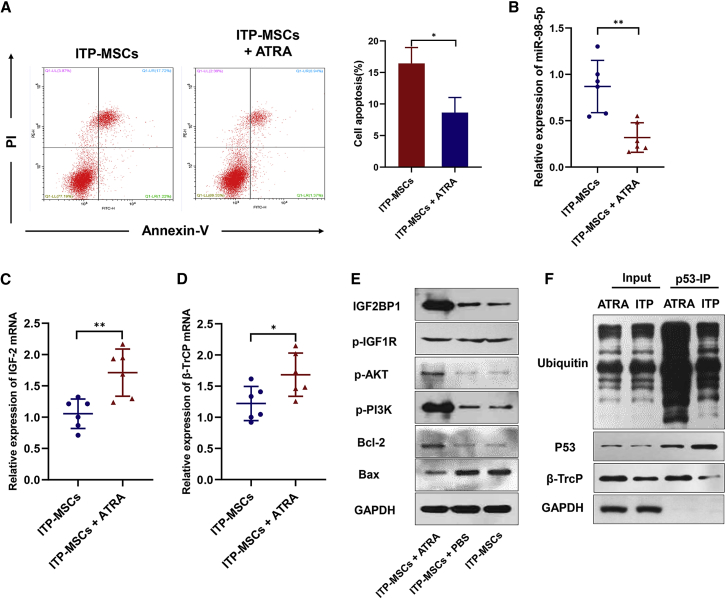
We have previously reported the efficacy of all-trans retinoic acid (ATRA) in treating corticosteroid-resistant or relapsed ITP patients.41 Several studies have demonstrated the mechanisms of ATRA in treating ITP.42,43 However, the regulatory effect of ATRA on ITP-MSCs has not been thoroughly studied. To address this question, we exposed ITP-MSCs to ATRA in the culture medium, as previously reported.10 As expected, ATRA treatment reduced apoptosis of ITP-MSCs (Figure 6A). We further detected the expression of miR-98-5p and its downstream signaling molecules. To our surprise, miR-98-5p expression was significantly decreased after ATRA treatment (Figure 6B). Accordingly, IGF-2 and β-TrCP mRNA increased after ATRA was administered (Figures 6C and 6D). PI3K/Akt signaling was activated (Figure 6E), and β-TrCP-mediated p53 ubiquitination was also upregulated (Figure 6F). These data provide evidence that ATRA protected ITP-MSCs from apoptosis by downregulating miR-98-5p in vitro. Taken together, ATRA protects ITP-MSCs from apoptosis most likely by targeting miR-98-5p, thus being a potential therapeutic approach for ITP patients.
Figure 6.
ATRA Protects ITP-MSCs from Apoptosis
(A) Cell apoptosis of ATRA-treated ITP-MSCs was analyzed by flow cytometry (n = 6). (B) miR-98-5p expression in ATRA-treated ITP-MSCs was detected by qRT-PCR (n = 6). (C and D) IGF-2 (C) and β-TrCP (D) mRNA expression in ATRA-treated ITP-MSCs was detected by qRT-PCR (n = 6). (E) The activity of the IGF-2/PI3K/Akt pathway was detected by western blot (n = 6). (F) β-TrCP-dependent p53 ubiquitination was detected in ATRA-treated ITP-MSCs (n = 6). All data are shown as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Discussion
MSCs function in supporting thrombopoiesis and regulating immunological responses, thus playing a vital role in the pathogenesis of ITP.9,44 An increasing number of studies have demonstrated the therapeutic effects of MSCs in autoimmune diseases. Therefore, understanding the molecular regulation of MSC deficiency could be fundamental for revealing the precise pathogenesis of ITP. miRNAs and targeting mRNAs for cleavage or translational repression are critical regulators that specify cell differentiation and developmental patterning of MSCs.26 Recently, it has been reported that bone marrow MSCs derived from systemic lupus erythematosus patients display a distinct miRNA signature and that miR-663 was found to induce immune dysregulation in MSCs by targeting TGF-β1.45
In this study, we first reanalyzed our previous microarray data from ITP-derived MSCs and identified 62 miRNAs that were differentially expressed between ITP patients and healthy controls. After integrating the data with mRNA profiling, 32 miRNAs were identified to be distinct in ITP-MSCs compared with controls. The miRNA profile of ITP-MSCs was notably different from the profile of circulation and T cells of ITP.22,46 Five miRNAs (miR-98-5p, miR-20b-5p, miR-3148, miR-4284, and miR-3977) were finally verified to be upregulated in ITP-MSCs. miRNAs are regulated by multiple factors, and the potential mechanisms for the upregulation of miRNAs in ITP-MSCs could be multifarious. A common G/C polymorphism within the precursor (pre-)miR-146a sequence could decrease the generation of miR-146a,47 which suggests that single nucleotide polymorphisms might affect the upregulation of miRNAs in ITP-MSCs. Epigenetic control including DNA methylation and histone modifications as well as some transcription factors positively or negatively regulate miRNA expression.48, 49, 50, 51 In addition, posttranscriptional regulation, including the regulation of Drosha and Dicer and RNA tailing and editing, often controls miRNA production,48,52,53 and this might also contribute to the upregulation of miRNAs in ITP-MSCs. We then characterized miR-98-5p as a candidate miRNA contributing to ITP-MSC apoptosis, because it had the highest fold change in microarray analysis and because of its relevance to cell apoptosis. Studies have shown that miR-98-5p is dysregulated in cancers and is associated with cancer cell proliferation and metastasis. Moreover, miR-98-5p is involved in the myocardial differentiation of MSCs by regulating TBX5.27 However, the function of miR-98-5p in ITP-MSCs has not been determined.
In this study, we determined that miR-98-5p was associated with ITP-MSC apoptosis and that inhibition of the IGF-2/PI3K/Akt pathway and accumulation of p53 were involved in the process. IGF2BP1, belonging to a highly conserved protein family, is predicted to be one of the potential targets of miR-98-5p. miR-98-5p was reported to inhibit cell proliferation and induce apoptosis in hepatocellular carcinoma by targeting IGF2BP1.31 Consistent with this report, our data showed that IGF2BP1 was potentially posttranscriptionally repressed by miR-98-5p via binding to the 3′ UTR of IGF2BP1 mRNA. IGF2BP1 has been found to play important roles in cell proliferation and growth of normal tissues and tumor tissues, as well as in tumor cell apoptosis.54 Furthermore, IGF2BP1 expression contributes to the stemness of MSCs and is essential for MSC proliferation; knockdown of IGF2BP1 significantly inhibits the proliferation of MSCs.55,56 Herein, we demonstrated that miR-98-5p induces MSCs apoptosis by downregulating IGF2BP1.
IGF2BP1 functions by binding to the mRNAs of certain genes, such as PTGS2, PTEN, and IGF-2, thus stabilizing IGF-2 and other transcripts.34,54,57 In the present study, we found that IGF-2 was decreased in ITP-MSCs as a consequence of the downregulation of IGF2BP1. IGF-2 can signal via paracrine or autocrine routes to interact with IGF1R, which is a receptor tyrosine kinase, while IGF2R is not.32 Upon ligand binding, IGF1R activates the PI3K/Akt pathway, thus inducing transcriptional activity to promote survival, self-renewal, and differentiation of MSCs.58 IGF-2 was recently demonstrated to be a key factor mediating the anti-inflammatory effect of human MSCs.59 Considering that IGF1R has the highest binding affinity toward IGF-1, followed by IGF-2, we detected both IGF-1 and IGF-2 concentrations in the bone marrow serum and cultured medium. The results showed that IGF-1 displayed no difference between ITP patients and healthy controls, but IGF-2 expression was significantly lower in ITP-MSCs. Importantly, the PI3K/Akt pathway in ITP-MSCs was predictably inhibited, which was a downstream effect of miR-98-5p targeting IGF2BP1. By adding exogenous IGF-2 to the culture medium, ITP-MSC apoptosis was considerably alleviated.
As a tumor suppressor, p53 plays an important regulatory role in apoptosis.60 We have previously demonstrated that the upregulation of p53 was responsible for the enhanced apoptosis of ITP-MSCs.9 In this study, we demonstrated that miR-98-5p contributed to the accumulation of p53 by downregulating IGF2BP1. β-TrCP, a critical factor of the SCFβ-TrCP ubiquitin ligase complex, mediates p53 ubiquitination and promotes proteasome degradation of p53.35,36 As a mRNA-binding protein, IGF2BP1 inhibits β-TrCP mRNA degradation and thus maintains the stability of β-TrCP.37,38 In addition to the accumulation of p53 in ITP-MSCs, we also detected the reduction in β-TrCP in ITP-MSCs. The ubiquitination assay reflected that the β-TrCP-dependent ubiquitination of p53 was inhibited, thus resulting in p53 upregulation in ITP-MSCs; this upregulation was found to be a consequence of the reduction in IGF2BP1 mediated by miR-98-5p. As a key regulator of apoptosis, p53 interacts with many signaling pathways through induction of target genes or transcription-independent mechanisms.61 Typically, p53 suppresses IGF1R gene transcription, leading to abrogation of the IGF signaling network.39,40 In this study, we revealed that p53 suppressed the activity of the IGF-2/PI3K/Akt pathway by suppressing IGF1R expression.
To further support our findings, we showed that in active ITP mice, the therapeutic effect of MSC transfusion was impaired when miR-98-5p was overexpressed in MSCs. ATRA is currently used as a chemotherapeutic agent in the treatment of acute promyelocytic leukemia.62 In addition, ATRA has recently been demonstrated to inhibit T helper 17 (Th17) cell polarization, enhance FoxP3 expression, and modulate regulatory T cells in human inflammatory diseases.63, 64, 65 The therapeutic efficacy of ATRA in ITP patients was reported in our previous study,41 and we showed that ATRA could protect MSCs from ITP patients by regulating the complement-IL-1β loop.43 It has been shown that ATRA treatment leads to the expression of a selected set of miRNAs in regulatory T cells.66 In this study, we showed that ATRA treatment improved ITP-MSCs by downregulating miR-98-5p.
In conclusion, our results demonstrate that miR-98-5p targeting IGF2BP1 plays a pro-apoptotic role in ITP-MSCs, and the resulting IGF-2/PI3K pathway inhibition and p53 accumulation are involved in ITP-MSC apoptosis. ATRA protects ITP-MSCs from apoptosis, most likely by inhibiting miR-98-5p expression, which presents a potential therapeutic approach for ITP.
Materials and Methods
Patient Samples
MSCs from four ITP patients and four healthy controls were used for miRNA microarray analysis, as previously mentioned.18 Another 12 newly diagnosed ITP patients (5 males and 7 females; age range, 19–49 years; median age, 35 years) and 12 matched healthy controls (6 males and 6 females; age range, 18–45 years; median age, 32 years) were selected for isolating and expanding MSCs to perform the following experiments. All patients were diagnosed based on previously published criteria for ITP.67 Patients more than 18 years old at diagnosis with platelet counts <30 × 109/L between December 2018 and February 2019 were enrolled. The clinical characteristics of the 12 ITP patients are presented in Table S1. All of the patients and controls provided written consent to participate in the study, which was approved by the Ethics Committee of the Peking University People’s Hospital, and the study was conducted in accordance with the Declaration of Helsinki.
Animal Model and Treatment
WT C57BL/6J mice that were used as platelet donors and splenocyte transfer recipients were purchased from Beijing Vital River Laboratory Animal Technology (Beijing, China). C57BL/6J CD61 knockout (KO) mice (B6.129S2-Itgb3tm1Hyn/JSemJ; stock no. 008819) were provided by Dr. Junling Liu from the Shanghai Jiaotong University School of Basic Medicine. All animal experiments were approved by the Animal Ethics Committee of Peking University People’s Hospital.
To establish an active murine model of ITP, we washed WT platelets and adjusted them to a concentration of 109 cells/mL. CD61 KO mice were transfused with 100 μL of 108 WT platelets weekly for 3 weeks, as previously described.68 The immunized CD61 KO mice were then killed, and their spleens were removed and prepared into a splenocyte suspension. Finally, 5 × 106 splenocytes were transfused into WT mice to establish the active ITP model.69 To observe the effects of miR-98-5p on MSCs in vivo, MSCs from healthy controls were transfected with pri-miR-98-5p plasmid vectors to induce overexpression of miR-98-5p. Then, 6 × 106 transfected MSCs were injected into ITP mice intravenously for 2 days after the splenocyte transfer. NC plasmid vector-transfected MSCs (6 × 106) were injected into ITP mice to serve as the control group. Platelet counts were recorded every 4 days, and mice were sacrificed for further sera cytokine detection and MSC experiments on day 12.
miRNA Microarray Analysis
The miRNA microarray analysis was conducted as in our previous study.18 In the present study, we reanalyzed the miRNA microarray data. The miRNA functions and miRNA target mRNAs were identified using the miRbase (http://www.mirbase.org), TargetScan, and Miranda algorithms.
Isolation, Expansion, and Characterization of MSCs
Samples of bone marrow were taken from the iliac crest of participants after informed consent had been obtained. Bone marrow mononuclear cells were isolated using 1.073 g/mL Ficoll separation medium (Solarbio, China) and were cultured in low-glucose Dulbecco’s modified Eagle’s medium (L-DMEM) (Life Technologies, USA) supplemented with 10% defined fetal bovine serum (FBS) (Gibco, USA) and 100 U/mL penicillin/streptomycin (Life Technologies). The cultures were maintained at 37°C in a humidified 5% CO2 incubator. Medium containing nonadherent cells was replaced after 48 h and then every 3 days. Cells were detached using 0.25% trypsin-EDTA (Life Technologies) when cultures reached 90% confluence, and then they were seeded in flasks at a density of 1 × 106 cells/25 cm2 and cultured for another 4–5 days to obtain the next passage of MSCs.
To confirm the MSC phenotype, passage 4 plastic adherent cells were analyzed by flow cytometry (FCM) (Beckman Coulter, USA) based on positive staining (>95%) for CD29, CD105, CD90, CD73, and CD166 and negative staining (<2%) for CD45, CD14, CD31, human leukocyte antigen (HLA)-DR, and CD133 (Figure S5). The following antibodies were applied: fluorescein isothiocyanate (FITC)-conjugated anti-human CD14 and CD29, allophycocyanin (APC)-conjugated CD45 and CD31, peridinin chlorophyll protein (PerCP)-conjugated CD105 and CD90, phycoerythrin (PE)-conjugated CD73, and HLA-DR. Their isotypes were used as controls.
Cell Transfection Assay
Plasmids vectors (Figure S2A) containing miR-98-5p NC (miR-98-5p-NC), pri-miR-98-5p (miR-98-5p-M), and inhibitor-miR-98-5p (miR-98-5p-I) were purchased from Invitrogen/Life Technologies). Before transfection, MSCs were seeded on plates and incubated overnight. According to the manufacturer’s instructions, transient transfection of MSCs with these plasmids was performed by electroporation using a 4D-Nucleofector system.
The siRNA-IGF2BP1 was transfected into MSCs to silence IGF2BP1 expression. As controls, cells were transfected with a scrambled siRNA sequence. The sequences of miR-98-5p-mimic, miR-98-5p-inhibitor, siRNA-IGF2BP1, and scrambled siRNA are presented in Table S2. All siRNAs were transfected into MSCs using the transfection reagent (Roche Applied Science, Germany) according to the manufacturer’s instructions. At 24, 48, and 72 h after transfection, cells were harvested to detect miR-98-5p and IGF2BP1 expression, thus determining the optimal transfection time.
PFT-α and ATRA Treatment
Cells were detached by incubation with 0.25% trypsin-EDTA for 1 min, and then they were seeded in flasks at 5 × 105 cells/mL and treated with PFT-α (50 μM) or ATRA (50 μg/mL) for 48 h. Then, cells were prepared for western blotting and qRT-PCR analysis.
Luciferase Reporter Assay
According to the manufacturer’s protocol, WT and MUT IGF2BP1 3′ UTRs were generated using the QuickChange site-directed mutagenesis kit (Stratagene, USA). The 3′ UTRs were then integrated into the plasmid. LM3 cells were cotransfected with 200 ng of the vector carrying the WT or MUT IGF2BP1 3′ UTR and 100 nM pri-miR-98-5p plasmids. Forty-eight hours after transfection, LM3 cells were washed and lysed using lysis buffer. Then, a Dual-Luciferase reporter assay system (Promega, USA) was used to determine the firefly luciferase and Renilla luciferase activities. Renilla luciferase activity was normalized to obtain the relative reporter activity.
qRT-PCR
The total RNA from MSCs was extracted from MSCs using TRIzol reagent (Life Technologies). The RNA was reverse transcribed into cDNA according to the manufacturer’s instructions. qRT-PCR was performed on an ABI 7500 Fast real-time PCR detection system (Applied Biosystems, USA). A Mir-X miRNA qRT-PCR TB Green kit (Takara, Japan) was used to analyze the expression of miRNAs, and a PrimeScript RT reagent kit (Takara) was used to analyze the expression of mRNAs. Internal housekeeping controls were U6 and GAPDH. All primers for miRNA and other genes were purchased from Guangzhou RiboBio (China). The relative expressions of miRNAs and mRNAs were calculated using the 2−ΔΔCt method. Primers for qRT-PCR are presented in Table S3.
Cell Proliferation and Apoptosis Assays
The proliferation of MSCs was determined using a CCK-8 assay kit (Beyotime, China) in accordance with the manufacturer’s protocol. Briefly, cells were seeded into a 96-well plate at a concentration of 5 × 103 cells per well for growth overnight. CCK-8 reagents were added to a subset of wells and incubated for 2 h at 37°C. The absorbance of each well was quantified at a wavelength of 450 nm.
For the apoptosis assay, an annexin V-FITC apoptosis detection kit (Sigma, USA) was used. Generally, cells were washed twice with phosphate-buffered saline (PBS) and incubated with 5 μL of annexin V-FITC and 5 μL of propidium iodide (PI) for 30 min at 4°C in dark conditions. Then, samples were analyzed by FCM (Beckman Coulter).
Western Blot Analysis and ELISA Assay
Cells were incubated in radioimmunoprecipitation assay buffer containing proteinase inhibitor and PhosSTOP (Roche, Switzerland). A bicinchoninic acid (BCA) kit (Sigma) was used to analyze the total protein concentration. Proteins were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and were then transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were then blocked with 5% BSA at room temperature for 2 h and incubated overnight with antibodies against IGF2BP1 (Abcam, ab82968, UK), β-TrCP (CST, 4394, USA), IGF1R (CST, 9750), p-IGF1R (Tyr1135) (CST, 3918), PI3K (Proteintech, 60225-1-Ig, USA), phosphorylated (p-)PI3K p85 (Tyr607) (Abcam, ab182651), Akt (CST, 4691), p-Akt (Ser473) (CST, 4060), Bcl-2 (CST, 15071), Bax (CST, 2772), caspase-3 (Proteintech, 66470-2-Ig), p53 (Abcam, ab26), ubiquitin (Abcam, ab7780), and GAPDH (CST, 2118) at dilutions specified by each manufacturer’s instructions. The membranes were then washed with Tris-buffered saline with Tween 20 (TBST) and incubated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibodies for 1 h. The blots were developed and proteins were semiquantitatively analyzed using an enhanced chemiluminescence kit (Pierce, USA).
We detected IGF-1 and IGF-2 concentrations in the bone marrow serum and cultured medium using an IGF-1 ELISA kit (R&D, DG100, USA) and an IGF-2 ELISA kit (BioVision, K4412, USA), respectively, according to the manufacturer’s instructions. The serum IFN-γ, IL-10, and TGF-β of mice were determined using the corresponding ELISA kits (Proteintech, KE10001, KE10008 and KE10005).
Coimmunoprecipitation and Ubiquitination Assay
For the detection of ubiquitinated p53 protein, cells were treated with 20 μM MG132 (Sigma) for 8 h. Then, cells were collected in PBS and resuspended in 1 mL of lysis buffer and centrifuged. Subsequently, the cell lysates were immunoprecipitated with mouse anti-p53 antibody and protein A-Sepharose (GE Healthcare, USA), and the precipitates were analyzed by immunoblot with the indicated antibodies after six washes with PBS with Tween 20 (PBST).
Statistical Analysis
The data are shown as the mean ± SEM and were analyzed using GraphPad Prism 8.0. Comparisons between two groups were performed using the Student’s t test, while comparisons among three or more groups were conducted using one-way ANOVA. p < 0.05 was considered statistically significant.
Author Contributions
X.Z., Y.W., and J.Z. designed the research. Y.W., Y.S., and G.Z. conducted the experiments. M.L., Y.C., J.P., and M.H. participated in the study design. Y.W. analyzed the data and wrote the manuscript. C.W., X.L., and Q.C. contributed to the bioinformatics analyses. X.Z. and X.H. contributed to the scientific supervision. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported by the Beijing Natural Science Foundation (nos. 7171013 and H2018206423); the Key Program of National Natural Science Foundation of China (no. 81730004); the National Natural Science Foundation of China (nos. 81670116 and 81970113); the Beijing Municipal Science and Technology Commission (no. Z171100001017084); and the National Key Research and Development Program of China (nos. 2017YFA0105500 and 2017YFA0105503).
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtn.2020.04.013.
Supplemental Information
References
- 1.De Botton S., Sabri S., Daugas E., Zermati Y., Guidotti J.E., Hermine O., Kroemer G., Vainchenker W., Debili N. Platelet formation is the consequence of caspase activation within megakaryocytes. Blood. 2002;100:1310–1317. doi: 10.1182/blood-2002-03-0686. [DOI] [PubMed] [Google Scholar]
- 2.Reems J.A., Pineault N., Sun S. In vitro megakaryocyte production and platelet biogenesis: state of the art. Transfus. Med. Rev. 2010;24:33–43. doi: 10.1016/j.tmrv.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broudy V.C., Lin N.L., Kaushansky K. Thrombopoietin (c-mpl ligand) acts synergistically with erythropoietin, stem cell factor, and interleukin-11 to enhance murine megakaryocyte colony growth and increases megakaryocyte ploidy in vitro. Blood. 1995;85:1719–1726. [PubMed] [Google Scholar]
- 4.Cho K.A., Lee J.K., Kim Y.H., Park M., Woo S.Y., Ryu K.H. Mesenchymal stem cells ameliorate B-cell-mediated immune responses and increase IL-10-expressing regulatory B cells in an EBI3-dependent manner. Cell. Mol. Immunol. 2017;14:895–908. doi: 10.1038/cmi.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jorgensen C., Djouad F., Apparailly F., Noël D. Engineering mesenchymal stem cells for immunotherapy. Gene Ther. 2003;10:928–931. doi: 10.1038/sj.gt.3302019. [DOI] [PubMed] [Google Scholar]
- 6.Ma J., Ning Y.N., Xu M., Hou Y., Wang N., Hou X.Y., Yu Y.Y., Li H., He W.D., Shao L.L. Thalidomide corrects impaired mesenchymal stem cell function in inducing tolerogenic DCs in patients with immune thrombocytopenia. Blood. 2013;122:2074–2082. doi: 10.1182/blood-2013-03-491555. [DOI] [PubMed] [Google Scholar]
- 7.Pérez-Simón J.A., Tabera S., Sarasquete M.E., Díez-Campelo M., Canchado J., Sánchez-Abarca L.I., Blanco B., Alberca I., Herrero-Sánchez C., Cañizo C., San Miguel J.F. Mesenchymal stem cells are functionally abnormal in patients with immune thrombocytopenic purpura. Cytotherapy. 2009;11:698–705. doi: 10.3109/14653240903051558. [DOI] [PubMed] [Google Scholar]
- 8.Zhang D., Li H., Ma L., Zhang X., Xue F., Zhou Z., Chi Y., Liu X., Huang Y., Yang Y., Yang R. The defective bone marrow-derived mesenchymal stem cells in patients with chronic immune thrombocytopenia. Autoimmunity. 2014;47:519–529. doi: 10.3109/08916934.2014.938320. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J.M., Feng F.E., Wang Q.M., Zhu X.L., Fu H.X., Xu L.P., Liu K.Y., Huang X.J., Zhang X.H. Platelet-derived growth factor-BB protects mesenchymal stem cells (MSCs) derived from immune thrombocytopenia patients against apoptosis and senescence and maintains MSC-mediated immunosuppression. Stem Cells Transl. Med. 2016;5:1631–1643. doi: 10.5966/sctm.2015-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He Y., Xu L.L., Feng F.E., Wang Q.M., Zhu X.L., Wang C.C., Zhang J.M., Fu H.X., Xu L.P., Liu K.Y. Mesenchymal stem cell deficiency influences megakaryocytopoiesis through the TNFAIP3/NF-κB/SMAD pathway in patients with immune thrombocytopenia. Br. J. Haematol. 2018;180:395–411. doi: 10.1111/bjh.15034. [DOI] [PubMed] [Google Scholar]
- 11.Xu L.L., Fu H.X., Zhang J.M., Feng F.E., Wang Q.M., Zhu X.L., Xue J., Wang C.C., Chen Q., Liu X. Impaired function of bone marrow mesenchymal stem cells from immune thrombocytopenia patients in inducing regulatory dendritic cell differentiation through the Notch-1/Jagged-1 signaling pathway. Stem Cells Dev. 2017;26:1648–1661. doi: 10.1089/scd.2017.0078. [DOI] [PubMed] [Google Scholar]
- 12.Le Blanc K., Frassoni F., Ball L., Locatelli F., Roelofs H., Lewis I., Lanino E., Sundberg B., Bernardo M.E., Remberger M., Developmental Committee of the European Group for Blood and Marrow Transplantation Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 13.Quevedo H.C., Hatzistergos K.E., Oskouei B.N., Feigenbaum G.S., Rodriguez J.E., Valdes D., Pattany P.M., Zambrano J.P., Hu Q., McNiece I. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc. Natl. Acad. Sci. USA. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D., Li J., Zhang Y., Zhang M., Chen J., Li X., Hu X., Jiang S., Shi S., Sun L. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: a multicenter clinical study. Arthritis Res. Ther. 2014;16:R79. doi: 10.1186/ar4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang P., Zhang G., Liu X., Liu H., Yang P., Ma L. Mesenchymal stem cells improve platelet counts in mice with immune thrombocytopenia. J. Cell. Biochem. 2019;120:11274–11283. doi: 10.1002/jcb.28405. [DOI] [PubMed] [Google Scholar]
- 16.Xiao J., Zhang C., Zhang Y., Zhang X., Zhao J., Liang J., Zhong X., Chen Y. Transplantation of adipose-derived mesenchymal stem cells into a murine model of passive chronic immune thrombocytopenia. Transfusion. 2012;52:2551–2558. doi: 10.1111/j.1537-2995.2012.03642.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang X., Yin X., Sun W., Bai J., Shen Y., Ao Q., Gu Y., Liu Y. Intravenous infusion umbilical cord-derived mesenchymal stem cell in primary immune thrombocytopenia: a two-year follow-up. Exp. Ther. Med. 2017;13:2255–2258. doi: 10.3892/etm.2017.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J.M., Zhu X.L., Xue J., Liu X., Long Zheng X., Chang Y.J., Liu K.Y., Huang X.J., Zhang X.H. Integrated mRNA and miRNA profiling revealed deregulation of cellular stress response in bone marrow mesenchymal stem cells derived from patients with immune thrombocytopenia. Funct. Integr. Genomics. 2018;18:287–299. doi: 10.1007/s10142-018-0591-2. [DOI] [PubMed] [Google Scholar]
- 19.Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen N., Liang D., Tang Y., de Vries N., Tak P.P. MicroRNAs—novel regulators of systemic lupus erythematosus pathogenesis. Nat. Rev. Rheumatol. 2012;8:701–709. doi: 10.1038/nrrheum.2012.142. [DOI] [PubMed] [Google Scholar]
- 21.Zhao S., Wang Y., Liang Y., Zhao M., Long H., Ding S., Yin H., Lu Q. MicroRNA-126 regulates DNA methylation in CD4+ T cells and contributes to systemic lupus erythematosus by targeting DNA methyltransferase 1. Arthritis Rheum. 2011;63:1376–1386. doi: 10.1002/art.30196. [DOI] [PubMed] [Google Scholar]
- 22.Jernås M., Nookaew I., Wadenvik H., Olsson B. MicroRNA regulate immunological pathways in T-cells in immune thrombocytopenia (ITP) Blood. 2013;121:2095–2098. doi: 10.1182/blood-2012-12-471250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edelstein L.C., Bray P.F. MicroRNAs in platelet production and activation. Blood. 2011;117:5289–5296. doi: 10.1182/blood-2011-01-292011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H., Zhao H., Wang D., Yang R. MicroRNA regulation in megakaryocytopoiesis. Br. J. Haematol. 2011;155:298–307. doi: 10.1111/j.1365-2141.2011.08859.x. [DOI] [PubMed] [Google Scholar]
- 25.Garzon R., Pichiorri F., Palumbo T., Iuliano R., Cimmino A., Aqeilan R., Volinia S., Bhatt D., Alder H., Marcucci G. MicroRNA fingerprints during human megakaryocytopoiesis. Proc. Natl. Acad. Sci. USA. 2006;103:5078–5083. doi: 10.1073/pnas.0600587103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark E.A., Kalomoiris S., Nolta J.A., Fierro F.A. Concise review: microRNA function in multipotent mesenchymal stromal cells. Stem Cells. 2014;32:1074–1082. doi: 10.1002/stem.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun H.H., Sun P.F., Liu W.Y. miR-98-5p regulates myocardial differentiation of mesenchymal stem cells by targeting TBX5. Eur. Rev. Med. Pharmacol. Sci. 2018;22:7841–7848. doi: 10.26355/eurrev_201811_16409. [DOI] [PubMed] [Google Scholar]
- 28.Liu W., Wang P., Xie Z., Wang S., Ma M., Li J., Li M., Cen S., Tang S., Zheng G. Abnormal inhibition of osteoclastogenesis by mesenchymal stem cells through the miR-4284/CXCL5 axis in ankylosing spondylitis. Cell Death Dis. 2019;10:188. doi: 10.1038/s41419-019-1448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim B.S., Jung J.Y., Jeon J.Y., Kim H.A., Suh C.H. Circulating hsa-miR-30e-5p, hsa-miR-92a-3p, and hsa-miR-223-3p may be novel biomarkers in systemic lupus erythematosus. HLA. 2016;88:187–193. doi: 10.1111/tan.12874. [DOI] [PubMed] [Google Scholar]
- 30.Horiguchi H., Kobune M., Kikuchi S., Yoshida M., Murata M., Murase K., Iyama S., Takada K., Sato T., Ono K. Extracellular vesicle miR-7977 is involved in hematopoietic dysfunction of mesenchymal stromal cells via poly(rC) binding protein 1 reduction in myeloid neoplasms. Haematologica. 2016;101:437–447. doi: 10.3324/haematol.2015.134932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang T., Li M., Li Q., Guo Z., Sun X., Zhang X., Liu Y., Yao W., Xiao P. MicroRNA-98-5p inhibits cell proliferation and induces cell apoptosis in hepatocellular carcinoma via targeting IGF2BP1. Oncol. Res. 2017;25:1117–1127. doi: 10.3727/096504016X14821952695683. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Youssef A., Aboalola D., Han V.K. The roles of insulin-like growth factors in mesenchymal stem cell niche. Stem Cells Int. 2017;2017:9453108. doi: 10.1155/2017/9453108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Youssef A., Han V.K. Low oxygen tension modulates the insulin-like growth factor-1 or -2 signaling via both insulin-like growth factor-1 receptor and insulin receptor to maintain stem cell identity in placental mesenchymal stem cells. Endocrinology. 2016;157:1163–1174. doi: 10.1210/en.2015-1297. [DOI] [PubMed] [Google Scholar]
- 34.Dai N., Christiansen J., Nielsen F.C., Avruch J. mTOR complex 2 phosphorylates IMP1 cotranslationally to promote IGF2 production and the proliferation of mouse embryonic fibroblasts. Genes Dev. 2013;27:301–312. doi: 10.1101/gad.209130.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia Y., Padre R.C., De Mendoza T.H., Bottero V., Tergaonkar V.B., Verma I.M. Phosphorylation of p53 by IκB kinase 2 promotes its degradation by β-TrCP. Proc. Natl. Acad. Sci. USA. 2009;106:2629–2634. doi: 10.1073/pnas.0812256106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verma S., Ali A., Arora S., Banerjea A.C. Inhibition of β-TrcP-dependent ubiquitination of p53 by HIV-1 Vpu promotes p53-mediated apoptosis in human T cells. Blood. 2011;117:6600–6607. doi: 10.1182/blood-2011-01-333427. [DOI] [PubMed] [Google Scholar]
- 37.Noubissi F.K., Elcheva I., Bhatia N., Shakoori A., Ougolkov A., Liu J., Minamoto T., Ross J., Fuchs S.Y., Spiegelman V.S. CRD-BP mediates stabilization of βTrCP1 and c-myc mRNA in response to β-catenin signalling. Nature. 2006;441:898–901. doi: 10.1038/nature04839. [DOI] [PubMed] [Google Scholar]
- 38.Elcheva I., Goswami S., Noubissi F.K., Spiegelman V.S. CRD-BP protects the coding region of βTrCP1 mRNA from miR-183-mediated degradation. Mol. Cell. 2009;35:240–246. doi: 10.1016/j.molcel.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Werner H., Sarfstein R., LeRoith D., Bruchim I. Insulin-like growth factor 1 signaling axis meets p53 genome protection pathways. Front. Oncol. 2016;6:159. doi: 10.3389/fonc.2016.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bruchim I., Sarfstein R., Werner H. The IGF hormonal network in endometrial cancer: functions, regulation, and targeting approaches. Front. Endocrinol. (Lausanne) 2014;5:76. doi: 10.3389/fendo.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng F.E., Feng R., Wang M., Zhang J.M., Jiang H., Jiang Q., Lu J., Liu H., Peng J., Hou M. Oral all-trans retinoic acid plus danazol versus danazol as second-line treatment in adults with primary immune thrombocytopenia: a multicentre, randomised, open-label, phase 2 trial. Lancet Haematol. 2017;4:e487–e496. doi: 10.1016/S2352-3026(17)30170-9. [DOI] [PubMed] [Google Scholar]
- 42.Feng Q., Xu M., Yu Y.Y., Hou Y., Mi X., Sun Y.X., Ma S., Zuo X.Y., Shao L.L., Hou M. High-dose dexamethasone or all-trans-retinoic acid restores the balance of macrophages towards M2 in immune thrombocytopenia. J. Thromb. Haemost. 2017;15:1845–1858. doi: 10.1111/jth.13767. [DOI] [PubMed] [Google Scholar]
- 43.Zhu X., Wang Y., Jiang Q., Jiang H., Lu J., Wang Y., Kong Y., Chang Y., Xu L., Peng J. All-trans retinoic acid protects mesenchymal stem cells from immune thrombocytopenia by regulating the complement-interleukin-1β loop. Haematologica. 2019;104:1661–1675. doi: 10.3324/haematol.2018.204446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khodadi E., Asnafi A.A., Shahrabi S., Shahjahani M., Saki N. Bone marrow niche in immune thrombocytopenia: a focus on megakaryopoiesis. Ann. Hematol. 2016;95:1765–1776. doi: 10.1007/s00277-016-2703-1. [DOI] [PubMed] [Google Scholar]
- 45.Geng L., Tang X., Zhou K., Wang D., Wang S., Yao G., Chen W., Gao X., Chen W., Shi S. MicroRNA-663 induces immune dysregulation by inhibiting TGF-β1 production in bone marrow-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Cell. Mol. Immunol. 2019;16:260–274. doi: 10.1038/cmi.2018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garabet L., Ghanima W., Rangberg A., Teruel-Montoya R., Martinez C., Lozano M.L., Nystrand C.F., Bussel J.B., Sandset P.M., Jonassen C.M. Circulating microRNAs in patients with immune thrombocytopenia before and after treatment with thrombopoietin-receptor agonists. Platelets. 2020;31:198–205. doi: 10.1080/09537104.2019.1585527. [DOI] [PubMed] [Google Scholar]
- 47.Jazdzewski K., Murray E.L., Franssila K., Jarzab B., Schoenberg D.R., de la Chapelle A. Common SNP in pre-miR-146a decreases mature miR expression and predisposes to papillary thyroid carcinoma. Proc. Natl. Acad. Sci. USA. 2008;105:7269–7274. doi: 10.1073/pnas.0802682105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim V.N., Han J., Siomi M.C. Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 2009;10:126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 49.Krol J., Loedige I., Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 50.Ando T., Yoshida T., Enomoto S., Asada K., Tatematsu M., Ichinose M., Sugiyama T., Ushijima T. DNA methylation of microRNA genes in gastric mucosae of gastric cancer patients: its possible involvement in the formation of epigenetic field defect. Int. J. Cancer. 2009;124:2367–2374. doi: 10.1002/ijc.24219. [DOI] [PubMed] [Google Scholar]
- 51.Davis-Dusenbery B.N., Hata A. Mechanisms of control of microRNA biogenesis. J. Biochem. 2010;148:381–392. doi: 10.1093/jb/mvq096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cai Y., Yu X., Hu S., Yu J. A brief review on the mechanisms of miRNA regulation. Genomics Proteomics Bioinformatics. 2009;7:147–154. doi: 10.1016/S1672-0229(08)60044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ha M., Kim V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 54.Huang X., Zhang H., Guo X., Zhu Z., Cai H., Kong X. Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) in cancer. J. Hematol. Oncol. 2018;11:88. doi: 10.1186/s13045-018-0628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahaira L.G., Katsara O., Pappou E., Iliopoulou E.G., Fortis S., Antsaklis A., Fotinopoulos P., Baxevanis C.N., Papamichail M., Perez S.A. IGF2BP1 expression in human mesenchymal stem cells significantly affects their proliferation and is under the epigenetic control of TET1/2 demethylases. Stem Cells Dev. 2014;23:2501–2512. doi: 10.1089/scd.2013.0604. [DOI] [PubMed] [Google Scholar]
- 56.Katsara O., Mahaira L.G., Iliopoulou E.G., Moustaki A., Antsaklis A., Loutradis D., Stefanidis K., Baxevanis C.N., Papamichail M., Perez S.A. Effects of donor age, gender, and in vitro cellular aging on the phenotypic, functional, and molecular characteristics of mouse bone marrow-derived mesenchymal stem cells. Stem Cells Dev. 2011;20:1549–1561. doi: 10.1089/scd.2010.0280. [DOI] [PubMed] [Google Scholar]
- 57.Manieri N.A., Drylewicz M.R., Miyoshi H., Stappenbeck T.S. Igf2bp1 Is required for full induction of Ptgs2 mRNA in colonic mesenchymal stem cells in mice. Gastroenterology. 2012;143:110–121.e10. doi: 10.1053/j.gastro.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zandstra P.W., Nagy A. Stem cell bioengineering. Annu. Rev. Biomed. Eng. 2001;3:275–305. doi: 10.1146/annurev.bioeng.3.1.275. [DOI] [PubMed] [Google Scholar]
- 59.Du L., Lin L., Li Q., Liu K., Huang Y., Wang X., Cao K., Chen X., Cao W., Li F. IGF-2 preprograms maturing macrophages to acquire oxidative phosphorylation-dependent anti-inflammatory properties. Cell Metab. 2019;29:1363–1375.e8. doi: 10.1016/j.cmet.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 60.Zuckerman V., Wolyniec K., Sionov R.V., Haupt S., Haupt Y. Tumour suppression by p53: the importance of apoptosis and cellular senescence. J. Pathol. 2009;219:3–15. doi: 10.1002/path.2584. [DOI] [PubMed] [Google Scholar]
- 61.Chipuk J.E., Green D.R. Dissecting p53-dependent apoptosis. Cell Death Differ. 2006;13:994–1002. doi: 10.1038/sj.cdd.4401908. [DOI] [PubMed] [Google Scholar]
- 62.Cicconi L., Lo-Coco F. Current management of newly diagnosed acute promyelocytic leukemia. Ann. Oncol. 2016;27:1474–1481. doi: 10.1093/annonc/mdw171. [DOI] [PubMed] [Google Scholar]
- 63.Lu L., Lan Q., Li Z., Zhou X., Gu J., Li Q., Wang J., Chen M., Liu Y., Shen Y. Critical role of all-trans retinoic acid in stabilizing human natural regulatory T cells under inflammatory conditions. Proc. Natl. Acad. Sci. USA. 2014;111:E3432–E3440. doi: 10.1073/pnas.1408780111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Povoleri G.A.M., Nova-Lamperti E., Scottà C., Fanelli G., Chen Y.C., Becker P.D., Boardman D., Costantini B., Romano M., Pavlidis P. Human retinoic acid-regulated CD161+ regulatory T cells support wound repair in intestinal mucosa. Nat. Immunol. 2018;19:1403–1414. doi: 10.1038/s41590-018-0230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elias K.M., Laurence A., Davidson T.S., Stephens G., Kanno Y., Shevach E.M., O’Shea J.J. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008;111:1013–1020. doi: 10.1182/blood-2007-06-096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schiavinato J.L.D.S., Haddad R., Saldanha-Araujo F., Baiochi J., Araujo A.G., Santos Scheucher P., Covas D.T., Zago M.A., Panepucci R.A. TGF-beta/atRA-induced Tregs express a selected set of microRNAs involved in the repression of transcripts related to Th17 differentiation. Sci. Rep. 2017;7:3627. doi: 10.1038/s41598-017-03456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cooper N., Ghanima W. Immune thrombocytopenia. N. Engl. J. Med. 2019;381:945–955. doi: 10.1056/NEJMcp1810479. [DOI] [PubMed] [Google Scholar]
- 68.Chow L., Aslam R., Speck E.R., Kim M., Cridland N., Webster M.L., Chen P., Sahib K., Ni H., Lazarus A.H. A murine model of severe immune thrombocytopenia is induced by antibody- and CD8+ T cell-mediated responses that are differentially sensitive to therapy. Blood. 2010;115:1247–1253. doi: 10.1182/blood-2009-09-244772. [DOI] [PubMed] [Google Scholar]
- 69.Ma L., Simpson E., Li J., Xuan M., Xu M., Baker L., Shi Y., Yougbaré I., Wang X., Zhu G. CD8+ T cells are predominantly protective and required for effective steroid therapy in murine models of immune thrombocytopenia. Blood. 2015;126:247–256. doi: 10.1182/blood-2015-03-635417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.