Abstract
Background:
Hematoma is the chief culprit in brain injury following intracranial cerebral hemorrhage (ICH). Noninvasive hematoma clearance could be an option to prevent and alleviate early brain injury after ICH. Peroxisome proliferator-activated receptor γ (PPAR-γ) and nuclear factor-erythroid 2 related factor-2 (Nrf2) facilitate removal of hematoma in ICH. Monascin acts as the natural Nrf2 activator with PPAR-γ agonist, and the long-term effects of monascin following ICH have not been elucidated.
Methods:
ICH in rats was induced by stereotactic, intrastriatal injection of type IV collagenase. Monascin was administered twice daily by gastric perfusion for 14 days after ICH induction. Long-term neurological scores (T maze, Garcia scales, rotor rod test, and Morris water maze), hematoma volume, as well as iron overload around hematoma and brain atrophy were evaluated at 7, 14, and 28 days after ICH.
Results:
The results showed that monascin improved long-term neurological deficits, spatial memory performance, learning ability, and brain shrinkage after ICH. Monascin also reduced hematoma volume at 7 days and iron content at 7 and 14 days after ICH.
Conclusion:
PPAR γ and Nrf2 play a crucial role in hematoma clearance after ICH in rat. As a dual agonist of PPAR γ and Nrf2, monascin improved long-term outcomes by facilitating hematoma clearance, and by attenuating iron overload and brain atrophy after experimental ICH.
Keywords: dual receptor agonist, hematoma clearance, intracerebral hemorrhage, monascin, peroxisome proliferator-activated receptor γ/nuclear factor-erythroid 2 related factor-2
Introduction
Brain injury after intracerebral hemorrhage (ICH) initially occurs within the first few hours as a result of mass effects due to hematoma formation. Continued insults after primary hemorrhage are believed to be caused by intraparenchymal blood lysis.1,2 Since hematoma is the chief culprit of brain injury following ICH, appropriate removal of hematomas is crucial to prevent and alleviate early brain insults after ICH. However, surgical removal of hematomas has not achieved the expected results.3 Although preliminary studies suggest that minimally invasive hematoma removal may reduce secondary neurotoxicity,4,5 the effects of hematoma shrinkage on clinical outcome remain unclear.6 Therefore, targeting hematoma clearance by promoting an endogenous garbage scavenging system is promising for ICH.
Peroxisome proliferator-activated receptor (PPAR) -γ, which is a part of the nuclear hormone receptor superfamily, can regulate the expression of CD36 and CD163, which participate in phagocytosis.7–9 In addition, PPAR-γ agonist enhanced CD36 and CD163 expression, accelerated hematoma resolution, then improved neurological deficits.9–11 Nuclear factor-erythroid 2-related factor 2 (Nrf2) and PPAR-γ play similar roles in phagocytosis and hematoma resolution following ICH.8,12–14 Monascin is a natural dual activator of both PPAR-γ and Nrf2, and is produced using a unique and natural fermentation process in China. Monascin constitutes one of the azaphilonoid pigments in the extracts of Monascus pilosus-fermented rice (red mold rice).15 Our previous study demonstrated that monascin was neuroprotective by facilitating hematoma clearance through the haptoglobin-hemoglobin-CD163 pathway in ICH.9,16 This study aims to further evaluate the long-term effects of monascin in an experimental ICH.
Materials and methods
Animals were randomized to control and experimental groups. Neurobehavioral testing as well as quantitative data collection were conducted in a blinded fashion (animals and sample tubes were marked by numbers, without any identifiers of group allocation).
Animals
All procedures in this study were approved by Animal Utilization and Management Committee at Shanxi Medical University, and comply with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. Male SD rats (12 weeks old, weight 275 g ± 25 g, Animal Experimental Center of Shanxi Medical University) were given free access to food and water.
ICH model
The procedure for inducing ICH by stereotactic injection of bacterial collagenase (0.5 U typeIV dissolved in 2 μl saline, Sigma-Aldrich, St. Louis, MO, USA) into the basal ganglia was performed as described in our previous publication.2 Sham-operated rats were subjected to needle insertion only. Vital signs were monitored throughout surgery and recovery period. Neurofunctional testing was performed at preselected time points after ICH induction (7, 14, and 28 days). After neurofunctional testing, animals were euthanized, and brain tissues were collected for measurements of hematoma volume, hemoglobin levels, iron staining, iron contents, weights of right and left hemispheres, and cerebral atrophy.
Experimental design
A total of 72 male SD rats were used in the experiment. Animals were added if they died before the research endpoint. There were no significant differences in the mortality rates between the different experimental groups. The 72 rats were divided equally into three groups: sham, ICH+ vehicle (sterile normal saline), and ICH+ monascin (10 mg/kg). The rats in each group were further divided equally into three subgroups according to the different time point of evaluation and then euthanasia (7, 14, or 28 days). Monascin was administered twice daily by gastric perfusion at 6 h after ICH for 14 days. Long-term neurofunctional testing (modified Garcia scale, T-maze, and rotor rod tests ) were evaluated at 14 days and 28 days after ICH; Morris water maze tests were conducted between 23 and 28 days after ICH induction. Hemoglobin levels, iron contents, and weights of right and left cerebral hemispheres were measured at 7, 14, and 28 days after ICH.
Neurobehavioral tests
Animals were subjected to neurofunctional assessments using the modified Garcia scale, T-maze tests, and rotor rod tests. Neurobehavior tests were assessed by an independent researcher blinded to treatment allocation. The modified Garcia scale and Morris water maze were described previously.2,17 Each test was repeated three times, and an average score was taken.
Modified Garcia test
The modified Garcia test involves a 21-point sensorimotor assessment that includes seven individual tests.2 Each test has a score ranging from 0 to 3, with a maximum score of 21.
T-maze test
The rats were placed in the startbox of the T-maze shown in Figure 1 and allowed to consume 2.5 ml of 25% w/v sucrose solution from a small dish at the end of the choice alley. At the beginning of each trial, the rat was placed head first through the entry door of the startbox, so that it could freely enter either choice alley. A choice accuracy and time of duration was recorded by the rat’s entry into a choice compartment. Regardless of whether the choice was correct or incorrect, the rat was then removed and placed in its home cage for the appropriate delay interval.18 Each rat repeated the trial six times.
Figure 1.

The T-maze consisted of a gray wooden startbox flanked on either side by a wooden choice chamber. The left compartment was painted white and the right side was black. One-way doors made of clear plastic permitted entry into the choice chambers and prevented retracing. The lids of all chambers were made of clear plastic and the floors of hardware cloth.
Rotor-rod test
The rats were placed on a rotating rod with either constant rotation or a steady acceleration; the latency to fall was recorded. In the fixed rotation protocol, the rats were placed on a rod that accelerates and then rotates constantly at 10 rpm. In the accelerating protocol, the rats were placed on a rod that accelerates quickly from 0 to 5 rpm and then gradually from 5 to 20 rpm. A trial is completed when the animal falls or the time period ends. The rats were tested for the two trials at 14 and 28 days post-surgery.
Morris water maze test
The Morris water maze test was performed to evaluate the effects of the treatment on spatial memory and learning ability following ICH. This test was conducted as previously described.17 After adaptation for two consecutive days, rats were tested from 25 to 28 days after ICH.
Hemoglobin assay
The procedure was performed as previously described.17 Drabkin’s reagent was added to 0.1 ml supernatant aliquots and allowed to rest for 15 min at room temperature, protected from light. This reaction converts hemoglobin to cyanomethemoglobin, which has an absorbance peak at 540 nm, and whose concentration can then be assessed by measuring the optical density (OD) of the solution at ≈550 nm wavelength. OD was measured and recorded at 540 nm with a spectrophotometer. These procedures yielded a linear relationship between measured hemoglobin concentrations in perfused brain tissues and the volume of added blood on a standard absorbance curve. To better illustrate the levels of hemoglobin compared with the sham (hematoma is zero), the data was showed as a ratio (the preset value of the sham was 1).17
Iron staining and iron contents
Iron staining and iron contents were evaluated as previously described.2,17 Brain sections were rinsed in distilled water and immersed in Perls’ solution for 20 min. All slides were rinsed with distilled water, then counterstained with nuclear fast red dye for 5 min. Iron contents were assayed as previously performed according to instructions of Iron Assay protocol.17 Iron values were calculated using a Bio-Rad iMarkTM Microplate Absorbance Reader. Total iron contents of the samples were acquired from the standard curve.
Brain weight analysis
Brain sections were stained by a hematoxylin-eosin staining kit. The rats were euthanized, then the brain was removed. After removal of the cerebellum and brainstem, the brain was split in half along the midline and the weight of the right and left hemispheres was recorded. The difference in weight between the left and right hemisphere was calculated.
Statistical analysis
Quantitative data is presented as mean ±SD. One-way ANOVA for multiple comparisons with Tukey’s post hoc test was used to determine the differences of hemoglobin levels, iron contents, and brain weight between right/left hemisphere among all groups at each time point. Two-way ANOVA with Bonfferoni post-test was used to assay the differences of neurological deficits. p < 0.05 was considered statistically significant.
Results
Mortality
All sham-operated rats survived. The total operative mortality of rats subjected to ICH was 6.94% (n = 5). The mortality was not significantly different among the experimental groups (Supplementary tables).
PPAR-γ/Nrf2 agonist monascin improved long-term neurological deficits following ICH
ICH+ vehicle animals scored significantly lower than sham animals in the neurofunctional tests performed at each time point, except for the T-maze test duration (p < 0.05, Supplemental Figure A–G). Compared with the vehicle, PPAR-γ/Nrf2 agonist monascin led to significant improvement in neurofunctional deficits evaluated with the modified Garcia scale, T-maze test, and rotor rod test at 14 and 28 days after ICH (p < 0.05, Supplemental Figure A–G ).
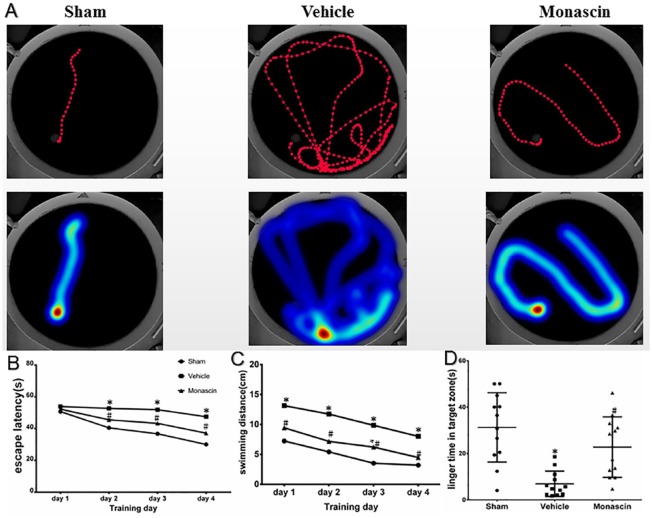
Results of the Morris water maze test showed that monascin improved spatial memory after ICH (p < 0.05, Figure 2a–d). Monascin-treated ICH rats showed shorter routes travelled compared with vehicle animals (Figure 2a). During the first day of testing, all rats showed a similar latency to reach the platform. In subsequent testing, monascin-treated ICH animals showed a shorter latency to escape onto the platform on the third and fourth day of the water maze experiments compared with vehicle animals (Figure 2b). Monascin-treated rats demonstrated shorter swimming distances before escaping onto the hidden platform on the second and fourth day of testing (Figure 2c). In the probe trial on the sixth day of testing, monascin-treated rats traveled significantly more within the target quadrant than vehicle animals (Figure 2d). Furthermore, no significantly different neurological test scores were found between the sham and monascin-treated ICH rats except modified Garcia scales at 28 days after surgery (Supplemental Figure A–G and Figure 2).
Figure 2.
Morris water maze test was applied in all groups from 25 to 28 days after ICH (n = 6/group). (a) Visualization of the swimming track and heat map. (b) Mean latency to escape onto the platform. (c) Mean swimming distances before escaping onto the platform. and (d) Mean platform searching times within the target quadrant. *p < 0.05 versus sham, #p < 0.05 versus ICH+vehicle, 2-way ANOVA, mean ± SD, analysis by repeated measures ANOVA.
ANOVA, analysis of variance; ICH, intracranial cerebral hemorrhage; SD, standard deviation.
PPAR-γ/Nrf2 agonist monascin decreased hematoma volume following ICH
Monascin-treated ICH rats exhibited decreased hemoglobin levels in the ipsilateral brain hemispheres compared with vehicle group at 7 days after surgery (p < 0.05, Figure 3). Compared with the sham group, the level of hemoglobin was significantly increased at 7 days and 14 days in the ICH+ vehicle group, whereas at 7 days in monascin-treated ICH rats after surgery (p < 0.05, Figure 3a,b), there was no significant difference between the sham and monascin treated ICH at 14 days and 28 days after surgery; no differences were found among the three study groups at 28 days after surgery (Figure 3).
Figure 3.
Hemorrhage slices (a) and hemoglobin levels (b) at 7, 14, and 28 days after ICH. To better illustrate the levels of hemoglobin compared with the sham (hematoma is zero), data are showed as a ratio (the preset value of the sham was 1). One-way ANOVA followed by Tukey tests were used. *p < 0.05 versus Sham, #p < 0.05 versus ICH+Vehicle.
ANOVA, analysis of variance; ICH, intracranial cerebral hemorrhage.
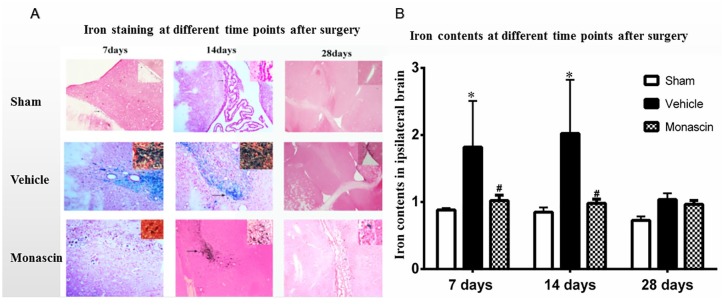
PPAR-γ/Nrf2 agonist monascin reduced iron deposits following ICH
Monascin-treated ICH rats exhibited lower iron contents in the ipsilateral brain hemispheres compared with vehicle group at 7 days and 14 days after surgery (p < 0.05, Figure 4). The vehicle group showed a significant increase in iron contents when compared with sham at 7 days and 14 days after surgery (p < 0.05, Figure 4). There was no significant difference between the sham and monascin-treated ICH, and no differences were found among all the groups at 28 days after surgery (p > 0.05, Figure 4). The results showed that iron accumulation had cleared by 4 weeks after ICH.
Figure 4.
(a) Iron staining and (b) iron contents assays at 7, 14, and 28 days after ICH induction. *p < 0.05 versus Sham, #p < 0.05 versus ICH+Vehicle, n = 6/group, One-way ANOVA, Tukey’s multiple comparison test, mean ± SD.
ANOVA, analysis of variance; ICH, intracranial cerebral hemorrhage; SD, standard deviation.
PPAR-γ/Nrf2 agonist monascin ameliorated hemisphere atrophy following ICH
Monascin-treated ICH rats showed less weight differences between the two brain hemispheres compared with the vehicle group at 28 days after surgery (p < 0.05, Figure 5). Compared with the sham group, the weight differences between the two brain hemispheres increased significantly in the ICH+ vehicle group at 28 days after surgery. There was no significant weight differences in two sides between the sham and monascin-treated ICH, no differences were found between the sham and ICH+ vehicle group at 7 days and 14 days after surgery (p > 0.05, Figure 5).
Figure 5.
(a) Hematoxylin-eosin staining and (b) brain weight differences between the left and right hemisphere at 7, 14, and 28 days after surgery. *p < 0.05 versus Sham, #p < 0.05 versus ICH+Vehicle, n = 6/group, One-way ANOVA, Tukey’s multiple comparison test, mean ± SD; values were calculated by subtracting weight of the right from the left hemisphere.
ANOVA, analysis of variance; ICH, intracranial cerebral hemorrhage; SD, standard deviation.
Discussion
In this study, we made the following observations: (1) PPAR-γ/Nrf2 agonist monascin significantly improved motor disability at 14 and 28 days after ICH, as assessed by modified Garcia scales and rotor-rod test. (2) Monascin also improved spatial memory and learning ability at 14 and 28 days following ICH, as evaluated by T-maze test and Morris water maze. (3) Monascin significantly attenuated hematoma volume at 7 days and iron contents at 7 and 14 days after ICH. (4) Monascin alleviated ipsilateral hemisphere atrophy at 28 days after ICH. These results suggest that PPAR-γ/Nrf2 is involved in hematoma scavenging in ICH. The PPAR-γ/Nrf2 agonist monascin prevented/improved long-term neurological deficits and complications by facilitating hematoma clearance and iron deposits after ICH.
It is well known that hematoma is a primary and vital culprit in ICH, so facilitating hematoma absorption or clearance with non-invasive methods is crucial to prevent early brain insults following ICH. As previously mentioned, Nrf2 and PPAR-γ play similar roles in promoting phagocytosis and hematoma resolution following ICH.8,12–14
Monascin, a yellow pigment isolated from Monascus-fermented product, is produced by a unique fermentation technology from red yeast rice in China.15 Monascin is a natural dual activator of PPAR-γ and Nrf2.19 Our previous study has also demonstrated that monascin exerts neuroprotective roles by facilitating hematoma clearance through the haptoglobin-hemoglobin-CD163 pathway in ICH.9,16 But no data were available on the long-term efficacy of monascin in ICH. We studied the long-term roles of monascin and described its beneficial neurobehavioral effects in ICH. It is well known that the hematoma degradation products hemoglobin, heme, and iron result in secondary insults after ICH.1,2,9,17 In the present study, we found that monascin prevented or ameliorated encephalatrophy in the late stage by reducing hemoglobin levels and iron deposits induced by ICH, then improved motor disability, spatial memory, and learning ability following ICH.
In conclusion, our study highlights the long-term effects of monascin, a dual agonist of PPAR-γ and Nrf2, in the setting of experimental rat ICH. Monascin facilitates hematoma and iron scavenging and prevents or alleviates encephalatrophy induced by ICH, which results in beneficial effects of long-term outcomes after ICH. The efficacy of monascin in clinical practice should be addressed in future studies.
Supplemental Material
Supplemental material, Supplementary_materials for Long-term outcomes of monascin – a novel dual peroxisome proliferator-activated receptor γ/nuclear factor-erythroid 2 related factor-2 agonist in experimental intracerebral hemorrhage by Pengcheng Fu, Jiachen Liu, Qinqin Bai, Xingang Sun, Zhenjia Yao, Lirong Liu, Cuimei Wu and Gaiqing Wang in Therapeutic Advances in Neurological Disorders
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the grant from National Natural Science Foundation of China (81771294).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Gaiqing Wang  https://orcid.org/0000-0002-8977-1383
https://orcid.org/0000-0002-8977-1383
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Pengcheng Fu, Department of Neurology, Longhua District Central Hospital, Shenzhen, Guangdong, 187 Guanlan St., 518110, China.
Jiachen Liu, Clinical Medicine, Xiangya Medical College of Central South University, Changsha, Hunan, China.
Qinqin Bai, Shanxi Medical University, Taiyuan, Shanxi, China; Pengcheng Fu and Jiachen Liu contributed equally to the manuscript.
Xingang Sun, Shanxi Medical University, Taiyuan, Shanxi, China; Pengcheng Fu and Jiachen Liu contributed equally to the manuscript.
Zhenjia Yao, Shanxi Medical University, Taiyuan, Shanxi, China; Pengcheng Fu and Jiachen Liu contributed equally to the manuscript.
Lirong Liu, Shanxi Medical University, Taiyuan, Shanxi, China; Pengcheng Fu and Jiachen Liu contributed equally to the manuscript.
Cuimei Wu, Shanxi Medical University, Taiyuan, Shanxi, China; Pengcheng Fu and Jiachen Liu contributed equally to the manuscript.
Gaiqing Wang, Department of Neurology, SanYa Central Hospital (The Third People’s Hospital of HaiNan Province), 146 Jiefang forth Rd, SanYa, HaiNan, 372000, China; Shanxi Medical University, Taiyuan, Shanxi, 030001, China.
References
- 1. Babu R, Bagley JH, Di C, et al. Thrombin and hemin as central factors in the mechanisms of intracerebral hemorrhage-induced secondary brain injury and as potential targets for intervention. Neurosurg Focus 2012; 32: E8. [DOI] [PubMed] [Google Scholar]
- 2. Wang G, Hu W, Tang Q, et al. Effect comparison of both iron chelators on outcomes, iron deposit, and iron transporters after intracerebral hemorrhage in rats. Mol Neurobiol 2016; 53: 3576–3585. [DOI] [PubMed] [Google Scholar]
- 3. Mendelow AD, Gregson BA, Rowan EN, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet 2013; 382: 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mould WA, Carhuapoma JR, Muschelli J, et al. Minimally invasive surgery plus recombinant tissue-type plasminogen activator for intracerebral hemorrhage evacuation decreases perihematomal edema. Stroke 2013; 44: 627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vespa P, Hanley D, Betz J, et al. ICES (intraoperative stereotactic computed tomography-guided endoscopic surgery) for brain hemorrhage: a multicenter randomized controlled trial. Stroke 2016; 47: 2749–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hanley DF, Thompson RE, Rosenblum M, et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet 2019; 393: 1021–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tontonoz P, Nagy L, Alvarez JG, et al. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 1998; 93: 241–252. [DOI] [PubMed] [Google Scholar]
- 8. Wang G, Wang L, Sun XG, et al. Haematoma scavenging in intracerebral haemorrhage: from mechanisms to the clinic. J Cell Mol Med 2018; 22: 768–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang G, Li T, Duan SN, et al. PPAR-gamma promotes hematoma clearance through haptoglobin-hemoglobin-CD163 in a rat model of intracerebral hemorrhage. Behav Neurol 2018; 2018: 7646104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao X, Grotta J, Gonzales N, et al. Hematoma resolution as a therapeutic target: the role of microglia/macrophages. Stroke 2009; 40: S92–S94. [DOI] [PubMed] [Google Scholar]
- 11. Zhao X, Sun G, Zhang J, et al. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann Neurol 2007; 61: 352–362. [DOI] [PubMed] [Google Scholar]
- 12. Zhao X, Sun G, Ting SM, et al. Cleaning up after ICH: the role of Nrf2 in modulating microglia function and hematoma clearance. J Neurochem 2015; 133: 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sugiyama T, Imai T, Nakamura S, et al. A novel Nrf2 activator, RS9, attenuates secondary brain injury after intracerebral hemorrhage in sub-acute phase. Brain Res 2018; 1701: 137–145. [DOI] [PubMed] [Google Scholar]
- 14. Durocher M, Ander BP, Jickling G, et al. Inflammatory, regulatory, and autophagy co-expression modules and hub genes underlie the peripheral immune response to human intracerebral hemorrhage. J Neuroinflammation 2019; 16: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Akihisa T, Tokuda H, Ukiya M, et al. Anti-tumor-initiating effects of monascin, an azaphilonoid pigment from the extract of Monascus pilosus fermented rice (red-mold rice). Chem Biodivers 2005; 2: 1305–1309. [DOI] [PubMed] [Google Scholar]
- 16. Wang J, Wang G, Yi J, et al. The effect of monascin on hematoma clearance and edema after intracerebral hemorrhage in rats. Brain Res Bull 2017; 134: 24–29. [DOI] [PubMed] [Google Scholar]
- 17. Wang G, Guo Z, Tong L, et al. TLR7 (Toll-like receptor 7) facilitates heme scavenging through the BTK (Bruton tyrosine kinase)-CRT (Calreticulin)-LRP1 (Low-density lipoprotein receptor-related protein-1)-Hx (Hemopexin) pathway in murine intracerebral hemorrhage. Stroke 2018; 49: 3020–3029. [DOI] [PubMed] [Google Scholar]
- 18. Lett BT. Long delay learning in the T-maze. Learn Motiv 1975; 6: 80–90. [Google Scholar]
- 19. Hsu WH, Pan TM. A novel PPARgamma agonist monascin’s potential application in diabetes prevention. Food Funct 2014; 5: 1334–1340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_materials for Long-term outcomes of monascin – a novel dual peroxisome proliferator-activated receptor γ/nuclear factor-erythroid 2 related factor-2 agonist in experimental intracerebral hemorrhage by Pengcheng Fu, Jiachen Liu, Qinqin Bai, Xingang Sun, Zhenjia Yao, Lirong Liu, Cuimei Wu and Gaiqing Wang in Therapeutic Advances in Neurological Disorders