Abstract
Background:
The critical shoulder angle (CSA) is the angle between the superior and inferior bony margins of the glenoid and the most lateral border of the acromion. Although studies have reported that the CSA is associated with rotator cuff tears (RCTs), few studies have examined the accuracy of the CSA for predicting RCTs in patients with shoulder pain.
Purpose:
To investigate the accuracy of the CSA for predicting RCTs among patients with nontraumatic shoulder pain.
Study Design:
Cross-sectional study; Level of evidence, 3.
Methods:
Data were retrospectively collected from 301 patients who had RCTs and underwent arthroscopic rotator cuff repair between January 2014 and December 2018 (RCT group). During that same period, we also included 300 patients with shoulder pain but without RCTs, confirmed through ultrasound (non-RCT group). Baseline demographic data, the CSA, and the acromion index (AI) were compared using an independent t test. Categorical variables were analyzed using the chi-square test. Receiver operating characteristic (ROC) curve analysis was performed to investigate the accuracy of the CSA and AI for predicting RCTs, and the optimal cutoff point was determined using the Youden index. Multiple stepwise and binary logistic regressions were used to determine the predictors of RCTs.
Results:
A total of 301 patients (123 males, 178 females) and 300 patients (116 males, 184 females) were included in the RCT and non-RCT groups, respectively. The RCT group had a higher CSA (P < .001) than the non-RCT group. The area under the ROC curve (AUC) was 70.5% (P < .001) for the CSA, but there was no significance for the AI, with an AUC of 47.7% for predicting RCTs in patients. Stepwise logistic regression revealed the CSA as an independent predictor of RCTs, with an adjusted odds ratio of 1.295 (95% CI, 1.019-1.571; P = .006). For patients with a CSA greater than 37.52°, binary logistic regression revealed an adjusted odds ratio of 3.92 (95% CI, 2.79-5.51; P < .001) for the presence of an RCT.
Conclusion:
The CSA was an objective assessment tool to identify patients with shoulder pain who may have RCTs. Our study indicated that the CSA predicted RCTs more accurately than did the AI for patients with shoulder pain.
Keywords: shoulder, rotator cuff tear, critical shoulder angle, acromial index
A rotator cuff tear (RCT) is a disorder characterized by shoulder pain and dysfunction. RCTs are common, found in approximately 15%-20%, 26%-30%, and 36%-50% of people aged 60, 70, and 80 years, respectively.10,24 Disorders of the rotator cuff account for 30% to 70% of cases of painful shoulders, and the prevalence of RCTs has been reported to be 22.1% in the general population.10 Patients with RCTs can be asymptomatic, and this may have led to the varying prevalence among different studies. RCTs may be more prevalent than reported because of asymptomatic cases. Surgery is required for severe RCTs to restore shoulder function.
RCTs have multiple causes, which can be classified as intrinsic (degeneration, microtrauma, and hypoperfusion) or extrinsic factors (chronic impingement syndrome and overuse).23 Recently, anatomic factors, such as the critical shoulder angle (CSA) and acromion index (AI), have become key associated aspects of RCTs and the prognosis of repair surgery.8 The CSA is the angle formed from the line of the superior to inferior bony margin of the glenoid and inferior bony margin of the glenoid to the lateral margin of the acromion. The AI is defined as the distance from the glenoid plane to the acromial lateral border, divided by the distance from the glenoid plane to the lateral aspect of the humeral head. The CSA can indicate glenoid inclination and lateral extension of the acromion. The AI reveals lateral extension of the acromion.7 Previous studies6,14,21 have reported that a higher CSA and AI are associated with full-thickness RCTs. Another study14 indicated that patients with degenerative RCTs had a higher CSA than did those without RCTs.
Although the CSA and AI have been mentioned as radiographic assessment measures and are associated with RCTs, there are no relevant studies that have investigated their accuracy for predicting RCTs.6,14,21 In addition, for clinical practice, early detection of RCTs is important for effective treatment. Therefore, we investigated the sensitivity and specificity of CSA and AI measurements for detecting patients with RCTs among a population with nontraumatic shoulder pain.
Methods
Study Design and Participants
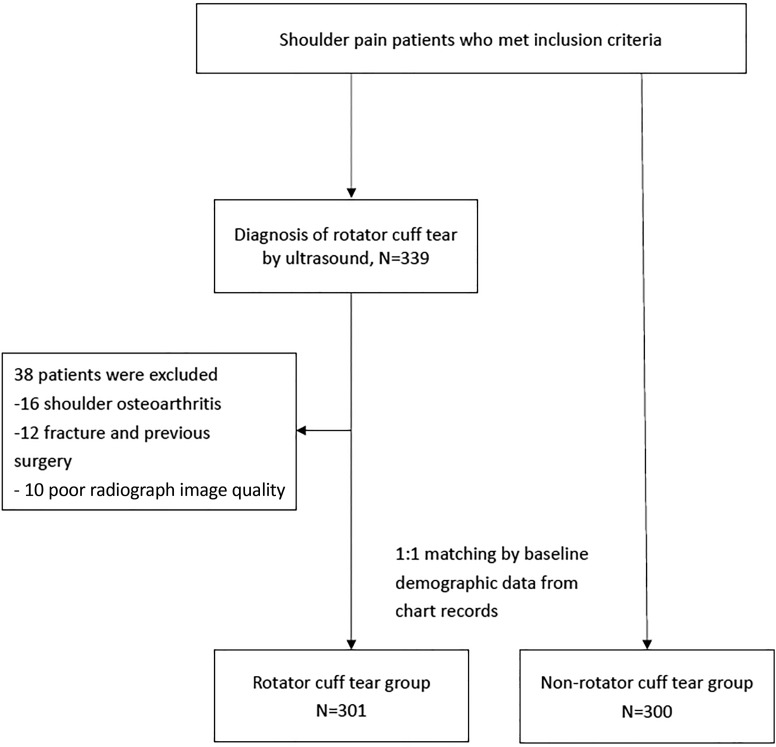
This study was a retrospective, case-control, cross-sectional investigation conducted at a medical university hospital from January 2014 to December 2018. All participants were recruited from the orthopaedic and rehabilitation outpatient departments, and this study was approved by the institutional review board of the medical university. Inclusion criteria were patients (1) aged 20 to 80 years, (2) experiencing shoulder pain with motion and who attended outpatient department visits in the past 3 months, and (3) undergoing a shoulder radiographic evaluation and shoulder ultrasound. Exclusion criteria were (1) previous surgery around the shoulder; (2) history of traumatic injuries in the past 6 months; (3) glenohumeral osteoarthritis or acromioclavicular arthritis, which could influence CSA and AI measurements; and (4) poor-quality shoulder radiographic images for assessment. After the radiographic evaluation, patients also underwent ultrasound testing. Based on shoulder ultrasound findings, participants were subdivided into the RCT and non-RCT groups. When shoulder ultrasound found RCTs in patients, they were classified into the RCT group. However, when shoulder ultrasound did not find RCTs but presented other types of rotator cuff disease such as bursitis or tendinitis, patients were classified into the non-RCT group. Baseline demographic data (namely age, sex, dominant/nondominant side, and body mass index [BMI]), diabetes mellitus, hypertension, hyperlipidemia, visual analog scale (VAS) score, steroid use (using oral steroids for more than 3 days according to medication history), and gout (based on medical chart records or medication history) were recorded by a research assistant who was blind to radiographic and ultrasound findings. With consideration of the potential selection bias caused by a retrospective case-control study and comparison with the study group, we matched patients without RCTs with similar baseline variables in a 1:1 ratio to patients with RCTs by propensity score matching using SPSS software (Version 19.0; IBM). The study flowchart is presented in Figure 1.
Figure 1.
Study flowchart.
Radiographic Evaluation of CSA and AI
After collecting demographic data, conventional anterior-posterior shoulder radiography was conducted on the same day as the outpatient department visit. Images were taken in an upright standing position, and with a descending beam tilted to 20° to ensure accuracy of the CSA assessment, the shoulder anterior-posterior image was obtained following a standardized protocol. We adopted the CSA measurement protocol used by Blonna et al.1 When radiographic images were not affected by the rotation and overlapping of the anterior and posterior edges of the glenoid cavity, we regarded them as having sufficient image quality for the CSA assessment. The CSA was measured as the angle formed by a line connecting the superior and inferior bony margins of the glenoid and a line from the inferior bony margin of the glenoid to the most lateral border of the acromion (Figure 2). To prevent the effect of differences in scapular rotation and beam projection angle, we adopted standardized measurement protocols, performing imaging of the shoulder in a true anterior-posterior view with a digitally embedded tool . There were 2 observers who independently measured the CSA and AI for all participants. To achieve consistent measurements, these 2 observers were instructed in the standardized evaluation technique and protocol by one of the authors. Each image was measured 3 times, and the mean values were obtained for subsequent analysis.
Figure 2.
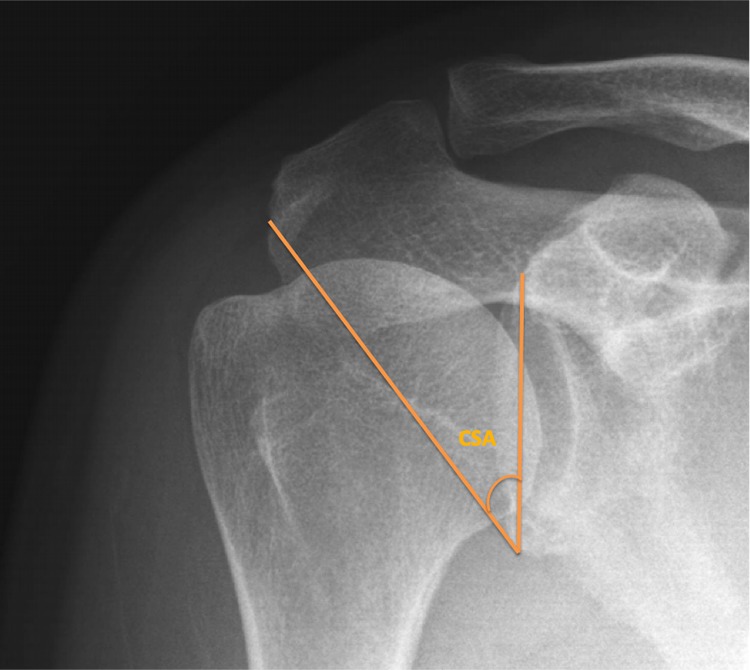
The critical shoulder angle (CSA) is formed from a line connecting the inferior and superior borders of the glenoid fossa and another line connecting the inferior border of the glenoid with the inferior lateral border of the acromion.
The AI was evaluated as the ratio of the distance between the glenoid plane and the lateral border of the acromion (GA) to the distance between the glenoid plane and the lateral aspect of the humeral head (GH), as illustrated in Figure 3.
Figure 3.
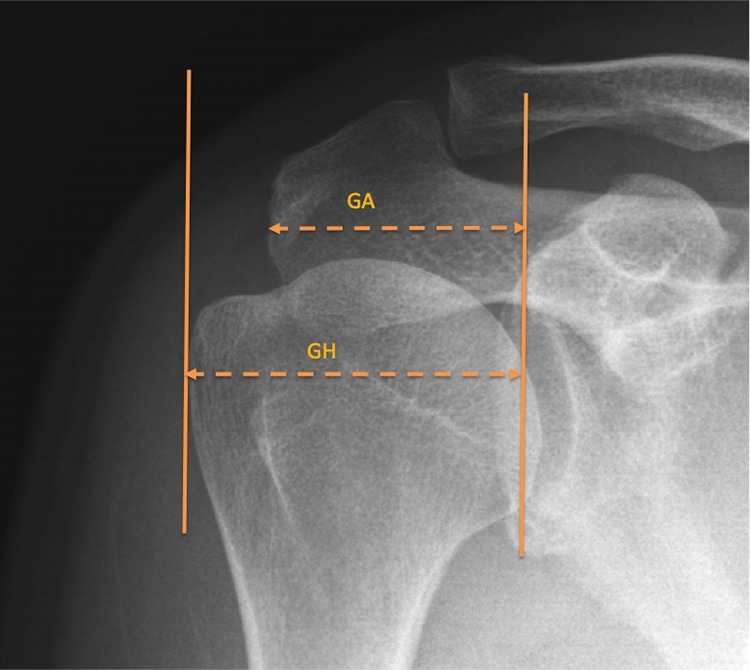
The acromion index (AI) is the ratio of the distance from the glenoid plane to the lateral border of the acromion (GA) to the distance from the glenoid plane to the most lateral aspect of the humeral head (GH). AI = GA/GH.
Ultrasound Evaluation of RCTs
The confirmation of an RCT was determined using ultrasound of the supraspinatus, infraspinatus, and subscapularis tendons after radiographic evaluation. An experienced physician performed the ultrasound evaluation for RCTs as routine practice and was unaware of the radiographic CSA and AI measurements because of the retrospective nature of the study. Based on a previous study,17 the diagnostic accuracy of ultrasound for full-thickness RCTs was more than 0.90 for sensitivity and specificity. For partial-thickness RCTs, the specificity was higher than 0.90.
Statistical Analysis
From the ultrasound findings of RCTs, we subdivided all participants into the RCT and non-RCT groups. The variables of age, sex, BMI, affected side, diabetes mellitus, hyperlipidemia, gout, steroid use, VAS score, CSA, GA, GH, and AI were presented as means with standard deviations or numbers.3,4 Continuous variables were compared using the independent Student t test between the RCT and non-RCT groups. The chi-square test was used for categorical variable comparisons between the 2 groups. To estimate the accuracy of the CSA and AI for predicting RCTs, we performed receiver operating characteristic (ROC) curve analyses of the CSA and AI. Cutoff points for the optimal sensitivity and specificity of the CSA and AI were determined using the Youden index. We used binary logistic regression for predicting the likelihood of RCTs when the CSA or AI values surpassed the cutoff points. Stepwise logistic regression was used for identifying independent predictors of RCTs. All statistical analyses were performed using SPSS (Version 19.0), and P < .05 was considered statistically significant.
Results
Initially, 339 participants met the inclusion criteria of this study. Of these, 16 participants were excluded because of osteoarthritis, 12 because of fractures, and 10 because of poor image quality of the CSA. Ultimately, 301 participants (98 partial-thickness tears and 203 full-thickness tears) were included in the RCT group (123 males, 178 females), and 300 participants whose data were collected from imaging and chart records were included in the non-RCT group (116 males, 184 females) by propensity score matching (Figure 1). Age, sex, BMI, diabetes mellitus, hyperlipidemia, gout, steroid use, and VAS score did not significantly differ between these 2 groups (Table 1).
TABLE 1.
Demographic Characteristicsa
| RCT (n = 301) | Non-RCT (n = 300) | P | |
|---|---|---|---|
| Age, y | 65.7 ± 9.6 | 65.2 ± 10.1 | .619 |
| Female sex, n | 178 | 184 | .233 |
| Affected dominant side, n | 210 | 212 | .816 |
| BMI, kg/m2 | 24.9 ± 3.5 | 25.7 ± 4.0 | .070 |
| Diabetes mellitus, n | 86 | 72 | .700 |
| Hyperlipidemia, n | 36 | 36 | .722 |
| Gout, n | 14 | 14 | >.999 |
| Steroid use, n | 25 | 23 | >.999 |
| VAS score | 3.6 ± 1.5 | 3.5 ± 1.4 | .408 |
aData are shown as mean ± SD unless otherwise indicated. The P value was calculated using the Student t test for continuous variables and the chi-square test for categorical variables. BMI, body mass index; RCT, rotator cuff tear; VAS, visual analog scale.
As indicated in Table 2, the RCT group had a higher CSA (P < .001), GA (P < .001), and GH (P < .001) than did the non-RCT group.
TABLE 2.
Radiographic Findingsa
| RCT (n = 301) | Non-RCT (n = 300) | P | |
|---|---|---|---|
| CSA | 38.90 ± 3.88 | 35.99 ± 4.03 | <.001b |
| GA | 3.97 ± 0.44 | 3.78 ± 0.38 | <.001 |
| GH | 5.19 ± 0.45 | 4.93 ± 0.54 | <.001 |
| AI | 0.76 ± 0.08 | 0.77 ± 0.08 | .497 |
aData are shown as mean ± SD. AI, acromion index; CSA, critical shoulder angle; GA, distance from the glenoid plane to the lateral border of the acromion; GH, distance from the glenoid plane to the most lateral aspect of the humeral head; RCT, rotator cuff tear.
bP < .05 (independent t test).
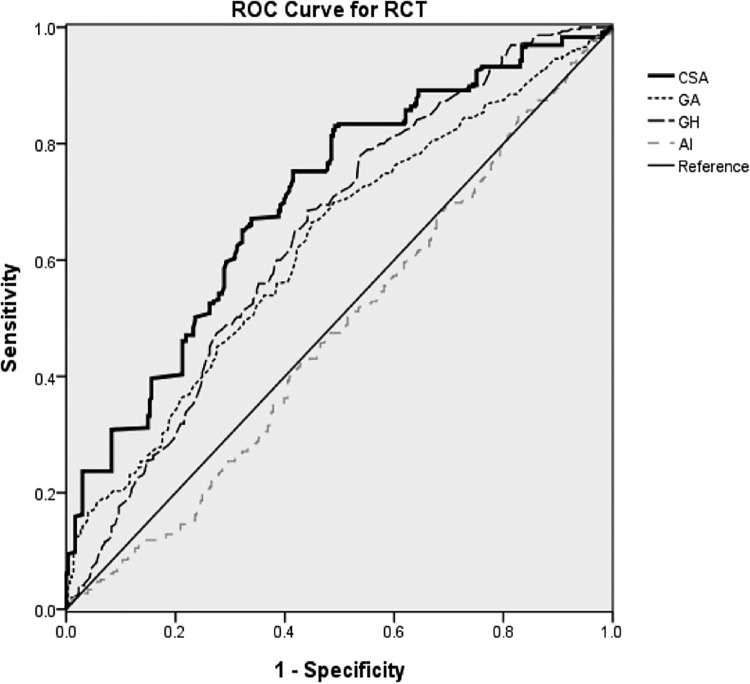
The ROC curve is presented in Figure 4, with the area under the curve (AUC) of the CSA being 70.5% (P < .001), the GA being 62.4% (P < .001), and the GH being 64.4% (P < .001). The AI had an AUC of 47.7% (P = .322) and had no significance for predicting RCTs in patients. According to the Youden index, the cutoff point of the CSA was 37.52° with 65.8% sensitivity and 67.8% specificity.
Figure 4.
Receiver operating characteristic curve analysis of the critical shoulder angle (CSA), distance from the glenoid plane to the lateral border of the acromion (GA), distance from the glenoid plane to the most lateral aspect of the humeral head (GH), and acromion index (AI) for predicting rotator cuff tears in patients with shoulder pain.
For patients with shoulder pain with a CSA higher than 37.52°, binary logistic regression revealed an adjusted odds ratio of 3.92 (95% CI, 2.79-5.51; P < .001) for the occurrence of RCTs. Stepwise logistic regression revealed the CSA as an independent predictor of RCTs, with an adjusted odds ratio of 1.295 (95% CI, 1.019-1.571; P = .006) (Table 3).
TABLE 3.
Predictors of RCTs by Multiple Stepwise Logistic Regressiona
| Odds Ratio (95% CI) | P | |
|---|---|---|
| Age | 0.652 (0.483-1.432) | .791 |
| CSA | 1.295 (1.019-1.571) | .006b |
| AI | 0.115 (0.004-3.315) | .207 |
aAI, acromion index; CSA, critical shoulder angle; RCT, rotator cuff tear.
bP < .05.
Discussion
When patients visit clinics for shoulder pain, a radiographic evaluation is usually the initial assessment tool; some clinics are not equipped with ultrasound capabilities. Radiographic measurement parameters could help clinicians determine the requirement of a further ultrasound evaluation for patients with shoulder pain and RCTs. Our study revealed that compared with the AI, the CSA could predict RCTs for patients with shoulder pain with better accuracy. A high CSA indicated high acromial coverage and glenoid inclination and contributed to the vulnerability of RCTs from impingement. In a biomechanical study, Gerber et al2 evaluated and compared the joint reaction force of a CSA of more than 38° with that of more than 33° in patients with RCTs. They reported that an increased CSA could lead to decreased superoinferior joint stability and that shoulder instability led to compensatory increasing loads on the supraspinatus tendon.2 High-loaded supraspinatus tendons could incur repetitive shoulder active adduction, leading to overloading of rotator cuffs and causing tears.2,24
The pathogenesis of RCTs is typically classified as having extrinsic (overuse, chronic impingement syndrome, and multifactorial causes) or intrinsic factors (degeneration, hypoperfusion, microtrauma, and apoptosis).18,23 The higher risk of RCTs among patients with a high CSA may be explained by extrinsic impingement and high tensile stress overloading. Previous studies5,9,15 have noted that tensile stress overloading on rotator cuff tendons is a pathogenesis of RCTs. Moor et al12,13 reported that the CSA could contribute to the risk of degenerative RCTs by affecting glenoid inclination and lateral extension of the acromion. Previous biomechanical studies2,13 have confirmed that a high CSA was associated with overloaded tensile stress on rotator cuff tendons, which then led to RCTs.
The mean CSA data from our study were similar to those in other studies in East Asia. Shinagawa et al20 reported that the mean CSA of full-thickness and partial-thickness RCTs was 34.3° ± 4.2° and 32.6° ± 3.2°, respectively. Seo et al19 presented similar results, which revealed that the mean CSA for articular-side RCTs was 34°. The mean CSA in our study was higher than that in these Asian studies. We believe that this could be explained by a higher proportion of full-thickness tears in our study group. The cutoff value of the CSA was 37.52° in our study. Pandey et al16 reported a cutoff value of 39.3°, which was more than our cutoff value. Racial differences should be considered for the clinical application of the CSA for RCTs in patients with shoulder pain.
Although previous studies have reported that the AI was associated with full-thickness RCTs, in our study, we observed more accuracy of the CSA in predicting RCTs in patients with shoulder pain. The AI indicated the ratio of lateral extension to the acromion and humeral lateral border. More lateral extension of the acromion leads to more vertical ascending force of the middle deltoid and impingement. However, a previous study11 investigated the association between the AI and RCTs among Brazilian and Japanese people and reported that the AI could predict RCTs in the Brazilian but not Japanese population. Our study was performed with a single ethnic population, and we found that the AI was not an accurate prediction tool for RCTs. Both the GA and GH were larger in the RCT group than the non-RCT group, which was likely because of patients with RCTs having a lateral (larger) acromion and humeral head. We conjectured that several biomechanical mechanisms beyond the AI and impingement that are still under investigation may explain our study findings.
This study revealed that the CSA could be an objective assessment tool for predicting RCTs among patients with shoulder pain, as it had better accuracy than the AI for the prediction of RCTs. Nevertheless, our study has several limitations in terms of clinical applications. First, we enrolled only patients with shoulder pain who underwent ultrasonography in this retrospective case-control study. Asymptomatic patients with RCTs were not included in this study, which limits our data because only patients with shoulder pain were included. Future prospective large-scale studies should screen for asymptomatic patients with RCTs. Second, all participants were from a single Asian race, and the cutoff CSA value for predicting RCTs in patients could vary across races. Third, multifactorial causes include extrinsic and intrinsic factors for RCTs, and the CSA is associated with impingement, which is an extrinsic factor of RCTs. Although we controlled for possible risk factors, such as diabetes mellitus, steroid use, age, and gout, contributing factors such as exercise, acromial shape, and shoulder biomechanical loads from daily life were not considered in this study. Finally, scapular and planar positioning could influence the reliability of CSA assessments.22 To reduce the risk of bias of radiographic image assessments, we adopted a standard protocol of shoulder radiographic imaging.
Conclusion
Patients with RCTs presented a higher CSA than did patients without RCTs, and the CSA could be an objective assessment tool to predict RCTs for patients with shoulder pain. Furthermore, our study revealed that the CSA had better accuracy for predicting RCTs than did the AI for patients. When the CSA surpasses the cutoff value on radiographic assessments, further ultrasonography is recommended to detect RCTs.
Footnotes
Final revision submitted January 22, 2020; accepted February 1, 2020.
One or more of the authors declared the following potential conflict of interest or source of funding: This work was supported by grants from the Ministry of Science and Technology of Taiwan (No. 108-2314-B-038-029), Taipei Medical University, and Shuang Ho Hospital (Nos. 108TMU-SHH-07 and TMU108-AE1-B01). AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the Taipei Medical University-Joint Institutional Review Board.
References
- 1. Blonna D, Giani A, Bellato E, et al. Predominance of the critical shoulder angle in the pathogenesis of degenerative diseases of the shoulder. J Shoulder Elbow Surg. 2016;25(8):1328–1336. [DOI] [PubMed] [Google Scholar]
- 2. Gerber C, Snedeker JG, Baumgartner D, Viehofer AF. Supraspinatus tendon load during abduction is dependent on the size of the critical shoulder angle: a biomechanical analysis. J Orthop Res. 2014;32(7):952–957. [DOI] [PubMed] [Google Scholar]
- 3. Huang SW, Wang WT, Chou LC, Liou TH, Chen YW, Lin HW. Diabetes mellitus increases the risk of rotator cuff tear repair surgery: a population-based cohort study. J Diabetes Complications. 2016;30(8):1473–1477. [DOI] [PubMed] [Google Scholar]
- 4. Huang SW, Wu CW, Lin LF, Liou TH, Lin HW. Gout can increase the risk of receiving rotator cuff tear repair surgery. Am J Sports Med. 2017;45(10):2355–2363. [DOI] [PubMed] [Google Scholar]
- 5. Inoue A, Chosa E, Goto K, Tajima N. Nonlinear stress analysis of the supraspinatus tendon using three-dimensional finite element analysis. Knee Surg Sports Traumatol Arthrosc. 2013;21(5):1151–1157. [DOI] [PubMed] [Google Scholar]
- 6. Kim JR, Ryu KJ, Hong IT, Kim BK, Kim JH. Can a high acromion index predict rotator cuff tears? Int Orthop. 2012;36(5):1019–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee M, Chen JY, Liow MHL, Chong HC, Chang P, Lie D. Critical shoulder angle and acromial index do not influence 24-month functional outcome after arthroscopic rotator cuff repair. Am J Sports Med. 2017;45(13):2989–2994. [DOI] [PubMed] [Google Scholar]
- 8. Li H, Chen Y, Chen J, Hua Y, Chen S. Large critical shoulder angle has higher risk of tendon retear after arthroscopic rotator cuff repair. Am J Sports Med. 2018;46(8):1892–1900. [DOI] [PubMed] [Google Scholar]
- 9. Luo ZP, Hsu HC, Grabowski JJ, Morrey BF, An KN. Mechanical environment associated with rotator cuff tears. J Shoulder Elbow Surg. 1998;7(6):616–620. [DOI] [PubMed] [Google Scholar]
- 10. Minagawa H, Yamamoto N, Abe H, et al. Prevalence of symptomatic and asymptomatic rotator cuff tears in the general population: from mass-screening in one village. J Orthop. 2013;10(1):8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miyazaki AN, Itoi E, Sano H, et al. Comparison between the acromion index and rotator cuff tears in the Brazilian and Japanese populations. J Shoulder Elbow Surg. 2011;20(7):1082–1086. [DOI] [PubMed] [Google Scholar]
- 12. Moor BK, Bouaicha S, Rothenfluh DA, Sukthankar A, Gerber C. Is there an association between the individual anatomy of the scapula and the development of rotator cuff tears or osteoarthritis of the glenohumeral joint? A radiological study of the critical shoulder angle. Bone Joint J. 2013;95(7):935–941. [DOI] [PubMed] [Google Scholar]
- 13. Moor BK, Kuster R, Osterhoff G, et al. Inclination-dependent changes of the critical shoulder angle significantly influence superior glenohumeral joint stability. Clin Biomech (Bristol, Avon). 2016;32:268–273. [DOI] [PubMed] [Google Scholar]
- 14. Moor BK, Wieser K, Slankamenac K, Gerber C, Bouaicha S. Relationship of individual scapular anatomy and degenerative rotator cuff tears. J Shoulder Elbow Surg. 2014;23(4):536–541. [DOI] [PubMed] [Google Scholar]
- 15. Nakajima T, Rokuuma N, Hamada K, Tomatsu T, Fukuda H. Histologic and biomechanical characteristics of the supraspinatus tendon: reference to rotator cuff tearing. J Shoulder Elbow Surg. 1994;3(2):79–87. [DOI] [PubMed] [Google Scholar]
- 16. Pandey V, Vijayan D, Tapashetti S, et al. Does scapular morphology affect the integrity of the rotator cuff? J Shoulder Elbow Surg. 2016;25(3):413–421. [DOI] [PubMed] [Google Scholar]
- 17. Roy JS, Braen C, Leblond J, et al. Diagnostic accuracy of ultrasonography,MRI and MR arthrography in the characterisation of rotator cuff disorders: a systematic review and meta-analysis. Br J Sports Med. 2015;49(20):1316–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seitz AL, McClure PW, Finucane S, Boardman ND 3rd, Michener LA. Mechanisms of rotator cuff tendinopathy: intrinsic, extrinsic, or both? Clin Biomech (Bristol, Avon). 2011;26(1):1–12. [DOI] [PubMed] [Google Scholar]
- 19. Seo J, Heo K, Kwon S, Yoo J. Critical shoulder angle and greater tuberosity angle according to the partial thickness rotator cuff tear patterns. Orthop Traumatol Surg Res. 2019;105(8):1543–1548. [DOI] [PubMed] [Google Scholar]
- 20. Shinagawa K, Hatta T, Yamamoto N, et al. Critical shoulder angle in an East Asian population: correlation to the incidence of rotator cuff tear and glenohumeral osteoarthritis. J Shoulder Elbow Surg. 2018;27(9):1602–1606. [DOI] [PubMed] [Google Scholar]
- 21. Spiegl UJ, Horan MP, Smith SW, Ho CP, Millett PJ. The critical shoulder angle is associated with rotator cuff tears and shoulder osteoarthritis and is better assessed with radiographs over MRI. Knee Surg Sports Traumatol Arthrosc. 2016;24(7):2244–2251. [DOI] [PubMed] [Google Scholar]
- 22. Suter T, Gerber Popp A, Zhang Y, Zhang C, Tashjian RZ, Henninger HB. The influence of radiographic viewing perspective and demographics on the critical shoulder angle. J Shoulder Elbow Surg. 2015;24(6):e149–e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Via AG, De Cupis M, Spoliti M, Oliva F. Clinical and biological aspects of rotator cuff tears. Muscles Ligaments Tendons J. 2013;3(2):70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Viehofer AF, Gerber C, Favre P, Bachmann E, Snedeker JG. A larger critical shoulder angle requires more rotator cuff activity to preserve joint stability. J Orthop Res. 2016;34(6):961–968. [DOI] [PubMed] [Google Scholar]