Abstract
Majority of patients infected with the COVID 19 virus display a mild to moderate course of disease and spontaneously recover at 14–20 days. However, about 15% of patients progress to severe stages and 2.5% of these patients succumb to this illness. Most patients with severe disease belong to the elderly age group (<65 years of age) and have multiple associated co-morbidities. The immune responses induced by the COVID 19 virus, during the incubation and non-severe stages, requires the early initiation of a specific adaptive immune response to eliminate the virus and prevent the progress to severe stages. In patients with a dysfunctional bridge adaptive immunity, the innate immune response becomes exaggerated due to the lack of feedback from the adaptive immune cells. The resultant cytokine storm is responsible for the severe lung injury leading to acute respiratory distress syndrome seen in COVID 19 patients.
Mesenchymal stem cells are known to suppress overactive immune responses as well as bring about tissue regeneration and repair. This immuno-modulatory effect of MSCs could hold potential to manage a patient with severe symptoms of COVID 19 infection due to a dysfunctional adaptive immune system.
Introduction
Individuals infected by the COVID 19 virus display a clinical course ranging from asymptomatic carriers, mildly symptomatic individuals to those with severe respiratory distress and death, based on age and other co-morbid features [1], [2], [3].
About 80–85% of patients infected with the COVID 19 virus display a mild to moderate course of the disease and spontaneously recover at 14–20 days from the point of the first contact. However, about 15% of patients progress to severe stages of the disease, often requiring ICU admission and mechanical ventilation with around 2.5% succumbing to this illness [1], [2]. Research has shown that most of the patients who progress to severe stages of disease belong to the elderly age group < 65 years of age and have multiple associated co-morbidities [1].
This paper serves to bring out the immunological responses of patients with severe disease, in order to design pertinent interventions to decrease morbidity and mortality of COVID 19.
Functional bridge adaptive immunity in patients with favourable outcomes with COVID 19
The immune responses induced by the COVID 19 virus, during the incubation and non-severe stages, requires the early initiation of a specific adaptive immune response to eliminate the virus and prevent the progression to severe stages [4]. This is carried out by neutralization of free virus particles and termination of viral replication by antiviral cytotoxic T cells (CTLs).
The Innate cytokine responses, like Interferon (IFN) alpha/beta or IFN-gamma have roles in controlling and initiating downstream adaptive immune responses [4]. Interferon-alpha and beta are synthesized by most virally infected cells whereas IFN-γ is synthesized only by certain cells of the immune system like the natural killer (NK) cells, CD4 Th1 cells, and CD8 cytotoxic suppressor cells.
A decrease in lymphocytic count is a deterrent to the adaptive immune response. Mild to moderate lymphocytopenia is a common haematological finding (about 44% of all patients) in individuals with COVID 19 infections. Studies have shown an absolute lymphocytic count of about 1–1.2 × 109/L [5], [6].
Early recruitment of CD4+ and CD8+ cells is crucial in the recovery of patients with COVID 19 Infections. Studies have shown that although CD4+ and CD8+ expression is lesser in patients with COVID 9 than healthy individuals, there is a steady increase in expression upto 4–5 days [6], [7], [8].
Individuals that recovered from the disease with only mild to moderate symptoms show activated CD4+ and CD8+ T cells, (co-expression of CD38 and HLA-DR), along with IgM and IgG viral-specific antibodies [4], [7], [8]. The emergence and rapid increase in activated T cells, especially CD8+ T cells, at days 7–9 has been shown to precede the resolution of symptoms. Following this, there is a rapid decline CD8+ cells and minor decline in CD4+ cell population upto day 20 [4]. This coincides with complete recovery of the patient, thus establishing the importance of a functional bridge adaptive immunity.
Dysfunctional bridge adaptive immunity in COVID 19 patients with severe disease
In cases that progress to severe or critical stages, counts of peripheral CD4+ and CD8+ T cells were substantially reduced around Day 4–5. Jing Liu et al. [6] demonstrated a significant reduction in CD8 cell counts with an insignificant decrease in CD4 counts in peripheral blood in severe cases when compared to mild cases. The T cells, although reduced in number, show hyperactivity as evidenced by the high proportions of HLA-DR and CD38 expression. Additionally, CD8+ cells contain higher concentrations of cytotoxic granules of perforin and granulysin but are grossly reduced in number [4], [6]. This implies an over-activation of T cells, and high cytotoxicity of CD8 T cells leading to an exaggerated immune response. This is substantiated by the increased expression of IL6, IL10, IL2 and IFN-γ in the sera of severe cases when compared with that of the mild cases [6].
The levels of inflammatory cytokines (IL1, TNF alpha, IL6) are high in the lungs of COVID-19 patients and these cytokines are strong inducers of hyaline production in lung alveolar epithelial cells, and fibroblasts [8], [9]. Lung inflammation is the main cause of life-threatening respiratory disorders and the severity of pulmonary immune injury correlates with extensive infiltration of neutrophils and macrophages in the lungs [10].
More pertinently, it has been demonstrated that when T cell counts drop to their lowest levels at day 4–6, the serum IL10, IL2, IL4, TNF-α and IFN-γ levels reach their peaks thereby initiating a cytokine storm [6]. This excessive, ineffective host immune response may lead to severe lung injury leading to Acute respiratory distress syndrome (ARDS) [7].
As the infection progresses and becomes severe in intensity, the remaining T cells are unable to sustain long term activation, leading to immune exhaustion and decline in effector activity and proliferation [11]. The overstimulated innate immune response, without feedback from adaptive immune system clearly demonstrates the dysfunctional bridge adaptive immunity.
Basis for mesenchymal stem cells (MSCs) in treatment of severe COVID 19 illness
Mesenchymal cells derived from umbilical cord, dental pulp, bone marrow etc, have been used for immune modulation in various autoimmune disorders [12].
Mesenchymal stem cells are known to suppress overactive immune responses as well as maintain a pro-inflammatory phenotype when inflammatory responses are absent. MSCs also bring about tissue regeneration and repair [12], [13]. The plasticity of these mesenchymal cells according to the presence or absence of pro inflammatory cytokines has been described and is being intensively studied [14].
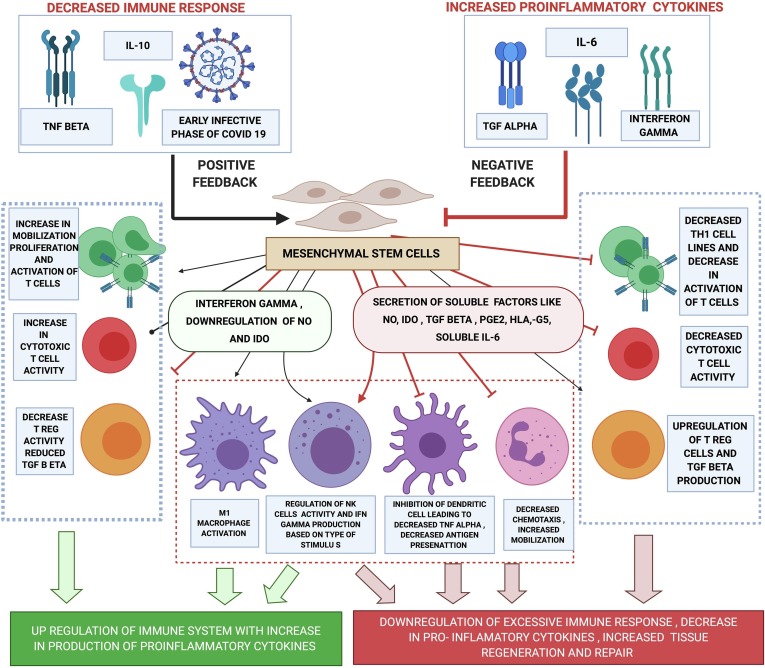
This immunomodulatory effect of MSCs could hold the potential to manage a patient at risk of manifesting the severe symptoms of COVID 19 infection due to a dysfunctional adaptive immune system (Fig. 1 ). A few reports of use of mesenchymal stromal/stem cell in the treatment of COVID 19 has emerged in recent times with promising results [15]. Wilson et al conducted a study to investigate the effectiveness and safety of the use of mesenchymal stem cells for ARDS with favourable results [16].
Fig. 1.
mechanism of action of mesenchymal stem cells for immunomodulation.
MSCs are non-immunogenic due to their low expression of Major histocompatibility Complex (MHC). This makes it well suited for therapeutic interventions using allogenic transfers without HLA matching [17]. In the presence of an exaggerated immune response as seen in COVID 19 patients with cytokine storm and ARDS the pro-inflammatory cytokines bring out an anti-inflammatory effect on the MSCs. The MSCs secrete several soluble factors such as Nitric oxide (NO) Transforming growth factor (TGF) beta Prostaglandin E2 (PGE2) indoleamine 2 3-dioxygenase (IDO) HLA -G5 and soluble interleukin 6. These molecules then inhibit the proliferation and activation of TH1 and TH17 cell lines thereby decreasing the production of interferon-gamma and interleukin 17. They also inhibit the activation of cytotoxic CD8+ cells thereby reducing direct injury to lung parenchyma. The activity and maturity of dendritic cells are also reduced via PGE2 pathways hence decreasing TNF alpha and increasing IL10 which is anti-inflammatory. This then activates T reg cells that regulate the T cell activity and further increase the production of IL10. The NK cells are suppressed both by the secretion of these soluble molecules as well as contact-mediated communication with MSCs. MSCs also bring about increased mobilization and decreased chemotaxis of neutrophils [12], [13], [14].
Mesenchymal stem cells may be lysed or inactivated by the already activated NK cells. This phenomenon can be controlled by priming the MSCs with interferon-gamma before transfusion [8], [12], [13].
To explore the use of mesenchymal stem cells in the treatment of COVID positive patients with severe disease, we have initiated a clinical trial (IMD/CoV/001/2020) to transfuse Allogeneic mesenchymal stem cells derived from donated bone marrow or umbilical cord administered via intrapulmonary implantation where possible or administered via the intravenous route.
In order to offset the neutralization of MSCs, we have proposed priming of MSC with a cytokine cocktail derived from Th1 cells of healthy donors to suppress the hyperactive immune response and promote tissue repair.
Declaration by authors
No conflict of interest between the authors.
No financial affiliations/external funding agency involved.
IRB Ethical clearance has been obtained for the study.
*The study mentioned has been registered under CTRI and Clinical Trials.
Acknowledgments
Acknowledgement
Anand Subash MS, DNB.
Consultant, Department of Head and Neck Oncology.
HealthCare Global Enterprises Ltd.
Dr.Ankita Kar MDS.
Program Director of Research, Grants and Publications.
Department of Head and Neck Surgical Oncology.
HCG Cancer Centre, Bangalore, India.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mehy.2020.109845.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020:15. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Zhe. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thevarajan I., Nguyen T.H.O., Koutsakos M. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat Med. 2020 doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan B.E., Chong V.C.L., Chan S.S.W., Lim G.H., Lim K.G.E., Tan G.B. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020 doi: 10.1002/ajh.25774. [DOI] [PubMed] [Google Scholar]
- 6.Liu Jing, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. Lancet Infect Dis (PRE PRINT). [DOI] [PMC free article] [PubMed]
- 7.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi Y., Wang Y., Shao C. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020 doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet Infect Dis. Doi:10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed]
- 10.de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiappelli Francesco, Khakshooy Allen, Greenberg Gillian. CoViD-19 immunopathology & immunotherapy. Bioinformation. 2020;16(3):219–222. doi: 10.6026/97320630016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Blanc K., Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 13.Regmi Shobha, et al. Mesenchymal stem cell therapy for the treatment of inflammatory diseases: challenges, opportunities, and future perspectives. Eur J Cell Biol. Doi:10.1016/j.ejcb.2019.04.002. [DOI] [PubMed]
- 14.Wang Y., Chen X., Cao W. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 15.Leng Z, et al. MSC transplantation improves COVID-19 patient outcome. Aging Dis 11; 2020. [DOI] [PMC free article] [PubMed]
- 16.Wilson JG, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respiratory Med 3(1): 24–32. doi:10.1016/s2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed]
- 17.Walter J., Ware L.B., Matthay M.A. Mesenchymal stem cells: mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respiratory Med. 2014;2(12):1016–1026. doi: 10.1016/s2213-2600(14)70217-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.