Abstract
The development of gastroretentive systems have been growing lately due to the high demand for carriers that increase drug bioavailability and therapeutic effectiveness after oral administration. Most of systems reported up to now are based on chitosan (CS) due to its peculiar properties, such as cationic nature, biodegradability, biocompatibility and important mucoadhesiveness, which make CS a promising biopolymer to design effective gastroretentive systems. In light of this, we reported in this review the CS versatility to fabricate different types of nano- and microstructured gastroretentive systems. For a better understanding of the gastric retention mechanisms, we highlighted expandable, density-based, magnetic, mucoadhesive and superporous systems. The biological and chemical properties of CS, anatomophysiological aspects related to gastrointestinal tract (GIT) and some applications of these systems are also described here. Overall, this review may assist researchers to explore new strategies to design safe and efficient gastroretentive systems in order to popularize them in the treatment of diseases and clinical practices.
Keywords: Gastroretentive systems, Chitosan, Drug delivery systems, Stomach, Biopolymer
Graphical abstract

1. Introduction
Chitosan (CS) is a biopolymer obtained from the deacetylation of chitin that presents several applications in photography [1], cosmetic/skin regeneration [2], food and nutrition [3], environmental protection [4] and drug delivery systems [5]. Specifically, CS is very useful for pharmaceutical applications due its non-toxic nature, biodegradability, bioadhesivity, biocompatibility besides the capability to form films, gels, nano and microparticles [6,7]. Thus, floating, mucoadhesive, porous and gastric pH resistant pharmaceutical systems may be originated using pristine CS or associated with other materials [8,9].
Investigations have been carried out with the purpose of suggesting, developing and testing microspheres, micelles, hydrogels and nanoparticles using this biopolymer [[10], [11], [12]]. An innovative application of CS is to develop systems with the capacity to remain and release drugs in the stomach. These systems are labelled gastroretentive drug delivery systems (GRDDSs), which demarcate an important frontier in gastric and drug transport therapies that require gastric absorption and/or local action. Consequently, these systems circumvent the short retention time caused by gastric emptying and some physicochemical limitation, such as low solubility of some drugs.
Despite the numerous advantages of controlled drug delivery systems, many drugs with high solubility at stomach pH, instability in intestinal pH, as well as those with narrow absorption window and that are intended for the local treatment of stomach disorders, require additional mechanisms for spatial control of the release, for which GRDDSs classification has been attributed. This topic has been extremely relevant according to the number of preclinical and clinical [13,14] studies in which their effectiveness have been demonstrated by increasing the drugs absorption despites the low absorption window [15].
Many characteristics attributed to CS are directly related to the preservation of protonable amino groups in the polymeric chain, which are nucleophilic and reactive regions of the molecule. Therefore, being able to interact with a large number of materials, and therefore be widely applied in the most diverse fields, from water treatment to the development of controlled drug release systems, its versatility can be explored individually or in combination with other materials [16].
This review highlights recent works addressing to the CS-based materials to fabricate GRDDSs. In the Scopus database, when requested to search using the keywords “chitosan”, “gastroretentive” and “drug delivery”, 1544 documents were found from 2009 to 2019, (Fig. 1 ). It is worth noting that since 2013 the number of publications on this topic has increased, showing great interest on the part of scientific community.
Fig. 1.

Publications from the last 10 years containing the terms “chitosan”, “gastroretentive” and “drug delivery”.
2. Chitin and chitosan
Discovered in 1811 by Henri Braconot, chitin is a polysaccharide formed by β(1 β(1 → 4)-2-acetamide-2-deoxy-d-glucopyranose units [17]. The orientation of these polymeric chains gives rise to different allomorphs of chitin, forming anti-parallel and parallel arrangements known as alpha, beta and gamma-chitin. Chitin is a hydrophobic polymer, with fewer applications than CS, because it is not dispersible in aqueous, acids and alkaline media, being possible its dispersion only in hexafluoroisopropanol and chloroalcohols. Chitin is usually found in exoskeletons of invertebrate animals as crabs, lobsters, shrimps, cockroaches and scorpions. In addition to these sources, chitin and CS also occur naturally in some fungi like Mucor rouxii (30% of total mass) and Choanephora cucurbitarum (28% of total mass) [18,19].
CS was first observed in 1859 by Rouget [20]. Later, authors showed that CS is obtained by alkaline deacetylation of chitin (Fig. 2 ) under heating [20]. CS consists of monomeric units of β(1 → 4)-2-acetamide-2-deoxy-d-glucopyranose and β(1 → 4)-2-amino-2-deoxy-d-glucopyranose, predominantly the last one [17,21,22].
Fig. 2.
Deacetylation of chitin producing CS.
CS presents interesting characteristics like biocompatibility, biodegradability and low toxicity being a versatile biomaterial used in many research areas like textiles, food industry, environmental, agriculture, medical products, cosmetics and mainly, in pharmaceutical applications [[23], [24], [25], [26]].
In relation to the different CS applications, its dispersion in acidified aqueous media promotes the increase of liquid formulations viscosity, making CS also applicable as a thickener. Additionally, the high viscosity of these systems associated with their polycationic nature gives to the CS impressive mucoadhesive properties. This can be attributed to the CS interaction with sialic acid, an anionic component presents in the mucin [27]. The properties above mentioned make CS a special biopolymer used to develop specific (mucoadhesive) drug delivery systems [28].
CS amino groups in acidified aqueous media can become protonated, giving rise to a positively charged material, which can interact with negative charges from anionic polymers, cell membranes, mucus and complex reactive oxygen species (ROS). When not protonated, amine groups can still complex metallic ions like Cu2+ and are mainly used in water treatment and clarification of beverages [[29], [30], [31]].
For biological applications, CS also requires dispersion in acidic aqueous solutions with pH values lower than 6.5. In this condition, the CS amino groups (NH2) present in the N-acetyl-d-glucosamine units act as nucleophiles becoming progressively protonated due to the ionization of acids present in the medium, forming a positively charged polyelectrolyte, as shown in Eq. (1) [32].
| (1) |
Changes in the CS chain can directly affect its physicochemical properties, leading to different technological applications changing also its biological activity [33,34]. Some types of CS have different degrees of deacetylation (DD) and molecular weight (MW), which are determinant for their polycationic nature at pH < 6.5. Variations, such as high DD and low MW can increase inter- and intra-chain electrostatic repulsion, favoring biopolymer-water interactions and reducing biopolymer-biopolymer interactions. This effect is also responsible for increasing interactions with negative surfaces like lipids, cells, drugs and anionic molecules, as well as mucus conferring mucoadhesive properties [35].
Interestingly, the CS interaction with anionic polymers can lead to gels formation by complex coacervation [36]. Interaction with smaller polyanions such as sodium tripolyphosphate leads to the formation of bridges (junction zones) between the polymer chains forming gel by ionotropic gelation [37]. These kinds of interactions make protonated CS interesting biopolymer for the development of pharmaceutical systems such as micro- and nanoparticles, gels and films [38].
The ability to form particles applied as GRDDSs is associated with characteristics of the polymeric chain, such as DD and MW, as well as pH value of the dispersion medium. CSs with low DD are more efficiently dispersible at high pH due to their high charge density that favors more intensely interactions with polymers, drugs and cross-linking agents, such as sodium tripolyphosphate. On the other hand, CS with high MW shows greater capacity than low MW to increase the media viscosity even at low concentrations, making the diluted/semi-diluted dispersion concentration regime suitable for particles formation [37].
The knowledge about structural and biological properties may provide a basis for exploring the potential and versatility of CS-based materials applications as smart drug delivery systems. For instance, changes in CS rheological behavior in response to pH changes can favor the fabrication of suitable GRDDSs [39]. At acidic pH, CS can increase the viscosity in the medium, promoting good adhesion. Beyond that, the modification of CS structure can increase the possibilities of its biological applications [6] as shown in Table 1 .
Table 1.
Biological applications of distinct and modified CS-based materials.
| Applications | Materials/systems | Remarks | Refs. |
|---|---|---|---|
| Antimicrobial | HTCC (N-(2-hydroxypropyl)-3-trimethylammonium chitosan chloride), degree of substitution of 57, 62, 63 and 77%. | The inhibitory effect of HTCC against SARS-CoV-2 and MERS-CoV viruses was tested in vitro with Vero and Vero 6 cells, as well as ex vivo on human airway epithelium (HAE).
|
[40] |
| CS nano- and microparticles | Low MW and DD presents to higher antimicrobial activity. At basic pH, CS loses antimicrobial activity. | [41] | |
| CS dispersed-Different DD (51.04%–100%), and ultra high MW CS (Mw > 106 g mol−1) | Optimal pH value was 6.0 for the highest bactericidal activity, high DD more effective against E. coli and S. aureus. MIC for DD 81% was 0.0625% (E. coli) and 0.0313% (S. aureus) MIC for DD 100% was 0.0156% for both microorganisms. |
[42] | |
| Anti-inflammatory | CS with different MWs and chitooligosaccharide (COS) | Larger MW (>29.2 kDa) CS usually exhibit anti-inflammatory activity. CS bind to CR3 receptor and TLR4 and CR3 receptors in macrophages down-regulated the phosphorylation of MAPK signaling proteins ERK, JNK, and p38, inhibited the LPS-induced NF-κB activation, abolished the production of TNF-α and IL-6 decreasing NO production, reducing inflammation. |
[43] |
| In-vivo (rats) test of anti-inflammatory activity in ulcer model of CS high and low MW. | Anti-ulcerative and wound healing abilities. LMW CS reduced ulcerative wounds. HMW CS helped retain the stomachal folds rendering a gastro protective effect, also can stimulate collagen synthesis. |
[44] | |
| Antioxidant | CS of MW (0.5–1000 kDa) and DD (50% -low then 10%) | Reduction of both DD and MW make CS more efficient in removing in vitro reactive oxygen species (ROS). | [45] |
| Gamma irradiated (5–50 KGy) and not irradiated CS DD (72–75%), dispersed in lactic acid | Reduction of CS molecular weight by gamma irradiation at 50KGy, increased antioxidant capacity of CS dispersion | [46] | |
| Lipid-lowering effects | Dietary supplementation with CS DD (70.03%) and MW (estimated 500–1000 kDa) | Comparison of the lipid-lowering and intestinal morphological effects of cholestyramine, CS and oat gum in rats. CS has hypolipidemic effects similar to cholestyramine without deleterious changes in the intestinal mucosa. Oat gum was less effective | [47] |
| Dietary supplementation with CS (4% DD) chitooligosaccharides COS < 1000 g mol−1, 4% DD COS < 3000 g mol−1, 9% DD |
CS and chitooligosaccharides have the ability to regulate the body weight, liver and cardiac indices, fat/body ratio, as well as serum, liver, and fecal lipids. Simultaneously, maintain the appropriate activity of liver and serum superoxide dismutase (SOD), alanine aminotransferase (ALT), aspartate aminotransferase (AST), as well as liver and fecal total bile acids (TBA) | [48] | |
| Scaffold for tissue regeneration | Simvastatin-loaded CS nanoparticles | CS induced new osteoid tissue formation, showing biodegradability and controlled simvastatin release. After 14 days, CS promoted increase of the enzyme ALP (indicator of osteoblast mineralization). CS nanostructures presented porosity suitable for angiogenesis and bone nutrition | [49] |
| CS-based hyaluronan hybrid polymer fiber | Fibroblasts from patellar tendon of Japanese white rabbit. Fibroblasts had adhesion onto hybrid fibers and produced collagen fibers after 14 days of culture | [50] | |
| CS | Compare polyvinyl alcohol (PVA), CS, and polycarbonate (PC) as scaffold for culture of embryonal submandibular gland (SMG). Best results observed in CS scaffold secreted extracellular matrices distributed in a reticular manner and formed thicker fibers beyond the extents of cell attachment and were able to further enhance SMG branching | [51] | |
| Anticancer | CS and chitooligosaccharides | Reduction of DD and MW of CS and its derivatives exhibiting good in vivo results, been efficient against prostate cancer, carcinomic human alveolar basal epithelial cells, and hepatocellular carcinoma, been nontoxic for healthy cells | [52] |
| Crab (Chionoecetes opilio) CS and Shiitaki mushroom (Lentinula edodes) CS | Shiitake CS had the best inhibitory effect on the growth of the human neuroblastoma cell line (IMR 32, BCRC 60014) and the human liver hepatocellular carcinoma cell line (Hep G2, BCRC 60025), occurring because Shitake CS is more deacetylated | [53] | |
| Hemostatic | CS hydrogel containing nano bioglass | Coagulation was twice faster than pure blood in in vitro hemocompatibility assay. In vivo test revealed the reduction to half the blood loss and coagulation time for both organs and arteries |
[54] |
| CS-based hemostatic dressing ChitoGauze® | A commercial hemostatic dressing for temporary external control of wounds with severe bleeding. It is a CS dressing composed of a non-woven medical gauze of polyester/rayon mixture coated with CS (HemCon Medical Technologies, Portland, OR, USA). Used by US military forces, emergency medical services | [55] |
Friedman et al. [56], demonstrated that CS anti-inflammatory activity is related to the blocking of NK-κB in human type 1 mast cells inhibiting the production of pro-inflammatory cytokines. This effect can occur due to a downregulation of Ca2+-dependent pathways [57]. In addition, CS antioxidant activity is directly associated with the chemical reactivity of the hydroxyl and amino groups from the C-6 and C-2 carbons, respectively [58]. Substitutions performed on CS by reducing the DD promote the exposure of more amino groups in the polymer chain increasing its antioxidant activity. The biopolymer chain reduction (MW) also exerts a positive effect on the CS antioxidant activity. Smaller biopolymer chains are very rigid, so the intra-chain hydrogen bonds that involve hydroxyl groups and amino acids are less intense than the same interactions in larger chains with more conformational freedom [45]. Thus, smaller polymer chains can interact more efficiently with oxidant molecules inhibiting their action.
CS hypolipidemic activity is associated with its polycationic nature, interacting with dietary lipids inhibiting their absorption. Additionally, CS can reduce the liver damage associated with the oxidation of fatty acids, leading to a considerable reduction in liver enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT) and elevation in activities of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px). Beyond that, diets rich in CS and chitooligosaccharides have shown the reduction of serum triglyceride and LDL-cholesterol levels as well as regulate the expression of liver peroxisome proliferator-activated receptor α (PPARα) [48,59].
Most of studies have demonstrated that CS and their derivatives display anticancer properties. These biopolymers show low toxicity reducing side effects and tumor size by inhibiting tumor cells proliferation and, consequently, provoking apoptosis [52,60].
Considering CS applicable as new biomaterials, this biopolymer could be used to fabricate scaffolds for regenerative medicine [61]. CS-based scaffolds exhibit advantages as biocompatibility, biodegradability and malleability. In addition, these biomaterials can easily interact with growth factors, glycosaminoglycans, and DNA making CS-based scaffolds excellent for bone, cartilage and intervertebral discs regeneration [62]. CS-based scaffolds allow an efficient angiogenic response, favoring the oxygen supply to cells, nutrients and chemical mediators for cells proliferation and differentiation, promoting osteogenesis in vitro and in vivo. CS hydrophilicity promotes cells adhesiveness by reducing immune reactions when applicable in implants [63].
NH2 groups available in CS interact with erythrocytes promoting the aggregation and the formation of a physical barrier that prevents the blood loss without rejection by the organism [64]. CS with low DD and dispersed in acidified water can induce the complement pathway by recruiting more erythrocytes, promoting rapid coagulation. Additionally, the CS MW showed less impact on the coagulant effect. In this scenario, CS with MW between 105 and 106 Da displayed less coagulant capacity whereas CS with higher MW in physiological saline solution were more efficient in inducing erythrocyte aggregation. The effect of MW in CS suggests that long chains can interact with more platelets, promoting greater efficiency in hemostasis. Therefore, when DD is high the influence of MW on platelet aggregation is reduced. This effect is more pronounced by reducing DD, indicating that DD is the most important factor for platelet aggregation [54,65]. In light of this, the importance of CS to build GRDDSs orally administered is evident. However, considering the harsh barriers imposed by the gastrointestinal tract (GIT).
An important aspect about the use of CS to develop drug delivery systems for GIT consists on understand CSgastrointestinal digestion process in humans. This process still lacks in-depth studies that describe it systematically. However, up to now, some studies have shown that CS cannot be digested in the upper GIT by human digestive enzymes whereas CS digestion can occur mainly in the intestine, through depolymerization catalyzed by enzymes [[66], [67], [68], [69], [70], [71]].
In 1990, Hirano et al. [67], from in vitro assays, showed that CS can be degraded by chitosanase enzymes produced and released by bacteria from the intestinal microbiota. Later, Varum et al. [68] demonstrated that CS can also be depolymerized in humans by the lysozyme present in the serum.
The digestion by enzymes action from intestine is related to the degree of deacetylation (DD) and molecular weight (MW) of CS. Authors reported that as higher the CS DD, lower is its degradability [68]. However, new studies have already shown that there are enzymes, but only present in vegetables and some bacteria, capable of cleaving the glycosidic bond, both in CS with high and low DD including chitin [72].
Another important factor in the CS degradability and digestibility is MW. A study performed by Chae et al. [73], in which it was administered orally 20 mg kg−1 marked (fluorescent) CS varying MW and DD in rats, exhibited that CS with 230 kDa presentedabsorption 20 times lower than CS of 3.8 kDa. These results indicate that greater CS digestibility and consequent absorption could be achieved decreasing CS MW.
Considering above mentioned properties, CS is widely used to design colon-specific systems. Unlike when CS is associated with GRDDSs that release the drug in gastric media, in colon-specific systems the dissolution of CS in acidic pH is overcome by association or coating with other polymers. These systems pass through the stomach quickly without significant drug release, protecting the drugs until they reach the intestine. In this organ, CS undergoes the action of enzymes from the intestinal microbiota and from its degradation can release the drug [74].
Thus, a deep anatomophysiology knowledge of the different portions that compose GIT is essential for the correct performance of GRDDSs.
3. Anatomical and physiological aspects of the stomach
CS-based systems that present mucoadhesive, swelling, and acid erosion properties emerge as an alternative to explore different delivery strategies for the gastric environment. The oral route is the most used and convenient because it is a noninvasive way to access the stomach, being a safe administration route that allows self-administration and consequently, greater acceptance from the patient to the treatment [75]. However, the bioavailability of orally administered drugs may be influenced by several parameters such as gastric emptying and gastro intestinal transit, gastric mucosa status, stomach pH [76].
The stomach is located in the upper left part of the abdominal cavity below the diaphragm. Stomach size can change according to the amount of distention. After meal, approximately 1500 mL whereas the empty volume range from 25 to 50 mL [77]. Gastric pH in healthy conditions during fasting and fed state are 1.1 ± 0.15 and 3.6 ± 0.4, respectively. The pH returns to basal level between 2 and 4 h [78]. Anatomically, the stomach is divided in two parts: the proximal stomach, consisting of fundus and body, and the distal stomach, consisting of pylorus. The proximal stomach acts as a food reservoir while the distal stomach has a role in food processing, forming the chymus, which will be released later into the small intestine. The distal stomach also acts as a propelling pump that assists in gastric emptying (GE). The rate of GE is influenced by both food volume and gastric content composition [79,80]. Table 2 describes the main characteristics of upper GIT.
Table 2.
Main characteristics of upper GIT.
| Length (m) | Transit time (h) | pH | |
|---|---|---|---|
| Stomach | 0.2 | 0.5–2 | <3 |
| Small intestine | 6–10 | 3–4 | 5–8 |
GE occurs during fasting and in the fed state. However, the motility pattern is different in both states. During fasting, a series of interdigestive electrical events occur every 2 or 3 h [81]. As described by Wilson and Washington [82], this process is called migratory myoelectric cycle (MMC), which is divided into the following 4 phases, illustrated in Fig. 3 :
-
1.
Phase I (basal phase) lasts from 30 to 60 min with rare contractions;
-
2.
Phase II (preburst phase) lasts from 20 to 40 min with progressive high frequency and intensity contractions;
-
3.
Phase III (burst phase) lasts from 10 to 20 min. Intense contractions occur and all undigested material is carried from the stomach to the small intestine;
-
4.
Phase IV lasts from 0 to 5 min and occurs between phases III and I.
Fig. 3.
Schematic representation of the gastric emptying stages.
After meal, the contraction pattern changes from the fasting state to the fed state. It is known as digestive motility and comprises continuous contractions as in phase II. These contractions result in size reduction of the food particles, which are propelled into the suspended pylorus. During the onset of the fed state, MMC is delayed resulting in decreased emptying rate [83].
The short gastric retention time of dosage forms and the unpredictable rate of GE are limiting factors that can be overcome by GRDDSs. These systems can increase gastric retention time contributing to an enhancement of drugs solubility and, consequently, bioavailability [79].
4. Mechanisms of gastroretention
During the planning of GRDDSs using CS, some particularities should be taken into account like length of retention on the drug/dosage form in the stomach, penetration in the gastric mucosal layer, stomach pH and stability of the drug/pharmaceutical system and CS protonation, drug's molecule physicochemical parameters, as well as its solubility. Another important factor is the presence of lipids in the stomach. CS may interact with these molecules and cause changes in the drug release profile or even not release it [84].
The use of CS to obtain these types of systems has already been shown to be effective in terms of improving the gastric distribution of some drugs. Modi et al. [85] developed mucoadhesive CS nanoparticles (NPs) with ketoconazole and observed a 5-hour increase in residence and release time and, consequently, absorption of this drug by the stomach. In other study, Arora et al. [86] designed CS NPs containing alginate and pluronic F-127 for gastric amoxicillin release. The authors observed that the drug was protected from acid degradation, CS NPs showed adherence and mucopenetration releasing amoxicillin to deep regions of gastric mucus, increasing its therapeutic effectiveness. In addition, several research have already proven that CS is a polysaccharide that promotes the improvement of gastric delivery of the most diverse drugs, such as beberine [87], ranitidine hydrochloride [88], carvendilol [89] and moxifloxacin hydrochloride [90].
One of the main challenges that motivate the development of new GRDDSs is associated to the drugs physicochemical characteristics such as narrow absorption windows, local activity in the stomach, instability in the colon or distal small intestine and with low solubility at high pH [91]. The use of GRDDSs can circumvent these limitations mainly when associated with biopolymers as CS. This kind of association leads to the design of promising controlled drug delivery systems [92].
GRDDSs in contact with gastric medium can release the drug into stomach. GRDDSs can furnish controlled release profile, more efficient and adapted to different needs, promoting important advances in pharmacotechnical aspects [92].
Regarding the design of pharmaceutical systems for gastric diseases treatment, controlled drug release, specific absorption in stomach and pharmaceutical dosage form with tunable retention time are required. One of the strategies comprises the fabrication of GRDDSs with mucoadhesive properties. These systems can be obtained by using CS or CS combined with other polymers (polymeric blends). CS displays important and desirable characteristics for these smart systems such as biocompatibility, biodegradability, mucoadhesiveness, buoyancy, resistance to gastric pH and, in some cases, pharmacological activity [18,77,[92], [93], [94]].
4.1. GRDDSs mucus/bioadhesiveness
Bioadhesive systems may be comprised of natural or synthetic polymers that can interact attractively with a biological substrate. When the substrate is the mucus or mucosa, such systems are called mucoadhesive [95]. CS has become a popular component of this type of system because it is able to establish various types of mucus interactions with numerous hydrogen bonds and electrostatic interactions, promoting the dosage form adhesion [96]. The application of this system comes with several advantages: gastric drug release, including increased bioavailability, pharmaceutical dosage form (PDF) residence time in the stomach and reduction of first pass metabolism [89].
For the adhesion process, the GRDDSs and mucus molecules must interact at the interface. These interactions can occur through ionic and covalent bonds, Van der Waals interactions, hydrogen bonds and hydrophobic interactions [97]. The adhesion is marked by two main steps, contact and consolidation stages, as shown in Fig. 4 .
Stage I - The initial contact between the PDF and the mucus begins with interpenetration of the chains in the mucus and adhesion to the surface [97]. At this stage, the polymer chains begin to interact with the medium and mucus. In cases where GRDDSs is developed with CS, it is essential that the acidic medium solution acts by protonating and hydrating the CS chains expecting more effective interactions with mucus and PDF.
Stage II - PDF adhesion consolidation occurs because the polymers constituting the system are mostly hydrated, in the case of CS, protonated and, therefore, presenting greater conformational freedom, being able to establish cooperative interactions with the mucus. The main interactions that occur in this stage are the hydrogen bonds, Van der Waals and electrostatic interactions [97]. Due to the high conformational freedom of the polymer chains and the close contact of the mucus, these chains diffuse through the mucosal layer, deepening and establishing more lasting interactions. Other important contribution to this stage is the suction-type effect promoted using polymers with high affinity for water that causes dehydration of the mucus, promoting the adhesion of the system; this mechanism is explained by the dehydration theory [98].
Fig. 4.
Schematic representation of mucoadhesion stages.
Nowadays, the phenomenon of mucoadhesion can be explained in part by six theories that individually or together are not able to exhaust discussions on this topic, since it is a complex phenomenon [99]. For CS-based mucoadhesive systems, the principal theories that explain adhesion are wetting, diffusion, electronic interlocking, adsorption and fracture, which are briefly described below.
4.1.1. Wetting theory
This approach is applicable to low viscosity liquid and semi-solid mucoadhesive systems. This theory considers the interfacial tension to estimate the spreadability degree of the system in the mucus. After the spreading phenomenon, the system penetrates into deformations and mucus recesses modifying the surface characteristic, interactions and interfacial tension leading to the adhesion phenomenon [99].
A method to estimate whether a liquid will have good spreadability and adhesion on a surface is the scattering coefficient (S b) measure, using the Eq. (2) where: energies of interfaces are represented by γ B and γ T - interfacial tension between the system and the mucus, and the interfacial tensions of each of the phases by γ B - surface tension of the system and γ T - surface mucosal tension [97].
As the contact angle is easily calculated, a relation is established between it and the system-mucus interfacial tension. In this technique, a system with equal or close contact angles of 0° is sought [100].
| (2) |
4.1.2. Diffusion theory
This theory proposes that adhesion is a two-way process (Fig. 5 ) due to the diffusion of the polymer chains into the mucus glycoprotein network, as well as mucus diffusion into mucoadhesive system [100]. It is a process in which the penetration rate depends on the diffusion coefficient, MW, cross-linking density, chain mobility/flexibility and expansion capacity of both [101].
Fig. 5.
Schematic representation of diffusion and polymer interpenetration.
From results based on rheological analysis and spectroscopic techniques, the diffusion process is interpreted as a kinetic process, i.e., time-dependent, so that it is possible to estimate the time required for maximum adhesion between two substrates during interpenetration [102], as depicted in Eq. (3).
| (3) |
where (L) is the depth of interpenetration, and (Db) the diffusion coefficient.
The adhesion strength of a polymer is maximal when the penetration is close to the size of its chain. It is assumed that the depth of interpenetration required to produce an efficient mucoadhesion bond lies in the range 0.2–0.5 μm [99].
4.1.3. Electronic theory
In this theory, when chains with different electrical charges, solvated or not, approach to form ionic pairs, the mucoadhesive process is established. However, the most accepted approach is the occurrence of bidirectional [103,104] electrons transference from the mucus to the polymers. This bidirectional electronic transfer at Fermi level is responsible for promoting the formation of an attractive electric double layer at the muco-polymer interface. The strength of adhesion is proportional to the attraction intensity exerted on this interface [99,104,105].
4.1.4. Adsorption theory
According to this theory, after contact between the interfaces, adhesion occurs as the product of two groups of forces, primary and secondary. The primary ones are ionic and covalent, while the secondary forces are Van der Waal's forces, hydrogen bonding, and hydrophobic forces [106].
Interface characteristics determine which types of forces will predominate in mucoadhesion. In most systems, adhesion occurs through secondary type forces, which are individually weak, but due to their high number they become globally significant [107].
4.1.5. Fracture theory
This theory is substantially different from the others, because it measures the necessary force to separate one material from the other one, correlating it with the intensity of the adhesive forces. Thus, it is assumed that adhesion failure occurs between interfaces, ignoring the fact that failure usually occurs due to the low cohesion within one of the components involved in the adhesion process [97,108] (Fig. 6 ). Larger chains as well as systems with lower cross-linking densities have higher fracture forces, indicating higher adhesion strength [109].
Fig. 6.
Schematic representation of the fracture of the hydrated layer of the system.
The adhesion force is calculated indirectly by Eq. (4), where: Sm is the adhesion force, Fm is the force required for the detachment of the materials and A is the total area of contact between the interfaces.
| (4) |
It is a very suitable approach for solid and semi-solid systems in which there is little penetration of the mucus chains [110].
4.2. CS-related factors that interfere with mucoadhesion
The theoretical approach to be considered when working with mucoadhesive systems will depend on factors that may influence PF adhesion, and it is necessary to take into account some parameters for the development of the system. In this session, we will discuss the main factors to consider when the polymer used is CS.
4.2.1. Concentration
During the development phase of the mucoadhesive systems it is important to establish the optimum concentration of the polymer used, especially in semi-solid systems [111]. In solid systems, it is observed that, to some extent, the adhesion force increases proportionally with the polymers concentration. Systems obtained with reduced polymer concentrations can lead to poor adhesion and short duration due to the reduced number of interactions between the systems and polymer. Very high concentrations of polymer impart an excessive stability to PF, in which the chains are folded and less accessible to the solvent, reducing the freedom of the chains, in order to reduce the adhesion [112,113].
4.2.2. Crosslinking
The crosslinking degree is an important factor to consider when using CS to fabricate mucoadhesive systems, because the main method of obtaining solid systems such as nano and microparticles is ionotropic gelation using sodium tripolyphosphate (TPP) or glutaraldehyde [[114], [115], [116], [117]]. A high crosslinking density immobilizes the polymer chain, reducing its conformational freedom, affecting its flexibility and in turn on penetration and entanglement with the mucus. When CS is crosslinked, it can hydrated and swell. Swelling is positive for the adhesion of these systems by increasing the area of contact with the mucus, it can additionally provide control characteristics in the drug release [98] pH/DA and hydration.
Acidic media (pH < pKa 6.5) exert a strong effect on the adhesiveness of CS-containing mucoadhesive systems. This is due to the polycatalytic CS character, which contributes to the establishment of electrostatic interactions and hydrogen bonding with solvent and mucus. The CS DD is the main factor responsible for the cationic nature of this polymer, so that the reduction of the DD is directly associated with the increase in the positive charge's density of the chain, which contributes to the inter- and intra-chain CS repulsion. Thus, in acid media, the chains with the lowest DD will be more protonated and separated from each other, because of the electrostatic repulsion. The polymer protonation exposes groups previously inaccessible to the solvent, increasing the capacity to establish more hydrogen bonds, having an impact on PF hydration. The hydrated chains swell, increasing the adhesion area, interpenetration and interaction with mucus and water, through electrostatic interactions and hydrogen bonds. These interactions are the result of a pronounced increase in the conformational freedom of these hydrated chains [112,115].
4.2.3. Molecular weight (MW), shape and flexibility of the polymer chains
The increase in polymers MW can enhance the adhesiveness of mucoadhesive pharmaceutical systems as well as chains flexibility [118]. Long and flexible polymer chains such as high MW CS are able to enter deeper into mucus and establish more interactions because it presents greater mobility than polymers of very short and excessively enveloped chains. However, the use of polymers with excessively long chains is deleterious to mucoadhesive systems because they lose the ability to diffuse and entangle the mucus. In the development of these systems, it is essential to establish an optimum size for each polymer, since the shape and flexibility of the polymer chain is also a factor to be considered along with the size [97,107].
Table 3 lists some mucoadhesive GRDDSs obtained from CS and their particularities.
Table 3.
Mucoadhesive CS-based GRDDSs.
| Polymer(s)/drug | Objective | Methods | Results | Refs. |
|---|---|---|---|---|
| CS/Scorpion Venom Mesobuthus eupeus | Obtain CS nanoparticles containing scorpion venom in order to promote the animals hyperimmunization. | Nanoparticles (NP) were fabricated by ionotropic crosslinking with TPP in different CS concentrations. NPs were characterized. In vitro release assays were performed and the efficiency and encapsulation capacity determined. |
Particles obtained using CS 2 mg mL−1 and CS/TPP mass ratio of 2 containing 500 μg mL−1 of M. eupeus venom presented encapsulation efficiency of 91.1%, loading capacity of 76.3% and size mean of the 300–400 nm range (polydispersity index: 0.429). The release occurred in two stages: an initial stage with release of 60% of the venom in the first 10 h, followed by the controlled release for 60 h. |
[119] |
| CS, Tween 80, pluronic F127/Emodin | Obtain, characterize and test the inhibition effect of nanomicelles loaded with mucoadhesive beads (NFM-Beads) on tumor cells. | CS-coated emodin-loaded pluronic F127/Tween 80 mixed nanomicelles were prepared by thin film hydration method, tested against human gastric carcinoma. NFM-Beads were prepared by ionotropic gelation using sodium carboxymethylcellulose (CMC) and aluminum chloride. Release, floating and mucoadhesion of the samples were evaluated in vitro and gastric retention evaluated in vivo. |
The encapsulation efficiency was approximately 97.74% using 140 mg Pluronic F127 and 10 mg Tween 80. The size of the nanomicelles were in a range of 206.8–288.6 nm, PDI of 0.162–0.295, and their zeta potential was around +30.76 mV, suggesting physical stability. Emodin-loaded nanomicelles demonstrated better antitumor efficacy compared to corresponding emodin suspensions. NFM-Beads showed suitable buoyancy. Emodin release occurred by an anomalous process guided by swelling and erosion. In vivo gastroretention assays showed that the systems were maintained in the stomach of the rats after 8 h of administration, indicating strong adhesion to the gastric mucosa. |
[120] |
| CS-HPMC/flavonoid taxofolin-Syloid AL-1 | Obtain, characterize and test CS mucoadhesive GRDDS microparticles in the inclusion complex of Taxofolin (TAX) and Syloid® AL-1, with proton pump inhibitory action. | Inclusion complex taxofolin-Syloid AL-1 was prepared using the solvent evaporation method, in Tax:Syloid® AL-1 ratio of 30:70, w/w Microparticles were prepared using spray-dried method, in 6:1 ratio, CS:HPMC. Mucoadhesion and the drug release profile from the systems were tested. |
In vitro 90% of the TAX permeated pig mucus in 2 h, during the 45 to 120 min interval, the average permeation rate was 3.3 mg cm−2 min Ex vivo The average flux calculated across the mucosa in the interval of 45–120 min was 0.8 10−3 mg cm−2min. CS microparticles released taxifolin for 5 h in simulated gastric fluid. Microparticles adhered to gastric mucosa for 5 h avoiding TAX intestinal degradation. Microparticles favored Tax absorption in the stomach, achieving rapid therapeutic onset in the treatment of gastric ulcer, and made it possible to avoid Tax degradation in the small intestine. |
[121] |
| CS/Ranitidine | Preparation and characterization of CS mucoadhesive microparticles containing ranitidine. | The microparticles were obtained by means of CS ionotropic gelation using TPP, both in different concentrations. Size, shape, encapsulation efficiency, in vitro bioadhesion using agar plate method, in vitro release kinetics were successfully performed. |
The particles could be obtained with concentrations between 4–5% of TPP and 2% of CS showing an average size of 620–720 μm. These systems encapsulated between 41.67 and 87.58% of ranitidine. The bioadhesion test showed that between 62 and 83% of the particles remained adhered for 8 h. At least, 75% of the formulations showed buoyancy for 12 h. The release of ranitidine obeyed zero-order kinetics, with 86% of ranitidine being released in 10 h. | [122] |
| Micromotors coated with CS/Clarithromycin | Preparation and characterization of Clarithromycin (CLR)-loaded Mg-micromotors coated with CS in H. pylori treatment. | Mg-micromotor was prepared by an asymmetrical coating of the Mg microspheres with a thin TiO2 layer using atomic layer deposition, after Mg-TiO2 Janus microparticles were then coated with a PLGA film containing the CLR antibiotic payload. After the drug-loading step, the microparticles were coated with an outer thin CS layer (thickness ~ 100 nm). Micromotors were evaluated for drug carrying capacity. In vivo: Capacity to retain micromotors in the mouse stomach, anti-H. pylori therapeutic efficacy and toxicity assessment. |
The systems demonstrated security in animal model (mouse). Although the therapeutic efficacy of standard treatment and micromotors have been similar. The systems further reduced the bacterial load of H. pylori and showed an efficient distribution and retention in the mouse stomach. The micromotors were capable to load antibiotic cargo with high-loading efficiency- 1032 ± 37 μg per 2 mg micromotor. |
[123] |
| Interpolimeric blend (IPB) and Poly-x-lipo CS nanoparticles and Eudragit-enabled tablets (PXLNET)/l evodopa | Develop and test IPB gastroretentive system as well as nano-enabled gastroretentive levodopa delivery system. | Poly-x-lipo nanoparticles: Eudragit and CS were dispersed in HCl. After levodopa (L-dopa) and benserazide were added into dispersion, lecithin was dissolved in chloroform and added to the L-dopa-loaded polymeric solution. TPP dissolved in acetic acid was added under stirring and the nanoparticles produced were thereafter frozen and lyophilized. IPB- Dissolved methacrylate copolymer was added to the NaCMC solution under vigorous stirring with locust bean addition and nanoparticles incorporation. PXLNET was achieved by incorporating L-dopa-loaded nanoparticles into IPB with other components and compressed directly with carver hydraulic press. |
Both IPB and PXLNET matrices were mucoadhesive, the adhesion force and adhesion work for IPB matrices were found to be significantly more than the values observed for a PXLNET matrix. IPB presented triple mechanism of gastroretention (high density, swelling and gastro-adhesive). Levodopa release from IPB exhibited a more linear profile than Madopar® HBSa and Sinemet® CRb. In pH = 1.5 medium, IPB matrices released 90% of levodopa in 24 h vs Madopar® HBS 100% of the levodopa was released by the 16th h. In comparisons to the conventional dosage forms, both matrices have exhibited constant delivery over a prolonged time period and L-dopa-loaded and IPB matrices were the best fit for zero-order release. |
[124] |
ADMadopar® HBS - hydrodynamically balanced system Madopar (levodopa+benzerazide).
Sinemet® CR - controlled release Sinemet (carbidopa + levodopa).
Considering the Table 3, CS applicability is vast, and it can integrate different types of gastroretentive systems. Despite the use of this biopolymer as carrier since 1980s [125], recent advances in nanotechnology favored the development of efficient drug delivery systems such as CS-based micromotors and nanosystems. CS remains contemporaneous and promising biopolymer to develop gastroretentive/mucoadhesive systems with controlled release profile. Although CS gastroretention properties are usually ascribed to its mucoadhesivity, other action mechanisms will be discussed in the next sessions.
4.3. Expandable GRDDSs
Expandable gastroretentive systems were originally designed to treat veterinary pathologies with high dimensions of about 15 × 3 cm (length × diameter) by Laby [126] in order to avoid regurgitation after administration and arrival of the pharmaceutical dosage form in the rumen. Posteriorly, they were also adapted for treatment of humans by Johnson and Rowe [127]. Early, veterinary devices exploited the unfolding mechanism and had a ring-shape or multilayer insoluble polymeric sheet from Griffin and Brewer [128]. On the other hand, the first device for human use was a tablet based on thiolated gelatin which after hydration in the stomach and swelling to a larger size, making it impossible to pass through the pyloric sphincter (mean diameter, 12.8 ± 7 mm) [129]. However, some authors relate that the cutt-off size of pylorus cannot be determined exactly [130]. For example, Timmermans [131] stated that the size of GRDDSs can vary from 7 to 10 mm, while Khosla and Davis [132] noted that sizes up to 15 mm in diameter were not sufficient to provide gastric retention.
According to Klausner et al. [133], the expandable systems should combine a number of characteristics, of which retention in the stomach is cited after facilitated oral administration, high degradability of the dosage form preferentially in the stomach, no interference in the gastric motility, prolongation of the shelf-life, among others. These systems, also called plug type systems [134] receive this classification precisely because after administration they undergo a great expansion of their structure, which may be related to both a high swelling or unfolding, blocking their passage through the pylorus. In this way, the permanence of the system in the stomach is increased until drug release begins with subsequent reduction in size as the system is evacuated through the GIT.
It is important to point out the three basic configurations associated with expandable GRDDSs that make clear the understanding of its gastroretention mechanism. First one, an initial state represented by expandable GRDDSs reduced dimensions that facilitate oral administration, followed by a second state represented by its maximum state of expansion which is responsible for gastroretention, and then, the third and last state of minimal dimensions related to the release and depuration of the drug from the system [101,133,135].
Several mechanisms of gastroretention for expandable GRDDSs have been explored, but it should be mentioned that they all show many advantages and also disadvantages over each other [134]. Two basic mechanisms are responsible for the expansion of structures, which are swelling and unfolding, the former being related to an osmotic effect, and the latter to shape memory.
The main mechanism for swelling and drug release is diffusion through hydrophilic polymers, which can absorb water from the gastric fluid through the appearance of pores in the device surface, creating channels along its entire length, leading to a capillary effect [134]. In unfolding systems, the previously large system (uncompressed) with adequate mechanical properties is folded (compressed) to just open after reaching the stomach when capsules are dissolved in the gastric juice [133]. Different geometrical shapes have been attributed to unfolding systems, such as tetrahedron, ring or planner membrane [15].
A recurrent problem that can occur with unfolding systems is the fact that their shape memory is often not sufficient, in addition to being influenced by storage stress, and may lead to performance problems in the stomach [136]. Some strategies have emerged to overcome this problem, mainly by exploiting polymers that do not present plastic deformation, but only elastic deformation.
Other forms of expandable GRDDSs have been reported, such as the use of hydrogels that swell to the significant extent primarily as a function of polymer content, such as those made of polyvinyl pyrrolidone cross-linked with albumin [137]. Superporous hydrogels (those with pores of a size of hundreds of micrometers) have been also used as expandable GRDDSs. In this case, the high porosity of these systems has been related to a very rapid rate of swelling (generally 1 min and an increase of up to 1000× initial weight), as well as the ability to absorb a greater volume of liquids, both factors contributing effectively to a fast size-increasing [138].
Regardless of the mechanism responsible for the expansion, after suffering a large increase in size, these systems must have adequate mechanical properties so that immediate erosion does not occur as a result of loosening the polymer chains together with the intense peristalsis of the stomach. Thus, the extensive expansion for swelling GRDDSs, for example, must be attributed to the presence of physical/chemical crosslinking in the hydrophilic polymer network. These crosslinks prevent the dissolution of the polymer and hence maintain the physical integrity of the dosage form [139].
Despite the effectiveness of these systems in increasing the residence time in the stomach, they must have some properties to ensure patient safety. Therefore, such devices should not interfere with gastrointestinal motility, should not irritate the stomach mucosa due to its local retention, the device must have rounded edges, and re-administrations should only be performed after the biodegradation of the previous system present in the stomach to avoid accumulation with high doses.
As it is known, alendronate - a drug belonging to the class of bisphosphonates - is the drug of choice for treating osteoporosis, acting mainly through specific inhibition of bone resorption. However, its bioavailability after oral administration is significantly low (<1%) due to reduced intestinal absorption and the fact that when administered with food it leads to the formation of complexes that cannot be absorbed [140]. Although alendronate is mainly absorbed in the upper portions of the GIT, the short transit time further contributes to low absorption and bioavailability. Another marked limitation is the local irritation caused in the upper portions of the GIT. Thus, treatment requires daily a strict fasting state close to the medication schedule, reducing patient compliance. In this sense, it seems evident that the development of delivery systems that manage to overcome the rapid transit time of the upper GIT by increasing its residence time at the absorption site, should contribute significantly to improving the therapeutic efficacy of alendronate.
In line with this reasoning, Su and coworkers [8] developed hydrogels of CS with ring-opened polyvinyl pyrrolidone (CS/roPVP) as GRDDSs to enhance the bioavailability of alendronate for the treatment of osteoporosis. The excellent properties seen in CS, such as a high degree of swelling and mucoadhesiveness in gastric media, are decisive to delay clearance through the pyloric sphincter, as well as making the system's contact with the gastric mucosa more intimate, respectively, contributing to the enhancement of alendronate absorption. However, it is known that CS rapidly dissolves in the stomach, which would impair the prolongation of the alendronate release rates, reason why ring-opened (ro) polyvinyl pyrrolidone (PVP) was rationally added to the composition. In this way, gastroretentive properties were evaluated in terms of swelling ability and mucoadhesive measurements. Authors stated that tablets from CS/roPVP hydrogels showed an optimal swelling (up to 500%) which higher axial than lateral swelling ratio, which was attributed to the application of compression force axially. Likewise, increasing proportions of CS in CS/roPVP complex were responsible for the raise of forces of mucoadhesion. It was interesting to note that in vitro release profile of the reference FOSAMAX® was 100% in only 30 min, while the complexes promoted sustained release of alendronate. The set of acquired properties contributed to 3-fold enhancement of the oral alendronate bioavailability.
Although the scientific literature brings many studies on the development of gastroretentive systems exploring the high swelling capacity of CS, great care must be taken when classifying such systems as expandable. Since this mechanism acts to avoid the clearance of the pharmaceutical form through of the pylorus, a pronounced swelling is required which promotes a considerable increase in the size of the structures. This means that although many hydrophilic polymers undergo swelling when in contact with gastric fluid, it may not occur to the extent necessary to prevent its clearance from the upper GIT. In these cases, its importance is more related to the mucoadhesiveness and/or flutuation of the gel layer formed by CS swelling, as is the case with works that explore the dual mechanism of gastroretention, for example, floating and swelling [141]. The said gel layer of the swollen CS is also related to the decreased toxicity of some drugs interacting with their cationic groups by the presence of negative charges (alendronate sodium, for example).
4.4. Superporous hydrogels (SPHs)
Several studies have combined different strategies for development of gastroretention systems, in order to obtain a larger time of gastric residence [142]. developed CS superporous hydrogel with floating properties for sustained delivery of ranitidine hydrochloride. This system showed an increased gastric residence time of ranitidine hydrochloride with a floating and drug release time of 17 h.
Yin et al. [143] combined the mechanisms of swelling and mucoadhesion of the superporous hydrogels containing poly(acrylic acid-co-acrylamide)/O-carboxymethyl CS. The in vitro results demonstrated the swelling ratios, mechanical properties, mucoadhesive force and loading capacity were improved through varying the O-carboxymethyl CS content.
In another study, Park and Kim [144] developed glycol CS superporous hydrogels using a gas blowing method and glyoxal as the crosslinking agent. The results showed an increase in mechanical strength accompanied by the decrease in swelling kinetics without loss of water absorption capacity, important properties for the development of efficient gastric retention devices.
Superporous hydrogels of rosiglitazone loaded with CS were synthesized using glyoxal as a crosslinking agent for Gupta et al. [145]. The authors explored CS precisely because it is a polyelectrolyte that gives rise to pH-dependent hydrogels which, when in contact with acidic solutions, show a high swelling due to the presence of positive charges in the network, expanding its volume. Swelling rates were dependent on the extent of crosslinking, so that the higher the amount of crosslinking agent, the lower the swelling ratio. Ionic strength was also another factor that affected swelling, which ranged from 11% for HCl solution (pH 1.2) with 1 M NaCl to 156% for lowest ionic strength (0.0001 M).
4.5. Magnetic systems
Among the strategies used to increase the gastric residence time, magnetic systems are the only gastroretentive delivery systems based on the attraction between two magnets. This approach contain a small internal magnet present in dosage form, and an external magnet attached to the abdomen over the position of the stomach, which retains the system in the gastric region [101,139,146]. The extracorporeal magnet allows the control of gastrointestinal transit of the dosage form for a prolonged period of time [139].
Ito et al. [147] described the first magnetic system in 1990, performed for application to targeting therapy for esophageal cancer. Magnetic granules containing ultrafine ferrite (γ-Fe2O3) were guided to the esophagus with an external magnet for the initial 2 min and almost the entire amount of granules were retained in the region after 2 h of administration [147].
Although there are successful works, few systems with CS using the magnetic approach have been reported in literature. Magnetic hollow spheres coated with multilayer of CS/PAA developed by Zhang et al. [118] showed a sustained drug release and a strong magnetic response to magnetic field, which should provide the drug targeting to a desired tissue and/or organ through the external magnetic field [148].
The main advantages of magnetic systems are absolute drug targeting for target organs and tissues, the increase absorption and bioavailability of encapsulated drug, as well as the reduction of the concentration of the drug at non target sites [149]. However, the applicability of these systems depends of an external magnet, which must be positioned with high precision degree to allow drug release in the appropriate place, without compromising patient compliance [101,139]. Moreover, magnetic technical is expensive and requires specialized manufacturing, correct position degree on the stomach is very difficult to achieve and the magnets must have relatively constant gradients in order to avoid overdosing of toxic drug [149].
4.6. Density drug delivery-based systems
4.6.1. Floating drug delivery systems
Specifically, the design of controlled drug delivery systems (CDDSs) with efficient action on GIT is still a challenge due to the inability to restrain DDSs in specific regions of the GIT as well as the dependence of the unpredictable gastric emptying time [150]. These drawbacks can be overcome by GRDDSs, showing great potential for improving the bioavailability and site-specific absorption of drugs [150,151]. Among several GRDDSs approaches, floating drug delivery systems (FDDSs) are of particular interest due to their local active and narrow drugs absorption window for specific area.
FDDS was first reported by Coupe [152] and comprises a system with density lower (usually lower than 1 g cm−3) than gastric fluids that remain buoyant in the stomach for prolonged time and, consequently, leading to slow drug release at the specific rate. Additionally, gastric retention time is increased as well as the adsorbed drug concentration [150,151,153]. FDDS can be designed and classified according to the dosage forms and mechanism of buoyance: (1) single unit floating dosage forms comprising a) effervescent and b) non-effervescent systems; (2) multiple unit floating dosage forms comprising a) effervescent, b) non-effervescent and c) hollow microspheres systems and, finally, (3) raft forming systems [79,151,154].
4.6.1.1. Single unit dosage systems
Single unit dosage systems are easier fabricated than multiple unit dosage forms. However, the application of single unit dosage forms are less effective due to their all or nothing emptying process from stomach generating distinct bioavailability and high drug concentration delivered in others sites causing irritation [79,154].
Effervescent systems (or gas-generating systems) can be prepared by using swellable polymers and compounds that can generate gas bubbles [79,135,154]. The most common useful swellable polymers as matrix in these systems are methylcellulose, hydroxypropylmethylcellulose (HPMC), hydroxyethylcellulose (HEC), hydroxypropylcellulose (HPC), sodium carboxymethylcellulose (NaCMC), polyacrylated polymers, polyvinyl acetates, polycarbonates, agar, CS and alginate [79,135,154]. As effervescent compounds sodium bicarbonate, tartaric acid and citric acid are the most commonly chosen [79,135,154]. The mechanism of action occurs when the fabricated system (swellable polymer+gas generator compounds+drug) is placed in contact with the fluid gastric leading to CO2 elimination while the polymer swelling with water. The combination of these actions generate effective density less than gastric fluid increasing the time buoyancy besides to favor the controlled and site-specific drug delivery as shown in Fig. 7 [79,155].
Fig. 7.
Gastroretentive drug delivery system based on combination of polymer swelling and effervescence.
Jiménez-Martínez et al. [156] designed effervescent single unit dosage systems (SUFS) based on the introduction of captopril into Metolose SH 4000 SR (matrix) containing sodium bicarbonate (NaHCO3) as gas generator. Properties as compounds proportion (Metolose and sodium bicarbonate) and distinct compaction pressures can influence floating and drug release from proposed matrix. In vitro assays showed that at lower pressure (55 MPa) floating time higher than 8 h were achieved. The system density decreases by reducing the compaction pressure and increasing the sodium bicarbonate content. On the other hand, authors displayed that increasing sodium bicarbonate and polymer contents favor the reduction of drug dissolution rate from this system. Tadros [157] prepared effervescent SUFS using HPMC K15M and/or sodium alginate as release-retarding polymers and NaHCO3 or calcium carbonate (CaCO3) as a gas former agents containing ciprofloxacin hydrochloride (Cipro HCl). The tablet formulations (F7: HPMC K15M (21.42%, w/w), Na alginate (7.14%, w/w), NaHCO3 (20%, w/w) and F10: HPMC K15M (21.42%, w/w), Na alginate (7.14%, w/w), CaCO3 (20%, w/w)) exhibited good floating total and lag time, swelling ability, adhesion and sustained drug release rate acting as a promising GRDDS.
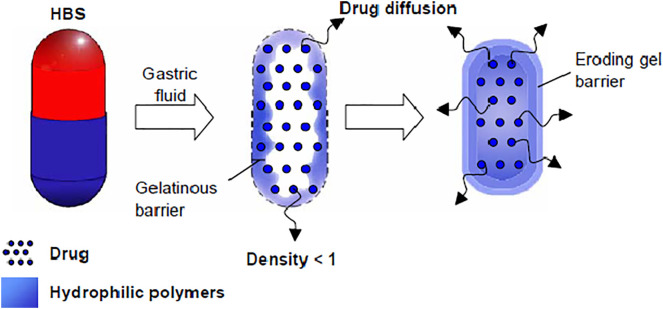
On the other hand, non-effervescent systems (such as hydrodynamically balanced systems, HBS) present one or more gel-forming or high swellable polymers. This system is prepared by mixing drug and polymer generally encapsulated by gelatin. The mechanism comprises fast dissolution of the capsule in gastric fluid followed by swelling of the polymers producing a buoyance gelatin mass with density lower than 1 g cm−3. The formed gelatinous barrier retains the capsule shape, avoiding the system disintegration promoting controlled drug release. The gelatinous surface erosion favors water permeation into inner layers preserving the surface hydration and the air trapped by the swollen polymers promotes buoyancy to the dosage forms, as depicted in Fig. 8 [79,135,151]. In addition, drug release is controlled by diffusion and dependent on the gel barrier erosion/dissolution. If the formulation is composed by fatty or viscous excipients, low-density systems could be achieved, decreasing water penetration and erosion process, which can reduce drug release rates [107,132].
Fig. 8.
Hydrodynamically balanced system (HBS). The gelatinous polymer barrier formation results from hydrophilic polymer swelling. Drug is released by diffusion and erosion of the gel barrier [135]. Copyright 2006.
Reproduced by permission of Elsevier Science Ltd.
Soni et al. [158] developed HSB systems by using natural and biodegradable polymer crushed puffed rice (CPR, as buoyance agent) combining with auxiliary polymers as high molecular weight CS (HMWCS, as swellable polymer) and HPMC (as gel-forming agent) as a single-unit floating capsule for Metoprolol Succinate (MS) sustained delivery. Authors reported that all evaluated samples displayed zero-order kinetics followed by Fickian diffusion model. This means that drug release from these HBS systems are controlled by drug diffusion through the gel barrier. Although the variation of pH and polymers could be evaluated, authors concluded that CPR associated with HPMC and HMWCS comprising a potential single-unit HBS systems for specific sustained release of hydrophilic drugs showing absorption window upper GIT.
The main drawback of single layer HBS consists on the dependence of the air arrested in the dry mass and the characteristics of the swellable polymers [135]. Considering the above mentioned, some approaches [135,[159], [160], [161]] have been developed to improve the efficiency of these dosage forms such as the design of bilayer formulations, as displayed in Fig. 9 . In these dosage forms, one layer promotes the fluctuation in gastric liquid whereas the other one control the drug release [135]. Oth et al. [161] fabricated a bilayer floating dosage unit containing misoprostol aiming to treat gastric and duodenal ulcers. As shown in Fig. 9A, both layers are composed by the same swellable polymer (in this case HPMC) but only one containing the misoprostol allowing this a bifunctional system performed buoyance and drug delivery independently in gastric media. Authors demonstrated that the production of large capsules lead to an increasing in gastric retention time (GRT). In vivo studies exhibited GRT dependent to the meal regimen. After a single meal, GRT was higher than 3 h whereas after several meals, the GRT was higher than 10 h. He et al. [160] produced bilayer floating tablet formulation (Fig. 9B) containing the combination of two distinct drugs such as metformin hydrochloride (MH) and pioglitazone hydrochloride (PG) to treat type 2 diabetes mellitus (T2DM). This formulation comprises the incorporation of each drug into two separate layers in order to achieve sustained MH release followed by immediate PG release. The bilayer tablet was fabricated by using wet granulation method with HPMC E5 for both layers as matrix and then compressed into tablet. In vitro assays showed buoyance time up to 24 h and floating lag time of 5 min besides sustained MH release controlled by diffusion manner (for 12 h) and fast PG release. In vivo tests performed in dogs suggested good absorption of PG and the enhancement of MH bioavailability with steady plasma concentration, decreased maximum plasma concentration and reduced time of maximum concentration appears as effective and promising GRDDSs to treat T2DM. Krögel and Bodmeier [159] developed multifunctional floating drug delivery systems using HPMC as hydrophilic polymer containing drugs (chlorpheniramine maleate or ibuprofen). One of the designed GRDDSs consists of two tablets (HPC + drug) fixed within two orifices of the cylinder (Fig. 9C) with air entrapped in the middle of the two tablets resulting in low density and, consequently, buoyance to the proposed system. Authors reported interesting GRDDSs that provides an enhancement on drug release as a function of HPMC viscosity and content, aqueous drug solubility and surface area of the matrix.
Fig. 9.
Three types of bilayer floating hydrodinamically balanced systems: (A) one layer with drug, (B) two layers containing drugs and (C) two layers containing drugs with gas entrapped.
Harrigan [162] described the fabrication of intragastric FDDSs with drugs present inside microporous compartment with pores along its top and bottom walls. In order to prevent a direct contact with the gastric surface, the peripheral walls of these systems are sealed aiming to avoid the direct contact of undissolved drugs with gastric mucosal surface. The containing entrapped air in the microporous compartment promotes buoyance over gastric media. The gastric fluid permeates the apertures favoring the drug dissolution and absorption in the stomach [139,163].
4.6.1.2. Multiple units dosage systems
Multiple units floating systems (MUFS) appear as an interesting way to overcome high variability of gastrointestinal transit time favoring a regular absorption. Beyond that, fast and high drug concentration release could be avoided and, consequently, gastrointestinal tract irritation [79,154]. Hooda et al. [122] reported the preparation of MUFS based on CS and sodium tripolyphosphate (TPP) via ionotropic gelation method containing Ranitidine hydrochloride (RHCl) as microspheres GRDDSs. RHCl has a narrow absorption window and is mainly absorbed in the proximal areas of GIT. Thus, conventional sustained-release dosage form reaches the colon, where it gets metabolized, resulting in low absorption and poor bioavailability (52%). In this way, Hooda et al. [122] proposed to design microspheres GRDDs based on chitosan (CS) and sodium tripolyphosphate (TPP) via ionotropic gelation containing RHCl. In vitro buoyance and mucoadhesive tests suggested that these microspheres present interesting floating (at least 12 h of buoyance) and bioadhesive properties. The above mentioned properties allowed the fabricated microspheres adhere to the gastric mucosal surface remaining prolonged period in stomach ensuring RHCl stability in gastric environment which eventually leads to better bioavailability at much lower dose. Additionally, in vitro drug release assays displayed a zero-order model suggesting that the drug (RHCl) transport across polymeric matrix occurred by a Fickian diffusion process [164].
Talukder [165] fabricated MUFS based on crosslinking reactions among sodium alginate, calcium chloride and low methoxylated pectin (anionic polysaccharide), in aqueous solution. The alginate beads can be achieved by dropping sodium alginate solution into aqueous solution of calcium chloride to precipitate calcium alginate beads. Separation and drying processes were performed by using air convection and freeze-drying. The alginate beads show porous structure that promotes buoyance for over 12 h besides increasing the residence time in gastric tract substantially (>5.5 h) [139,163]. Dey et al. [166] designed floating and mucoadhesive beads based on sodium alginate and hydroxypropyl methylcellulose (HPMC) as matrix polymers and CS as coating polymer. Sunflower oil was entrapped in this system to improve floating time. All these polymers were chosen to encapsulate amoxicillin trihydrate aiming to treat Helicobacter pylori infection. The floating and mucoadhesive system were prepared by ionotropic gelation method and CS used as polymer barrier promoting bioadhesion. Authors reported that these beads as interesting GRDDSs exhibiting good mucoadhesion properties, excellent efficiency of drug encapsulation increasing buoyance time and drug release for >24 h and 7 h, respectively [166].
As multiple units floating effervescent systems, Jyang et al. [167] fabricated a blend of Eudragit L100 and Eudragit RLPO containing dipyridamole (antithrombotic agent) via solid dispersion technique and afterwards, the dispersion was incorporated into alginate beads prepared by ionotropic gelation method using CaCO3 as gas-forming agent. Buoyance tests were performed showing that 92% of alginate beads remained floating after 9 h suggesting that drug encapsulated can be retained in the stomach indicating specific drug delivery. In vitro release studies displayed the controlled dipyridamole release using the proposed formulation (ratio of dipyridamole:Eudragit L100:Eudragit RLPO = 1:2:3) with 70% of release up to 9 h. In vivo results exhibited higher (about 2.52-fold) bioavailability for the FDDSs than commercial tablets corroborating this platform for stomach-specific drug delivery by the oral route.
4.6.1.3. Hollow microspheres systems
Hollow microspheres (or microballoons) comprise low-density polymers with low-density core relating to the shell. Most of them are MUFS fabricated by emulsion solvent diffusion method that entraps oil or air in hollow core [135,163]. Polymers such as polycarbonate, Eudragit S, cellulose acetate, calcium alginate, agar, CS and low methoxylated pectin are commonly used to fabricate hollow microspheres and foam-particles [168,169]. Great buoyancy and drug release from these systems depends on polymers amount, the plasticizer polymer ratio and the solvent used for formulations [135]. Fig. 10 displays hollow (low-density) microalloons (Fig. 10a) and foam-particles (Fig. 10b) obtained by solvent evaporation.
Fig. 10.
Microballoons (a) and foam-particles (b) as multiple units floating effervescent systems [135,168]. Copyright 2006.
Reproduced by permission of Elsevier Science Ltd.
Pawar et al. [153] formulated microballoons as GRDDS based on mucoadhesive and floating mechanisms to delivery norfloxacin (NFX). These systems were fabricated by using HPMC and ethylcellulose (EC) as core matrix by solvent evaporation method. CS coating was prepared via ionotropic gelation method to improve mucoadhesion properties and increasing gastroretention time of the microballoons. Good in vitro buoyance was achieved for these systems. Pharmacokinetics results displayed an enhancement of NFX bioavailability for these systems. In vivo assays showed that the microcarriers could prolong half-life of NFX and increasing plasma drug concentration.
4.6.1.4. Raft-forming systems
Another system that has attracted great attention in the administration of drugs to treat gastrointestinal infections is the raft forming systems. The mechanism of raft preparation consists on the formation of viscous cohesive gel containing entrapped CO2 gas in contact with gastric fluid [79,135]. Usually, the formulation containing gel forming agent and antiacids such as alkaline bicarbonates or carbonates to reduce gastric acidity. The buoyance of raft systems occurs by CO2 formation and elimination, acting as a blocker to preclude reflux of gastric media [79,135]. Fig. 11 depicted a schematic illustration of raft forming systems as barrier in specific stomach region.
Fig. 11.
Schematic illustration of the barrier formed by a raft-forming system.
Raft forming systems usually form a low-density layer on gastric media with high surface area regarding tablets, favoring drug release and the bioavailability enhancement. Beyond that, these GRDDSs show fast buoyance than other floating dosage forms, improving therapeutic efficacy been easily administrated (once a day) to the patient [139]. On the other hand, the stability of these GRDDSs are susceptible to chemical modifications, like: oxidation and hydrolysis. Exposure to the temperature and pH variation as well as types of radiation (ultraviolet-visible, X-ray, among others) could also promotes modification in these systems [139]. El Nabawari [170] demonstrated the preparation of a floating raft system with Mebeverine HCl (MbH) using sodium alginate, HPMC K100M and precirol. The optimized formulation (FRS-11: sodium alginate 3%/HPMC K100M 1%/precirol 2%) displayed floating lag time and total buoyance time near to 15 s and 12 h (in simulated gastric media), respectively. In contrast, FRS-11 formulation exhibited sustained drug release of 82% up to 8 h showing good MbH bioavailability compared to the commercial product. These results suggest the designed raft-forming system as a potential GRDDS to prolong drug action aiming clinical trials [170,171].
4.6.2. High-density drug delivery systems
Different of low-density systems, dosage forms with density next to 3 g mL−1 can sink to the bottom of the stomach to withstand in vivo peristaltic movement remaining intact independently of the GIT disturbance as depicted in Fig. 12 [110]. By using high-density systems (from 2.5 to 3.0 g mL−1) the gastric retention time can increase up to 25 h [155]. These systems are formulated with inorganic compounds such as barium sulphate, zinc oxide, iron powder and titanium dioxide increasing the density of the dosage form. However, up to now no effective action of these systems were detected in human just in pre-clinical assays [101,151,155]. In addition, the main drawbacks regarding high-density systems consist on the fabrication of pellets with high drug concentration and achieve systems with a density next to 3 g mL−1 [101].
Fig. 12.
Schematic illustration of the gastroretentive drug delivery system based on high-density dosage form.
5. Conclusions
CS is a biopolymer with a wide applicability, highlighted as a gastroretentive drug delivery system-forming material, because it brings together special and desirable biochemical properties in this type of system. These characteristics enable the fabrication of CS-based drug delivery systems for the stomach with mucoadhesive, floating, expandable, biocompatible, nontoxic and biodegradable properties. Advances in gastroretentive drug delivery system development technology associated with the versatility of CS will be able to deliver increasingly efficient and selective dosage forms with new mechanisms of gastroretention, as well as capable of increasing the capacity of drug internalization using lower drug doses to reach the therapeutic range, ensuring greater safety.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
This study is part of the National Institute of Science and Technology in Pharmaceutical Nanotechnology: a transdisciplinary approach INCT-NANOFARMA, which is supported by São Paulo Research Foundation (FAPESP, Brazil) Grant number:2014/50928-2, "Conselho Nacional de Desenvolvimento Científico e Tecnológico" (CNPq, Brazil) Grant number:465687/2014-8, "Coordenação de Aperfeiçoamento de Pessoal de Nível Superior" (Capes) Grant number:88887.199954/2018-00 and to Ministry of Education of Brazil.
References
- 1.Gupta K.C., Ravi Kumar M.N.V. An overview on chitin and chitosan applications with an emphasis on controlled drug release formulations. J. Macromol. Sci. Part C Polym. Rev. 2000;40:273–308. [Google Scholar]
- 2.Aranaz I., Acosta N., Civera C., Elorza B., Mingo J., Castro C., Gandía M.D. los L., Heras Caballero A. Cosmetics and cosmeceutical applications of chitin, chitosan and their derivatives. Polymers (Basel) 2018;10:213. doi: 10.3390/polym10020213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shahidi F., Arachchi J.K.V., Jeon Y.J. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999;10:37–51. [Google Scholar]
- 4.Rameshthangam P., Solairaj D., Arunachalam G., Ramasamy P. Chitin and chitinases: biomedical and environmental applications of chitin and its derivatives. J. Enzym. 2018;1:20. [Google Scholar]
- 5.Kumar M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000;46:1–27. [Google Scholar]
- 6.Kim S.-K. CRC Press; 2010. Chitin, Chitosan, Oligosaccharides and Their Derivatives: Biological Activities and Applications. [Google Scholar]
- 7.Croisier F., Jérôme C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013;49:780–792. [Google Scholar]
- 8.Su C., Ho H., Chen Y., Yu Y., Liu D., Chao F. 2018. Complex Hydrogels Composed of Chitosan with Ring-opened Polyvinyl Pyrrolidone as a Gastroretentive Drug Dosage Form to Enhance the Bioavailability of Bisphosphonates; pp. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porwal A., Dwivedi H., Pathak K. 2012. Decades of Research in Drug Targeting Using Gastroretentive Drug Delivery Systems for Antihypertensive Therapy; pp. 1–15. [Google Scholar]
- 10.Liechty W.B., Kryscio D.R., Slaughter B.V., Peppas N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010;1:149–173. doi: 10.1146/annurev-chembioeng-073009-100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SUDHA P.N., Gomathi T., Aisverya S. Recent research in the applications of chitin, chitosan and oligosaccharides. Green Polym. Environ. Pollut. Control. 2016:303. [Google Scholar]
- 12.Hamedi H., Moradi S., Hudson S.M., Tonelli A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: a review. Carbohydr. Polym. 2018;199:445–460. doi: 10.1016/j.carbpol.2018.06.114. [DOI] [PubMed] [Google Scholar]
- 13.Gupta R., Tripathi P., Bhardwaj P., Mahor A. Recent advances in gastro retentive drug delivery systems and its application on treatment of H. pylori infections. 2018;7:404–410. [Google Scholar]
- 14.Schneider F., Koziolek M., Weitschies W. In vitro and in vivo test methods for the evaluation of gastroretentive dosage forms. Pharmaceutics. 2019;11:416. doi: 10.3390/pharmaceutics11080416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nayak A.K., Maji R., Das B. Gastroretentive drug delivery systems: a review. Asian J. Pharm. Clin. Res. 2010;3:2–10. [Google Scholar]
- 16.Lopes E.C.N., Sousa K.S., Airoldi C. Chitosan—cyanuric chloride intermediary as a source to incorporate molecules—thermodynamic data of copper/biopolymer interactions. Thermochim. Acta. 2009;483:21–28. [Google Scholar]
- 17.Robert G.A.F. Vol. 1. Macmillan Education UK; London: 1992. Structure of Chitin and Chitosan; pp. 1–53. (Chitin Chem.). [Google Scholar]
- 18.Rinaudo M. Chitin and chitosan: properties and applications. Prog. Polym. Sci. 2006;31:603–632. [Google Scholar]
- 19.Peniche C., Argüelles-Monal W., Goycoolea F.M. Monomers, Polym. Compos. From Renew. Resour. Elsevier; 2008. Chitin and chitosan: major sources, properties and applications; pp. 517–542. [Google Scholar]
- 20.Winterowd J.G., Sandford P.A. Chitin and chitosan. FOOD Sci. Technol. YORK-MARCEL DEKKER. 1995:441. [Google Scholar]
- 21.Teng D. Chitosan-based Hydrogels Funct. Appl. CRC Press Boca; Raton, Florida: 2011. From chitin to chitosan; pp. 2–33. [Google Scholar]
- 22.Stephen A.M., Phillips G.O., Williams P.A. 2006. Food Polysaccharides and Their Applications. [Google Scholar]
- 23.Sharma S., Batra S. Mater. Biomed. Eng. Elsevier; 2019. Recent advances of chitosan composites in artificial skin: the next era for potential biomedical application; pp. 97–119. [Google Scholar]
- 24.Hidangmayum A., Dwivedi P., Katiyar D., Hemantaranjan A. Application of chitosan on plant responses with special reference to abiotic stress. Physiol. Mol. Biol. Plants. 2019;25:313–326. doi: 10.1007/s12298-018-0633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ways M., Mohammed T., Lau W.M., Khutoryanskiy V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers (Basel) 2018;10:267. doi: 10.3390/polym10030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali A., Ahmed S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018;109:273–286. doi: 10.1016/j.ijbiomac.2017.12.078. [DOI] [PubMed] [Google Scholar]
- 27.Maiti S., Jana S. Woodhead Publishing; 2019. Polysaccharide Carriers for Drug Delivery. [Google Scholar]
- 28.Kassem M.A.A., ElMeshad A.N., Fares A.R. Lyophilized sustained release mucoadhesive chitosan sponges for buccal buspirone hydrochloride delivery: formulation and in vitro evaluation. AAPS PharmSciTech. 2015;16:537–547. doi: 10.1208/s12249-014-0243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tavaria F.K., Costa E.M., Pina-Vaz I., Carvalho M.F., Pintado M.M. A quitosana como biomaterial odontológico: estado da arte. Rev. Bras. Eng. Biomédica. 2013;29:110–120. [Google Scholar]
- 30.Bina B., Mahdinezhad M.H., Nikaein M., Movahedian A.H. 2009. Effectiveness of Chitosan as Natural Coagulant Aid in Treating Turbid Waters. [Google Scholar]
- 31.Pontius F.W. Chitosan as a drinking water treatment coagulant. Am. J. Civ. Eng. 2016;4:205–215. [Google Scholar]
- 32.Goycoolea F.M., Milkova V. Electrokinetic behavoir of chitosan adsorbed on o/w nanoemulsion droplets. Colloids Surfaces A Physicochem. Eng. Asp. 2016;519:205–211. [Google Scholar]
- 33.Hirano S. Chitin biotechnology applications. Biotechnol. Annu. Rev. 1996;2:237–258. doi: 10.1016/s1387-2656(08)70012-7. [DOI] [PubMed] [Google Scholar]
- 34.Dotto G.L., Campana-Filho S.P., de Almeida Pinto L.A. Bentham Science Publishers; 2017. Frontiers in Biomaterials: Chitosan Based Materials and Its Applications. [Google Scholar]
- 35.Kim S. Competitive biological activities of chitosan and its derivatives: antimicrobial, antioxidant, anticancer, and anti-inflammatory activities. Int. J. Polym. Sci. 2018;2018 [Google Scholar]
- 36.Karabiyik Acar O., Kayitmazer A.B., Torun Kose G. Hyaluronic acid/chitosan coacervate-based scaffolds. Biomacromolecules. 2018;19:1198–1211. doi: 10.1021/acs.biomac.8b00047. [DOI] [PubMed] [Google Scholar]
- 37.Sreekumar S., Goycoolea F.M., Moerschbacher B.M., Rivera-Rodriguez G.R. Parameters influencing the size of chitosan-TPP nano-and microparticles. Sci. Rep. 2018;8:4695. doi: 10.1038/s41598-018-23064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henao E., Delgado E., Contreras H., Quintana G. Polyelectrolyte complexation versus ionotropic gelation for chitosan-based hydrogels with carboxymethylcellulose, carboxymethyl starch, and alginic acid. Int. J. Chem. Eng. 2018;2018 [Google Scholar]
- 39.Zargar V., Asghari M., Dashti A. 2015. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications; pp. 204–226. [Google Scholar]
- 40.Milewska A., Chi Y., Szczepanski A., Barreto-Duran E., Liu K., Liu D., Guo X., Ge Y., Li J., Cui L., et al. BioRxiv. 2020. HTCC as a highly effective polymeric inhibitor of SARS-CoV-2 and MERS-CoV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma Z., Garrido-maestu A., Casey K. Application, mode of action, and in vivo activity of chitosan and its micro- and nanoparticles as antimicrobial agents: a review. Carbohydr. Polym. 2017;176:257–265. doi: 10.1016/j.carbpol.2017.08.082. [DOI] [PubMed] [Google Scholar]
- 42.Li J., Wu Y., Zhao L. Antibacterial activity and mechanism of chitosan with ultra high molecular weight. Carbohydr. Polym. 2016;148:200–205. doi: 10.1016/j.carbpol.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 43.Chang S., Lin Y., Wu G., Huang C., Jane G. Effect of chitosan molecular weight on anti-inflammatory activity in the RAW 264.7 macrophage model. Int. J. Biol. Macromol. 2019;131:167–175. doi: 10.1016/j.ijbiomac.2019.02.066. [DOI] [PubMed] [Google Scholar]
- 44.Tavaria F., Jorge M.P., Ruiz L.T.G., Pintado M.E., Carvalho J.E., et al. Anti-proliferative, anti-inflammatory, anti-ulcerogenic and wound healing properties of chitosan. Curr. Bioact. Compd. 2016;12:114–122. [Google Scholar]
- 45.Anraku M., Gebicki J.M., Iohara D., Tomida H., Uekama K., Maruyama T., Hirayama F., Otagiri M. Antioxidant activities of chitosans and its derivatives in in vitro and in vivo studies. Carbohydr. Polym. 2018;199:141–149. doi: 10.1016/j.carbpol.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 46.García M.A., Pérez L., de la Paz N., González J., Rapado M., Casariego A. Effect of molecular weight reduction by gamma irradiation on chitosan film properties. Mater. Sci. Eng. C. 2015;55:174–180. doi: 10.1016/j.msec.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 47.Jennings C.D., Boleyn K., Bridges S.R., Wood P.J., Anderson J.W. A comparison of the lipid-lowering and intestinal morphological effects of cholestyramine, chitosan, and oat gum in rats. Proc. Soc. Exp. Biol. Med. 1988;189:13–20. doi: 10.3181/00379727-189-42773. [DOI] [PubMed] [Google Scholar]
- 48.Zou P., Yang X., Wang J., Li Y., Yu H., Zhang Y., Liu G. Advances in characterisation and biological activities of chitosan and chitosan oligosaccharides. Food Chem. 2016;190:1174–1181. doi: 10.1016/j.foodchem.2015.06.076. [DOI] [PubMed] [Google Scholar]
- 49.Xue Y., Wu M., Liu Z., Song J., Luo S., Li H., Li Y., Jin L. In vitro and in vivo evaluation of chitosan scaffolds combined with simvastatin-loaded nanoparticles for guided bone regeneration. J. Mater. Sci. Mater. Med. 2019;30:47–60. doi: 10.1007/s10856-019-6249-3. [DOI] [PubMed] [Google Scholar]
- 50.Funakoshi T., Majima T., Iwasaki N., Yamane S., Masuko T., Minami A., Harada K., Tamura H., Tokura S., Nishimura S.-I. Novel chitosan-based hyaluronan hybrid polymer fibers as a scaffold in ligament tissue engineering. J. Biomed. Mater. Res. Part A An Off. J. Soc. Biomater. Japanese Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2005;74:338–346. doi: 10.1002/jbm.a.30237. [DOI] [PubMed] [Google Scholar]
- 51.Yang T.-L., Young T.-H. The enhancement of submandibular gland branch formation on chitosan membranes. Biomaterials. 2008;29:2501–2508. doi: 10.1016/j.biomaterials.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 52.Kim S. 2018. Review Article Competitive Biological Activities of Chitosan and Its Derivatives: Antimicrobial, Antioxidant, Anticancer, and Anti-inflammatory Activities; p. 2018. [Google Scholar]
- 53.Chien R.-C., Yen M.-T., Mau J.-L. Antimicrobial and antitumor activities of chitosan from shiitake stipes, compared to commercial chitosan from crab shells. Carbohydr. Polym. 2016;138:259–264. doi: 10.1016/j.carbpol.2015.11.061. [DOI] [PubMed] [Google Scholar]
- 54.Sundaram M.N., Amirthalingam S., Mony U., Kerala P., Jayakumar R. Injectable chitosan-nano bioglass composite hemostatic hydrogel for effective bleeding control. Int. J. Biol. Macromol. 2019;129:936–943. doi: 10.1016/j.ijbiomac.2019.01.220. [DOI] [PubMed] [Google Scholar]
- 55.Bennett B.L., Littlejohn L.F., Kheirabadi B.S., Butler F.K., Kotwal R.S., Dubick M.A., Bailey J.A. Management of external hemorrhage in tactical combat casualty care: chitosan-based hemostatic gauze dressings-TCCC Guidelines-Change 13–05. J Spec Oper Med. 2014;14:40–57. doi: 10.55460/03VO-8FLO. [DOI] [PubMed] [Google Scholar]
- 56.Friedman A.J., Phan J., Schairer D.O., Champer J., Qin M., Pirouz A., Blecher-paz K., Oren A., Liu P.T., Modlin R.L., Kim J. Antimicrobial and anti-inflammatory activity of chitosan – alginate nanoparticles: a targeted therapy for cutaneous pathogens. J. Invest. Dermatol. 2013;133:1231–1239. doi: 10.1038/jid.2012.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eo S.S., Eong H.J., Hung H.C., Ee J.L., Ou Y.Y. Vol. 26. 2003. Inhibitory Effect of High Molecular Weight Water-soluble Chitosan on Hypoxia-induced Inflammatory Cytokine Production; pp. 717–721. [DOI] [PubMed] [Google Scholar]
- 58.Park P.-J., Koppula S., Kim S.-K. 18 antioxidative activity of chitosan, chitooligosaccharides and their derivatives, chitin, chitosan, oligosaccharides their deriv. Biol. Act. Appl. 2010:241. [Google Scholar]
- 59.Tao W., Sun W., Liu L., Wang G., Xiao Z., Pei X., Wang M. Chitosan oligosaccharide attenuates nonalcoholic Fatty liver disease induced by high fat diet through reducing lipid accumulation, inflammation and oxidative stress in C57BL/6 mice. Mar. Drugs. 2019;17:645–660. doi: 10.3390/md17110645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adhikari H.S., Yadav P.N. Anticancer activity of chitosan, chitosan derivatives, and their mechanism of action. Int. J. Biomater. 2018;2018 doi: 10.1155/2018/2952085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soundarya S.P., Menon A.H., Chandran S.V., Selvamurugan N. Bone tissue engineering: scaffold preparation using chitosan and other biomaterials with different design and fabrication techniques. Int. J. Biol. Macromol. 2018;119:1228–1239. doi: 10.1016/j.ijbiomac.2018.08.056. [DOI] [PubMed] [Google Scholar]
- 62.Dutta P.K. Springer; 2016. Chitin and Chitosan for Regenerative Medicine. [Google Scholar]
- 63.Li Z., Ramay H.R., Hauch K.D., Xiao D., Zhang M. Vol. 26. 2005. Chitosan – Alginate Hybrid Scaffolds for Bone Tissue Engineering; pp. 3919–3928. [DOI] [PubMed] [Google Scholar]
- 64.Balan V., Verestiuc L. Strategies to improve chitosan hemocompatibility: a review. Eur. Polym. J. 2014;53:171–188. [Google Scholar]
- 65.Yang J., Tian F., Wang Z., Wang Q., Zeng Y., Chen S. 2007. Effect of Chitosan Molecular Weight and Deacetylation Degree on Hemostasis; pp. 131–137. [DOI] [PubMed] [Google Scholar]
- 66.Baldrick P. The safety of chitosan as a pharmaceutical excipient. Regul. Toxicol. Pharmacol. 2010;56:290–299. doi: 10.1016/j.yrtph.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 67.Hirano S., Itakura C., Seino H., Akiyama Y., Nonaka I., Kanbara N., Kawakami T. Chitosan as an ingredient for domestic animal feeds. J. Agric. Food Chem. 1990;38:1214–1217. [Google Scholar]
- 68.Vårum K.M., Myhr M.M., Hjerde R.J.N., Smidsrød O. In vitro degradation rates of partially N-acetylated chitosans in human serum. Carbohydr. Res. 1997;299:99–101. doi: 10.1016/s0008-6215(96)00332-1. [DOI] [PubMed] [Google Scholar]
- 69.Bhattarai N., Gunn J., Zhang M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010;62:83–99. doi: 10.1016/j.addr.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 70.Park J.H., Saravanakumar G., Kim K., Kwon I.C. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv. Drug Deliv. Rev. 2010;62:28–41. doi: 10.1016/j.addr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 71.McConnell E.L., Murdan S., Basit A.W. An investigation into the digestion of chitosan (noncrosslinked and crosslinked) by human colonic bacteria. J. Pharm. Sci. 2008;97:3820–3829. doi: 10.1002/jps.21271. [DOI] [PubMed] [Google Scholar]
- 72.Kaczmarek M.B., Struszczyk-Swita K., Li X., Szczesna-Antczak M., Daroch M. Enzymatic modifications of chitin, chitosan and chitooligosaccharides. Front. Bioeng. Biotechnol. 2019;7:243. doi: 10.3389/fbioe.2019.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chae S.Y., Jang M.-K., Nah J.-W. Influence of molecular weight on oral absorption of water soluble chitosans. J. Control. Release. 2005;102:383–394. doi: 10.1016/j.jconrel.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 74.Gulbake A., Jain S.K. Chitosan: a potential polymer for colon-specific drug delivery system. Expert Opin. Drug Deliv. 2012;9:713–729. doi: 10.1517/17425247.2012.682148. [DOI] [PubMed] [Google Scholar]
- 75.Alexander K. Pharmacology. Elsevier; 2009. Dosage forms and their routes of administration; pp. 9–29. [Google Scholar]
- 76.Lipka E., Amidon G.L. Setting bioequivalence requirements for drug development based on preclinical data: optimizing oral drug delivery systems. J. Control. Release. 1999;62:41–49. doi: 10.1016/s0168-3659(99)00022-x. [DOI] [PubMed] [Google Scholar]
- 77.Gharge V., Pawar P. Vol. 2. 2017. Recent Trends in Chitosan Based Nanotechnology: A Reference to Ocular Drug Delivery System; pp. 98–105. [Google Scholar]
- 78.Niharika M.G., Krishnamoorthy K., Akkala M. Overview on floating drug delivery system. Int J App Pharm. 2018;10:65–71. [Google Scholar]
- 79.Chandel A., Chauhan K., Parashar B., Kumar H., Arora S. Floating drug delivery systems: a better approach. Int. Curr. Pharm. J. 2012;1:119–127. [Google Scholar]
- 80.Patil H., Tiwari R.V., Repka M.A. Recent advancements in mucoadhesive floating drug delivery systems: a mini-review. J. Drug Deliv. Sci. Technol. 2016;31:65–71. [Google Scholar]
- 81.Vantrappen G.R., Peeters T.L., Janssens J. The secretory component of the interdigestive migrating motor complex in man. Scand. J. Gastroenterol. 1979;14:663–667. doi: 10.3109/00365527909181934. [DOI] [PubMed] [Google Scholar]
- 82.Washington N., Washington C., Wilson C. CRC Press; 2000. Physiological pharmaceutics: barriers to drug absorption. [Google Scholar]
- 83.Gupta G., Singh A. A short review on stomach specific drug delivery system. Int. J. PharmTech Res. 2012;4:1527–1545. [Google Scholar]
- 84.Krajewska B. Wydro Pawełand, Hac-Wydro K. Chitosan as a lipid binder: a Langmuir monolayer study of chitosan- lipid interactions. Biomacromolecules. 2007;8:2611–2617. doi: 10.1021/bm700453x. [DOI] [PubMed] [Google Scholar]
- 85.Modi J., Joshi G., Sawant K. Chitosan based mucoadhesive nanoparticles of ketoconazole for bioavailability enhancement: formulation, optimization, in vitro and ex vivo evaluation. Drug Dev. Ind. Pharm. 2013;39:540–547. doi: 10.3109/03639045.2012.666978. [DOI] [PubMed] [Google Scholar]
- 86.Arora S., Gupta S., Narang R.K., Budhiraja R.D. Amoxicillin loaded chitosan—alginate polyelectrolyte complex nanoparticles as mucopenetrating delivery system for H. pylori. Sci. Pharm. 2011;79:673–694. doi: 10.3797/scipharm.1011-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chang C.-H., Huang W.-Y., Lai C.-H., Hsu Y.-M., Yao Y.-H., Chen T.-Y., Wu J.-Y., Peng S.-F., Lin Y.-H. Development of novel nanoparticles shelled with heparin for berberine delivery to treat Helicobacter pylori. Acta Biomater. 2011;7:593–603. doi: 10.1016/j.actbio.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 88.Darbasizadeh B., Motasadizadeh H., Foroughi-Nia B., Farhadnejad H. Tripolyphosphate-crosslinked chitosan/poly (ethylene oxide) electrospun nanofibrous mats as a floating gastro-retentive delivery system for ranitidine hydrochloride. J. Pharm. Biomed. Anal. 2018;153:63–75. doi: 10.1016/j.jpba.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 89.Radhakrishnan P., Singh S.K., Verma P.R.P. Pharmaceutical formulations to increase gastric residence time: concepts and strategies. Drug Deliv. Lett. 2017;7:190–200. [Google Scholar]
- 90.Praveen R., Verma P.R.P., Venkatesan J., Yoon D.-H., Kim S.-K., Singh S.K. In vitro and in vivo evaluation of gastro-retentive carvedilol loaded chitosan beads using GastroplusTM. Int. J. Biol. Macromol. 2017;102:642–650. doi: 10.1016/j.ijbiomac.2017.04.067. [DOI] [PubMed] [Google Scholar]
- 91.Wilson C.G., Crowley P.J. Springer; 2011. Controlled Release in Oral Drug Delivery. [Google Scholar]
- 92.McClements D.J. Advances in nanoparticle and microparticle delivery systems for increasing the dispersibility, stability, and bioactivity of phytochemicals. Biotechnol. Adv. 2018:0–1. doi: 10.1016/j.biotechadv.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 93.Chuah L.H., Billa N., Roberts C.J., Burley J.C., Manickam S. Curcumin-containing chitosan nanoparticles as a potential mucoadhesive delivery system to the colon. Pharm. Dev. Technol. 2013;18:591–599. doi: 10.3109/10837450.2011.640688. [DOI] [PubMed] [Google Scholar]
- 94.Spagnol C.M., Zaera A.M., Isaac V.L.B., Corrêa M.A., Salgado H.R.N. Release and permeation profiles of spray-dried chitosan microparticles containing caffeic acid. Saudi Pharm. J. 2018;26:410–415. doi: 10.1016/j.jsps.2017.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bruschi M.L. Woodhead Publishing; 2015. Strategies to Modify the Drug Release From Pharmaceutical Systems. [Google Scholar]
- 96.Sandri G., Rossi S., Bonferoni M.C., Ferrari F., Mori M., Caramella C. The role of chitosan as a mucoadhesive agent in mucosal drug delivery. J. Drug Deliv. Sci. Technol. 2012;22:275–284. [Google Scholar]
- 97.Smart J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005;57:1556–1568. doi: 10.1016/j.addr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 98.Andrews G.P., Laverty T.P., Jones D.S. Mucoadhesive polymeric platforms for controlled drug delivery. Eur. J. Pharm. Biopharm. 2009;71:505–518. doi: 10.1016/j.ejpb.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 99.Carvalho F.C., Bruschi M.L., Evangelista R.C., Palmira M., Gremião D. Vol. 46. 2010. Mucoadhesive Drug Delivery Systems; pp. 1–18. [Google Scholar]
- 100.Mansuri S., Kesharwani P., Jain K., Tekade R.K., Jain N.K. Mucoadhesion: a promising approach in drug delivery system. REACT. 2016;100:151–172. [Google Scholar]
- 101.Lopes C.M., Bettencourt C., Rossi A., Buttini F., Barata P. Overview on gastroretentive drug delivery systems for improving drug bioavailability. Int. J. Pharm. 2016;510:144–158. doi: 10.1016/j.ijpharm.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 102.Thakur S., Kesharwani P., Tekade R.K., Jain N.K. Impact of pegylation on biopharmaceutical properties of dendrimers. Polymer (Guildf) 2015;59:67–92. [Google Scholar]
- 103.Lowell J. Bidirectional charge transfer in repeated contacts to polymers. J. Electrost. 1987;20:233–238. [Google Scholar]
- 104.Lee L.-H. Dual mechanism for metal-polymer contact electrification. J. Electrost. 1994;32:1–29. [Google Scholar]
- 105.Khurana S., Madhav N.V.S., Tangri P. Mucoadhesive drug delivery: mechanism and methods of evaluation. Int J Pharm Biosci. 2011;2:458–467. [Google Scholar]
- 106.Ahuja A., Khar R.K., Ali J. Mucoadhesive drug delivery systems. Drug Dev. Ind. Pharm. 1997;23:489–515. [Google Scholar]
- 107.Shaikh R., Raj Singh T.R., Garland M.J., Woolfson A.D., Donnelly R.F. Mucoadhesive drug delivery systems. J. Pharm. Bioallied Sci. 2011;3:89–100. doi: 10.4103/0975-7406.76478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Khutoryanskiy V.V. John Wiley & Sons; 2014. Mucoadhesive Materials and Drug Delivery Systems. [Google Scholar]
- 109.Ahagon A., Gent A.N. Effect of interfacial bonding on the strength of adhesion. J. Polym. Sci. Polym. Phys. Ed. 1975;13:1285–1300. [Google Scholar]
- 110.HäGerström H., Edsman K., Strømme M. Low-frequency dielectric spectroscopy as a tool for studying the compatibility between pharmaceutical gels and mucous tissue. J. Pharm. Sci. 2003;92:1869–1881. doi: 10.1002/jps.10451. [DOI] [PubMed] [Google Scholar]
- 111.Ugwoke M.I., Agu R.U., Verbeke N., Kinget R. Nasal mucoadhesive drug delivery: background, applications, trends and future perspectives. Adv. Drug Deliv. Rev. 2005;57:1640–1665. doi: 10.1016/j.addr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 112.Boddupalli B.M., Mohammed Z.N.K., Nath R.A., Banji D. Mucoadhesive drug delivery system: an overview. J. Adv. Pharm. Technol. Res. 2010;1:381–387. doi: 10.4103/0110-5558.76436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Solomonidou D., Cremer K., Krumme M., Kreuter J. Effect of carbomer concentration and degree of neutralization on the mucoadhesive properties of polymer films. J. Biomater. Sci. Polym. Ed. 2001;12:1191–1205. doi: 10.1163/156856201753395743. [DOI] [PubMed] [Google Scholar]
- 114.Sinha V.R., Singla A.K., Wadhawan S., Kaushik R., Kumria R., Bansal K., Dhawan S. Chitosan microspheres as a potential carrier for drugs. Int. J. Pharm. 2004;274:1–33. doi: 10.1016/j.ijpharm.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 115.Dhawan S., Singla A.K., Sinha V.R. Evaluation of mucoadhesive properties of chitosan microspheres prepared by different methods. AAPS PharmSciTech. 2004;5:122–128. doi: 10.1208/pt050467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ekinci M., Ilem-Ozdemir D., Gundogdu E., Asikoglu M. Methotrexate loaded chitosan nanoparticles: preparation, radiolabeling and in vitro evaluation for breast cancer diagnosis. J. Drug Deliv. Sci. Technol. 2015;30:107–113. [Google Scholar]
- 117.Tripathy S., Mahapatra S.K., Chattopadhyay S., Das S., Dash S.K., Majumder S., Pramanik P., Roy S. A novel chitosan based antimalarial drug delivery against Plasmodium berghei infection. Acta Trop. 2013;128:494–503. doi: 10.1016/j.actatropica.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 118.Huang Y., Leobandung W., Foss A., Peppas N.A. Molecular aspects of muco-and bioadhesion: tethered structures and site-specific surfaces. J. Control. Release. 2000;65:63–71. doi: 10.1016/s0168-3659(99)00233-3. [DOI] [PubMed] [Google Scholar]
- 119.Mohammadpour Dounighi N., Eskandari R., Avadi M.R., Zolfagharian H., Mir Mohammad Sadeghi A., Rezayat M. Preparation and in vitro characterization of chitosan nanoparticles containing Mesobuthus eupeus scorpion venom as an antigen delivery system. J. Venom. Anim. Toxins Incl. Trop. Dis. 2012;18:44–52. [Google Scholar]
- 120.Chen N., Li Q., Li J., Ren Y., Wu G., Liu Y., Shi Y. Development and evaluation of a new gastroretentive drug delivery system: nanomicelles-loaded floating mucoadhesive beads. J. Drug Deliv. Sci. Technol. 2019;51:485–492. [Google Scholar]
- 121.Moura F.C.S., Perioli L., Pagano C., Vivani R., Ambrogi V., Bresolin T.M., Ricci M. Chitosan composite microparticles: a promising gastroadhesive system for taxifolin. Carbohydr. Polym. 2019;218:343–354. doi: 10.1016/j.carbpol.2019.04.075. [DOI] [PubMed] [Google Scholar]
- 122.Hooda A., Nanda A., Jain M., Kumar V., Rathee P. Optimization and evaluation of gastroretentive ranitidine HCl microspheres by using design expert software. Int. J. Biol. Macromol. 2012;51:691–700. doi: 10.1016/j.ijbiomac.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 123.de Ávila B.E.-F., Angsantikul P., Li J., Lopez-Ramirez M.A., Ramírez-Herrera D.E., Thamphiwatana S., Chen C., Delezuk J., Samakapiruk R., Ramez V., et al. Micromotor-enabled active drug delivery for in vivo treatment of stomach infection. Nat. Commun. 2017;8:272. doi: 10.1038/s41467-017-00309-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ngwuluka N.C., Choonara Y.E., Kumar P., Lisa C., Modi G., Pillay V. An optimized gastroretentive nanosystem for the delivery of levodopa. Int. J. Pharm. 2015;494:49–65. doi: 10.1016/j.ijpharm.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 125.Kas H.S. Chitosan: properties, preparations and application to microparticulate systems. J. Microencapsul. 1997;14:689–711. doi: 10.3109/02652049709006820. [DOI] [PubMed] [Google Scholar]
- 126.Laby R. 1974. Device for Administration to Ruminants. [Google Scholar]
- 127.Johnson R.H., Rowe E.L. 1971. Medicinal Dosage Forms of Unpolymerized Thiolated Gelatin With a Cross-linking Accelerating Agent Providing Slowly Released Medication From a Swollen Matrix. [Google Scholar]
- 128.Brewer M.D., Griffin G.J.L. 1980. Sustained Release Compositions. [Google Scholar]
- 129.Salessiotis N. Measurement of the diameter of the pylorus in man: part I. Experimental project for clinical application. Am. J. Surg. 1972;124:331–333. doi: 10.1016/0002-9610(72)90036-0. [DOI] [PubMed] [Google Scholar]
- 130.Streubel A., Siepmann J., Bodmeier R. Gastroretentive drug delivery systems. Expert Opin. Drug Deliv. 2006;3:217–233. doi: 10.1517/17425247.3.2.217. [DOI] [PubMed] [Google Scholar]
- 131.Timmermans J., Moës A.J. The cutoff size for gastric emptying of dosage forms. J. Pharm. Sci. 1993;82:854. doi: 10.1002/jps.2600820821. [DOI] [PubMed] [Google Scholar]
- 132.Khosla R., Davis S.S. The effect of tablet size on the gastric emptying of non-disintegrating tablets. Int. J. Pharm. 1990;62:R9–R11. [Google Scholar]
- 133.Klausner E.A., Lavy E., Friedman M., Hoffman A. Expandable gastroretentive dosage forms. J. Control. Release. 2003;90:143–162. doi: 10.1016/s0168-3659(03)00203-7. [DOI] [PubMed] [Google Scholar]
- 134.Tripathi J., Thapa P., Maharjan R., Jeong S.H. Current state and future perspectives on gastroretentive drug delivery systems. Pharmaceutics. 2019;11:193. doi: 10.3390/pharmaceutics11040193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bardonnet P.L., Faivre V., Pugh W.J., Piffaretti J.C., Falson F. Gastroretentive dosage forms: overview and special case of Helicobacter pylori. J. Control. Release. 2006;111:1–18. doi: 10.1016/j.jconrel.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 136.Pogany S.A., Zentner G.M. 1993. Poly (Orthocarbonate Acetal) Bioerodible Polymers. [Google Scholar]
- 137.Shalaby W.S.W., Blevins W.E., Park K. ACS Publications; 1991. Enzyme-degradable Hydrogels: Properties Associated With Albumin-cross-linked Polyvinylpyrrolidone Hydrogels. [Google Scholar]
- 138.Chen J., Park H., Park K. Synthesis of superporous hydrogels: hydrogels with fast swelling and superabsorbent properties. J. Biomed. Mater. Res. An Off. J. Soc. Biomater. Japanese Soc. Biomater. Aust. Soc. Biomater. 1999;44:53–62. doi: 10.1002/(sici)1097-4636(199901)44:1<53::aid-jbm6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 139.Prajapati V.D., Jani G.K., Khutliwala T.A., Zala B.S. Raft forming system—an upcoming approach of gastroretentive drug delivery system. J. Control. Release. 2013;168:151–165. doi: 10.1016/j.jconrel.2013.02.028. [DOI] [PubMed] [Google Scholar]
- 140.Pazianas M., Abrahamsen B., Ferrari S., Russell R.G.G. Eliminating the need for fasting with oral administration of bisphosphonates. Ther. Clin. Risk Manag. 2013;9:395. doi: 10.2147/TCRM.S52291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen Y.-C., Ho H.-O., Lee T.-Y., Sheu M.-T. Physical characterizations and sustained release profiling of gastroretentive drug delivery systems with improved floating and swelling capabilities. Int. J. Pharm. 2013;441:162–169. doi: 10.1016/j.ijpharm.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 142.Chavda H., Patel C. Chitosan superporous hydrogel composite-based floating drug delivery system: a newer formulation approach. J. Pharm. Bioallied Sci. 2010;2:124. doi: 10.4103/0975-7406.67010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yin L., Fei L., Cui F., Tang C., Yin C. Superporous hydrogels containing poly (acrylic acid-co-acrylamide)/O-carboxymethyl chitosan interpenetrating polymer networks. Biomaterials. 2007;28:1258–1266. doi: 10.1016/j.biomaterials.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 144.Park J., Kim D. Effect of polymer solution concentration on the swelling and mechanical properties of glycol chitosan superporous hydrogels. J. Appl. Polym. Sci. 2010;115:3434–3441. [Google Scholar]
- 145.Gupta N.V., Shivakumar H.G. Development of a gastroretentive drug delivery system based on superporous hydrogel. Trop. J. Pharm. Res. 2010;9 [Google Scholar]
- 146.Nayak B., Maji A.K., Das R. Gastroretentive drug delivery systems: a review. Asian J. Pharm. Clin. Res. 2010;3:2–10. [Google Scholar]
- 147.Ito R., Machida Y., Sannan T., Nagai T. Magnetic granules: a novel system for specific drug delivery to esophageal mucosa in oral administration. Int. J. Pharm. 1990;61:109–117. [Google Scholar]
- 148.Zhang Y., Li L., Tang F., Ren J. Controlled drug delivery system based on magnetic hollow spheres/polyelectrolyte multilayer core—shell structure. J. Nanosci. Nanotechnol. 2006;6:3210–3214. doi: 10.1166/jnn.2006.469. [DOI] [PubMed] [Google Scholar]
- 149.Garg T., Kumar A., Rath G., Goyal A.K. Gastroretentive drug delivery systems for therapeutic management of peptic ulcer. Crit. Rev. Ther. Drug Carr. Syst. 2014;31 doi: 10.1615/critrevtherdrugcarriersyst.2014011104. [DOI] [PubMed] [Google Scholar]
- 150.Singh B.N., Kim K.H. Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. J. Control. Release. 2000;63:235–259. doi: 10.1016/s0168-3659(99)00204-7. [DOI] [PubMed] [Google Scholar]
- 151.Kumar R., Philip A. Gastroretentive dosage forms for prolonging gastric residence time. Int. J. Pharm. Med. 2007;21:157–171. [Google Scholar]
- 152.Hwang S.-J., Park H., Park K. Gastric retentive drug-delivery systems. Crit. Rev. Ther. Drug Carr. Syst. 1998;15 [PubMed] [Google Scholar]
- 153.Pawar V.K., Kansal S., Garg G., Awasthi R., Singodia D., Kulkarni G.T. Gastroretentive dosage forms: a review with special emphasis on floating drug delivery systems. Drug Deliv. 2011;18:97–110. doi: 10.3109/10717544.2010.520354. [DOI] [PubMed] [Google Scholar]
- 154.Das P.S., Saha P. A review on approaches to achieve gastric retention of floating drug delivery system. World J. Pharm. Pharm. Sci. 2017;6:415–426. [Google Scholar]
- 155.Mandal U.K., Chatterjee B., Senjoti F.G. Gastro-retentive drug delivery systems and their in vivo success: a recent update. Asian J. Pharm. Sci. 2016;11:575–584. [Google Scholar]
- 156.Jiménez-Martínez I., Quirino-Barreda T., Villafuerte-Robles L. Sustained delivery of captopril from floating matrix tablets. Int. J. Pharm. 2008;362:37–43. doi: 10.1016/j.ijpharm.2008.05.040. [DOI] [PubMed] [Google Scholar]
- 157.Tadros M.I. Controlled-release effervescent floating matrix tablets of ciprofloxacin hydrochloride: development, optimization and in vitro—in vivo evaluation in healthy human volunteers. Eur. J. Pharm. Biopharm. 2010;74:332–339. doi: 10.1016/j.ejpb.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 158.Soni S., Ram V., Verma A. Crushed puffed rice-HPMC-chitosan based single-unit hydro-dynamically balanced system for the sustained stomach specific delivery of metoprolol succinate. J. Appl. Pharm. Sci. 2017;7:47–57. [Google Scholar]
- 159.Krögel I., Bodmeier R. Development of a multifunctional matrix drug delivery system surrounded by an impermeable cylinder. J. Control. Release. 1999;61:43–50. doi: 10.1016/s0168-3659(99)00096-6. [DOI] [PubMed] [Google Scholar]
- 160.He W., Li Y., Zhang R., Wu Z., Yin L. Gastro-floating bilayer tablets for the sustained release of metformin and immediate release of pioglitazone: preparation and in vitro/in vivo evaluation. Int. J. Pharm. 2014;476:223–231. doi: 10.1016/j.ijpharm.2014.09.056. [DOI] [PubMed] [Google Scholar]
- 161.Oth M., Franz M., Timmermans J., Möes A. The bilayer floating capsule: a stomach-directed drug delivery system for misoprostol. Pharm. Res. 1992;9:298–302. doi: 10.1023/a:1015870314340. [DOI] [PubMed] [Google Scholar]
- 162.Harrigan R.M. 1977. Drug Delivery Device for Preventing Contact of Undissolved Drug With the Stomach Lining. [Google Scholar]
- 163.Gupta M.E., Amulya C., Babu I.S. 2019. A Review on Floating Drug Delivery Systems. [Google Scholar]
- 164.Costa P., Sousa Lobo J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001;13:123–133. doi: 10.1016/s0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]
- 165.Talukder R., Fassihi R. Gastroretentive delivery systems: a mini review. Drug Dev. Ind. Pharm. 2004;30:1019–1028. doi: 10.1081/ddc-200040239. [DOI] [PubMed] [Google Scholar]
- 166.Dey S.K., De P.K., De A., Ojha S., De R., Mukhopadhyay A.K., Samanta A. Floating mucoadhesive alginate beads of amoxicillin trihydrate: a facile approach for H. pylori eradication. Int. J. Biol. Macromol. 2016;89:622–631. doi: 10.1016/j.ijbiomac.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 167.Jiang H., Tian R., Hu W., Jia Y., Yuan P., Wang J., Zhang L. Formulation and evaluation of gastroretentive floating drug delivery system of dipyridamole. Drug Dev. Ind. Pharm. 2015;41:674–680. doi: 10.3109/03639045.2014.893355. [DOI] [PubMed] [Google Scholar]
- 168.Streubel A., Siepmann J., Bodmeier R. Floating microparticles based on low density foam powder. Int. J. Pharm. 2002;241:279–292. doi: 10.1016/s0378-5173(02)00241-7. [DOI] [PubMed] [Google Scholar]
- 169.Datir S.K., Patil P.B., Saudagar R.B. Floating type drug delivery system: a review. J. Drug Deliv. Ther. 2019;9:428–432. [Google Scholar]
- 170.El Nabarawi M.A., Teaima M.H., El-Monem R.A.A., El Nabarawy N.A., Gaber D.A. Formulation, release characteristics, and bioavailability study of gastroretentive floating matrix tablet and floating raft system of Mebeverine HCl. Drug Des. Devel. Ther. 2017;11:1081. doi: 10.2147/DDDT.S131936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kumar M., Kaushik D. An overview on various approaches and recent patents on gastroretentive drug delivery systems. Recent Pat. Drug Deliv. Formul. 2018;12:84–92. doi: 10.2174/1872211312666180308150218. [DOI] [PubMed] [Google Scholar]