Abstract
The clinical-immunological spectrum of human Leishmania (L.) infantum chagasi-infection in Amazonian Brazil has recently been reviewed based on the combined use of the delayed-type hypersensitivity (DTH) and indirect fluorescence antibody test (IFAT-IgG/IgM), both with homologous L. (L.) infantum chagasi-antigens, and associated with the clinical evaluation of infected individuals. This diagnostic approach has allowed to identify the broadest clinical-immunological spectrum of human L. (L.) infantum chagasi-infection composed by five clinical-immunological profiles of infection: three asymptomatic, 1) Asymptomatic Infection (AI) [DTH+/++++, IFAT−], 2) Subclinical Resistant Infection (SRI) [DTH+/++++, IFAT+/++], and 3) Indeterminate Initial Infection (III) [DTH−, IFAT+/++], and two symptomatic ones, 4) Symptomatic Infection (SI) [=American visceral leishmaniasis - AVL] and, 5) Subclinical Oligosymptomatic Infection (SOI), both with the same immune profile [DTH−, IFAT+++/++++]. Herein, we confirm for the third time the preclinical diagnosis of AVL through IgM-antibody response in an early asymptomatic case of infection (profile III), a 17-year-old boy who evolved to AVL (=profile SI) six weeks after the initial infection diagnosis, confirming that the combined use of DTH and IFAT-(IgG/IgM) assays associated with the clinical evaluation of infected individuals is potentially useful for monitoring human L. (L.) infantum chagasi-infection in endemic areas as well as optimizing AVL control.
Keywords: Leishmania (L.) infantum chagasi, American visceral leishmaniasis, Preclinical diagnosis, Disease control, Amazonian Brazil
1. Introduction
American visceral leishmaniasis (AVL) is a non-contagious infectious disease caused by Leishmania (L.) infantum chagasi Lainson & Shaw 2005 (=Leishmania chagasi Cunha & Chagas 1937), a protozoan parasite of the order Trypanosomatida, family Trypanosomatidae, and genus Leishmania (Lainson, 2010; Lainson and Shaw, 2010; Marcili et al., 2014), which is principally transmitted by the infected female of the phlebotomine vector, Lutzomyia longipalpis (Lainson and Rangel, 2005). AVL is primarily considered a rural zoonosis in Brazil, but has been expanding to medium and large urban areas to become a growing public health problem in that country (Silveira et al., 2009; Silva et al., 2013). It is considered a dynamic disease, with the circumstances of its transmission continually changing, largely due to anthropic environmental and demographic impacts (Moreno et al., 2006; de Araújo et al., 2013).
AVL principally develops in 1 to 10 year-old children, although it also manifests itself in adults, principally males. Following an average incubation time of 2 to 3 months, the disease will suddenly (or gradually) make a clinical appearance in the form of a daily fever lasting up to two months, as well as weakness, indisposition, loss of appetite, weight loss, skin-mucous pallor, diarrhea, and abdominal distension. The latter symptom is caused by progressive enlargements of the liver and spleen, causing hepato-splenomegaly due to hyperplasia and hypertrophy of the mononuclear phagocytic system in the parenchyma of those organs. From an immunopathological point of view, there will be pancytopenia - anemia, leucopenia, and thrombocytopenia -, as well as the suppression of T-cell immune responses (mainly the CD4+/Th1 subtype). The latter condition is the main cause of intercurrent bronchopulmonary or intestinal infections that, together with hemorrhaging due to coagulation changes, account for deaths in advanced stages of the disease (Silveira et al., 2013, Silveira et al., 2016).
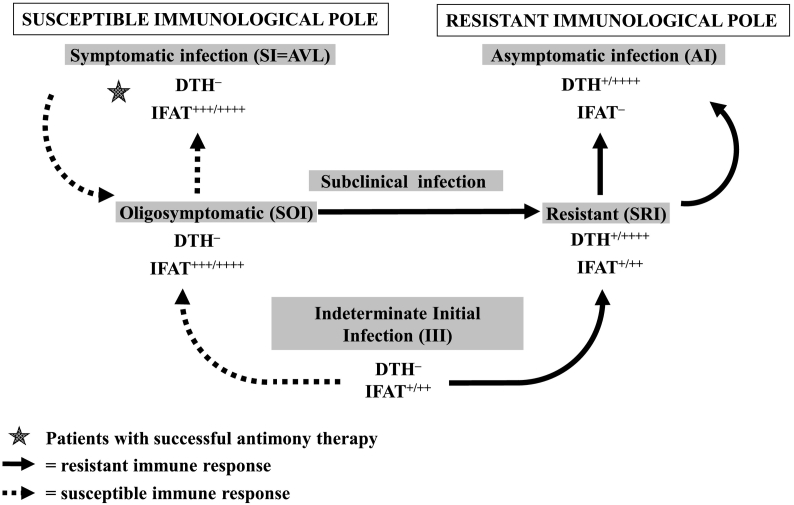
Although AVL is recognized as the most severe clinical-immunological manifestation of the interactions between the parasite L. (L.) infantum chagasi and the human immune responses [with a 98% probability of evolving to death in the absence of adequate diagnosis and treatment], recent studies carried out in Amazonian Brazil have shown that additional clinical-immunological stages resulting from those interactions can also make up part of the clinical-immunological spectrum of that infection. As such, the combined use of semi-quantitative delayed-type hypersensitivity (DTH) and indirect fluorescent antibody test (IFAT-IgG), associated with the clinical status of infected individuals, have allowed the diagnosis of the broadest clinical-immunological spectrum of infection composed of five profiles, such as: three asymptomatic, 1) Asymptomatic Infection (AI = DTH+/++++, IFAT−), 2) Subclinical Resistant Infection (SRI = DTH+/++++, IFAT+/++), and 3) Indeterminate Initial Infection (III = DTH−, IFAT+/++); and two symptomatic ones, 4) Symptomatic Infection (SI = AVL) and, 5) Subclinical Oligosymptomatic Infection (SOI), both with the same immune profile (DTH−, IFAT+++/++++). Thus, the three previously established profiles (AI, SI [=AVL] and SOI) were confirmed (Pearson and Sousa, 1996), as well as two more recent ones (Silveira et al., 2010a, Silveira et al., 2010b). This represents the broadest clinical-immunological spectrum of human L. (L.) infantum chagasi-infection known until then in Latin America.
With regards to the pathophysiology of those clinical-immunological infection profiles, it should be emphasized that the profile AI is characterized by positive DTH reactivity and the absence of an IgG-antibody response [below the IFAT-IgG dilution cut-off of 1:80] – which seems to be strongly linked to genetic resistance to infection (Jeronimo et al., 2007). The profile SI (=AVL), on the other hand, is strongly linked to genetic susceptibility to infection, with clear DTH inhibition and high expression of the IgG-antibody response. The SOI and SRI profiles represent borderline genetic expressions of disease susceptibility and resistance respectively; the former generally evolves into mild clinical signs of susceptibility [such as fever, asthenia, cutaneous pallor, and moderate enlargement of the spleen], but with spontaneous clinical resolution of infection after one to two months (Gama et al., 2004); the latter profile presents an asymptomatic stage evolving towards the AI resistant profile. Asymptomatic profile III represents the earliest stage of infection [which is not well-defined from an immune-genetic point of view], with the capacity to evolve to either the AI resistant profile or to the SI susceptible profile (=AVL). Therefore, depending on the genetic background of the infected individual, an infection can evolve to an AI resistant profile or to a SI susceptible profile (=AVL) after passing first through either the SRI or SOI profiles respectively (Fig. 1). Two prospective studies in Amazonian Brazil were therefore undertaken to focus on the dynamics of the clinical and immunological evolution of infection, and estimated that 1–3% of all III cases will evolve to classical AVL (= profile SI) (Silveira et al., 2010a, Silveira et al., 2010b).
Fig. 1.
Dynamics of the clinical and immunological evolution of human L. (L.) infantum chagasi-infection in Amazonian Brazil.
IFAT: indirect fluorescent antibody test (IgG); IFAT ++++: 5.120–10,240 (IgG); IFAT +++: 1.280–2.560 (IgG); IFAT ++: 320–640 (IgG); IFAT +: 80–160 (IgG); IFAT –: negative reaction (IgG); DTH: delayed-type hypersensitivity; DTH ++++: exacerbate reaction (≥ 16 mm); DTH +++: strong reaction (13–15 mm); DTH ++: moderate reaction (9–12 mm); DTH +: weak reaction (5–8 mm); DTH –: negative reaction (< 5 mm); AI: Asymptomatic infection; SI: Symptomatic infection (=AVL); SOI: Subclinical oligosymptomatic infection; SRI: Subclinical resistant infection; III: Indeterminate initial infection.
In terms of cytokine responses over the entire clinical-immunological spectrum of human L. (L.) infantum chagasi-infection in Amazonian Brazil, the roles of pro-inflammatory (TNF-α and IL-6) and anti-inflammatory (IL-4 and IL-10) cytokines were recently examined based on previous observations in the States of Piaui and Maranhão in northeastern Brazil (Costa et al., 2013; Gama et al., 2013), with the conclusion that the association between IL-6 and IL-10 cytokines apparently has a pivotal influence on the immunopathogenesis of AVL (Ramos et al., 2016).
Taking into account the above considerations, we present here our findings concerning the preclinical diagnosis of an early asymptomatic infection (profile III) that evolved to AVL (=profile SI) six weeks after an initial diagnosis. That observation was made during a prospective study (April/ 2017-June /2018) to evaluate by gene expression the innate and adaptive immune responses of human L. (L.) infantum chagasi-infection in the municipality of Bujarú, northeastern Pará State, in Amazonian Brazil. It represents the third such case study in the last 10 years – but of significant importance in terms of prospects for optimizing disease control. Systematic investigations of IgM-antibody responses in asymptomatic cases of profile III have allowed the preclinical diagnosis of human L. (L.) infantum chagasi-infection with genetic predisposition that would otherwise rapidly progress to AVL – allowing its early treatment and the prevention of the severe effects of its morbidity.
2. Methods
2.1. Study area
This study was carried out in a rural area [Tracuateua village] of the municipality of Bujarú (01° 30′ 54“ S: 48° 02’ 41” W), northeastern Pará State, in Amazonian Brazil [along the Guamá River, and about 72 km from the state capital at Belém]. The climate is typically equatorial, with a mean temperature of 28 °C and high humidity. The Tracuateua village currently has a significant number of AVL cases (= profile SI) that were diagnosed based on specific serological assays (IFAT-IgG/IgM) performed at the “Ralph Lainson Leishmaniasis Lab” in the Evandro Chagas Institute. Six cases were confirmed in 2010, fourteen in 2011, four in 2012, thirty four in 2013, and four cases in 2014 (Silveira et al., unpublished data).
2.2. Study population
The total population of the municipality of Bujarú is approximately 28.016 people, with 10.420 (37.2%) in urban areas and 17.596 (62.8%) in rural ones (IBGE, 2017). In the present work there were examined approximately 7.5% (1.320) of the rural population of the municipality of Bujarú, being 792 (60%) males and 528 (40%) females aged between 1 (minimum) and 76 (maximum) years old, with a median age of 20 years old.
2.3. Study design
As stated earlier, this was a prospective study (April/ 2017–June/2018) aimed to evaluate by gene expression the innate and adaptive immune responses of human L. (L.) infantum chagasi-infection in the municipality of Bujarú, northeastern Pará State, in Amazonian Brazil. For this reason, it was necessary to discriminate between the clinical-immunological infection profiles, mainly the profile III due to the high significance of its evolution towards the resistance pole of the infection [profile AI] or the susceptibility pole [profile SI = AVL]. In this sense, it seems critical to evaluate the innate and adaptive immune responses of the infection cases of profile III in order to better understanding the genetics of the immune response that evolves to the resistance or susceptibility poles of the infection. The criteria for discriminating between these clinical-immunological infection profiles, as well as the susceptible and resistant cases of profile III, can be seen in previous studies respectively (Crescente et al., 2009; Silveira et al., 2010a, Silveira et al., 2010b, Silveira et al., 2011; Lima et al., 2014).
2.4. Ethical approval
This study was approved by the Research Programs Evaluating Committee of the Faculty of Medicine, University of São Paulo, São Paulo State, Brazil, with the protocol number 127115/2016 and the approval number 3.156.918.
3. Results
Based on the criteria (DTH/IFAT-IgG/clinical evaluation of infected individuals) for the diagnosis of human, symptomatic (SI = AVL and SOI profiles) and asymptomatic (III, SRI and AI profiles), L. (L.) infantum chagasi-infection there was identified a total of 42 cases of profile III (30 in the first year [2017] of the study and 12 in the second [2018]), being 22 (52.4%) males and 20 (47.6%) females aged between 3 (minimum) and 56 (maximum) years old, with a median age of 26.2 years old.
Following the above diagnosis, all 42 cases of profile III underwent IFAT-IgM serological evaluation, revealing that all 30 cases from the first year (April–June/2017) were negative, while one (an 17-year-old boy) of the twelve new cases diagnosed in the second year (April–June/2018) showed a positive serological reaction with a titer of 80. However, there were not detect, a year earlier, any clinical signs and/or symptoms of AVL in any of the household members during our first visit to the boy's home (May 18, 2017), but nonetheless performed specific DTH and IFAT-IgG assays on four individuals (the 17-year-old boy, his 19-year-old brother, his 39-year-old mother, and his 50 year-old father). Their results were all negative at that time.
When we visited the same household for the second time in the following year (May 29, 2018), two of the four individuals were found to have anti-L. (L.) infantum chagasi IgG-antibody responses: the 17-year-old boy and his mother, with serological titers of 320 and 160 respectively. Neither showed any reactivity to DHT, which assigned them a status of profile III (Table 1). The other two individuals in the household did not show any reactivity to IFAT-IgG or DTH. Accordingly, we also performed an IFAT-IgM assay on the two IgG-antibody responding individuals, but only the boy showed an anti-L. (L.) infantum chagasi IgM-antibody response, with a serological titer of 80. The boy was then monitored on a weekly basis by local health care agents to detect any clinical signs and/or symptoms of AVL. After a six-week period, we were notified that the boy had had a fever with chills for seven days, as well as asthenia, weakness, and a loss of appetite. Another IFAT-IgG/IgM assays were then performed, revealing a quick up-tick of his serological titers to 1280 for both IgG and IgM antibodies, confirming the preclinical diagnosis of AVL predicted by the previous presence of an anti-L. (L.) infantum chagasi IgM-antibody response. Additional laboratory analysis indicated pancytopenia (3,150,000/mm3 red blood cells, hemoglobin 9.3 g/dL, 2900/mm3 white blood cells, and 92,000/mm3 platelets), while a physical examination of the patient likewise confirmed spleen enlargement (~3 cm below the left costal border). After the diagnostic confirmation of AVL, the boy was then hospitalized and received antimony therapy (15 mg/Sbv/kg) for twenty-five days – which resulted in the successful remission of all clinical signs and symptoms of the disease.
Table 1.
Personal characteristics (age and gender) and laboratory results (IFAT/DTH) in an early asymptomatic case of L. (L.) infantum chagasi-Indeterminate Initial Infection (profile III) that evolved to American visceral leishmaniasis (AVL) [Symptomatic Infection = profile SI] and its family contacts (mother, father and brother), in the municipality of Bujarú, Pará State, Amazonian Brazil.
| Cases | Gender | Age | 2017 (May) |
2018 (May) |
2018 (July) |
|||
|---|---|---|---|---|---|---|---|---|
| DTH | IFAT(IgG) | DTH | IFAT (IgG/IgM) |
DTH | IFAT (IgG/IgM) | |||
| 1 | Malea (patient) | 17 | Neg | Neg | Neg | 320/80 | – | 1280/1280 |
| 2 | Female (mother) | 39 | Neg | Neg | Neg | 160/Neg | – | |
| 3 | Male (father) | 50 | Neg | Neg | Neg | Neg/ - | – | – |
| 4 | Male (brother) | 19 | Neg | Neg | Neg | Neg/ - | – | – |
DTH = Delayed-type hypersensitivity.
IFAT = Indirect fluorescent antibody test.
IgG = Immunoglobulin G.
IgM = Immunoglobulin M.
= The case of profile III who developed to AVL.
4. Discussion
We confirm here the preclinical diagnosis of AVL in a 17 year-old boy with an early asymptomatic infection (profile III) by an IgM-antibody response to L. (L.) infantum chagasi-infection, who six weeks later developed clinical signs and/or symptoms of the disease. This represents the third preclinical diagnosis of AVL in an asymptomatic case of L. (L.) infantum chagasi-infection demonstrating clinical-immunological profile III that progressed to AVL (=profile SI) six weeks after the initial diagnosis of infection in our study area in northeastern of Pará State, in Amazonian Brazil. The first two cases were diagnosed in two girls, the youngest being three years old (Silveira et al., 2010a; Lima et al., 2014), while the present case was diagnosed in an 17-year-old boy native to the region – suggesting that under certain circumstances, not fully understood, some individuals residing in areas known to transmit the infection are not infected in their early childhood. Corroborating that observation, it should also be highlighted that among the four individuals residing in the home, only the boy and his mother were diagnosed as infected in 2018 (when he was 17 and his mother 40), as indicated by anti-L. (L.) infantum chagasi IgG-antibody responses, with serological titers of 320 and 160 respectively, although neither showed any reactivity to DHT. The other two household members, his 20-year-old brother and his 51-year-old father, had remained uninfected – indicating that infection transmission in the rural endemic area is not uniform, but rather sporadically dispersed or opportunist, possibly depending on environmental and/or social factors that include the densities of the main phlebotomine vector, Lutzomyia longipalpis (Laison & Rangel, 2005), the domestic infection reservoir (dogs) [Silveira et al., 2012], and the degree of individual exposure to the infection transmission in the home environment as was shown in previous research in that region (Silveira et al., 2009, Silveira et al., 2010a, Silveira et al., 2010b). Interestingly, even if this profile III case received a homologous L. (L.) infantum chagasi-antigenic stimulus for DTH a year earlier, it was not sufficient to develop any protective immune response against infection.
With respect to the clinical evolution of these cases, it is interesting to note that the incubation periods of the disease (counting from the initial diagnosis of infection, when the clinical-immunological diagnosis of profile III was established) were very similar in all three cases studied (ranging from six to eight weeks), which is absolutely compatible with the time frames referred to in basic texts on AVL (Pearson and Sousa, 1996; Lainson and Shaw, 2010; Silveira et al., 2013). The marked significance of that incubation period in those three profile III cases should be noted in terms of the accuracy of the diagnosis of human asymptomatic L. (L.) infantum chagasi-infection (profile III) in the endemic areas, allowing detection and monitoring of the clinical-immunological evolution of those profile III cases.
While those occurrences may appear to have inexpressive epidemiological significance in light of the high prevalence (12.7% to 17%) of human L. (L.) infantum chagasi-infection in that region (Silveira et al., 2010[a,b]), it should not be forgotten that this finding resulted from the examination of only about 7.5% (1.320) of the rural population (17.596) of Bujarú (IBGE, 2017). A projection could thus be made that up to 13.3 cases (almost 15) of profile III with susceptibility to develop AVL (=profile SI) could be expected to be diagnosed in the study area. It would therefore also be possible to project that if this diagnostic approach was applied on a wide or even medium scale, it would be a valuable tool for optimizing AVL control through the early diagnosis of a significant number of asymptomatic cases of infection (profile III) with a genetic predisposition to evolve to AVL. Unfortunately, however, that diagnostic approach would be difficult to apply in Brazil at the moment – and not only for technical reasons – but because of an unjust political system that prioritizes the business class and makes a Single Health System (Ministry of Health, Brazil) based on the municipalization of health care unviable, and the surveillance and control of the serious endemic diseases such as AVL nearly impossible. Within that scenario, it is difficult to control endemic diseases due to the misuse of financial resources and the lack of trained personnel. In northeastern of Pará State, in Amazonian Brazil (which represents the largest focal area of AVL in the region today), for example, there is no laboratory infrastructure in any of the 49 municipalities there with the capacity to perform serological tests for AVL (IFAT or ELISA). In these situations, the diagnosis of the disease has been based mainly on the “immunochromatographic rapid test” (OnSit™ – Bio Advance Diagnóstico), but with some diagnostic limitations which has required further confirmation (Brasil, 2018).
Furthermore, considering that there is currently no efficient vaccine available in Brazil that could be used to protect humans against L. (L.) infantum chagasi-infection, this diagnostic approach may prove useful for monitoring human infections and identifying profile III cases with a propensity for evolving towards full AVL. Additionally, the preclinical diagnosis of AVL in cases of the appearance of profile III by an IgM-antibody response to L. (L.) infantum chagasi-infection (Silveira et al., 2011; Lima et al., 2014) could help prevent the severe morbidity of this disease and the necessity of long-duration treatments.
Funding
This work was supported by Instituto Evandro Chagas (Secretaria de Vigilância em Saúde, Ministério da Saúde, Brazil); Núcleo de Medicina Tropical (Universidade Federal do Pará, Brazil); Laboratório de Investigação Médica (LIM)-50 (Hospital de Clínicas-Faculdade de Medicina-Universidade de São Paulo (USP), Brazil) and, Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP: 2014/50315-0).
Declaration of competing interest
The authors of this work have no conflicts of interest to declare.
Acknowledgments
Acknowledgments
For the technician team of “Ralph Lainson Leishmaniasis Lab” of the Evandro Chagas Institute.
Authors' contributions
LVRL, MBC, PKR, TVS and FTS: experiments design and development; CMCG, MDL, VM, CEPC and FTS: data analysis; LVRL, MBC, PKR, TVS and FTS: manuscript writing; CMCG, MDL, VM, CEPC and FTS: manuscript revision; MDL, CEPC and FTS: getting financial support. All authors read and approved the final version of the manuscript.
References
- de Araújo V.E., Pinheiro L.C., Almeida M.C., de Menezes F.C., Morais M.H., Reis I.A., Assunção R.M., Carneiro M. Relative risk of visceral leishmaniasis in Brazil: a spatial analysis in urban area. PLoS Negl. Trop. Dis. 2013;7(11):e2540. doi: 10.1371/journal.pntd.0002540. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil, 2018. Nota informativa no 3/2018-CGLAB/DEVIT/SVS/MS (saude.gov.br).
- Costa D.L., Rocha R.L., Carvalho R.M.A., Lima-Neto A.S., Harbay M.O., Costa C.H., Barral-Neto M. Serum cytokines associated with severity and complications of kala-azar. Pathog. Glob. Health. 2013;107(2):78–87. doi: 10.1179/2047773213Y.0000000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescente J.A.B., Silveira F.T., Lainson R., Gomes C.M.C., Laurenti M.D., Corbett C.E.P. A cross-sectional study on the clinical and immunological spectrum of human Leishmania (L.) infantum chagasi infection in the Brazilian Amazon region. Trans. Roy. Soc. Trop. Med. Hyg. 2009;103(12):1250–1256. doi: 10.1016/j.trstmh.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Gama M.E.A., Costa J.M.L., Gomes C.M.C., Corbett C.E.P. Subclinical form of the American visceral leishmaniasis. Mem. Inst. Oswaldo Cruz. 2004;99(8):889–893. doi: 10.1590/s0074-02762004000800018. [DOI] [PubMed] [Google Scholar]
- Gama M.E.A., Gomes C.M.C., Silveira F.T., Laurenti M.D., Gonçalves E. da G., Silva A.R., Corbett C.E.P. Severe visceral leishmaniasis in children: the relationship between cytokine patterns and clinical features. ver. Soc. Bras. Med. Trop. 2013;46(6):741–745. doi: 10.1590/0037-8682-0203-2013. [DOI] [PubMed] [Google Scholar]
- IBGE . 2017. Estimativas da população residente para os municípios e para as unidades da federação Brasileira com data de referência em 1° de julho de 2017. (11p) [Google Scholar]
- Jeronimo S.M.B., Holst A.K., Jamieson S.E., Francis R., Bezerra F.L., Ettinger N.A., Nascimento E.T. Genes at human chromosome 5q31.1 regulate delayed-type hypersensitivity responses associated with Leishmania chagasi infection. Genes Immun. 2007;8(7):539–551. doi: 10.1038/sj.gene.6364422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainson R. The Neotropical Leishmania species: a brief historical review of their discovery, ecology and taxonomy. Rev. Pan-Amaz. Saude. 2010;1(2):13–32. [Google Scholar]
- Lainson R., Rangel E.F. Lutzomyia longipalpis and the eco-epidemiology of American visceral leishmaniasis, with particular reference to Brazil – a review. Mem. Inst. Oswaldo Cruz. 2005;100(8):811–827. doi: 10.1590/s0074-02762005000800001. [DOI] [PubMed] [Google Scholar]
- Lainson R., Shaw J.J. New World Leishmaniasis. In: Collier L., Balows A., Sussman M., editors. Topley & Wilson’s Microbiology and Microbial Infections. 10th ed. Vol. 5. Parasitol. Arnold; London: 2010. pp. 313–349. [Google Scholar]
- Lima L.V.R., Ramos P.K.S., Campos M.B., dos Santos T.V., Gomes C.M.C., Laurenti M.D., Corbett C.E.P., Silveira F.T. Preclinical diagnosis of American visceral leishmaniasis during early onset of human Leishmania (L.) infantum chagasi-infection. Pathog. Glob. Health. 2014;108(8):381–384. doi: 10.1179/2047773214Y.0000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcili A., Sperança M.A., Costa A.P., Madeira M.F., Soares H.S., Sanches C.O.C., et A.L. Phylogenetic relationships of Leishmania species based on trypanosomatid barcode (SSU rDNA) and gGAPDH genes: taxonomic revision of Leishmania (L.) infantum chagasi in South America. Infec. Gen. Evol. 2014;25:44–51. doi: 10.1016/j.meegid.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Moreno E.C., Melo M.N., Lambertucci J.R., Serufo J.C., Andrade A.S., Antunes C.M. Diagnosing human asymptomatic visceral leishmaniasis in an urban area of the state of Minas Gerais, using serological and molecular biology techniques. Rer. Soc. Bras. Med. Trop. 2006;39(5):421–427. doi: 10.1590/s0037-86822006000500001. [DOI] [PubMed] [Google Scholar]
- Pearson R.D., Sousa A.Q. Clinical spectrum of Leishmaniasis. Clin. Infec. Dis. 1996;22(1):1–13. doi: 10.1093/clinids/22.1.1. [DOI] [PubMed] [Google Scholar]
- Ramos P.K.S., Carvalho K.I., Rosa D.S., Rodrigues A.P., Lima L.V.R., Campos M.B., et A.L. Serum cytokine responses over the entire clinical-immunological spectrum of Leishmania (L.) infantum chagasi-infection. Biomed. Res. Int. 2016;2016:6937980. doi: 10.1155/2016/6937980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva L.A., Romero H.D., Fagundes A., Nehme N., Fernandes O., Rodrigues V., Costa R.T., Prata A. Use of the polymerase chain reaction for the diagnosis of asymptomatic Leishmania infection in a visceral leishmaniasis-endemic area. Rev. Inst. Med. Trop. São Paulo. 2013;55(2):101–104. doi: 10.1590/s0036-46652013000200006. [DOI] [PubMed] [Google Scholar]
- Silveira F.T., Lainson R., Pereira E.A., de Souza A.A.A., Campos M.B., Chagas E.J. A longitudinal study on the transmission dynamics of human Leishmania (Leishmania) infantum chagasi infection in Amazonian Brazil, with special reference to its prevalence and incidence. Parasitol. Res. 2009;104(3):559–567. doi: 10.1007/s00436-008-1230-y. [DOI] [PubMed] [Google Scholar]
- Silveira F.T., Lainson R., de Souza A.A.A., Campos M.B., Carneiro L.A., Lima L.V.R. Further evidences on a new diagnosis approach for monitoring human Leishmania (L.) infantum chagasi infection in Amazonian Brazil. Parasitol. Res. 2010;106(2):377–386. doi: 10.1007/s00436-009-1672-x. [DOI] [PubMed] [Google Scholar]
- Silveira F.T., Lainson R., de Souza A.A.A., Crescente J.A.B., Campos M.B., Gomes C.M.C. A prospective study on the dynamics of the clinical and immunological evolution of human Leishmania (L.) infantum chagasi infection in the Brazilian Amazon region. Trans. R. Soc. Trop. Med. Hyg. 2010;104(8):529–535. doi: 10.1016/j.trstmh.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Silveira F.T., Lima L.V.R., Bittencourt L.F., Han A.P., Han C.P., Ramos P.K. IgM antibody response in symptomatic (visceral leishmaniasis) and asymptomatic (indeterminate initial infection) human Leishmania (L.) infantum chagasi-infection in Amazonian Brazil. Eur. J. Trop. Med. Int. Health. 2011;16(I):64. [Google Scholar]
- Silveira F.T., Carneiro L.A., Ramos P.K.S., Lima L.V.R., Campos M.B., Laurenti M.D. A cross-sectional study on canine Leishmania (L.) infantum chagasi infection in Amazonian Brazil ratifies a higher prevalence of specific IgG-antibody response than delayed-type hypersensitivity in symptomatic and asymptomatic dogs. Parasitol. Res. 2012;111(4):1513–1522. doi: 10.1007/s00436-012-2989-4. [DOI] [PubMed] [Google Scholar]
- Silveira F.T., Lainson R., de Souza A.A.A., Crescente J.A.B., Corbett C.E.P. RNG Leão, Medicina Tropical e Infectologia na Amazônia, v. 2, Belém, Pará, Samauma Editorial. 1th Ed. Instituto Evandro Chagas; Belém, Pará: 2013. Leishmaniose visceral americana; pp. 1245–1274. [Google Scholar]
- Silveira F.T., Lima L.V.R., Vasconcelos-dos-Santos T., Ramos P.K.S., Campos M.C. Reviewing the trajectory of American visceral leishmaniasis in the Amazon, Brazil: from Evandro Chagas to the present days. Rev. Pan-Amaz. Saúde. 2016;7:15–22. (special number) [Google Scholar]