Abstract
Catalyst-controlled C—H functionalization by means of the C—H insertion chemistry of rhodium carbenes has become a powerful synthetic method. The key requirements for the development of this chemistry are donor/acceptor carbenes and the chiral dirhodium tetracarboxylate catalysts. This perspective will describe the stages involved in developing this chemistry and illustrate the scope of the donor/acceptor carbene C—H functionalization.
Graphical Abstract

Introduction
C—H Functionalization has become a very active field of study over the last 25 years because it offers a new logic for organic synthesis.1 The most well-developed approaches to achieve site-selectivity rely on intramolecular reactions to place a specific C—H bond close to the reagent,2 using directing groups to coordinate to a metal catalyst and place it in proximity of a specific C—H bond,1d, 3 or radical chemistry, where specific C—H bonds are inherently more reactive than the other C—H bonds in the substrate.4 An intriguing alternative approach is to move away from the substrate being the dominant controlling element for the site selectivity and instead rely on catalysts and/or reagents that would select which C—H bond is functionalized.5 This perspective describes our development of a catalyst-controlled intermolecular C—H alkylation method using rhodium carbenes as the reactive intermediates (Scheme 1). To achieve such a transformation we required a new class of rhodium carbenes, the donor/acceptor carbenes, reactive enough to functionalize C—H bonds, but still capable of doing so in a selective manner. Also, a collection of new chiral dirhodium catalysts were needed with defined structural features to control which C—H bond is functionalized. This program began over 30 years ago and at the onset of the work, the intent was simply to explore new carbene chemistry. During the course of these studies, however, a number of the proposed reactions failed and surprising results were obtained. As the general area of chemistry was relatively unexplored, these “failures” opened up interesting new opportunities in synthesis, which ultimately led to the C—H functionalization method described herein. The key discoveries which ultimately resulted in the highly selective intermolecular C—H functionalization method will be highlighted in this perspective.
Scheme 1.
The first example of C—H functionalization by means of metal-carbene induced C—H insertion was reported by Scott in 1974 for a copper-catalyzed reaction of ethyl diazoacetate with cyclohexane as solvent.6 Soon, thereafter, Teyssié demonstrated that the dirhodium tetracarboxylates were even better catalysts for C—H insertions into alkanes, but generally the reactions gave mixtures of products when the substrates contained multiple C—H bonds.7 To overcome the site selectivity issue, the intramolecular version of the reaction was developed in the 1980’s and 1990’s, most notably by the groups of Taber, Doyle and Hashimoto, and this intramolecular version has become a broadly useful synthetic process (Scheme 2A).2a, 8 However, the intermolecular version remained relatively undeveloped because the site selectivity issues could not be effectively controlled (Scheme 2B).9 Indeed, in Doyle’s comprehensive book on the chemistry of diazo compounds, published in 1997, it was stated that “intermolecular C—H insertions are of mechanistic interest but are not synthetically useful”.10 Ironically, our first publication on enantioselective intermolecular C—H functionalization with rhodium carbenes was also published in the same year,11 but a considerable amount of earlier work brought us to the position of being able to solve the selectivity challenges.
Scheme 2.
Early Exposure to Rhodium Carbene Chemistry
The genesis of the ideas for an independent research career often occurs during one’s graduate or post-doctoral studies. In my case, it originated during my post-doctoral studies at Princeton University (19801983) with Professor E. C. Taylor, an expert in nitrogen rich heterocycles. During my time in his laboratory, he had two collaborative medicinal chemistry projects supported by Eli Lilly. The first was the study of pteridine derivatives, which ultimately resulted in the development of a major anticancer agent, Alimta (premetrexed).12 The second involved aza-β-lactams (1) and their conversion into aza analogues 2 of β-lactam antibiotics such as thienamycin (3) (Scheme 3). I chose to work on the second project because I was attracted to strained rings, but it quickly became apparent that the aza-β-lactam ring was likely to be too fragile to lead to a potential drug candidate, especially as the fusion of a second ring system onto it would add considerable strain to an already delicate system.
Scheme 3.
As the desired targets of the project were likely to be unreachable, a different outlook was needed for this project to be successful. I decided to apply the best annulation methods known at the time for the synthesis of fused β-lactams to the aza-β-lactam system because the contrast between successful reactions with β-lactams and the anticipated failed reactions with aza-β-lactams was likely to be mechanistically interesting and potentially insightful. Indeed, a number of unusual transformations were discovered,13 but in the context of this perspective, the most relevant one came during efforts to mimic the dirhodium-catalyzed carbene N—H insertion approach (4 to 5) developed by Merck,14 a transformation that was ultimately applied to the large scale synthesis of penem antibiotics (Scheme 4). The Merck industrial approach was audacious because it required large-scale utilization of diazo compounds and at that time, a relatively newly developed catalyst, dirhodium tetraacetate. Application of this method to the aza-β-lactam 6, generated none of the desired N—H insertion product 7.13a Instead, a mixture of the fused pyrrolidinone 8 and the tricyclic system 9 was obtained. The likely cause of the unexpected products is the preferential attack of the carbene by the more nucleophilic amine nitrogen to form ylide 10, which then underwent a Stevens rearrangement to form 8 or elimination of isocyanic acid to form a second ylide 11, which dimerized to form 9.
Scheme 4.
Even though the reaction outcome was a complete failure in terms of the synthetic goals of the project, it was intriguing to me for several reasons:
Rhodium acetate gives much cleaner reactions compared to the traditional copper catalysts, and so there appeared to be an opportunity for a new era of discovery in metal carbene chemistry.
The amide bond in the aza-β-lactams remained intact. When one is considering normal nucleophilic behavior, the weakest link in β-lactams is the amide bond, yet even though the formation of 8 and 9 involve elaborate rearrangement pathways, the amide bond never broke.
Carbenes derived from diazo esters have natural umpolung reactivity. The site next to the ester is electrophilic, which is likely to lead to unusual disconnection strategies for organic synthesis
Carbenes are prone to undergo unusual reactions, and this could lead to unexplored avenues for methodology development
Even though the carbene chemistry involves highly reactive organometallic intermediates, the chemistry is procedurally very simple and does not require any specialized equipment.
Discovery of Donor/Acceptor Carbenes
When I started my independent career at Wake Forest University in 1983, I decided to make rhodium-carbene chemistry the central focus of my research program. The first question that needed to be answered, however, was what specific carbene reaction would I examine? At that time, the vast majority of the metal-carbene chemistry was derived from diazo compounds substituted with one or two electron withdrawing groups, such as diazoacetates, diazomalonates or α-diazo-β-ketoacetates. I wanted to explore other carbene structural types and so, decided to examine whether vinylcarbene 12 could act as a 2π system similar to allyl cation 13, and be capable of undergoing [4+3] cycloadditions (Scheme 5).15 The first vinylcarbene precursor we made was diazo glutaconate 14 and we examined its rhodium acetate-catalyzed reaction with cyclopentadiene 15. The result was spectacular! The desired [4+3] cycloadduct 16 was formed in essentially quantitative yield, exclusively as the endo diastereomer. We were worried, however, because the result seemed too good to be true! Cycloaddition reactions, such as the Diels-Alder reaction, tend to form preferentially the endo diastereomer and the stereospecific nature of our reaction was highly unusual. Therefore, we began to consider whether the reaction may have occurred by a different mechanism, an initial cyclopropanation, followed by a Cope rearrangement. It is well known that the Cope rearrangement of divinylcyclopropanes proceeds through a boat transition state, and thus, it would explain why the formation of 16 is stereospecific. This hypothesis was tested by conducting the reaction with the cis-substituted vinyldiazoacetate 17 because cis substituents are known to sterically interfere and slow down the rate of divinylcyclopropane rearrangements. This control experiment resulted in the clean formation of the cisdivinylcyclopropane 18, which on heating in refluxing toluene underwent the Cope rearrangement to form the formal [4+3] cycloadduct 19 as a single diastereomer. Thus, we had obtained exactly what we had proposed from the vinylcarbene 2π system, but the reaction actually proceeds by a totally different mechanism.
Scheme 5.
Even though the control experiment was very informative, it raised another area of concern. The cyclopropanation must have been highly diastereoselective, yet the literature precedence showed that this was not typically the case for rhodium-catalyzed cyclopropanations. For example, the rhodium-catalyzed cyclopropanation of styrene (20) with ethyl diazoacetate (21) gave the cyclopropane 22 as a 3:2 mixture of diastereomers (Scheme 6A).16 Therefore, we examined the reaction of styrene (20) with 14 and found that cyclopropanation was much more diastereoselective.17 The cyclopropane 23 was produced as a 8:1 diastereomeric mixture. When the reaction was conducted with styryldiazoacetate 24, which has a stronger donor group, the cyclopropane 25 was formed in a highly diastereoselective manner with >20:1 d. r. Furthermore, the carbene dimerization problem that is often seen in reactions with ethyl diazoacetate were greatly reduced with the vinyldiazoacetate 14 and 24. This led to the realization that the presence of the donor group has a major influence on the reactivity/stereoselectivity profile of the carbenes and this could open up interesting opportunities for the discovery of selective carbene transformations. Thus donor/acceptor carbenes became the central focus of my research program. We introduced a classification system for the three major types of metal carbene intermediates: acceptor, acceptor/acceptor and donor/acceptor (Scheme 6B), because the three types of carbenes have very different reactivity profiles.18 We have emphasized this classification system in the various reviews we have published on rhodium carbene chemistry, and it has become a commonly used terminology for the field.19 All three types of carbenes are very reactive, but the donor group attenuates the reactivity of the donor/acceptor carbenes, leading to more selective transformations, especially in the case of intermolecular reactions.
Scheme 6.
Development of the Tandem Cyclopropanation/Cope Rearrangement and Some Unexpected Observations
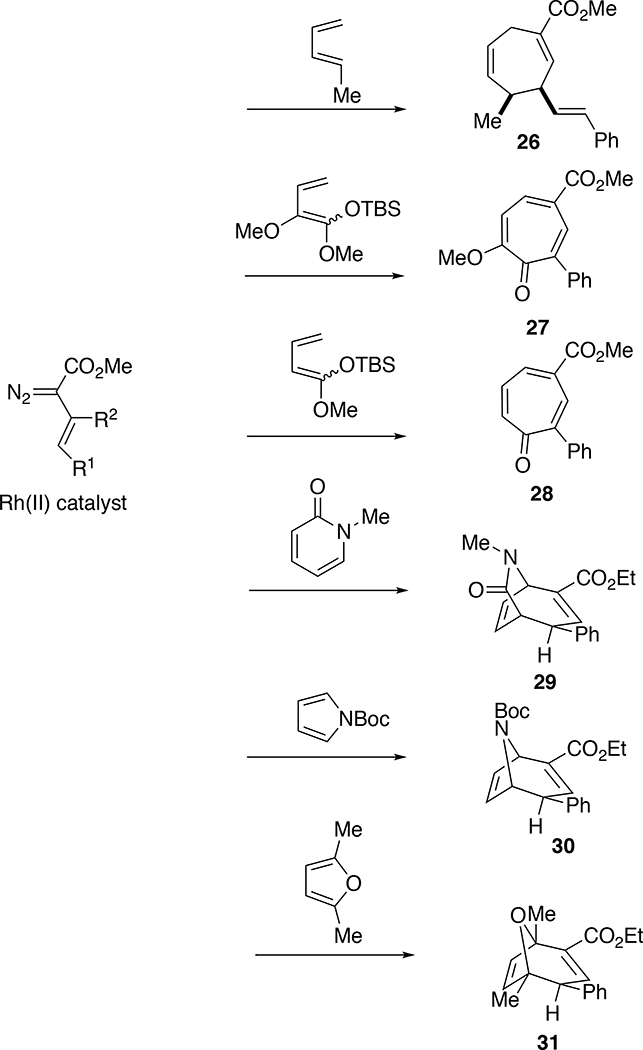
The tandem cyclopropanation/Cope rearrangement can be widely applied to the synthesis of seven membered rings, generating up to three stereogenic centers in a stereospecific manner.20 We conducted an extensive study on the scope of the reactions and showed a variety of dienes are compatible with the chemistry as illustrated in the formation of 26–31 (Scheme 7). The reaction with oxygenated dienes can lead to the synthesis of tropones21 or tropolones.22 A range of aromatic heterocycles are compatible with the chemistry,23 including furans,24 pyrroles25 and pyridinones.26 Even benzene is prone to the tandem cyclopropanation/Cope rearrangement although electron-rich aromatic rings lead to alkylation products formed by means of zwitterionic intermediates instead of initial cyclopropanation.27
Scheme 7.
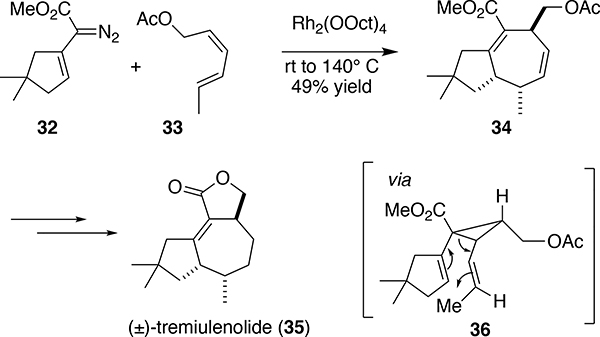
During the studies of the reactions with dienes we learned that donor/acceptor carbenes unexpectedly displayed demanding steric effects. The regiochemistry of the formal [4+3] cycloaddition depends on which double bond of the diene is cyclopropanated.20a An unsubstituted double bond is easily cyclopropanated as well as an internally substituted double bond. A cis disubstituted double bond is less reactive than a mono-substituted double bond, whereas reaction at a trans double bond is considerably less favorable. An example, illustrating the significance of the steric effect, was seen in the reaction of the cyclopentenyldiazoacetate 32 the (E,Z)-hexadiene 33, which generated the cycloheptadiene 34 as a key step in the synthesis of (±)-tremulenolide (35) (Scheme 8).28 The reaction proceeds via the intermediacy of the divinylcyclopropane 36 in which the cis alkene has been cyclopropanated selectively, setting up both the high regiocontrol and diastereocontrol of the reaction. The high regioselectivity observed with these donor/acceptor carbenes is far more pronounced than what is typically seen in rhodium catalyzed cyclopropanations with the acceptor carbene derived from ethyl diazoacetate.
Scheme 8.
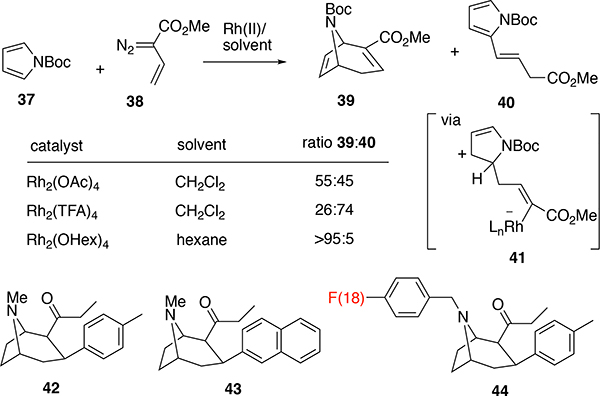
Another surprising discovery during the early development of the vinylcarbenes is the tendency of the unsubstituted vinylcarbenes to react at the vinylogous position rather than the carbene site.29 This type of reactivity was later developed by us30 and others31 into a useful synthetic transformation but that topic is outside the scope of this perspective. An early example of this phenomenon is the reaction of N-Boc-pyrrole (37) with the vinyldiazoacetate 38, which gave a mixture of the formal [4+3] cycloadduct 39 and the vinylogous alkylation product 40 (Scheme 9).29b Fortunately, the ratio of the products can be controlled by the right combination of catalyst and solvent. The formation of the vinylogous product is considered to involve the zwitterionic intermediate 41 and is enhanced when a relatively polar solvent such as dichloromethane and an electron-deficient catalyst such as Rh2(TFA)4 are used. The selectivity for reactions at the carbene site is enhanced when a non-polar solvent is used, such as pentanes or hexanes. The effective control of site selectivity of unsubstituted vinyl carbenes was important because the reaction with pyrrole became a useful entry to molecular probes and potential therapeutic agents for cocaine addiction.32 For example the tolyl derivative 42 has been extensively studied as a potential therapeutic agent to block cocaine selfadministration,32b, 32c the 1-naphthyl derivative 43 is highly potent and has been used as a chemical knockout of the dopamine transporter,32d and 44 has been used as a PET radioligand.32e Ironically, due to the enhancement of the carbene reactivity over vinylogous reactivity, pentane and hexanes became the routine solvent for the rhodium-catalyzed reactions of donor/acceptor carbenes. The donor/acceptor carbenes are sufficiently selective to react cleanly with the π-system substrates without interference from the hydrocarbon solvents.
Scheme 9.
Dirhodium Tetraprolinates as Chiral Catalysts for Donor Acceptor Carbenes
Even though dirhodium tetracarboxylates quickly became widely used as the catalysts of choice for the reactions of diazo compounds, in the early 1990’s their lantern structure (45) was not considered to be particularly promising for the design of chiral catalysts.33 The carbene would have two possible binding sites and the carboxylate ligands would be pointing away from the carbene (Scheme 10). A study of enantioselective cyclopropanation of styrene with ethyl diazoacetate with 40 different chiral dirhodium catalysts gave a maximum of 12% ee,34 and the only reasonable enantioselective reactions with dirhodium tetracarboxylates that were known at that time were intramolecular reactions.35 Chiral catalysis for intermolecular carbene chemistry were dominated by C2 symmetric copper catalysts and dirhodium tetracarboxamidate catalysts.19a We became interested in achieving asymmetric transformations with the donor/acceptor carbenes but when these types of catalysts were applied to vinyldiazoacetate cyclopropanation, none of the cyclopropane was formed.36 Instead the vinyldiazoacetate 24 underwent a thermal 6π electrocyclization to form pyrazole 46 because the catalysts were not sufficiently active to generate a rhodium carbene intermediate before the thermal cyclization occurred. Therefore, we needed to explore another approach to achieve asymmetric induction. Chiral auxiliaries had not been particularly successful in carbene chemistry before but we discovered methyl (S)-lactate (47a) and (R)pantolactone (47b) were particularly effective with donor/acceptor carbenes as illustrated in the formation of the cyclopropanes 48a and 48b.36 A model (49) was proposed to explain the asymmetric induction in which the carbonyl of the ester auxiliary interacts with the carbene to block one face of attack but the carbonyl interactions is limited and does not go all the way to an ylide intermediate. Even though the chiral auxiliary approach was successful with a variety of substrates,37 the timing of this work was not ideal. It occurred during the era where the use of chiral catalysis was starting to dominate over the use of chiral auxiliaries, and so we still needed to solve the chiral catalysis problem.
Scheme 10.
The copper and rhodium carboxamidate catalysts are sluggish compared to the dirhodium tetracarboxylate catalysts.36 Therefore, we needed to return to the dirhodium tetracarboxylates as the scaffold for chiral catalysis, even though the established precedence was not promising. We made the hypothesis that the background precedence on acceptor carbenes may not be relevant to the reactions of donor/acceptor carbenes because the reactivity profile between the two classes of carbenes is very different. This turned out to be a good decision because when we conducted the first cyclopropanation with the N-phenylsulfonyl prolinate dirhodium catalyst Rh2(S-BSP)4 (50) in dichloromethane as solvent (Scheme 11), the product was formed in 74% ee!38 We quickly discovered that the enantioselectivity was far superior in non-polar solvents such as pentane and consequently, we developed the tert-butylphenyl derivative Rh2(STBSP)4 (51) and then the dodecylsulfonylprolinate Rh2(S-DOSP)4 (52), which are soluble in hydrocarbons even at low temperatures. The solvent change resulted in the formation of the cyclopropane 25 in 90% ee at rt and 98% ee when the reaction was started at −78 °C and allowed to gradually warm to rt. Highly enantioselective cyclopropanations are possible with a variety of alkenes and vinyldiazoacetates, aryldiazoacetates, hetereoaryldiazoacetates and alkynyldiazoacetates.18, 39 The alkenes need to be 1substituted, 1,1-disubstituted39c of cis-1,2-disubstituted as trans-1,2-disubstituted39e or more highly substituted alkenes are generally too sterically crowded for cyclopropanation. Particularly noteworthy is the reaction of 1,1-diphenylethylene which is highly enantioselective and as will be described later, became the foundation of a new class of catalysts that are very important for site-selective C—H functionalization. It has also been widely used in enantioselective versions of the tandem cyclopropanation/Cope rearrangement, leading to the formation of the cycloheptadiene derivatives with high asymmetric induction.40
Scheme 11.
Rh2(S-DOSP)4 routinely gives high asymmetric induction as long as the donor/acceptor carbenes has a methyl ester as the acceptor group.19a Another catalyst, Rh2(S-PTAD)4 (53) was developed and it is capable of high asymmetric induction with donor/acceptor carbenes containing a variety of acceptor groups (Scheme 12).41 Rh2(S-PTAD)4 is related to the series of phthalimido catalysts developed by Hashimoto,42 but the adamantyl group in Rh2(S-PTAD)4 tends to give higher asymmetric induction than the Hashimoto catalysts, which have smaller alkyl groups. Excellent asymmetric induction was obtained in cyclopropanations with aryldiazophosphonates 54,41 aryldiazoacetonitriles 55,43 and trifluoromethylphenyldiazomethanes 56,44 and aryldiazoketones 57.45
Scheme 12.
Cyclopropanation with aryldiazoacetates can be conducted with very low catalyst loadings. Most of the exploratory reactions were conducted with 1 mol % catalyst loading but much lower catalyst loading could be used if desired. In the original studies with Rh2(S-DOSP)4, high asymmetric induction was still obtained with 0.1 mol % catalysts loading but dropped to below 60% ee with 0.01 mol % catalyst loading.38b Later it was shown that in the cyclopropanation of styrene with methyl phenyldiazoacetate (58) to form 59, the bridged prolinate catalyst Rh2(S-biTISP)2 (60), maintained high asymmetric induction (92% yield and 85% ee) with a catalyst loading of 0.001 mol % (Scheme 13).46 Subsequent studies revealed that much lower catalyst loadings could be used if the reactions were conducted without solvent. The lowest catalyst loadings were obtained with Rh2(S-PTAD)4 as catalyst, p-methoxyphenyldiazoacetate 61 as the carbene precursor, and cyclopentadiene as a very reactive trapping agent.47 The stronger donor group is considered to stabilize the carbene, allowing productive reactions with the reactive trapping agent to occur rather than unimolecular destruction of the rhodium carbene complex. This system was effective at a catalyst loading on 0.00006 mole % and under these conditions, the cyclopropane 62 was formed in 83% yield and 76% ee (1,300,000 turnovers). Even though these are impressive TON’s, the reaction still has some significant challenges that need to be overcome before carbene reactions with extremely low catalyst loadings become routine. The enantioselectivity tends to drop under high TON reaction conditions especially if a less reactive trapping agents is used. Also, the optimum conditions using no solvent would not be safe on large scale.
Scheme 13.
Once Rh2(S-DOSP)4 was established as an outstanding chiral catalysts for the reactions of donor/acceptor carbenes, we needed to rationalize how the asymmetric induction occurred. We proposed in 1996 that the arylsulfonylprolinate ligand was too big to exist in the periphery of the dirhodium tetracarboxylate lantern structure.38b It would then be forced to be either on the top face or the bottom face of the catalyst. If all four ligands have a similar constraint, then we recognized that the complex could adopt a higher symmetry structure than the ligands itself as illustrated in Scheme 14. The complex could be either D2 symmetric (63), C4 symmetric (64) or C2 symmetric (65). We proposed that Rh2(S-DOSP)4 adopted a D2 symmetric structure, primarily because of the extreme solvent effect where the highest asymmetric induction is obtained in non-polar solvent and the polar N-sulfonyl groups would tend to cancel each other out in the D2-symmetric agents. A predictive model was then developed (66) to explain why the Re face of the carbene is always attacked when Rh2(S-DOSP)4 is used as catalyst. Even though this model is highly predictive, the D2 symmetric structure of Rh2(S-DOSP)4 has not been validated experimentally or computationally and recent studies indicate that the N-arylsulfonyl groups are not that big and could align in the periphery of the complex.48 However, the concept of generating higher symmetry complexes depending on how the lower symmetry ligands align has become a central design element for generating new chiral dirhodium tetracarboxylate catalysts,49 as will become clear later in this perspective when our second and third generation chiral catalysts are introduced.
Scheme 14.
Early Collaborations with Synthetic Organic Chemists to Enhance the Scope of the Tandem Cyclopropanation Cope rearrangement
Having invested extensively into the cyclopropanation chemistry of donor/acceptor carbenes, we wished to demonstrate the broader utility of the chemistry. Certainly, we have applied the chemistry in total synthesis independently,28, 40b but we began collaborating with other synthetic experts to maximize the potential of our chemistry. Our first collaboration was with Dr. Steven Diver, in which we combined his enyne metathesis chemistry to make dienes with subsequent tandem cyclopropanation/Cope rearrangement reactions of those dienes with our vinylcarbenes (Scheme 15).50 This led to some interesting observations as illustrated in the conversion of the diene 69 to the cycloheptadiene 70. The diene 69 was prepared from the enyne metathesis of alkyne 67 with vinyl ether 68 and is formed as a racemate and a 1.2:1 E/Z mixture. Even so, the Rh2(R-DOSP)4-catalyzed reaction results not only in kinetic resolution but also with selective reaction with the E-diene of 69, forming 70 in 95% ee and 9:1 dr. The Z-diene of 69 was considered to be unable to remain planar and thus, the alkene undergoing the initial cyclopropanation in the E-diene would be more electron rich than the corresponding alkene in the Z-diene. This collaboration was a great learning experience, showing that beneficial collaborations can arise between organic chemists with relatively similar skill sets, especially when the project builds on combining the expertise of the two groups.
Scheme 15.
When it comes to total synthesis, our interest is to demonstrate the utility of donor/acceptor carbene chemistry in a key step or steps in the synthetic endeavor rather than necessarily be the first to achieve the synthesis of a particular target. Consequently collaborating with experts in total synthesis to bring such applications to fruition is beneficial to our program. An illustration of this approach is the total synthesis of epi-vibsanin E (71), which we conducted in collaboration with Dr. Craig Williams (Scheme 16). The Rh2(R-PTAD)4 catalyzed tandem cyclopropanation/Cope rearrangement between the diene 72 and the vinyldiazoacetate 73 generate the cycloheptadiene 74 with good asymmetric induction of the key quaternary stereocenter.51,52 In a few steps, 74 was readily converted to the tricyclic system 75 on a multigram scale. Completion of the synthesis required a conjugate addition to a sterically challenging unsaturated ketone followed by trapping of the resulting enolate. As the Williams group had explored related chemistry on other members of the vibsanin class of natural products,53 we combined forces to complete successfully the total synthesis of 71.
Scheme 16.
Another collaborative study is the synthesis of bakerolide (76) with Dr. Richmond Sarpong (Scheme 17).54 The Sarpong group had been interested in using asymmetric reactions of donor/acceptor carbenes with chiral dienes to generate either diastereomer of the product, controlled by the chiral catalyst.55 We knew we had exceptional systems for conducting such reactions, so we combined resources to carry out model studies on elaborate chiral dienes available from the Sarpong lab and then, we showcased the chemistry in the total synthesis of barekoxide (76).54 The key step is the Rh2(R-PTAD)4 catalyzed reaction of the chiral diene 77 with the vinyldiazoacetate 73 to form 78. This is the miss-matched reaction but 78 could still be formed in a 6:1 dr and could be recrystallized to the pure diastereomer in 47% overall yield. In a few additional steps, 78 was readily converted to bakerolide 76.
Scheme 17.
The positive experiences in collaborating with biologists using tropanes as molecular probes and potential medications for cocaine addiction, followed by fruitful collaborations with other synthetic organic chemists convinced me of the value of the team approach to chemistry research. It led to my belief that in general, chemists can tackle bigger problems working together than when they are working alone, and ultimately became the inspiration for proposing the NSF Center for Selective C—H Functionalization.
Discovery of Intermolecular C—H Functionalization with Donor/Acceptor Carbenes
During the mid 1990’s as the group transitioned to the State University on New York at Buffalo, it was routine for us to carry out the reactions of donor/acceptor carbenes in hydrocarbon solvents, such as pentane or hexanes because the chiral rhodium prolinate catalysts gave much higher levels of enantioselectivity in hydrocarbon solvents. Furthermore, the undesired reactions at the vinylogous position of the vinylcarbenes could be blocked when non-polar solvents were used. Ironically, I believed at that time that one of the reasons why cyclopropanations with donor/acceptor carbenes were so clean was because these carbenes do not readily undergo C—H insertion reactions. This prevailing view changed quickly during model studies directed towards the total synthesis of CP-225,917 (79), which in the mid 1990’s was a very popular natural product target because it had promising biological activity and had an unusual structure, containing an anti-Bredt double bond (Scheme 18).56 We became intrigued with this compound because we envisioned that a type 2 intramolecular reaction between a diene and a vinylcarbene could potentially lead to the bicyclic core of the natural product. Indeed we were successful in an intramolecular reaction with furan 80 (although the product 81 was too labile to lead to a serious campaign to the natural product).57 However, when we conducted the reaction with the diene 82 in hexanes as solvent, none of the desired product 83 was obtained.57 The NMR of the crude material showed the diene and the vinyl group of the carbene remained intact but there were some broad undefined peaks between 1 and 2 ppm.58 These broad peaks were an indication that the carbene may have reacted with the hydrocarbon solvent to form the mixture 84, the first time we had seen any evidence of intermolecular C—H insertion with the donor/acceptor carbenes.
Scheme 18.
We then conducted a survey to determine what types of substrates would be susceptible to C—H functionalization with donor/acceptor carbenes.11, 59 It quickly became apparent that the donor/acceptor carbenes were far superior to the acceptor carbenes at intermolecular C—H insertion. Illustrative examples from the early studies are the Rh2(S-DOSP)4-catalyzed reactions of phenyldiazoacetate 58 to form 85–88 (Scheme 19). Cycloalkanes were readily functionalized and electronically activated C—H bonds, such as allylic sites and C—H bonds adjacent to oxygen or nitrogen were particularly favorable. A competition study was conducted between some simple substrates, which gave an indication of the relative reactivity of C—H bonds. Cyclohexadiene was 28,000 times more reactive than cyclohexane. Tetrahydrofuran and N-Boc-pyrrolidine were 2700 and 1,800 times more reactive than cyclohexane. In contrast, 2-methylbutane was 1/10th as reactive as cyclohexane. Taking into account the number of reactive C—H bonds, the tertiary C—H bond in 2-methylbutane is about as reactive as the secondary C—H bond in cyclohexane. If the tertiary bond is more sterically crowded, as seen in 2,3-dimethylbutane, the substrates is a further factor of 10 less reactive.
Scheme 19.
Four advantageous features of the donor/acceptor carbenes became readily apparent form the early studies on their C—H functionalization reactions:
Their C—H functionalization reactions are much more selective than the corresponding reactions of acceptor carbenes.
C—H functionalization is feasible in the presence of functional groups.
Donor/acceptor carbenes are far less prone to dimerization than acceptor carbenes, causing the trapping of the carbene by the substrate to be more efficient.
Rh-DOSP-catalyzed reactions with the donor/acceptor carbenes derived from methyl aryldiazoacetates generally proceed in 85–95% ee as long as the reaction is conducted in nonpolar solvents.
The C—H functionalization at benzylic sites further revealed the role of sterics on the reaction of donor/acceptor carbenes.60 When a benzene ring is 1,4disubstituted, the ring is sterically protected and the C—H functionalization is cleanly formed as illustrated in the reaction with p-ethylanisole (89) with the pbromophenyl diazoacetate 90 to form 91. In contrast, with mono-substituted benzenes 92, C—H functionalization to form 93 is competing with double cyclopropanation of the aromatic ring to form 94 (Scheme 20). The influence of the steric effect is seen on comparing the reactions of toluene (92a), ethylbenzene (92b) and isopropyl benzene (92c). All three substrates have a competing double cyclopropanation reaction (two regioisomers with toluene (92a)) with the C—H functionalization but the amount of C—H functionalization is greatest for ethyltoluene, suggesting that the methylene benzylic site is the preferred site for C—H functionalization. Indeed, in the Rh-DOSP catalyzed reactions, C—H functionalization of a methylene site is generally preferred because it is a good balance between an electronically preferred site versus a sterically accessible site. A similar steric protection of an aromatic ring has been seen in other substrates such as N,N-dimethylanilines61 and indole derivatives.62 A less substituted substrate is prone to cyclopropanation but as soon as the ring is at least 1,4-disubstituted the ring is sterically protected.
Scheme 20.
C—H Functionalization offers an opportunity to generate new disconnection strategies for the synthesis of complex targets. In order to demonstrate how C—H functionalization could be considered to be a strategic reaction, we have reported several examples of the carbene-induced C—H functionalization acting as a surrogate to some of the classic reactions. C—H functionalization at sites a to oxygen would generate protected b-alkoxycarboxylates 95, the typical products of an aldol reaction (Scheme 21). 63,64 Particularly useful substrates are silyl allyl ethers (96),63 tetraalkoxy silanes (97)64b and alkyl silyl ethers (98),64a although the yields were relatively low for the alkyl silyl ethers. In each case the products 99–101 were formed with excellent diastereocontrol, which is typical for C—H functionalization at methylene sites in which there is considerable difference in size between the two substituents. The allyl silyl ether 96 is a very active substrate but the enantioselectivity is relatively moderate. The reaction with butyl silyl ether 98 is the least effective, proceeding in relatively low yield but with excellent stereocontrol. The roles of steric and electronic factors were also seen in these reactions. Tetraethoxysilane was much more reactive that tetramethoxysilane and tetratisopropoxysilane. Also, even though E-allyl silyl ethers were excellent substrates for C—H functionalization, unsubstituted and internally substituted allyl silyl ethers preferentially underwent cyclopropanation whereas Z-allyl silyl ethers generated a mixture of the cyclopropane and the C—H insertion.
Scheme 21.
C—H Functionalization a to nitrogen generates bamino esters 102, the products typical of a Mannich reaction (Scheme 22).61, 65 The Rh2(S-DOSP)4catalyzed reaction with N-Boc-pyrrolidine is highly stereoselective favoring the erythro diastereomer and can even be used in kinetic resolution reactions of 2substituted pyrrolidines as illustrated in the conversion of 103 to 104.65a, 65c The Rh2(S-DOSP)4-catalyzed reaction with N-Boc-piperidine (105) gives a mixture of threo and erythro diastereomers 106. Studies have been conducted independently by Winkler66 and us65a, 65c to improve the diastereoselectivity to favor the threo diastereomer because this would represent a quick enantioselective entry to threo-methylphenidate (Ritalin, 106). Improved diastereoselectivity was obtained using the bridged catalyst Rh2(S-biDOSP)265a, 65c and a chiral dirhodium tetracarboxamidate catalyst developed by Doyle, favoring the threo isomer. A bissilyl protected methylamine 107 is also an effective substrates leading to the synthesis of beta-amino carboxylates 108 with high enantioselectivity.65d This transformation has been used for the asymmetric synthesis of the antidepressant venlafaxine.
Scheme 22.
C—H Functionalization at allylic C—H bonds gives rise to γ,δ-unsaturated esters 109, products equivalent to a Claisen rearrangement (Scheme 23).67 Exceptionally favorable substrates are cyclohexadiene and cycloheptatriene because the buildup of positive charge during the C–H functionalization is favored. The reaction with 1-silylcyclohexanes 110 to form 111 illustrates the influence of steric factors on the diastereoselectivity of the C—H functionalization. The reaction with cyclohexene gives a mixture of the allylic C—H functionalization and cyclopropanation whereas 1-substituted cyclohexenes give only the C—H functionalization products. As the size of the substituent increases from TMS to TBDPS the diastereoselectivity of 111 improves from 70:30 dr to 96:4 dr. The reaction can also be conducted on apinene, leading to kinetic resolution when racemic (±)α-pinene (112) are used, as illustrated in the conversion of 112 to 113 in 99% ee.
Scheme 23.
Two other example of transformations, which are surrogate to conventional chemistry is the reaction with silyl enol ethers 114 to form 115, which correspond to a Michael addition equivalent68 and the C—H functionalization of acetals 116 to form 117, which corresponding to the Claisen condensation equivalent (Scheme 24).69 An advantage of the C—H functionalization approach is that it can generate products that would not be feasible using the conventional transformation. For example the enone required to form formal Michael adduct 115 would be the tautomer of 1-naphthol, which is not a viable substrate. In the Claisen condensation equivalent the product 117 has the keto carbonyl protected as a ketal and so it is not prone to facile epimerization as would be the case for an unprotected β-keto ester.
Scheme 24.
In general the C—H functionalization with Rh2(S-DOSP)4 preferentially occurs at secondary sites although it is possible to have selective C—H functionalization at primary sites if the position is sufficiently activated and there are not competing secondary sites. C—H Functionalization at tertiary sites is limited with Rh2(S-DOSP)4 and does not proceed with high asymmetric induction. One notable exception is the reaction of styryldiazoacetate 24 with adamantane 118 which produced 119 in 95% ee (Scheme 25).41 Indeed this particular reaction was the inspirations for the synthesis of the secondary generation catalyst Rh2(R-PTAD)4 because oxidative cleavage of the alkene in 119 followed by a Curtius rearrangement lead to the formation of the required adamantyl amino acid derivative. This is an early example of how we tend to approach the design of new chiral catalysts by maximizing the opportunities of our new enantioselective reactions.
Scheme 25.
Detailed mechanistic studies on the rhodium-catalyzed reactions of diazo compounds are difficult because the reactions are so fast and for quite some time there was even a question about the structure of the rhodium carbene intermediates and they were called rhodium carbenoids because of the ambiguity. In 2013, we were able in a collaborative study with Dr. John Berry to isolate the rhodium carbene intermediate 120 and obtained considerable spectral data that helped to confirm it did indeed have a rhodium carbene structure (Scheme 26).70 Since then, Fürstner has obtained crystal structures of several rhodium carbene complexes, demonstrating convincingly that a metal bound carbene is involved in these reactions.71 We have conducted computational studies in collaboration with Dr. Jochen Autschbach on the rhodium carbene intermediates and shown that the C—H functionalization proceeds by a hydride transfer event leading to build-up of positive charge on the carbon of the C—H bonds.72 This resulted in the development of model 121 for the relative and absolute stereochemistry of the C—H functionalization. These calculations also showed that the donor/acceptor carbenes are much more stable than the acceptor carbenes and the activation energy for the C—H functionalization, covers a range of values from 5 kcal/mole for the reaction with cyclohexadiene to 15 kcal/mole for the reaction with cyclopentane. In contrast, the activation energy for C—H functionalization with the acceptor carbene from ethyl diazoacetate is much less covering a range 2–5 kcal/mole. Thus, it is much harder to have selective C—H functionalization with acceptor carbenes because the C—H functionalization activation energies are so small, analogous to the difference in selectivities for free radical bromination versus free radical chlorination.
Scheme 26.
Combined C—H Functionalization/Cope Rearrangement: Discovery and Applications
When we developed Rh2(S-DOSP)4 as a chiral catalyst, we had already established the tandem cyclopropanation/Cope rearrangement as a general method for the synthesis of cycloheptadiene. Consequently, we had an obvious study that needed to be completed, the enantioselective version of the tandem cyclopropanation/Cope rearrangement to form 122 (Scheme 27). Three students were given a set of diene substrates each to explore and in a matter of weeks the study was complete.40c However, one substrate did not behave as planned. The reaction with 1,3-cyclohexadiene (123) resulted in the formation of an unexpected major product, the 1,4-cyclohexadiene 124, in which the new C-C bond had been formed at the vinylogous position of the vinylcarbene.73 Initially, we considered that the reaction was a C—H functionalization followed by a Cope rearrangement, but control experiments quickly ruled out this possibility because on heating, 124 rearranged to the formal C—H functionalization product 125, which shows that 125 is the thermodynamic product.
Scheme 27.
When first discovered, the reaction was described as a combined C—H activation/Cope rearrangement (later changed to “combined C—H functionalization/Cope rearrangement”) because it had the essence of both types of reactions.74 Later computational studies revealed that the competition between the direct C—H functionalization and the combined C—H functionalization/Cope rearrangement involved a bifurcating process (Scheme 28).75 It was initiated by a hydride transfer event to form the rhodium bound allyl anion and the allyl cation (126), followed by rapid C-C bond formation to form 127 and 128. Thus, even though the reaction has an apparent step-wise process, the intermediates do not have time to equilibrate and the reaction proceeds with very high stereocontrol.
Scheme 28.
The combined C—H functionalization/Cope rearrangement starting from E-styryldiazoacetate 24 was applied to a variety of substrates. Some representative examples are shown in Scheme 29.74 In all cases the products 129–131 were generated with extremely high enantioselectivity and diastereoselectivity, consistent with a reaction proceeding though a chair transition state.
Scheme 29.
Most examples of the combined C—H functionalization/Cope rearrangement especially with E-vinyldiazoacetates proceed through a chair TS.74 However, this ensures that bulky functionality points away from the rhodium catalyst surface. However, in certain systems, it is possible to achieve a complete switch of the diastereoselectivity by causing the reaction to proceed through a boat transition state. An illustration of this effect is seen in Scheme 30.76 The Rh2(S-PTAD)4-catalyzed reaction of the vinyldiazoacetate 132 with the siloxycyclohexene 133 generates after silyl hydrolysis and a diazo transfer reaction the product 134 as a single diastereomer in 89% ee. In contract when the same reaction is conducted on the silylcyclopentene 135, the product 136 is produced in the opposite diastereomeric series. The carbene produced from 132, will adopt the s-cis configuration and will react with 133, so that the cyclohexene ring is pointing away from the catalysts surface, leading to a chair TS 137. In the case of 132, the methyl group preferentially points away from the catalyst, whereas the cyclopentane ring can be accommodated and the reaction proceeds through the boat TS 138.
Scheme 30.
A synthetically useful class of substrates are (±)-4-methyl-1,2-dihydronapthalenes (Scheme 31).77 As illustrated for the parent system (±)-139, the Rh2(S-DOSP)4-catalyzed reaction of the vinyldiazoacetate 24 results in kinetic resolution and the formation of two products 141 and 142. The R enantiomer of 139, preferentially undergoes the combined C—H functionalization/Cope rearrangement to form 141 as a single diastereomer in 98% ee. In contrast (S)-139 preferentially undergoes cyclopropanation to form 142 in 98% ee.
Scheme 31.
Once the enantiomer differentiation methodology was established, several natural products became accessible using this chemistry. The first natural product we examined was erogorgiaene (145), and this total synthesis became a folklore of the group (Scheme 32).77 The whole total synthesis, from the 5-step synthesis of the dihydronaphthalene, the key enantiomer differentiation step and then the end game strategy was accomplished in a total of 10 days – truly a “time economical synthesis”! In the Rh2(R-DOSP)4catalyzed reaction of the dihydronaphthalene (±)-143 with the vinyldiazoacetate 140, the S enantiomer of 143 preferentially underwent the combined C—H functionalization/Cope rearrangement and after hydrogenation and lithium aluminum hydride reduction, the alcohol 144 was isolated in 31% yield from (±)-143 and 90% ee. Oxidation of the alcohol in 144 to the aldehyde followed by a Wittig reaction resulted in the formation of 145.
Scheme 32.
A more elaborate example is the total synthesis of (−)colombiasin A (148) and (+)-elisapterosin B (149) (Scheme 33)78 In this case the highly functionalized dihydronaphthalene (±)-146 is required. The additional functionality in (±)-146 does not interfere with the site for C—H functionalization and the desired carbene-induced reaction proceeds in a similar way to form the key intermediate 147 in >95% ee. The completion of the total syntheses follows strategies previously developed by Nicolaou,79 with a late stage intramolecular [4+3] cycloaddition used to form (−)-colombiasin A (148) and a late stage intramolecular [5+2] cycloaddition used to form (+)-elisapterosin B (149). The distinctive feature of our C—H functionalization strategy is the ready onestep synthesis of three stereogenic centers, which had been very challenging using more conventional methodology.
Scheme 33.
Development of Second and Third Generation Chiral Catalysts for Site Selective C—H Functionalization
The Rh2(DOSP)4-catalyzed C—H functionalization with donor/acceptor carbenes has resulted in wide range of synthetic application,19a but it does suffer from the fact the methodology needs to be matched to suitable substrates. The C—H functionalization often works well on substrates with an activated secondary C—H bond (allylic, benzylic, alpha to O or N), but if a substrate failed to give a clean reaction, then there were very few options available to improve the selectivity. An additional limitation is that high asymmetric induction requires the use of a non-polar solvent. Hexanes or pentanes can be used when the trapping agent is very reactive but with less active trapping agents 2,2-dimethylbutane, a very expensive solvent needs to be used. Rh2(DOSP)4 has been designed to be hydrocarbon soluble but many of the substrates will not be. A blend of hydrocarbon and trifluorotoluene can be used to mitigate solubility issues but invariably the enantioselective is slight lowered in a mixed solvent.
After moving to Emory University in 2008, we began the Phase I NSF Center for Chemical Innovation on Selective C—H Functionalization, which grew into a phase II Center in 2012, consisting of 26 professors from 15 universities.80 The central goal of the Center has been to develop methods that would allow C—H functionalization to be under catalyst control. Consequently, we became interested in developing new catalysts of different sizes and shapes, in order to be able to control which C—H bond in a substrate will react by simply choosing the appropriately designed catalyst (Scheme 34).81 We worked on the hypothesis that the steric environment around the dirhodium catalysts could be very influential. A less bulky catalyst may favor reaction at a tertiary C—H bond, which is electronically the preferred site of attack. However, if a catalyst is very bulky, it could overcome the electronic preference for tertiary C—H bonds and cause the reaction to occur preferentially at secondary or even primary C—H bonds.
Scheme 34.
The second generation catalyst Rh2(S-PTAD)4 has been useful for a range of enantioselective cyclopropanation reactions but has not been especially useful in controlling C—H functionalization reactions. Charette and Fox have shown that the related tert-butyl catalyst is conformationally mobile but the corresponding tetrachlorphthalimido catalyst is much more rigid.49b, 49c We have found the same characteristic is also the case with Rh2(S-TCPTAD)4 (150) and it adopts a C4 symmetric structure (Scheme 35).82 Another important new catalyst is Rh2(S-TPPTTL)4 (151).83 It also adopts a C4 symmetric arrangement but it has an additional stereochemical feature. The phenyl groups are all tilted and in the X-ray structure of the catalyst, 12 are tiled one way and 4 are tilted the other way, but computational studies in collaboration with the Musaev group, reveal that the structure with all 16 phenyl groups tilting one way is the most preferred arrangement. This means the fixed stereogenic centers in the ligands have induced a propeller like chirality in the complex due to the arrangement of the phenyl rings.
Scheme 35.
The third generation catalysts have triarylcyclopropane carboxylate ligands and are much more sterically demanding than the other two classes of catalysts (Scheme 36).84 The ligands are readily generated from the asymmetric cyclopropanation by aryldiazoacetates 152 of 1,1-diphenylethylene (153) to form 154 followed by enantioenrichment through recrystallization, ester hydrolysis and then ligand exchange to form the chiral dirhodium complex. Further catalyst diversification is possible through multifold Suzuki coupling on the preformed catalyst to form 155. At this stage, five distinctive members of the TPCP catalysts have been developed. The first two members of this class were Rh2(R-p-BrTPCP)4 (156) and Rh2(R-p-PhTPCP)4 (157). The former catalyst adopts a D2 symmetric structure, whereas the latter adopts a C2 symmetric structure due to p-stacking between the biphenyl groups.84a Further refinement of this general structures led to the development of another D2 symmetric catalyst Rh2(R-3,5-di(p-tBuC6H4)TPCP)4 (158), which is selective for C—H functionalization at the most sterically accessible methylene sites.84b
Scheme 36.
Another class of TPCP catalysts that exhibits special features are those with an o-Cl substituent on the C1aryl ring (Scheme 37). The original member of this class was85 Rh2(S-o-ClTPCP)4 (159) but Rh2(S-2-Cl-5BrTPCP)4 (160) is considerably better at achieving high asymmetric induction. These catalysts adopt a C4 symmetric arrangement in which the fixed stereogenic center of the ligand induces a center of axial chirality in the complex, because of hindered rotation between the cyclopropane and the o-Cl-aryl ring. The o-ClTPCP catalysts are very effective at C—H functionalization at the most accessible methylene site.
Scheme 37.
An even more sterically demanding catalysts can be generated by a 12 fold Suzuki coupling of a preformed catalysts. Rh2[R-tris(p-tBuC6H4)TPCP]4 (161) has 12 biphenyl groups (Scheme 38). Computational studies in collaboration with the Houk group showed that due to the pi-stacking between these groups, the C4 symmetry is broken and the catalyst adopts a C2 symmetric shape instead.86 It is so sterically demanding that it will cause C—H functionalization to occur preferentially at a primary C—H bond.
Scheme 38.
A further advantage of all the second and third generation catalysts is that they are much more rigid compared to Rh2(DOSP)4. They no longer require the use of hydrocarbon solvents for high asymmetriuc induction. Dichloromethane is generally the optimum solvent, which expands the overall practicality of the chemistry.
Catalyst-Controlled Site-Selective C—H Functionalization
Once the TPCP catalysts became available, they were evaluated in C—H functionalization reactions. It became readily apparent they behaved as sterically crowded catalysts. Applying these catalysts to site selective C—H functionalization at benzylic sites resulted in a dramatic effect, as illustrated in Scheme 39.87 The Rh2(R-DOSP)4-catalyzed reaction of methyl aryldiazoacetate 90 with p-ethyltoluene (162) resulted in clean C—H functionalization at the secondary C—H bond to form 163a. However, when the reaction was catalyzed by Rh2(p-PhTPCP)4 the major product 164a was derived from primary C—H functionalization. Even further enhancement of the site selectivity could be achieved by using the trichloroethyl aryldiazoacetate 165 in which case, 164b was formed with a 13:1 r.r. and 99% ee.
Scheme 39.
Having established that catalyst-control could have a dramatic influence on site selectivity, we initiated a campaign to develop protocols for site selective C—H functionalization occurring at unactivated C—H bonds. Even though we discovered the intermolecular C—H functionalization when hexanes were used as solvent, we had avoided an extensive study on reactions with alkanes as substrates because we did not have the tools for clean site selective reactions. With the development of the new bulky catalysts and the advantages of using carbene precursors containing trichloroethyl esters instead of methyl esters, we finally were in a position to take on the challenge of catalyst-controlled site selective functionalization of unactivated tertiary, secondary or primary C—H bonds. C—H functionalization at tertiary C—H bonds would be electronically preferred but by steadily increasing the sterically crowding around the catalyst, we would expect to block the electronically preferred sites and instead functionalize the most sterically accessible sites.
Even though C—H functionalization at a tertiary site is electronically preferred, it is still challenging because tertiary sites are a bit crowded for the rhodium-bound donor/acceptor carbenes. This effect was observed in an early study, in which effective C—H functionalization of unactivated tertiary sites could only be achieved with a limited range of substrates, as illustrated in the reaction of 166 with methyl aryldiazoacetate 58 to form 167 (Scheme 40).88 Under normal conditions with solvent, the yields were very low so these reactions needed to be carried under virtually neat conditions and relatively high temperatures. The reactions catalyzed by either Rh2(S-DOSP)4 or Rh2(S-PTAD)4 generated 167 with good site selectivity but in relatively low yields and poor enantioselectivity.
Scheme 40.
Recently, a much better system for tertiary C—H functionalization was identified, trifluoroethyl aryldiazoacetates with the conformationally much more rigid catalyst. Rh2(S-TCPTAD)4.82 Some examples of the types of products that could be generated using this combination are shown in Scheme 41. In addition to the reaction with simple alkanes, some functional groups were compatible with the chemistry, as illustrated in the formation of 168–173. Most notably the reaction could be carried out on elaborate substrates containing many tertiary C—H bonds, such as cholesteryl acetate 174, and the C—H functionalization occurred cleanly at the most accessible tertiary C—H bond to form 175.
Scheme 41.
A selective C—H functionalization at an unactivated secondary C—H bond would require a sterically bulky catalyst to overcome the electronic preference for reactions occurring at tertiary sites. The D2 symmetric catalyst, Rh2(R-3,5-di(p-tBuC6H4)TPCP)4 (158), was found to be an exceptional catalyst for site selective reactions at the most accessible methylene site (Scheme 42).84b In the model studies, the catalyst was shown to be effective at functionalization of the C2 of pentane, with no competing reactions occurring at the C3 methylene to form 173. Other representative examples are the reaction with 1-bromohexane, 1-TMS-pentane and 1-acetoxyhexane to form 174–176. These reactions proceed with good site selectivity, diastereoselectivity and enantioselectivity, demonstrating the remarkable control exhibited by this catalyst.
Scheme 42.
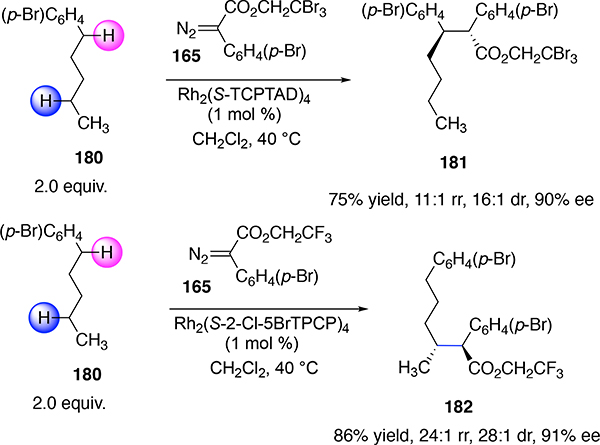
Later, the o-ClTPCP catalysts were found to display even better site selectivity for the most accessible secondary C—H bond.85a Further studies revealed that Rh2(2-Cl-5-BrTPCP)4 was the better catalyst because it gave higher levels of asymmetric induction than Rh2(o-ClTPCP)4).85b Its subtle control was demonstrated in the reaction of n-alkylbenzenes (Scheme 43). The Rh2(S-TCPTAD)4-catalyzed reaction gave a strong preference for benzylic C—H functionalization with excellent diastereocontrol and enantiocontrol as illustrated in the conversion of 180 to 181. In contrast the Rh2(S-2-Cl-5BrTPCP)4-catalyzed reaction gave a clean reaction at the most accessible methylene site to form 182, again with excellent stereocontrol. The general approach was applicable to a range of aryl and heteroaryl-substituted alkanes.
Scheme 43.
The synthetic utility of the Rh2(2-Cl-5-BrTPCP)4-catalyzed reaction has been demonstrated in collaboration with the Stoltz group with a direct synthesis of the paracyclophane core 183 of the cylindrocyclophane class of natural products (Scheme 44).85b The palladium catalyzed C—H functionalization between the aryl iodide 184 and the diazoacetate 185 generated the carbene precursor 186. The Rh2(R-2-Cl5-BrTPCP)4-catalyzed reaction of 186 with the aryl iodide 184 occurred cleanly at the most accessible methylene site of 184 to form 187 in 83% yield, 91% ee and excellent site and diastereocontrol. A further palladium catalyzed C—H functionalization on 187 generate a second carbene precursor 188, which on Rh2(R-2-Cl-5-BrTPCP)4-catalyzed reaction underwent a clean intramolecular reaction to form 183. As the minor enantiomer in 187 would form preferentially a diastereomer product to 183, the final product is formed with very high levels of enantioselectivity (>99% ee). The overall scheme illustrates the potential of catalyst- controlled C—H functionalization to generate complex structures with multiple stereogenic centers in a very direct way.
Scheme 44.
The C—H functionalization of primary C—H bonds would have to overcome the inherent tendency to react at tertiary or secondary C—H bonds. Rh2[R-tris(p-tBuC6H4)TPCP]4 (161) was found to be a sufficiently crowded catalyst to force the C—H functionalization to occur preferentially at primary C—H bonds as illustrated in Scheme 45.86 The enantiomerically pure substrates 189 and 190 have two methyl groups but the reaction occurs only at the most accessible methyl group. The catalyst (S)-161) can achieve very high asymmetric induction leading to the diastereomers 191 and 192. Clear demonstration that the reaction is under catalyst control is seen when (R)-161) because in this case the other diastereomers 193 and 194 are formed.
Scheme 45.
An impressive example illustrating the effectiveness of the catalyst control is seen in the reaction with the steroid 195 (Scheme 46).86 Even though this compound has several allylic sites, these sites are not functionalized because they are too sterically crowded. When Rh2(R-TCPTAD)4 (150) and the trifluoroethyl aryldiazoacetate 196 were used, the reaction occurs at the most accessible tertiary site to form 197, whereas when Rh2[S-tris(p-tBuC6H4)TPCP]4 (161) and trifluoroethyl aryldiazoacetate 198 were used, the most accessible primary C—H bond is the preferred site to form 199. These reactions also proceed with high asymmetric induction. The site selectivity is primarily controlled by the nature of the catalyst although the nature of the trihaloethyl ester also has some influence.
Scheme 46.
Having established catalysts that are capable of selecting between primary, secondary and tertiary C— H bonds, we have begun examining the possibility of distinguishing between similar unactivated secondary C—H bonds. A particularly impressive example is the Rh2(S-TPTTTL)4 (151)-catalyzed reaction of aryldiazoacetates with alkylcyclohexanes, which results in desymmetrization of the cyclohexane with a clean reaction at the equatorial C3 C—H bond (Scheme 47).83 Illustrative examples are the reactions to form 200–203. The reactions are routinely highly enantioselective and diastereoselective at the C—H functionalization site and the dr in this case represents the kinetic resolution (reactions at C3 equatorial versus C5 equatorial). As expected the kinetic resolution in the reaction of tertbutylcyclohexane to form 200 is greater than in the case of methylcyclohexane to form 201. Even though p-bromophenyldiazoacetate is the standard reference reagent used to evaluate the C—H functionalization selectivity, a range of aryl and hetereoaryldiazoacetates are compatible as illustrated in the formation of 202 and 203. The site selectivity control exhibited by Rh2(STPTTTL)4 (151) is different from the other chiral catalysts. It is not simply due to the steric environment around the carbene because it behaves as if it is not that sterically crowded. An additional controlling factor is how the substrate fits within the pocket during the C—H functionalization.
Scheme 47.
The Rh2(S-TPTTTL)4 (151)-catalyzed reaction with 1,4-dimethylcyclohexanes 204 and 205 gives an indication of the rate difference between C—H functionalization occurring at equatorial versus axial positions (Scheme 48).83 Both 204 and 205 are capable of undergoing. C—H functionalization at tertiary C—H bonds, as illustrated in the formation of 206 and 207. However, competition experiments reveal that the reaction with the cis isomer 204 is 70 times fasters than the trans isomer 205, which indicates that attack at the equatorial position is 140 times more favorable than attack at the equatorial position.
Scheme 48.
The site selectivity exhibited by the various chiral dirhodium catalysts prepared to date is considered to be primarily due to steric factors. In collaborations with Dr. John Bacsa we have been obtained extensive crystallographic information on a large number of the catalysts and combined with extensive computational studies in collaboration with Dr. Djamalddin Musaev and more recently with Dr. Ken Houk, we have gained insight about the dynamics of the catalysts and the catalyst-carbene complexes. A complete understanding of how these catalysts perform is challenging because the complexes are so large, but an illustration of the proposed models for how two of the catalysts are discussed below.
Rh2(S-TCPTAD)4 (150) adopts a C4 symmetric bowl-like structure (Scheme 35).82 The wall of the “bowl” spreads outward such that once the carbene is bound, a tertiary C-H bond, which is electronically preferred, can still approach closely to the carbene, but only if is uncrowded, such as an isopropyl group (Scheme 49). C4 symmetric bowl-shaped catalysts would not normally be considered ideal for asymmetric induction, but in this case the donor group of the carbene is involved in π-stacking to a phthalimido group, Two π-stacking complexes A and B can be formed and the preferred orientation B leads to the observed asymmetric induction (Re face attack). The TPCP catalysts can adopt different symmetries but in each case the carbene will be contained within a sterically crowded The catalyst wall in these cases is more directly upwards and the steric environment is much more severe. Rh2(R-3,5-di(p-tBuC6H4)TPCP)4 (158) and Rh2(S-2-Cl-5-BrTPCP)4 (160) (Schemes 36 and 37) have a strong preference for the most sterically accessible secondary site and Rh2[R-tris(ptBuC6H4) TPCP]4 (161) (Scheme 38) causes reaction to occur at the most accessible primary site.
Scheme 49.
The controlling elements in Rh2(S-TPTTTL)4 (151) is much subtler because it is not that sterically demanding at the carbene site.83 The site selectivity come from how the substrate fits into the pocket and what features of the substrate interferes with the wall of the catalyst. Rh2(STPTTTL)4 (151) is also C4 symmetric but has the additional feature of the indicated propeller chirality due to the tilting of the 16 phenyl ring (Scheme 35). The catalyst is relatively rigid but is still capable of some flexibility to allow the carbene to bind and then allow the substrate to approach the bound carbene complex (Scheme 50). Structure C shows the lowest energy form of the rhodium carbene complex and structure D, shows the lowest energy transition state for the tert-butylcyclohexane reacting with the carbene. The attack of the carbene is occurring at the equatorial C-3 position of the cyclohexane and the red tert-butyl group is pointing out of the pocket. The corresponding model of the complex with attack occuring at C-4 of the cyclohexane cannot be made because the tert-butyl group would have a serious steric class with the wall of the catalyst.
Scheme 50.
Alternative Precursors to Donor/Acceptor Carbenes
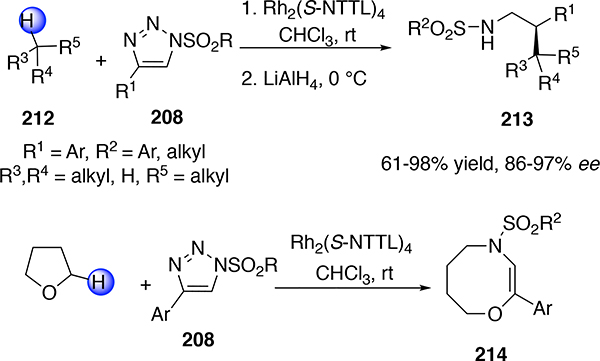
Diazo compounds have been shown to be ideal precursors to the metal carbene intermediates, especially when conducting challenging C—H functionalization reactions because the nitrogen byproduct does not interfere with the reactions. However, there is considerable interest in developing alternative strategies to the carbene intermediates because this could broaden the scope of the type of donor/acceptor carbenes that could be accessed. One particularly effective approach has been the ringopening reaction of N-sulfonyltriazoles, initially developed by Fokin89 and then popularized by several research groups (Scheme 51).90 The N-sulfonyltriazoles 208 on heating in the presence of a dirhodium catalysts undergoes ring opening to a diazo compound 209, which then loses nitrogen to generate the rhodiumbound imino carbene 210. The best catalyst for asymmetric induction with this system is Rh2(S-NTTL)4 (211), a catalyst originally developed by Muller.91
Scheme 51.
This approach has been widely used in a range of carbene reactions but its application in C—H functionalization reactions is still underdeveloped. Folkin showed that the Rh2(S-NTTL)4-cataltzed reaction was effective in enantioselective C—H functionalization of alkanes 212, followed by reduction, to form the sulfonylated amines 213 (Scheme 52).89 However, the initial studies revealed that extension to other substrates was limited as illustrated, in the reaction with tetrahydrofuran to form the ring expanded product 214.92 This is considered to occur first by oxonium ylide formation followed by 2,3-sigmatropic rearrangement. In contrast, in the case of aryldiazoacetates, there is evidence to suggest that the reaction of the carbene with heteroatoms can be reversible,93 which explains why a range of functional groups are compatible with aryldiazoacetates even when functionalization is occurring at unactivated C—H bonds.
Scheme 52.
Recently, we have demonstrated that the N-sulfonyl triazoles can be applied to C—H functionalization reactions with more elaborate substrates than just alkanes, as illustrated in Scheme 53.94 The Rh2(S-NTTL)4-catalyzed reaction of the trans alkene 215 with the triazole 216 results in clean allylic C—H functionalization at the tertiary site to form 217 in 84% yield after reduction. Ozonolysis of 217 followed by reductive amination generated the pyrrolidine 218. The four steps could be conducted in a one-pot process to generate 218 in 71% overall yield from the triazole 215. Another effective substrate is silacyclobutanes as illustrated by the Rh2(S-NTTL)4-catalyzed conversion of 219 to 220.95 So far, Rh2(S-NTTL)4 has been shown to be the superior chiral catalyst for the N-sulfonyltriazole system, and so, the option for effective catalyst-controlled site selectivity has not yet been realized in this system.
Scheme 53.
Enhancement of the Practicality of Rhodium-Catalyzed Reactions of Donor/Acceptor Carbenes
The rhodium-catalyzed reactions of donor/acceptor carbenes have resulted in a number of synthetically useful transformations. Even so, it is important to consider the practicality of these reactions generally because the dirhodium catalysts are expensive and diazo compounds have high energy and are potentially explosive. The Rh-DOSP-catalyzed enantioselective cyclopropanation of styrene 221 to form 222, a key intermediate in the synthesis of the antiviral drug, Beclabuvir (223), has been conducted safely on a multi-Kilogram scale by keeping the styryldiazoacetate 24 as a 10% solution in toluene (Scheme 54).96 We have already demonstrated that the rhodium catalysts can be used with very low catalyst loading, which mitigates the high cost of these catalysts.
Scheme 54.
Another way to enhance the practicality of the chemistry has been to conduct the reactions with immobilized catalysts either in batch or in flow. In collaboration with the Jones group both Rh-DOSP and Rh-TPCP related catalysts have been immobilized and demonstrated that they are capable of a range of C—H functionalization reactions in flow, including reactions at unactivated C—H bonds.97 An even more advantageous protocol would be the generation of the diazo compounds in flow because this would avoid the buildup of large quantities of it, as illustrated in Scheme 55.97c A potassium iodosulfonamide resin (PSSO2NIK) was found to be an effective oxidant for the conversion of a hydrazone 224 to the diazo compound 225 in flow, and after passing through a column containing a drying agent and sodium thiosulphite as a reductant, the diazo compound 225 can be directly reacted either in batch or in flow in subsequent C—H functionalization reactions, as illustrated in the benzylic primary C—H functionalization of 226 to form 227. In the current system an immobilized version of Rh2(pBrTPCP)4 (228) was used as the catalyst, The site- and stereoselectivity of the flow reactions compares very favorable with the results obtained with the corresponding homogeneous catalyst in batch.
Scheme 55.
Summary and Future Outlook
In conclusion, this perspective illustrates the stages involved in the development of catalyst-controlled site-selective C—H functionalization reactions of donor/acceptor carbenes. Research progress rarely follows a straight line as exemplified by this research program. It began focusing on the discovery of new reactions of rhodium carbene intermediates, which lead to the recognition that donor/acceptor carbenes have modulated reactivity compared to the standard carbene intermediates lacking the donor group. This opened up the possibility for the development of highly selective synthetic methods and effective chiral dirhodium catalysts. A curious series of events led to the use of hydrocarbons as the routine solvents for these carbene reactions. For several years, it was not realized that these more stabilized carbenes would still be able to react with hydrocarbon solvents. However, once a failed reaction revealed that reactions with hydrocarbons were indeed possible, the program of selective intermolecular C—H functionalization with donor/acceptor carbenes began in earnest and continues unabated to this day.
Even though considerable progress has been made in catalyst controlled C—H functionalization, many challenges still remain. The remarkable selectivity requires donor/acceptor carbene reagents so a major challenge is to broaden the scope of the chemistry. What other types of carbenes or other group transfer agents can be used? Can the donor group be expanded to amino or alkoxy leading to the formation of general useful chiral α-amino- and α-alkoxy acids? The catalysts so far simply rely on steric factors to control site selectivity. Is it possible to introduce other controlling elements into the catalyst structures, such as polar functionality or directing groups? The key take-home message so far is that exquisite site selectivity can be obtained using transition metal catalysts, and hopefully this will be an inspiration for others to identify other systems capable of exceptional catalyst-controlled C—H functionalization.
ACKNOWLEDGMENT
Financial support was provided by NSF under the CCI Center for Selective C–H Functionalization (CHE-1700982). Additional support was provided by NSF (CHE 1465189) and NIH (GM-099142). The chemistry of donor/acceptor carbenes has been the dominant theme of my research program and a large number of highly dedicated post-doctoral associates, graduate students and undergraduates have contributed to the success of the program. I am greatly indebted to their fantastic engagement and enthusiasm. I have been fortunate to collaborate with a large number of professors, both in the US and internationally, and I thank them for their passionate involvement in our joint projects. I also want to acknowledge the great comradery and support of my faculty colleagues at the three universities I have taught. Finally, I want to thank all of the members of the NSF Center for C—H functionalization, past and present, who have enriched the breadth of our program through extensive discussions and collaborations. I would like to that my post-doctoral associate, Dr. Zhi Ren, for his assitance in preparing the visuals of the catalysts.
Biography

Huw M. L. Davies was born in Aberystwyth, Wales, UK. He received his B. Sc. degree from University Cllege Cardiff, Wales (1977) and his Ph. D degree from the University of East Anglia (1980). After a post-doctoral position at Princeton University (1980–1983), he joined the faculty at Wake Forest University (18831995). After being promoted to full professor he moved to the University at Buffalo, the State University of New York (1995–2008) where he held the positions of UB Distinguished Professor and Larkin Professor of Organic Chemistry. In 2008 he moved to Emory University as the Asa Griggs Candler Professor of Chemistry. He is currently the Director of the NSF Center for Chemical Innovation for Selective C—H Functionalization.
Footnotes
The author is a named inventor on a patent entitled, Dirhodium Catalyst Compositions and Synthetic Processes Related Thereto (US 8,974,428, issued March 10, 2015).
REFERENCES
- (1).(a) Abrams DJ; Provencher PA; Sorensen EJ, Recent applications of C-H functionalization in complex natural product synthesis. Chem. Soc. Rev 2018, 47, 8925–8967; [DOI] [PubMed] [Google Scholar]; (b) Gutekunst WR; Baran PS, C-H functionalization logic in total synthesis. Chem. Soc. Rev 2011, 40, 1976–1991; [DOI] [PubMed] [Google Scholar]; (c) BrUckl T; Baxter RD; Ishihara Y; Baran PS, Innate and Guided C-H Functionalization Logic. Acc. Chem. Res 2012, 45, 826–839; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Saint-Denis TG; Zhu R-Y; Chen G; Wu Q-F; Yu J-Q, Enantioselective C(sp3)-H bond activation by chiral transition metal catalysts. Science 2018, 359, 759; [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Cernak T; Dykstra KD; Tyagarajan S; Vachal P; Krska SW, The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev 2016, 45, 546–576. [DOI] [PubMed] [Google Scholar]
- (2).(a) Doyle MP; Liu Y; Ratnikov M, Catalytic, asymmetric, intramolecular carbon-hydrogen insertion. Org. React 2013, 80, 1–131; [DOI] [PubMed] [Google Scholar]; (b) Stateman LM; Nakafuku KM; Nagib DA, Remote CH Functionalization via Selective Hydrogen Atom Transfer. Synthesis 2018, 50, 1569–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).(a) Sambiagio C; Schonbauer D; Blieck R; Dao-Huy T; Pototschnig G; Schaaf P; Wiesinger T; Zia MF; WencelDelord J; Besset T; Maes BUW; Schnurch M, A comprehensive overview of directing groups applied in metal-catalyzed C-H functionalization chemistry. Chem. Soc. Rev 2018, 47, 6603–6743; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Engle KM; Mei T-S; Wasa M; Yu J-Q, Weak Coordination as a Powerful Means for Developing Broadly Useful C-H Functionalization Reactions. Acc. Chem. Res 2012, 45, 788–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).(a) Angnes RA; Li Z; Correia CRD; Hammond GB, Recent synthetic additions to the visible light photoredox catalysis toolbox. Org. Biomol. Chem 2015, 13, 9152–9167; [DOI] [PubMed] [Google Scholar]; (b) Gao P; Gu Y-R; Duan X-H, Direct C-H Functionalization of Heteroarenes via Redox-Neutral Radical Process: A Facile Route to C-C Bonds Formation. Synthesis 2017, 49, 3407–3421. [Google Scholar]
- (5).(a) White MC; Zhao J, Aliphatic C-H Oxidations for LateStage Functionalization. J. Am. Chem. Soc 2018, 140, 13988–14009; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hartwig JF; Larsen MA, Undirected, Homogeneous C-H Bond Functionalization: Challenges and Opportunities. ACS Cent. Sci 2016, 2, 281–292; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hartwig JF, Catalyst-Controlled Site-Selective Bond Activation. Acc. Chem. Res 2017, 50, 549–555; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Davies HML; Manning JR, Catalytic C-H functionalization by metal carbenoid and nitrenoid insertion. Nature 2008, 451, 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Scott LT; DeCicco GJ, Intermolecular carbon-hydrogen insertion of copper carbenoids. J. Amer. Chem. Soc 1974, 96, 322–3. [Google Scholar]
- (7).Demonceau A; Noels AF; Hubert AJ; Teyssie P, Transition metal-catalyzed reactions of diazoesters. Insertion into carbon-hydrogen bonds of paraffins by carbenoids. J. Chem. Soc., Chem. Commun 1981, 688–9. [Google Scholar]
- (8).Doyle MP; Duffy R; Ratnikov M; Zhou L, Catalytic Carbene Insertion into C-H Bonds. Chem. Rev 2010, 110, 704–724. [DOI] [PubMed] [Google Scholar]
- (9).Caballero A; Diaz-Requejo MM; Fructos MR; Olmos A; Urbano J; Perez PJ, Catalytic functionalization of low reactive C(sp3)-H and C(sp2)-H bonds of alkanes and arenes by carbene transfer from diazo compounds. Dalton Trans. 2015, 44, 20295–20307. [DOI] [PubMed] [Google Scholar]
- (10).Doyle MP; McKervey MA; Ye T, Modern Catalytic Methods for Organic Synthesis with Diazo Compounds: From Cyclopropanes to Ylides. Wiley: 1998. [Google Scholar]
- (11).Davies HML; Hansen T, Asymmetric Intermolecular Carbenoid C-H Insertions Catalyzed by Rhodium(II) (S)-N-(pDodecylphenyl)sulfonylprolinate. J. Am. Chem. Soc 1997, 119, 9075–9076. [Google Scholar]
- (12).Taylor EC In The discovery of alimta (pemetrexed), Wiley-VCH Verlag GmbH & Co. KGaA: 2015; pp 157–180. [Google Scholar]
- (13).(a) Taylor EC; Davies HML, Approaches to the synthesis of aza analogs of the β-lactam antibiotics: some anomalous rhodium(II)-catalyzed carbene insertion reactions. J. Org. Chem 1984, 49, 113–16; [Google Scholar]; (b) Taylor EC; Davies HML, Synthesis of fused 1,2-diazetidinones via an intramolecular Horner-Emmons reaction. J. Org. Chem 1986, 51, 1537–40; [Google Scholar]; (c) Taylor EC; Davies HML; Hinkle JS, Synthesis and reactions of some 1,2disubstituted 1,2-diazetidin-3-ones: an intramolecular aldol approach to bicyclic systems. J. Org. Chem 1986, 51, 1530–6; [Google Scholar]; (d) Taylor EC; Davies HML; Lavell WT; Jones ND, N- vs. O-acylation of 4,5-dihydro-1,3-oxadiazin-6-ones by ring enlargement. J. Org. Chem 1984, 49, 2204–8. [Google Scholar]
- (14).(a) Ratcliffe RW; Salzmann TN; Christensen BG, Novel synthesis of the carbapen-2-em ring system. Tetrahedron Letters 1980, 21, 31–34; [Google Scholar]; (b) Karady S; Amato JS; Reamer RA; Weinstock LM, Stereospecific conversion of penicillin to thienamycin. J. Am. Chem. Soc 1981, 103, 6765–6767. [Google Scholar]
- (15).Davies HML; Smith HD; Korkor O, Tandem cyclopropanation/Cope rearrangement sequence. Stereospecific [3 + 4] cycloaddition reaction of vinylcarbenoids with cyclopentadiene. Tetrahedron Lett. 1987, 28, 1853–6. [Google Scholar]
- (16).(a) Doyle MP; Dorow RL; Buhro WE; Griffin JH; Tamblyn WH; Trudell ML, Stereoselectivity of catalytic cyclopropanation reactions. Catalyst dependence in reactions of ethyl diazoacetate with alkenes. Organometallics 1984, 3, 44–52; [Google Scholar]; (b) Doyle MP, Catalytic methods for metal carbene transformations. Chem. Rev 1986, 86, 919–40. [DOI] [PubMed] [Google Scholar]
- (17).Davies HML; Clark TJ; Church LA, Stereoselective cyclopropanations with vinylcarbenoids. Tetrahedron Lett. 1989, 30, 5057–60. [Google Scholar]
- (18).Davies HML; Bruzinski PR; Fall MJ, Effect of diazoalkane structure on the stereoselectivity of rhodium(II) (S)-N(arylsulfonyl)prolinate catalyzed cyclopropanations. Tetrahedron Lett. 1996, 37, 4133–4136. [Google Scholar]
- (19).(a) Davies HML; Antoulinakis EG, Intermolecular metal-catalyzed carbenoid cyclopropanations. Org. React 2001, 57, 1–326; [Google Scholar]; (b) Davies HML; Beckwith REJ, Catalytic enantioselective C-H activation by means of metal-carbenoidinduced C-H insertion. Chem. Rev 2003, 103, 2861–2903; [DOI] [PubMed] [Google Scholar]; (c) Davies HML; Walji AM In Rhodium(II)-stabilized carbenoids containing both donor and acceptor substituents, Wiley-VCH Verlag GmbH & Co. KGaA: 2005; pp 301–340; [Google Scholar]; (d) Davies HML; Pelphrey PM, Intermolecular C-H insertions of carbenoids. Org. React) 2011, 75, 75–211. [Google Scholar]
- (20).(a) Davies HML, Tandem cyclopropanation/Cope rearrangement: a general method for the construction of sevenmembered rings. Tetrahedron 1993, 49, 5203–23; [Google Scholar]; (b) Davies HML, [3+4] Annulations between rhodium-stabilized vinylcarbenoids and dienes. Adv. Cycloaddit. 1999, 5, 119–164. [Google Scholar]
- (21).Davies HML; Clark TJ; Kimmer GF, Versatile synthesis of tropones by reaction of rhodium(II)-stabilized vinylcarbenoids with 1-methoxy-1-[(trimethylsilyl)oxy]buta-1,3diene. J. Org. Chem 1991, 56, 6440–7. [Google Scholar]
- (22).Davies HML; Clark TJ, Synthesis of highly functionalized tropolones by rhodium(II)-catalyzed reactions of vinyldiazomethanes with oxygenated dienes. Tetrahedron 1994, 50, 9883–92. [Google Scholar]
- (23).Davies HML; Hedley SJ, Intermolecular reactions of electron-rich heterocycles with copper and rhodium carbenoids. Chem. Soc. Rev 2007, 36, 1109–1119. [DOI] [PubMed] [Google Scholar]
- (24).(a) Davies HML; Clark DM; Smith TK, [3 + 4] Cycloaddition reactions of vinyl carbenoids with furans. Tetrahedron Lett. 1985, 26, 5659–62; [Google Scholar]; (b) Davies HML; Clark DM; Alligood DB; Eiband GR, Mechanistic aspects of formal [3 + 4] cycloadditions between vinyl carbenoids and furans. Tetrahedron 1987, 43, 4265–70. [Google Scholar]
- (25).Davies HML; Young WB; Smith HD, Novel entry to the tropane system by reaction of rhodium(II) acetate stabilized vinylcarbenoids with pyrroles. Tetrahedron Lett. 1989, 30, 4653–6. [Google Scholar]
- (26).Davies HML; Hodges LM, Rhodium Carboxylate Catalyzed Decomposition of Vinyldiazoacetates in the Presence of Heterodienes: Enantioselective Synthesis of the 6Azabicyclo[3.2.2]nonane and 6-Azabicyclo[3.2.2]nonanone Ring Systems. J. Org. Chem 2002, 67, 5683–5689. [DOI] [PubMed] [Google Scholar]
- (27).Davies HML; Smith HD; Hu B; Klenzak SM; Hegner FJ, Divergent reaction pathways between rhodium(II)-stabilized vinylcarbenoids and benzenes. J. Org. Chem 1992, 57, 6900–3. [Google Scholar]
- (28).Davies HML; Doan BD, Total Synthesis of (±)-Tremulenolide A and (±)-Tremulenediol A via a Stereoselective Cyclopropanation/Cope Rearrangement Annulation Strategy. J. Org. Chem 1998, 63, 657–660. [DOI] [PubMed] [Google Scholar]
- (29).(a) Davies HML; Saikali E; Clark TJ; Chee EH, Anomalous reactivity of mono substituted rhodium stabilized vinylcarbenoids. Tetrahedron Lett. 1990, 31, 6299–302; [Google Scholar]; (b) Davies HML; Saikali E; Young WB, Synthesis of (±)-ferruginine and (±)-anhydroecgonine methyl-ester by a tandem cyclopropanation/Cope rearrangement. J. Org. Chem 1991, 56, 5696–700; [Google Scholar]; (c) Davies HML; Hu B; Saikali E; Bruzinski PR, Carbenoid versus Vinylogous Reactivity in Rhodium(II)-Stabilized Vinylcarbenoids. J. Org. Chem 1994, 59, 4535–41. [Google Scholar]
- (30).(a) Smith AG; Davies HML, Rhodium-catalyzed enantioselective vinylogous addition of enol ethers to vinyldiazoacetates. J. Am. Chem. Soc 2012, 134, 18241–18244; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Guzman PE; Lian Y; Davies HML, Reversal of the Regiochemistry in the Rhodium-Catalyzed [4+3] Cycloaddition between Vinyldiazoacetates and Dienes. Angew. Chem., Int. Ed 2014, 53, 13083–13087; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lian Y; Davies HML, Rh2(SbiTISP)2-Catalyzed Asymmetric Functionalization of Indoles and Pyrroles with Vinylcarbenoids. Org. Lett 2012, 14, 1934–1937; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Valette D; Lian Y; Haydek JP; Hardcastle KI; Davies HML, Alkynoate Synthesis through the Vinylogous Reactivity of Rhodium(II) Carbenoids. Angew. Chem., Int. Ed 2012, 51, 8636–8639, S8636/1–S8636/69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).(a) Xu XF; Doyle MP, The 3+3 -Cycloaddition Alternative for Heterocycle Syntheses: Catalytically Generated Metalloenolcarbenes as Dipolar Adducts. Accounts of Chemical Research 2014, 47, 1396–1405; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Xu XF; Doyle MP, Recent Developments in the Synthetic Uses of Silyl-protected Enoldiazoacetates for Heterocyclic Syntheses. Aust. J. Chem 2014, 67, 365–373; [Google Scholar]; (c) Cheng Q-Q; Yu Y; Yedoyan J; Doyle MP, Vinyldiazo Reagents and Metal Catalysts: A Versatile Toolkit for Heterocycle and Carbocycle Construction. ChemCatChem 2018, 10, 488–496. [Google Scholar]
- (32).(a) Davies HML; Saikali E; Huby NJS; Gilliatt VJ; Matasi JJ; Sexton T; Childers SR, Synthesis of 2β-Acyl-3βaryl-8-azabicyclo[3.2.1]octanes and Their Binding Affinities at Dopamine and Serotonin Transport Sites in Rat Striatum and Frontal Cortex. J. Med. Chem 1994, 37, 1262–8; [DOI] [PubMed] [Google Scholar]; (b) Porrino LJ; Migliarese K; Davies HML; Saikali E; Childers SR, Behavioral effects of the novel tropane analog, 2β-propanoyl-3β-(4toluyl)-tropane (PTT). Life Sci. 1994, 54, PL511–PL517; [DOI] [PubMed] [Google Scholar]; (c) Nader MA; Grant KA; Davies HML; Mach RH; Childers SR, The reinforcing and discriminative stimulus effects of the novel cocaine analog 2β-propanoyl-3β-(4-tolyl)-tropane in rhesus monkeys. J. Pharmacol. Exp. Ther 1997, 280, 541–550; [PubMed] [Google Scholar]; (d) Daunais JB; Hart SL; Smith HR; Letchworth SR; Davies HML; Sexton T; Bennett BA; Childers SR; Porrino LJ, Long-acting blockade of biogenic amine transporters in rat brain by administration of the potent novel tropane 2β-propanoyl-3β-(2-naphthyl)-tropane. J. Pharmacol. Exp. Ther 1998, 285, 1246–1254; [PubMed] [Google Scholar]; (e) Mach RH; Nader MA; Ehrenkaufer RL; Gage HD; Childers SR; Hodges LM; Hodges MM; Davies HML, Fluorine-18labeled tropane analogs for PET imaging studies of the dopamine transporter. Synapse 2000, 37, 109–117. [DOI] [PubMed] [Google Scholar]
- (33).Doyle MP, Chiral catalysts for enantioselective carbenoid cyclopropanation reactions. Recl. Trav. Chim. Pays-Bas 1991, 110, 305–16. [Google Scholar]
- (34).Brunner H; Kluschanzoff H; Wutz K, Enantioselective catalysis. 47. Rhodium(II)-carboxylate complexes and their use in enantioselective cyclopropanation. Bull. Soc. Chim. Belg 1989, 98, 63–72. [Google Scholar]
- (35).(a) Kennedy M; McKervey MA; Maguire AR; Roos GHP, Asymmetric synthesis in carbon-carbon bond forming reactions of α-diazoketones catalyzed by homochiral rhodium(II) carboxylates. J. Chem. Soc., Chem. Commun 1990, 361–2; [Google Scholar]; (b) McKervey MA; Ye T, Asymmetric synthesis of substituted chromanones via carbon-hydrogen insertion reactions of α-diazo ketones catalyzed by homochiral rhodium(II) carboxylates. J. Chem. Soc., Chem. Commun 1992, 823–4. [Google Scholar]
- (36).Davies HML; Huby NJS; Cantrell WR Jr.; Olive JL, α-Hydroxy esters as chiral auxiliaries in asymmetric cyclopropanations by rhodium(II)-stabilized vinylcarbenoids. J. Am. Chem. Soc 1993, 115, 9468–79. [Google Scholar]
- (37).(a) Davies HML; Matasi JJ; Thornley C, Control of carbenoid reactivity through neighboring group participation. Tetrahedron Lett. 1995, 36, 7205–8; [Google Scholar]; (b) Davies HML; Ahmed G; Churchill MR, Asymmetric Synthesis of Highly Functionalized 8-Oxabicyclo[3.2.1]octene Derivatives. J. Am. Chem. Soc 1996, 118, 10774–10782; [Google Scholar]; (c) Davies HML; Matasi JJ; Hodges LM; Huby NJS; Thornley C; Kong N; Houser JH, Enantioselective Synthesis of Functionalized Tropanes by Rhodium(II) Carboxylate-Catalyzed Decomposition of Vinyldiazomethanes in the Presence of Pyrroles. J. Org. Chem 1997, 62, 1095–1105; [Google Scholar]; (d) Savinov SN; Austin DJ, Modular Evolution of a Chiral Auxiliary for the 1,3-Dipolar Cycloaddition of Isomuenchnones with Vinyl Ethers. Org. Lett 2002, 4, 1415–1418; [DOI] [PubMed] [Google Scholar]; (e) Moore JD; Hanson PR, Substituent effects in the double diastereotopic differentiation of α-diazophosphonates via intramolecular cyclopropanation. Tetrahedron: Asymm. 2003, 14, 873–880. [Google Scholar]
- (38).(a) Davies HML; Hutcheson DK, Enantioselective synthesis of vinylcyclopropanes by rhodium(II) catalyzed decomposition of vinyldiazomethanes in the presence of alkenes. Tetrahedron Lett. 1993, 34, 7243–6; [Google Scholar]; (b) Davies HML; Bruzinski P; Hutcheson DK; Kong N; Fall MJ, Asymmetric Cyclopropanations by Rhodium(II) N-(Arylsulfonyl)prolinate Catalyzed Decomposition of Vinyldiazomethanes in the Presence of Alkenes. Practical Enantioselective Synthesis of the Four Stereoisomers of 1-Amino-2-phenylcyclopropanecarboxylic acid. J. Am. Chem. Soc 1996, 118, 6897–6907. [Google Scholar]
- (39).(a) Davies HML; Rusiniak L, Effect of catalyst on the diastereoselectivity of methyl phenyldiazoacetate cyclopropanations. Tetrahedron Lett. 1998, 39, 8811–8812; [Google Scholar]; (b) Davies HML; Boebel TA, Asymmetric synthesis of 1-alkynylcyclopropane-1carboxylates. Tetrahedron Lett. 2000, 41, 8189–8192; [Google Scholar]; (c) Davies HML; Nagashima T; Klino JL III, Stereoselectivity of Methyl Aryldiazoacetate Cyclopropanations of 1,1-Diarylethylene. Asymmetric Synthesis of a Cyclopropyl Analogue of Tamoxifen. Org. Lett 2000, 2, 823–826; [DOI] [PubMed] [Google Scholar]; (d) Davies HML; Townsend RJ, Catalytic asymmetric cyclopropanation of heteroaryldiazoacetates. J. Org. Chem 2001, 66, 6595–6603; [DOI] [PubMed] [Google Scholar]; (e) Ventura DL; Li Z; Coleman MG; Davies HML, Intermolecular C-H functionalization versus cyclopropanation of electron rich 1,1-disubstituted and trisubstituted alkenes. Tetrahedron 2009, 65, 3052–3061; [Google Scholar]; (f) Fu L; Mighion JD; Voight EA; Davies HML, Synthesis of 2,2,2-Trichloroethyl Aryl- and Vinyldiazoacetates by Palladium-Catalyzed CrossCoupling. Chem. - Eur. J 2017, 23, 3272–3275. [DOI] [PubMed] [Google Scholar]
- (40).(a) Reddy RP; Davies HML, Asymmetric Synthesis of Tropanes by Rhodium-Catalyzed [4 + 3] Cycloaddition. J. Am. Chem. Soc 2007, 129, 10312–10313; [DOI] [PubMed] [Google Scholar]; (b) Olson JP; Davies HML, Asymmetric [4 + 3] Cycloadditions between Benzofuranyldiazoacetates and Dienes: Formal Synthesis of (+)Frondosin B. Org. Lett 2008, 10, 573–576; [DOI] [PubMed] [Google Scholar]; (c) Davies HML; Stafford DG; Doan BD; Houser JH, Tandem Asymmetric Cyclopropanation/Cope Rearrangement. A Highly Diastereoselective and Enantioselective Method for the Construction of 1,4-Cycloheptadienes. J. Am. Chem. Soc 1998, 120, 3326–3331. [Google Scholar]
- (41).Reddy RP; Lee GH; Davies HML, Dirhodium Tetracarboxylate Derived from Adamantylglycine as a Chiral Catalyst for Carbenoid Reactions. Org. Lett 2006, 8, 3437–3440. [DOI] [PubMed] [Google Scholar]
- (42).Anada M; Hashimoto S, Enantioselective intramolecular C-H insertion route to a key intermediate for the synthesis of trinem antibiotics. Tetrahedron Lett. 1998, 39, 9063–9066. [Google Scholar]
- (43).Denton JR; Cheng K; Davies HML, Stereoselective construction of nitrile-substituted cyclopropanes. Chem. Commun 2008, 1238–1240. [DOI] [PubMed] [Google Scholar]
- (44).Denton JR; Sukumaran D; Davies HML, Enantioselective Synthesis of Trifluoromethyl-Substituted Cyclopropanes. Org. Lett 2007, 9, 2625–2628. [DOI] [PubMed] [Google Scholar]
- (45).Denton JR; Davies HML, Enantioselective Reactions of Donor/Acceptor Carbenoids Derived from α-Aryl-α-Diazoketones. Org. Lett 2009, 11, 787–790. [DOI] [PubMed] [Google Scholar]
- (46).Davies HML; Venkataramani C, Dirhodium Tetraprolinate-Catalyzed Asymmetric Cyclopropanations with High Turnover Numbers. Org. Lett 2003, 5, 1403–1406. [DOI] [PubMed] [Google Scholar]
- (47).Pelphrey P; Hansen J; Davies HML, Solvent-free catalytic enantioselective C-C bond forming reactions with very high catalyst turnover numbers. Chem. Sci. 2010, 1, 254–257. [Google Scholar]
- (48).Ren Z; Sunderland TL; Tortoreto C; Yang T; Berry JF; Musaev DG; Davies HML, Comparison of Reactivity and Enantioselectivity between Chiral Bimetallic Catalysts: BismuthRhodium- and Dirhodium-Catalyzed Carbene Chemistry. ACS Catal. 2018, 8, 10676–10682. [Google Scholar]
- (49).(a) Hansen J; Davies HML, High symmetry dirhodium(II) paddlewheel complexes as chiral catalysts. Coord. Chem. Rev 2008, 252, 545–555; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) DeAngelis A; Dmitrenko O; Yap GP; Fox JM, Chiral crown conformation of Rh(2)(SPTTL)(4): enantioselective cyclopropanation with alpha-alkyl-alphadiazoesters. J. Am. Chem. Soc 2009, 131, 7230–1; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lindsay VNG; Lin W; Charette AB, Experimental Evidence for the All-Up Reactive Conformation of Chiral Rhodium(II) Carboxylate Catalysts: Enantioselective Synthesis of cis-Cyclopropane alphaAmino Acids. J. Am. Chem. Soc 2009, 131, 16383−+. [DOI] [PubMed] [Google Scholar]
- (50).Deng L; Giessert AJ; Gerlitz OO; Dai X; Diver ST; Davies HML, Metal carbene-promoted sequential transformations for the enantioselective synthesis of highly functionalized cycloheptadienes. J. Am. Chem. Soc 2005, 127, 1342–1343. [DOI] [PubMed] [Google Scholar]
- (51).Williams CM, The Story Behind the Total Synthesis of Vibsanin E and 5-epi-Vibsanin E In Strategies and Tactics in Organic Synthesis, Vol 8, Harmata M, Ed. 2012; Vol. 8, pp 395–412. [Google Scholar]
- (52).Schwartz BD; Denton JR; Lian Y; Davies HML; Williams CM, Asymmetric [4 + 3] Cycloadditions between Vinylcarbenoids and Dienes: Application to the Total Synthesis of the Natural Product (−)-5-epi-Vibsanin E. J. Am. Chem. Soc 2009, 131, 8329–8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Schwartz BD; Williams CM; Bernhardt PV, End game strategies towards the total synthesis of vibsanin E, 3hydroxyvibsanin E, furanovibsanin A, and 3-Omethylfuranovibsanin A. Beilstein J. Org. Chem 2008, 4, No. 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Lian Y; Miller LC; Born S; Sarpong R; Davies HML, Catalyst-Controlled Formal [4 + 3] Cycloaddition Applied to the Total Synthesis of (+)-Barekoxide and (−)-Barekol. J. Am. Chem. Soc 2010, 132, 12422–12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Miller LC; Ndungu M; Sarpong R, Parallel kinetic resolution approach to the cyathane and cyanthiwigin diterpenes using a cyclopropanation/Cope rearrangement. Angew. Chem., Int. Ed 2009, 48, 2398–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Nicolaou KC; Baran PS, The CP molecule labyrinth: A paradigm of how endeavors in total synthesis lead to discoveries and inventions in organic synthesis. Angew. Chem., Int. Ed 2002, 41, 2678–2720. [DOI] [PubMed] [Google Scholar]
- (57).Davies HML; Calvo R; Ahmed G, Type II intramolecular annulations between vinylcarbenoids and furans. Tetrahedron Lett. 1997, 38, 1737–1740. [Google Scholar]
- (58).Calvo R; Davies HML, unpublished results.
- (59).Davies HML; Hansen T; Churchill MR, Catalytic Asymmetric C-H Activation of Alkanes and Tetrahydrofuran. J. Am. Chem. Soc 2000, 122, 3063–3070. [Google Scholar]
- (60).Davies HML; Jin Q; Ren P; Kovalevsky AY, Catalytic Asymmetric Benzylic C-H Activation by Means of Carbenoid-Induced C-H Insertions. J. Org. Chem 2002, 67, 4165–4169. [DOI] [PubMed] [Google Scholar]
- (61).Davies HML; Jin Q, Double C-H activation strategy for the asymmetric synthesis of C2-symmetric anilines. Org. Lett 2004, 6, 1769–1772. [DOI] [PubMed] [Google Scholar]
- (62).Davies HML; Hedley SJ; Bohall BR, Asymmetric Intermolecular C-H Functionalization of Benzyl Silyl Ethers Mediated by Chiral Auxiliary-Based Aryldiazoacetates and Chiral Dirhodium Catalysts. J. Org. Chem 2005, 70, 10737–10742. [DOI] [PubMed] [Google Scholar]
- (63).Davies HML; Antoulinakis EG; Hansen T, Catalytic Asymmetric Synthesis of Syn-Aldol Products from Intermolecular CH Insertions between Allyl Silyl Ethers and Methyl Aryldiazoacetates. Org. Lett 1999, 1, 383–385. [Google Scholar]
- (64).(a) Davies HML; Beckwith REJ; Antoulinakis EG; Jin Q, New Strategic Reactions for Organic Synthesis: Catalytic Asymmetric C-H Activation α to Oxygen as a Surrogate to the Aldol Reaction. J. Org. Chem 2003, 68, 6126–6132; [DOI] [PubMed] [Google Scholar]; (b) Davies HML; Antoulinakis EG, Asymmetric Catalytic C-H Activation Applied to the Synthesis of Syn-Aldol Products. Org. Lett 2000, 2, 4153–4156. [DOI] [PubMed] [Google Scholar]
- (65).(a) Davies HML; Hansen T; Hopper DW; Panaro SA, Highly Regio-, Diastereo-, and Enantioselective C-H Insertions of Methyl Aryldiazoacetates into Cyclic N-Boc-Protected Amines. Asymmetric Synthesis of Novel C2-Symmetric Amines and threoMethylphenidate. J. Am. Chem. Soc 1999, 121, 6509–6510; [Google Scholar]; (b) Davies HML; Venkataramani C, Catalytic enantioselective synthesis of β2-amino acids. Angew. Chem., Int. Ed 2002, 41, 21972199; [PubMed] [Google Scholar]; (c) Davies HML; Venkataramani C; Hansen T; Hopper DW, New Strategic Reactions for Organic Synthesis: Catalytic Asymmetric C-H Activation α to Nitrogen as a Surrogate for the Mannich Reaction. J. Am. Chem. Soc 2003, 125, 6462–6468; [DOI] [PubMed] [Google Scholar]; (d) Davies HML; Ni A, Enantioselective synthesis of β-amino esters and its application to the synthesis of the enantiomers of the antidepressant Venlafaxine. Chem. Commun 2006, 3110–3112. [DOI] [PubMed] [Google Scholar]
- (66).Axten JM; Ivy R; Krim L; Winkler JD, Enantioselective Synthesis of D-threo-Methylphenidate. J. Am. Chem. Soc 1999, 121, 6511–6512. [Google Scholar]
- (67).Davies HML; Ren P; Jin Q, Catalytic Asymmetric Allylic C-H Activation as a Surrogate of the Asymmetric Claisen Rearrangement. Org. Lett 2001, 3, 3587–3590. [DOI] [PubMed] [Google Scholar]
- (68).Davies HML; Ren P, Catalytic Asymmetric C-H Activation of Silyl Enol Ethers as an Equivalent of an Asymmetric Michael Reaction. J. Am. Chem. Soc 2001, 123, 2070–2071. [DOI] [PubMed] [Google Scholar]
- (69).Davies HML; Yang J; Nikolai J, Asymmetric C-H insertion of Rh(II) stabilized carbenoids into acetals: A C-H activation protocol as a Claisen condensation equivalent. J. Organomet. Chem 2005, 690, 6111–6124. [Google Scholar]
- (70).Kornecki KP; Briones JF; Boyarskikh V; Fullilove F; Autschbach J; Schrote KE; Lancaster KM; Davies HML; Berry JF, Direct Spectroscopic Characterization of a Transitory Dirhodium Donor-Acceptor Carbene Complex. Science 2013, 342, 351–354. [DOI] [PubMed] [Google Scholar]
- (71).(a) Werle C; Goddard R; Fuerstner A, The First Crystal Structure of a Reactive Dirhodium Carbene Complex and a Versatile Method for the Preparation of Gold Carbenes by Rhodium-to-Gold Transmetalation. Angew. Chem., Int. Ed 2015, 54, 15452–15456; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Werle C; Goddard R; Philipps P; Fares C; Fuerstner A, Stabilization of a Chiral Dirhodium Carbene by Encapsulation and a Discussion of the Stereochemical Implications. Angew. Chem., Int. Ed 2016, 55, 10760–10765. [DOI] [PubMed] [Google Scholar]
- (72).Hansen J; Autschbach J; Davies HML, Computational Study on the Selectivity of Donor/Acceptor-Substituted Rhodium Carbenoids. J. Org. Chem 2009, 74, 6555–6563. [DOI] [PubMed] [Google Scholar]
- (73).Davies HML; Stafford DG; Hansen T, Catalytic asymmetric synthesis of diarylacetates and 4,4-diarylbutanoates. A formal asymmetric synthesis of (+)-sertraline. Org. Lett 1999, 1, 233–236. [DOI] [PubMed] [Google Scholar]
- (74).Davies HML; Lian Y, The Combined C-H Functionalization/Cope Rearrangement: Discovery and Applications in Organic Synthesis. Acc. Chem. Res 2012, 45, 923–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Hansen JH; Gregg TM; Ovalles SR; Lian Y; Autschbach J; Davies HML, On the Mechanism and Selectivity of the Combined C-H Activation/Cope Rearrangement. J. Am. Chem. Soc 2011, 133, 5076–5085. [DOI] [PubMed] [Google Scholar]
- (76).Lian Y; Hardcastle KI; Davies HML, Computationally Guided Stereocontrol of the Combined C-H Functionalization/Cope Rearrangement. Angew. Chem., Int. Ed 2011, 50, 9370–9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Davies HML; Walji AM, Direct synthesis of (+)erogorgiaene through a kinetic enantiodifferentiating step. Angew. Chem., Int. Ed 2005, 44, 1733–1735. [DOI] [PubMed] [Google Scholar]
- (78).Davies HML; Dai X; Long MS, Combined C-H Activation/Cope Rearrangement as a Strategic Reaction in Organic Synthesis: Total Synthesis of (−)-Colombiasin A and (−)-Elisapterosin B. J. Am. Chem. Soc 2006, 128, 2485–2490. [DOI] [PubMed] [Google Scholar]
- (79).Nicolaou KC; Vassilikogiannakis G; Magerlein W; Kranich R, Total synthesis of colombiasin A. Angew. Chem., Int. Ed 2001, 40, 2482–2486. [DOI] [PubMed] [Google Scholar]
- (80).Davies HML; Morton D, Collective Approach to Advancing C-H Functionalization. ACS Cent. Sci 2017, 3, 936–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Davies HML; Liao K, Dirhodium tetracarboxylates as catalysts for selective intermolecular C-H functionalization. Nat. Rev. Chem 2019, 3, 347–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Liao K; Pickel TC; Boyarskikh V; Bacsa J; Musaev DG; Davies HML, Site-selective and stereoselective functionalization of non-activated tertiary C-H bonds. Nature 2017, 551, 609–613. [DOI] [PubMed] [Google Scholar]
- (83).Fu J; Ren Z; Bacsa J; Musaev DG; Davies HML, Desymmetrization of cyclohexanes by site- and stereoselective C-H functionalization. Nature 2018, 564, 395–399. [DOI] [PubMed] [Google Scholar]
- (84).(a) Qin C; Boyarskikh V; Hansen JH; Hardcastle KI; Musaev DG; Davies HML, D2-Symmetric Dirhodium Catalyst Derived from a 1,2,2-Triarylcyclopropanecarboxylate Ligand: Design, Synthesis and Application. J. Am. Chem. Soc 2011, 133, 19198–19204; [DOI] [PubMed] [Google Scholar]; (b) Liao K; Negretti S; Musaev DG; Bacsa J; Davies HML, Site-selective and stereoselective functionalization of unactivated C-H bonds. Nature 2016, 533, 230–234. [DOI] [PubMed] [Google Scholar]
- (85).(a) Liao K; Liu W; Niemeyer ZL; Ren Z; Bacsa J; Musaev DG; Sigman MS; Davies HML, Site-Selective Carbene-Induced C-H Functionalization Catalyzed by Dirhodium Tetrakis(triarylcyclopropanecarboxylate) Complexes. ACS Catal. 2018, 8, 678–682; [Google Scholar]; (b) Liu W; Ren Z; Bosse AT; Liao K; Goldstein EL; Bacsa J; Musaev DG; Stoltz BM; Davies HML, Catalyst-Controlled Selective Functionalization of Unactivated C-H Bonds in the Presence of Electronically Activated C-H Bonds. J. Am. Chem. Soc 2018, 140, 12247–12255. [DOI] [PubMed] [Google Scholar]
- (86).Liao K; Yang Y-F; Li Y; Sanders JN; Houk KN; Musaev DG; Davies HML, Design of catalysts for site-selective and enantioselective functionalization of non-activated primary C-H bonds. Nat. Chem 2018, 10, 1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).(a) Guptill DM; Davies HML, 2,2,2-Trichloroethyl Aryldiazoacetates as Robust Reagents for the Enantioselective C-H Functionalization of Methyl Ethers. J. Am. Chem. Soc 2014, 136, 17718–17721; [DOI] [PubMed] [Google Scholar]; (b) Qin C; Davies HML, Role of Sterically Demanding Chiral Dirhodium Catalysts in Site-Selective C-H Functionalization of Activated Primary C-H Bonds. J. Am. Chem. Soc 2014, 136, 9792–9796. [DOI] [PubMed] [Google Scholar]
- (88).Nadeau E; Li Z; Morton D; Davies HML, Rhodium carbenoid induced intermolecular C-H functionalization at tertiary CH bonds. Synlett 2009, 151–154. [Google Scholar]
- (89).Chuprakov S; Malik JA; Zibinsky M; Fokin VV, Catalytic Asymmetric C-H Insertions of Rhodium(II) Azavinyl Carbenes. J. Am. Chem. Soc 2011, 133, 10352–10355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Davies HML; Alford JS, Reactions of metallocarbenes derived from N-sulfonyl-1,2,3-triazoles. Chem. Soc. Rev 2014, 43, 5151–5162. [DOI] [PubMed] [Google Scholar]
- (91).Fruit C; Mueller P, Asymmetric transfer of nitrenes catalyzed by chiral dirhodium(II) using aromatic sulfamate esters. Tetrahedron: Asymm. 2004, 15, 1019–1026. [Google Scholar]
- (92).Horneff T; Chuprakov S; Chernyak N; Gevorgyan V; Fokin VV, Rhodium-Catalyzed Transannulation of 1,2,3-Triazoles with Nitriles. J. Am. Chem. Soc 2008, 130, 14972–14974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Fu L; Guptill DM; Davies HML, Rhodium(II)-Catalyzed C-H Functionalization of Electron-Deficient Methyl Groups. J. Am. Chem. Soc 2016, 138, 5761–5764. [DOI] [PubMed] [Google Scholar]
- (94).(a) Kubiak RW; Mighion JD; Wilkerson-Hill SM; Alford JS; Yoshidomi T; Davies HML, Enantioselective Intermolecular C-H Functionalization of Allylic and Benzylic sp3 C-H Bonds Using N-Sulfonyl-1,2,3-triazoles. Org. Lett 2016, 18, 3118–3121; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kubiak RW; Davies HML, Rhodium-Catalyzed Intermolecular C-H Functionalization as a Key Step in the Synthesis of Complex Stereodefined β-Arylpyrrolidines. Org. Lett 2018, 20, 3771–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Garlets ZJ; Davies HML, Harnessing the β-Silicon Effect for Regioselective and Stereoselective Rhodium(II)-Catalyzed C-H Functionalization by Donor/Acceptor Carbenes Derived from 1Sulfonyl-1,2,3-triazoles. Org. Lett 2018, 20, 2168–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Bien J; Davulcu A; DelMonte AJ; Fraunhoffer KJ; Gao Z; Hang C; Hsiao Y; Hu W; Katipally K; Littke A; Pedro A; Qiu Y; Sandoval M; Schild R; Soltani M; Tedesco A; Vanyo D; Vemishetti P; Waltermire RE, The First Kilogram Synthesis of Beclabuvir, an HCV NS5B Polymerase Inhibitor. Org. Process Res. Dev 2018, 22, 1393–1408. [Google Scholar]
- (97).(a) Chepiga KM; Feng Y; Brunelli NA; Jones CW; Davies HML, Silica-Immobilized Chiral Dirhodium(II) Catalyst for Enantioselective Carbenoid Reactions. Org. Lett 2013, 15, 6136–6139; [DOI] [PubMed] [Google Scholar]; (b) Moschetta EG; Negretti S; Chepiga KM; Brunelli NA; Labreche Y; Feng Y; Rezaei F; Lively RP; Koros WJ; Davies HML; Jones CW, Composite Polymer/Oxide Hollow Fiber Contactors: Versatile and Scalable Flow Reactors for Heterogeneous Catalytic Reactions in Organic Synthesis. Angew. Chem., Int. Ed 2015, 54, 6470–6474; [DOI] [PubMed] [Google Scholar]; (c) Yoo C-J; Rackl D; Liu W; Hoyt CB; Pimentel B; Lively RP; Davies HML; Jones CW, An Immobilized-Dirhodium Hollow-Fiber Flow Reactor for Scalable and Sustainable C-H Functionalization in Continuous Flow. Angew. Chem., Int. Ed 2018, 57, 10923–10927. [DOI] [PubMed] [Google Scholar]