Abstract
Mucosal leishmaniasis (ML) is a rare clinical variant of tegumentary leishmaniasis in Mediterranean Europe. Here we report on three autochthonous cases of head and neck ML in patients living in Northeastern Italy. Patients presented with non-specific, long-standing symptoms of upper respiratory tract involvement, mimicking other diseases. Parasitological diagnosis was reached by histopathology, immunohistochemistry and molecular biology on tissue specimens. Leishmania infantum was identified by molecular typing in all three cases. All patients reached a complete remission with protracted multivalent antileishmanial drugs; in one case, a novel approach of combined medical and endoscopic surgical treatment was carried out. High clinical suspicion led to a prompt diagnosis and deployment of a multivalent treatment. ML should be considered in the differential diagnosis of nasal, oral, and pharyngolaryngeal lesions in endemic areas. A prompt diagnosis is mandatory to establish a correct management; different antileishmanial medications as well as endoscopic surgical options may be required to reach a complete remission.
Keywords: head and neck mucosal leishmaniasis, novel ways of administration of anti-leishmanial drugs, endoscopic surgical treatment
1. Introduction
Mucosal leishmaniasis (ML) is a rare clinical variant of tegumentary leishmaniasis in Mediterranean Europe [1]. In France, ML represents around 2% of autochthonous Leishmania cases [2], while its prevalence in other countries is unknown. ML is mainly caused by Leishmania infantum (L. infantum) and is transmitted by the bite of sandflies of the genus Phlebotomus. Clinical manifestations of ML are variable, including nodules, polypoid lesions or granular inflammation and may involve the buccal area, pharyngeal and laryngeal regions and, less frequently, the nose [3,4,5,6,7].
Mediterranean ML is usually not associated with previous cutaneous lesions, which differentiates this tegumentary manifestation from the more common New World mucocutaneous leishmaniasis (NWML) [1,3]. Other differences are reported between NWML and Mediterranean ML; for example, L. braziliensis or L. panamensis are causing NWML, while L. infantum is the usual agent of ML, although L. major and L. tropica can also be involved [1]. Furthermore, the nasal cavity is commonly affected in NWML cases, but, less frequently in Mediterranean ML cases, and patients with ML acquired in the Mediterranean region have a better prognosis than those who acquired NWML in Latin America [3]. In NWML, destructive lesions with few parasites and high levels of tumor necrosis factor-α (TNF-α) have been reported [3]. This suggests that an uncontrolled immune response with increased production of pro-inflammatory cytokines is responsible for the tissue damage. Conversely, the pathogenesis of Mediterranean ML is still obscure.
Despite the fact that there is no validated guideline for therapy of the rare ML in Mediterranean countries, miltefosine (50 mg tid × 28 days) and liposomal amphotericin B (L-Amb; 21–40 mg/kg total dose) are suggested as the first choices of treatment [4,7]. For NWML, pentavalent antimonials (Sb 20 mg/kg/day for 28–30 days) are still the gold standard of treatment, but also L-Amb (18–40 mg/kg total dose) can be used [1,3]. Pentoxyfilline (400 mg tid for 30 days) can be added to antimonials in NWML because of its capacity to downregulate TNF-α and inhibit leukocyte migration and adhesion [8,9]. Combining antimonials with pentoxifylline in NWML reduces the healing time significantly and prevents the need for further courses of Sb [10].
We recently observed an upsurge of visceral and cutaneous leishmaniasis in the Bologna province, Northeastern Italy [11,12]. Here, we report three cases of ML in patients living in this area.
2. Description of Cases
2.1. Case 1
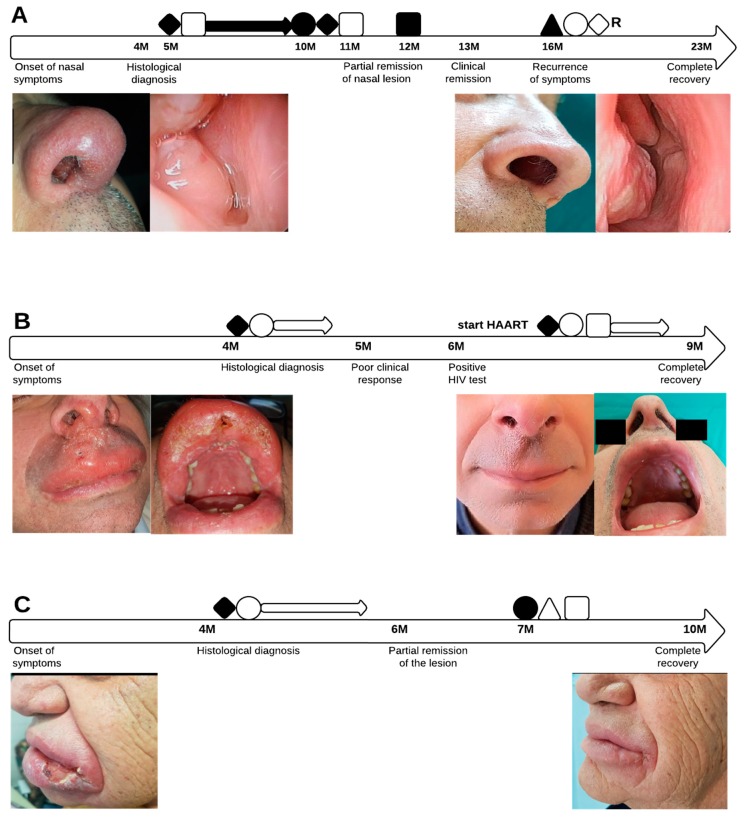
A 63-year-old, immunocompetent man presented with rhinitis, mild epistaxis and nasal obstruction. Travel to Croatia was reported six months before symptoms onset. Physical examination revealed a mild erythema on the top of the nose and an ulcerated obstructive mass involving the right nostril and the vestibule extending to the anterior nasal valve. He was clinically diagnosed with nasal polyposis (Figure 1A) and biopsied. The biopsy specimen was formalin fixed, embedded in paraffin and stained with hematoxylin and eosin (H&E) and Giemsa; histology revealed suspected leishmanial bodies (Figure 2A). Immunohistochemistry was performed on an automated Immunostainer (Tucson, Arizona, USA) applying an anti-CD1a antibody (clone EP3622, CELL MARQUE); microscopic examination confirmed an intense positivity for CD1a within the Leishmania amastigotes (Figure 2B). Leishmanial DNA was also detected in the paraffined-embedded biopsy by using two in-house, real-time PCR assays that amplified a segment of the small-subunit rRNA gene of Leishmania [13] as well as a segment of the kinetoplast DNA [14], respectively, as previously described [12]. For species identification, a region of the Internal Transcribed Spacer-1 (ITS-1) was amplified and sequenced according to El Tai et al. [15] and following the protocols described by Rugna et al. [16]; ITS-1 typing displayed the presence of L. infantum. The presence of anti-Leishmania IgM and IgG antibodies was investigated by employing the Leishmania ELISA IgG + IgM kit (Vircell, Granada, Spain), but specific antibodies were not detected in the patient’s serum. Treatment was started with the association of intralesional meglumine antimoniate (one injection per week for four weeks) plus fluconazole (100 mg twice a day for one month). As the nasal mass only partially decreased, miltefosine (50 mg three times a day for one month) was added. After six months, the patient underwent a second endonasal biopsy, which revealed an extensive granulomatous process with the persistence of CD1a-positive amastigotes and leishmanial DNA. The patient was then started on IV L-Amb 3 mg/kg/day at day 1 to 5, 14 and 21 following international guidelines [1]. After an apparent remission, the patient returned with recurrence of the endonasal mass two months later; both Leishmania amastigotes and Leishmania DNA were detected in the biopsy specimen. After consultation with otorhinolaryngologists, the patient underwent a surgical protocol including surgical debulk of the macroscopic mass and intralesional meglumine antimoniate submucosal injections with a syringe of 1 mL and an insulin needle. All these passages were made under local anesthesia with the topical application of 2% Xylocaine (2% Lidocaine Hydrochloride, AstraZeneca, Sweden) through rhino-endoscopy to reach the upper part of the anterior nasal valve. Rigid nasal endoscopy was performed using a 30° 4 mm diameter rigid nasal endoscope (Karl Stortz Sinuscope, KARL STORZ GmbH & Co. KG, Tuttlingen, Germany). The patient underwent one injection of 1 mL of meglumine per week for two weeks, associated with pentamidine isethionate (3 mg/kg, weekly IM injections for two weeks). The surgical–medical combined therapy led to a rapid improvement of the patient’s clinical status (Figure 1A), allowing better air flow through the nasal vestibule obstructed by the thickened infected tissue. At a 12-month follow-up the patient exhibited a complete clinical and endoscopic recovery.
Figure 1.
(A–C). Clinical course and treatment of mucosal leishmaniasis (ML) cases. (A) Case 1; clinical and endoscopic appearance of the nasal lesion before and after treatment. (B) Case 2; upper lip and nasal lesions before and after treatment. (C) Case 3; ulcerated lesion of the lower lip disappeared after treatment. ◆: intralesional meglumine antimoniate; ◊R: intralesional meglumine antimoniate via rhinoendoscopy; ●: miltefosine; o: pentamidine; ■: amphotericin B; □: oral fluconazole; ▲: surgical debridement; △: allopurinol.
Figure 2.
Histologic examination of ML bioptic specimen (Case 1). (A) Giemsa staining revealing a non-necrotizing granulomatous inflammatory infiltration, rich in plasma cells and epithelioid histiocytes containing eukaryotic elements, suspected for Leishmania. (B) Immunohistochemistry staining with CD1a confirmed an intense positivity for CD1a of Leishmania amastigotes in parasitized macrophages and in extracellular space.
2.2. Case 2
A 59-year-old patient presented with a six-month history of painful nasal and oral bleeding and chewing difficulties. He reported not travelling abroad in the last two years. Clinical examination revealed an edematous infiltration of the upper lip and the anterior buccal mucosa and an ulcerated-crusted lesion in the right nasal vestibule (Figure 1B). Biopsy revealed a mixed inflammatory infiltrate and numerous amastigotes in both H&E and Giemsa stained sections, confirmed by CD1a immunohistochemistry. Leishmania DNA was detected on the tissue specimen by the two abovementioned real time PCR assays and results of ITS-1 typing indicated L.infantum. Antileishmanial therapy was started with intralesional meglumine antimoniate (1 mL per week), and pentamidine isethionate (3 mg/kg, weekly IM injections for three weeks), with little or no response. Risk factors for immunosuppression were investigated, including HIV infection. The HIV test turned positive with a CD4+ count of 200/mmc and a viral load of 234.995 copies/mL. Peripheral blood and bone marrow aspirate were examined and tested negative for leishmanial DNA, ruling out visceral involvement. The patient started antiretroviral therapy with the combination of tenofovir/emtricitabine and darunavir/cobicistat and was again put on treatment for ML with oral fluconazole (200 mg once a day), intralesional meglumine antimoniate (one injection/week) and injections of pentamidine isethionate (3 mg/kg, weekly IM injections) for three weeks, with complete remission of the lesions (Figure 1B) confirmed at a six-month follow-up.
2.3. Case 3
A 77-year-old immunocompetent man presented with a massive ulcerated bleeding lesion on the lower lip extending to the oral cavity mucosa (Figure 1C). He was a heavily smoker with no history of recent travel abroad; he only reported a traumatic cut in the lesioned area. In the suspicion of malignancy, the lesion was biopsied; histology revealed an intense chronic flogistic reaction and dysplastic aspects, consistent with a traumatic lesion. Owing to the persistence of symptoms, the patient was re-biopsied; specimens showed a mixed inflammatory infiltrate with leishmanial amastigotes detected by H&E and Giemsa staining. CD1a immunohistochemistry showed numerous Leishmania amastigotes, and leishmanial DNA was also detected in the biopsy specimen by the two PCR assays amplifying a fragment of the small-subunit rRNA gene as well as of the kinetoplast DNA, respectively. Molecular typing indicated the presence of L. infantum. The patient underwent a multivalent therapy, including cryotherapy, intralesional meglumine antimoniate (one injection/week) and IM pentamidine isethionate injections (one per week, 3 mg/kg for six weeks), with only partial remission. Subsequent dermoscopy still showed pathological aspects and therapy was modified to oral miltefosine 150 mg/day for one month, allopurinol 300 mg/day for three months and fluconazole 200 mg/day for three weeks [17,18,19], with complete remission at a 3-month follow-up (Figure 1C).
2.4. Ethics
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the St. Orsola-Malpighi University Hospital (prot. n.3175/2018, 29 October 2018).
3. Discussion
An elevated circulation of Leishmania has been recently observed in Northeastern Italy [11,12]; therefore, it is not surprising that autochthonous cases of ML have emerged in the same area.
Because of the long incubation period and time to diagnosis, it may be difficult to distinguish whether cases of tegumentary leishmaniasis are autochthonous or imported; therefore, accurate patient history and parasite identification are crucial [20]. A lack of travel history in areas that are endemic for NWML and species identification as L. infantum strongly suggests an autochthonous origin of the current ML cases. All three cases presented with long-standing mucosal disease, with a duration of around 6 months from the onset of symptoms to the diagnosis. Delayed diagnosis is a common feature of ML and NWML [2,21,22], which is often misdiagnosed as other upper respiratory tract diseases. Since the overlap in clinical presentation (ulcerated and bleeding masses) of ML and neoplasia, leishmaniasis should be taken into consideration in the differential diagnosis of head and neck cancer in patients from endemic areas or who report travelling to endemic countries.
The diagnosis of ML was performed by histology and molecular methods with detection of leishmanial amastigotes in tissue sections as well as leishmanial DNA. Detection of parasites in tissue sections may be difficult when only a few parasites are present; in these cases, CD1a staining can enhance the sensitivity of histological examination [23,24]. In Case 1, the diagnosis of ML was brought up by immunohistochemical staining, while regular H&E and Giemsa staining produced less clear results. The diagnosis was then confirmed by molecular methods; in our study, real-time PCR enabled confirmation of ML diagnosis in all three cases. Evidence suggests that PCR is a highly sensitive and specific method for detecting leishmanial DNA in mucosal biopsies [2,5,25]. In addition, in one out of three cases serology tested negative (Case 1), and this is compatible with a low titer of circulating antibodies against Leishmania in ML [26].
At present, there is no standardized treatment for ML; the drug, dosage and duration of therapy should be individualized for each case, considering the clinical aspect of the lesions, the infecting Leishmania species and the immunological status of the patient. Specific treatment regimens are mainly guided by practical considerations and the personal experience of the treating physician [3,7]. A systemic treatment is considered mandatory to prevent morbidity (e.g., disfigurement) and mortality (e.g., aspiration pneumonia and respiratory obstruction). The examined patients reached complete remission by combining multivalent systemic and local treatment; drugs with distinct efficacy against Leishmania were used, including those targeting ergosterol biosynthesis (fluconazole), hampering thiol metabolism thereby inducing DNA fragmentation (meglumine antimoniate) and hindering DNA synthesis at mitochondrial level (pentamidine) [27].
Furthermore, in Case 1, a novel approach of combined medical and endoscopic treatment was successfully carried out; meglumine antimoniate injections through rhino-endoscopy were combined with surgical endoscopic debridement, which allowed coverage of all the internal areas of the lesion up to the nasal valve. To the best of our knowledge, this is the first ML case successfully treated with endonasal intralesional injection by rhino-endoscopy.
ML is often detected in immunocompromised individuals [1] presenting with extensive lesions and poor response to antiparasitic therapy. In Case 2, the remission was likely due to the introduction of antiretroviral therapy in combination with a multivalent antileishmanial treatment.
4. Conclusions
In conclusion, ML should be considered in the differential diagnosis of granulomatous plaques and nodules of the head and neck mucosa in immunocompetent and immunocompromised individuals living in endemic areas for leishmaniasis or reporting travels to endemic areas.
Combined dermatological and otolaryngologist examination is important for the clinical management of ML [28]. A biopsy of the lesion is mandatory to perform a parasitological diagnosis, thus allowing the prompt introduction of antileishmanial therapy. In some cases, as in Case 1 of this report, it may be necessary to introduce different methods of drug administration.
Acknowledgments
We thank Maria Pia Foschini and Giacomo Santandrea for their expertise in pathology; Anna Lanzoni for the clinical expertise; we also thank Paolo Gaibani, Margherita Ortalli, Giada Rossini and Caterina Vocale for the support in the molecular diagnosis of the cases.
Author Contributions
V.G., S.V. and G.M. contributed to the study conception and design. Patients’ data collection and analysis were performed by I.Z., F.L., N.S. and E.C. The first draft of the manuscript was written by V.G., I.Z. and S.V., and all authors commented on previous versions of the manuscript. Funding acquisition: A.P., S.V. and M.C.R. Resources: M.C.R. and A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Lab P3 funds from the Emilia-Romagna Region and by RFO 2014-2018 funds (to A.P., M.C.R. and S.V.) from the University of Bologna.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Gradoni L., López-Vélez R., Mokni M. Manual on Case Management and Surveillance of the Leishmaniases in the WHO European Region. World Health Organization; Copenhagen, Denmark: 2017. [Google Scholar]
- 2.Faucher B., Pomares C., Fourcade S., Benyamine A., Marty P., Pratlong L., Faraut F., Mary C., Piarroux R., Dedet J.-P., et al. Mucosal Leishmania infantum leishmaniasis: Specific pattern in a multicentre survey and historical cases. J. Infect. 2011;63:76–82. doi: 10.1016/j.jinf.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Blum J., Buffet P., Visser L., Harms G., Bailey M.S., Caumes E., Clerinx J., van Thiel P.P.A.M., Morizot G., Hata C., et al. LeishMan recommendations for treatment of cutaneous and mucosal leishmaniasis in travelers, 2014. J. Travel. Med. 2014;21:116–129. doi: 10.1111/jtm.12089. [DOI] [PubMed] [Google Scholar]
- 4.Aliaga L., Cobo F., Mediavilla J.D., Bravo J., Osuna A., Amador J.M., Martín-Sánchez J., Cordero E., Navarro J.M. Localized mucosal leishmaniasis due to Leishmania (Leishmania) infantum: Clinical and microbiologic findings in 31 patients. Medicine. 2003;82:147–158. doi: 10.1097/01.md.0000076009.64510.b8. [DOI] [PubMed] [Google Scholar]
- 5.Richter J., Hanus I., Häussinger D., Löscher T., Harms G. Mucosal Leishmania infantum infection. Parasitol Res. 2011;109:959–962. doi: 10.1007/s00436-011-2356-x. [DOI] [PubMed] [Google Scholar]
- 6.Mignogna M.D., Celentano A., Leuci S., Cascone M., Adamo D., Ruoppo E., Favia G. Mucosal leishmaniasis with primary oral involvement: A case series and a review of the literature. Oral. Dis. 2015;21:e70–e78. doi: 10.1111/odi.12268. [DOI] [PubMed] [Google Scholar]
- 7.Mosimann V., Blazek C., Grob H., Chaney M., Neumayr A., Blum J. Miltefosine for Mucosal and Complicated Cutaneous Old World Leishmaniasis: A Case Series and Review of the Literature. Open Forum. Infect. Dis. 2016;3:ofw008. doi: 10.1093/ofid/ofw008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bafica A., Oliveira F., Freitas L.A., Nascimento E.G., Barral A. American cutaneous leishmaniasis unresponsive to antimonial drugs: Successful treatment using combination of Nmethilglucamine antimoniate plus pentoxifylline. Int. J. Dermatol. 2003;42:203–207. doi: 10.1046/j.1365-4362.2003.01868.x. [DOI] [PubMed] [Google Scholar]
- 9.Lessa H.A., Machado P., Lima F., Cruz A.A., Bacellar O., Guerreiro J., Carvalho E.M. Successful treatment of refractory mucosal leishmaniasis with pentoxifylline plus antimony. Am. J. Trop. Med. Hyg. 2001;65:87–89. doi: 10.4269/ajtmh.2001.65.87. [DOI] [PubMed] [Google Scholar]
- 10.Machado P.R., Lessa H., Lessa M., Guimaraes L.H., Bang H., Ho J.L., Carvalho E.M. Oral pentoxifylline combined with pentavalent antimony: A randomized trial for mucosal leishmaniasis. Clin. Infect. Dis. 2007;44:788–793. doi: 10.1086/511643. [DOI] [PubMed] [Google Scholar]
- 11.Varani S., Cagarelli R., Melchionda F., Attard L., Salvadori C., Finarelli A.C., Gentilomi G.A., Tigani R., Rangoni R., Todeschini R., et al. Ongoing outbreak of visceral leishmaniasis in Bologna Province, Italy, November 2012 to May 2013. Eurosurveillance. 2013;18:20530. doi: 10.2807/1560-7917.ES2013.18.29.20530. [DOI] [PubMed] [Google Scholar]
- 12.Gaspari V., Ortalli M., Foschini M.P., Baldovini C., Lanzoni A., Cagarelli R., Gaibani P., Rossini G., Vocale C., Tigani R., et al. New evidence of cutaneous leishmaniasis in north-eastern Italy. J. Eur. Acad. Dermatol. Venereol. 2017;31:1534–1540. doi: 10.1111/jdv.14309. [DOI] [PubMed] [Google Scholar]
- 13.Wortmann G., Sweeney C., Houng H.S., Aronson N., Stiteler J., Jackson J., Ockenhouse C. Rapid diagnosis of leishmaniasis by fluorogenic polymerase chain reaction. Am. J. Trop. Med. Hyg. 2001;65:583–587. doi: 10.4269/ajtmh.2001.65.583. [DOI] [PubMed] [Google Scholar]
- 14.Mary C., Faraut F., Lascombe L., Dumon H. Quantification of Leishmania infantum DNA by a real-time PCR assay with high sensitivity. J. Clin. Microbiol. 2004;42:5249–5255. doi: 10.1128/JCM.42.11.5249-5255.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Tai N.O., El Fari M., Mauricio I., Miles M.A., Oskam L., El Safi S.H., Presber W.H., Schonian G. Leishmania donovani: Intraspecific polymorphisms of Sudanese isolates revealed by PCR-based analyses and DNA sequencing. Exp. Parasitol. 2001;97:35–44. doi: 10.1006/expr.2001.4592. [DOI] [PubMed] [Google Scholar]
- 16.Rugna G., Carra E., Corpus F., Calzolari M., Salvatore D., Bellini R., Di Francesco A., Franceschini E., Bruno A., Poglayen G., et al. Distinct Leishmania infantum Strains Circulate in Humans and Dogs in the Emilia-Romagna Region, Northeastern Italy. Vector Borne Zoonotic Dis. 2017;17:409–415. doi: 10.1089/vbz.2016.2052. [DOI] [PubMed] [Google Scholar]
- 17.Kirigi G., Mbuchi M.W., Mbui J.K., Rashid J.R., Kinoti D.M., Njoroge S.N., Basiye F., Magiri C., Wasunna M.K. A successful treatment of a Kenyan case of unresponsive cutaneous leishmaniasis with a combination of pentostam and oral allopurinol: Case report. East Afr. Med. J. 2010;87:521–524. [PubMed] [Google Scholar]
- 18.Yaich S., Charfeddine K., Masmoudi A., Masmoudi M., Zaghdhane S., Turki H., Hachicha J. Atypical presentation of cutaneous leishmaniasis in a renal transplant recipient successfully treated with allopurinol and fluconazole. Ann. Saudi. Med. 2013;33:187–191. doi: 10.5144/0256-4947.2012.01.7.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boecken G., Sunderkötter C., Bogdan C., Weitzel T., Fischer M., Müller A., Löbermann M., Anders G., von Stebut E., Schunk M., et al. Diagnosis and therapy of cutaneous and mucocutaneous Leishmaniasis in Germany. J. Dtsch. Dermatol. Ges. 2011;9:1–51. doi: 10.1111/j.1610-0379.2011.07820.x. [DOI] [PubMed] [Google Scholar]
- 20.Di Muccio T., Scalone A., Bruno A., Marangi M., Grande R., Armignacco O., Gradoni L., Gramiccia M. Epidemiology of Imported Leishmaniasis in Italy: Implications for a European Endemic Country. PLoS ONE. 2015;26:e0129418. doi: 10.1371/journal.pone.0129418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diniz J.L.C.P., Costa MO da R., Gonçalves D.U. Mucocutaneous Leishmaniasis: Clinical markers in presumptive diagnosis. Braz. J. Otorhinolaryngol. 2011;77:380–384. doi: 10.1590/S1808-86942011000300018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.da Costa D.C.S., Palmeiro M.R., Moreira J.S., Martins A.C.d.C., da Silva A.F., Madeira M.d.F., Quintella L.P., Confort E.M., Schubach A.d.O., da Conceicao Silva F., et al. Oral manifestations in the American tegumentary leishmaniasis. PLoS ONE. 2014;9:e109790. doi: 10.1371/journal.pone.0109790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez-Flores A., Rodriguez-Peralto J.L. Morphological and immunohistochemical clues for the diagnosis of cutaneous leishmaniasis and the interpretation of CD1a status. J. Am. Acad. Dermatol. 2016;74:536–543. doi: 10.1016/j.jaad.2015.09.038. [DOI] [PubMed] [Google Scholar]
- 24.Dias-Polak D., Geffen Y., Ben-Izhak O., Bergman R. The Role of Histopathology and Immunohistochemistry in the Diagnosis of Cutaneous Leishmaniasis Without “Discernible” Leishman–Donovan Bodies. Am. J. Dermatopathol. 2017;39:890. doi: 10.1097/DAD.0000000000000861. [DOI] [PubMed] [Google Scholar]
- 25.Strazzulla A., Cocuzza S., Pinzone M.R., Postorino M.C., Cosentino S., Serra A., Cacopardo B., Munnari G. Mucosal leishmaniasis: An underestimated presentation of a neglected disease. Biomed. Res. Int. 2013;2013:805108. doi: 10.1155/2013/805108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cobo F., Rodríguez-Granger J., Gómez-Camarasa C., Sampedro A., Aliaga-Martínez L., Navarro J.M., Fernández J.G. Localized mucosal leishmaniasis caused by Leishmania infantum mimicking cancer in the rhinolaryngeal region. Int. J. Infect. Dis. 2016;50:54–56. doi: 10.1016/j.ijid.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Singh O.P., Sundar S. Immunotherapy and targeted therapies in treatment of visceral leishmaniasis: Current status and future prospects. Front Immunol. 2014;5:296. doi: 10.3389/fimmu.2014.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boaventura V.S., de Oliveira J.G.S., Costa J.M.L., Novais F.O., de Oliveira C.I., Barral-Netto M., Barral A. The value of the otorhinolaryngologic exam in correct mucocutaneous leishmaniasis diagnosis. Am. J. Trop Med. Hyg. 2009;81:384–386. doi: 10.4269/ajtmh.2009.81.384. [DOI] [PubMed] [Google Scholar]