Abstract
Serum lipid abnormalities (dyslipidemias) are major risk factors for cardiovascular disease in type 2 diabetes mellitus. Recent findings provide new evidence that dyslipidemia characterized by elevated triglycerides and non‐high‐density lipoprotein cholesterol levels with a decreased high‐density lipoprotein cholesterol level are risk factors for cardiovascular disease in patients with type 2 diabetes. There are multiple therapeutic agents to help patients to achieve target lipid levels, but understanding the molecular mechanisms could provide useful information for new treatment strategies for diabetic dyslipidemia.
Serum lipid abnormalities (dyslipidemias) are major risk factors for cardiovascular disease in type 2 diabetes patients. The features of diabetic dyslipidemia are elevated plasma concentrations of triglyceride (TG)‐rich lipoproteins, small dense low‐density lipoprotein cholesterol (LDL‐C) and decreased plasma high‐density lipoprotein cholesterol (HDL‐C) concentration. Non‐HDL‐C is a better marker of cardiovascular risk than LDL‐C. A recent study reported that non‐HDL‐C was also a better predictor of diabetes than traditional cholesterol parameters in women with normal glucose tolerance, but not men 1 , suggesting the importance of non‐HDL‐C monitoring in women.
Diabetes frequently accompanies dyslipidemia and hypertension. A recent cross‐sectional study found that elevated LDL‐C/HDL‐C ratio and TG levels were significantly and independently associated with diabetes in hypertensive patients 2 . The interactive effects of increased TG and the LDL‐C/HDL‐C ratio suggest that dyslipidemia might exaggerate the risk of diabetes in hypertensive patients.
Metabolites have the potential to be useful biomarkers for the prediction of type 2 diabetes. A recent study found that insulin resistance (IR) was connected with tyrosine metabolism, and elevated tyrosine levels might inhibit insulin signaling 3 ; another found that high plasma tyrosine levels were associated with type 2 diabetes in Chinese individuals, particularly in the presence of low HDL‐C 4 . These findings suggested an interactive effect between high tyrosine and low HDL‐C for type 2 diabetes.
Dipeptidyl peptidase‐4 inhibitors are effective antihyperglycemic agents for type 2 diabetes. Treatment with the inhibitor, anagliptin, for 24 weeks to Japanese type 2 diabetes patients was shown to reduce plasma LDL‐C level, partly by suppressing apoB‐100 synthesis 5 . The effects were pronounced in type 2 diabetes patients with higher baseline LDL‐C levels, and a recent report suggested that it benefits lipid metabolism through dipeptidyl peptidase‐4‐dependent and glucagon‐like peptide‐1‐independent inhibition of intestinal cholesterol transport 6 .
Non‐alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease worldwide. It represents a metabolic syndrome affecting the liver and is a risk factor for IR, hyperlipidemia and type 2 diabetes. The coexistence of NAFLD and type 2 diabetes worsens metabolic profile and increases cardiovascular risk. The relationship between NAFLD and type 2 diabetes, and the natural history and clinical implications of NAFLD in patients with prediabetes or type 2 diabetes are largely unknown. Angiopoietin‐like protein 8 (ANGPTL‐8) is reportedly increased in NAFLD patients, and ANGPTL‐8 knockout mice show lower plasma TG levels as a result of enhanced TG clearance through increased lipoprotein lipase activity 7 . Hong et al. 8 investigated the association of plasma ANGPTL‐8 levels with hepatic lipid content and IR in patients with NAFLD. They observed a positive correlation between ANGPTL‐8 and hepatic lipid content, independent of obesity, IR, and liver injury. High serum ANGPTL‐8 presumably results from hepatic lipid accumulation, and ANGPTL‐8 might be a new and useful predictor of the NAFLD severity.
Inflammation plays an important role in the progression of NAFLD to non‐alcoholic steatohepatitis. The vagus nerve regulates inflammatory responses in macrophage and Kupffer cells through α7 nicotinic acetylcholine receptor (α7nAchR). Studies show that α7nAchR knockout mice have elevated pro‐inflammatory cytokines and develop IR when fed a high‐fat diet (HFD) 9 , and α7nAchR deficiency leads to inflammation and fibrosis, which exacerbate non‐alcoholic steatohepatitis 10 . Therefore, α7nAchR agonists are promising candidates for the treatment of non‐alcoholic steatohepatitis.
Overnutrition, hyperinsulinemia and hyperglycemia drive steatosis by promoting hepatic de novo lipogenesis (DNL), and researchers are investigating hepatic DNL regulating factors. Rho‐associated coiled‐coil‐containing kinase (ROCK1) is a serine/threonine‐protein kinase that regulates diverse cellular functions. Recently, Huang et al. reported that hepatic ROCK1 level and activity were enhanced by HFD feeding in mice, and HFD‐fed liver‐specific ROCK1 knockout mice lost bodyweight with decreasing lipid accumulation in the liver and adipose tissue, and increased energy expenditure, thus preventing diet‐induced obesity 11 . The endocannabinoid system contributes upstream signaling components that activate hepatic ROCK1 and contribute to HFD‐induced hepatic lipogenesis by inhibiting adenosine monophosphate‐activated protein kinase 12 . Hepatocyte growth factor (HGF) can stimulate hepatocyte regeneration, which is important for liver repair. Jing et al. 13 investigated the effect of HGF on hepatic lipid metabolism in HFD‐fed mice, and found that HGF reduced lipid content in the serum and liver, and ameliorated glucose intolerance 13 . These effects were mediated through farnesoid X receptor/small heterodimer partner axis‐dependent suppression of sterol regulatory element‐binding protein‐1c and activation of peroxisome proliferator‐activated receptor‐α in the liver. These studies suggest that regulation of hepatic DNL will reduce NAFLD. Hepatic ROCK1 inhibition and HGF treatment could be therapeutic targets for the prevention and treatment of NAFLD and associated metabolic disorders.
NAFLD is also characterized by atherogenic dyslipidemia. 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropanoic acid is a metabolite of furan fatty acids found in marine animals and is a possible biomarker of fish intake. A recent study in type 2 diabetes and normal glucose tolerant individuals showed that the circulating 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropanoic acid level was independently and negatively associated with TG, HDL‐C, aspartate aminotransferase, and alanine aminotransferase levels after adjusting for diabetes status 14 . This finding suggests that 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropanoic acid plays an important role in improving lipid and glucose metabolism and could be a new predictor of NAFLD development.
These findings provide new evidence that dyslipidemia characterized by elevated TGs and non‐HDL‐C levels with a decreased HDL‐C level are risk factors for cardiovascular disease in patients with type 2 diabetes. The underlying disturbances are activation of hepatic DNL, hepatic overproduction of large TG‐rich very LDL and delayed clearance of TG‐rich lipoproteins (Figure 1). There are multiple therapeutic agents to help patients to achieve target lipid levels, but understanding the molecular mechanisms could provide useful information for new treatment strategies for diabetic dyslipidemia.
Figure 1.
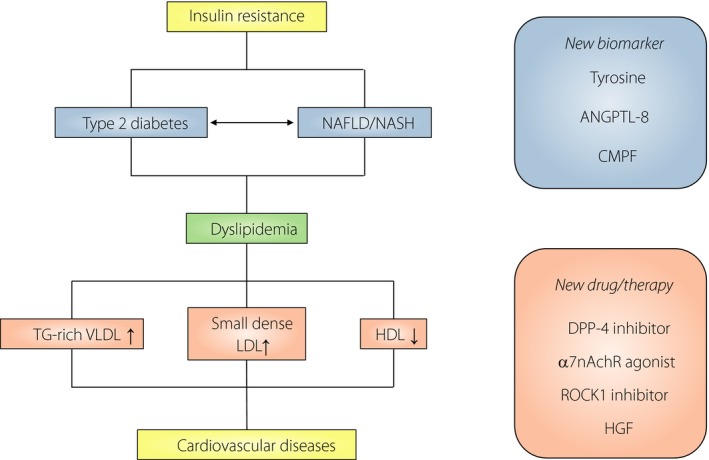
The pathogenic interplay between type 2 diabetes, non‐alcoholic fatty liver disease (NAFLD)/non‐alcoholic steatohepatitis (NASH), dyslipidemia and cardiovascular disease, and the potential clinical targets for these complications. Insulin resistance and excess insulin stimulate the production of triglycerides (TGs) in the liver, which promotes the serum lipid accumulation and the development of NAFLD. Increased secretion of these lipids into the bloodstream causes diabetic dyslipidemia, characterized by elevated serum concentrations of TG‐rich very low‐density lipoprotein cholesterol (VLDL) and small dense low‐density lipoprotein (LDL) cholesterol, and low concentrations of high‐density lipoprotein (HDL) cholesterol. Consequently, all of these factors, combined with hyperglycemia, increase the risk for cardiovascular diseases. Recent studies showed that serum levels of tyrosine, angiopoietin‐like protein 8 (ANGPTL‐8), and 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropanoic acid (CMPF), together with dyslipidemia, could be new biomarkers of type 2 diabetes and NAFLD. Recent studies also found dipeptidyl peptidase‐4 inhibitors, α7 nicotinic acetylcholine receptor (α7nAchR) agonist, Rho‐associated coiled‐coil‐containing kinase 1 (ROCK1) inhibitor, and hepatocyte growth factor (HGF) as novel and potential targets for the effective treatment of dyslipidemia and NAFLD in diabetes patients.
Disclosure
The authors declare no conflict of interest.
References
- 1. Liu L, Li Q, Yuan Z, et al Non‐high‐density lipoprotein cholesterol is more informative than traditional cholesterol indices in predicting diabetes risk for women with normal glucose tolerance. J Diabetes Investig 2018; 9: 1304–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hong M, Ling Y, Lu Z, et al Contribution and interaction of the low‐density lipoprotein cholesterol to high‐density lipoprotein cholesterol ratio and triglyceride to diabetes in hypertensive patients: a cross‐sectional study. J Diabetes Investig 2019; 10: 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferguson AA, Roy S, Kormanik KN, et al TATN‐1 mutations reveal a novel role for tyrosine as a metabolic signal that influences developmental decisions and longevity in Caenorhabditis elegans . PLoS Genet 2013; 9: e1004020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li J, Cao YF, Sun XY, et al Plasma tyrosine and its interaction with low high‐density lipoprotein cholesterol and the risk of type 2 diabetes mellitus in Chinese. J Diabetes Investig 2019; 10: 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kurozumi A, Okada Y, Arao T, et al Comparison of effects of anagliptin and alogliptin on serum lipid profile in type 2 diabetes mellitus patients. J Diabetes Investig 2018; 9: 360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goto M, Furuta S, Yamashita S, et al Dipeptidyl peptidase 4 inhibitor anagliptin ameliorates hypercholesterolemia in hypercholesterolemic mice through inhibition of intestinal cholesterol transport. J Diabetes Investig 2018; 9: 1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee YH, Lee SG, Lee CJ, et al Association between betatrophin/ANGPTL8 and non‐alcoholic fatty liver disease: animal and human studies. Sci Rep 2016; 6: 24013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hong BS, Liu J, Zheng J, et al Angiopoietin‐like protein 8/betatrophin correlates with hepatocellular lipid content independent of insulin resistance in non‐alcoholic fatty liver disease patients. J Diabetes Investig 2018; 9: 952–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang X, Yang Z, Xue B, et al Activation of the cholinergic antiinflammatory pathway ameliorates obesity‐induced inflammation and insulin resistance. Endocrinology 2011; 152: 836–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kimura K, Inaba Y, Watanabe H, et al Nicotinic alpha‐7 acetylcholine receptor deficiency exacerbates hepatic inflammation and fibrosis in a mouse model of non‐alcoholic steatohepatitis. J Diabetes Investig 2019; 10: 659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang H, Lee SH, Sousa‐Lima I, et al Rho‐kinase/AMPK axis regulates hepatic lipogenesis during overnutrition. J Clin Invest 2018; 128: 5335–5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miyamoto T, Matsuzaka T, Shimano H. Rho‐associated, coiled‐coil‐containing protein kinase 1 as a new player in the regulation of hepatic lipogenesis. J Diabetes Investig 2019; 10: 1165–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jing Y, Sun Q, Xiong X, et al. Hepatocyte growth factor alleviates hepatic insulin resistance and lipid accumulation in high‐fat diet‐fed mice. J Diabetes Investig 2019; 10: 251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dai J, Yi J, Zhang S, et al Serum 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropanoic acid is associated with lipid profiles and might protect against non‐alcoholic fatty liver disease in Chinese individuals. J Diabetes Investig 2019; 10: 793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]


