Abstract
Aims/Introduction
Our aims were to examine the add‐on effects of a sodium–glucose cotransporter 2 inhibitor, dapagliflozin, compared with existing antidiabetes treatments, on anthropometric/metabolic parameters, the levels of an endocrine regulator, fibroblast growth factor 21 (FGF21); a skeletal muscle mass (SMM) negative regulator, myostatin; and a metabolic regulator, irisin, in patients with type 2 diabetes.
Materials and Methods
A total of 54 patients with type 2 diabetes were randomly divided into dapagliflozin and control groups. The dapagliflozin group received dapagliflozin 5 mg/day in addition to conventional therapy for 24 weeks. The primary outcome was the change in the level of serum FGF21 from baseline. The secondary outcomes included changes from baseline in anthropometric/metabolic parameters and serum levels of myostatin and irisin.
Results
Bodyweight decreased in the dapagliflozin group compared with the control group (P < 0.001), but the changes in SMM were not significant between the groups (P = 0.611), thereby elevating the ratio of SMM‐to‐bodyweight in the dapagliflozin group (P = 0.028). Myostatin levels were significantly decreased (P = 0.010), and irisin levels showed a nearly significant reduction (P = 0.052) in the dapagliflozin group compared with the control group, whereas FGF21 levels did not change significantly from baseline to the end of the intervention in both the dapagliflozin (P = 0.673) and the control (P = 0.823) groups.
Conclusions
Dapagliflozin add‐on therapy in patients with type 2 diabetes reduced myostatin levels significantly and maintained SMM, without significant changes in FGF21 levels.
Keywords: Muscle mass, Sodium–glucose cotransporter 2 inhibitor, Type 2 diabetes
Add‐on effects of a sodium–glucose cotransporter 2 inhibitor, dapagliflozin, on type 2 diabetes were examined. Dapagliflozin reduced serum myostatin levels and elevated the SMM‐to‐bodyweight ratios with improving metabolic parameters. Dapagliflozin would contribute to reducing the risk of muscle loss.

Introduction
The prevalence of type 2 diabetes is increasing worldwide, and it is pathologically involved in accelerating the loss of muscle mass and strength1, 2, 3. Muscle atrophy is a high‐risk factor for physical disability and mortality1; therefore there is an urgent need to develop effective strategies to prevent and treat diabetes‐related muscle atrophy, including sarcopenia.
Dapagliflozin, a selective sodium–glucose cotransporter 2 inhibitor (SGLT2i), shows glucose‐lowering effects by inhibiting renal glucose reabsorption and increasing urinary glucose excretion4. Reportedly, SGLT2i exerted pleiotropic beneficial effects, such as reduction in bodyweight (BW), fat mass, and other risk factors for cardiovascular and kidney diseases5. Conversely, there are studies reporting that, regarding the effects of SGLT2i on skeletal muscle mass (SMM) in type 2 diabetes patients, SGLT2i significantly reduces muscle mass6, 7, whereas others show no significant effect8, 9. Accordingly, whether SGLT2i has deleterious, no substantial or beneficial effects on muscle mass remains controversial.
Fibroblast growth factor 21 (FGF21) is an endocrine regulator of glucose and lipid metabolism, and is produced by metabolically active tissues, such as the liver, muscle and adipose tissues10, 11. FGF21 targets these tissues, and improves glucose and/or lipid homeostasis11, 12; a state of FGF21 resistance has been observed in diabetes, because the circulating levels of FGF21 are elevated, and are correlated to the severity of muscle and hepatic insulin resistance in type 2 diabetes11, 13. Therefore, SGLT2i might modulate the function of FGF21 and/or improve the sensitivity of FGF21 in target tissues, thereby leading to the pleiotropic effects on metabolism. Conversely, the potential roles of FGF21 in maintaining SMM have not been validated. The circulating levels of FGF21 are elevated in patients with mitochondrial disorders affecting skeletal muscle14, and FGF21 counteracts muscle stress by enhancing mitochondrial activity15, 16. Thus, FGF21 might be involved in regulating SMM and in modulating metabolism. Accordingly, elucidating the effects of SGLT2i on FGF21 in type 2 diabetes would provide novel insights about the influence of SGLT2i on SMM and the mechanisms underlying the pleiotropic effects of SGLT2i.
Myostatin, a myokine mainly produced in skeletal muscle and also in adipose tissue at a low level, negatively regulates muscle growth17, 18, 19. Myostatin secreted into the circulation reflects its intramuscular levels20, 21, 22, and acts systemically to bind its receptors, causing muscle loss23. As skeletal muscle functions as the major site of insulin‐mediated glucose uptake, wasting muscle would aggravate insulin resistance, which would further lead to exacerbation of diabetes and cause diabetes‐related muscle loss3. In addition, skeletal muscle produces both myostatin and FGF21; thus, a functional cross‐talk might exist between these regulators. In patients with type 2 diabetes, a previous study reported that circulating myostatin levels were negatively associated with levels of fasting plasma glucose and triglycerides24, whereas another study observed no significant metabolic effect of circulating myostatin25. Therefore, the clinical significance of circulating myostatin on glucose metabolism and muscle mass, and the effects of SGLT2i on myostatin in type 2 diabetes have not been fully elucidated.
Preclinical studies have shown that myostatin also plays a role in modulating the function of adipose tissue26. Myostatin is involved in suppressing the secretion of irisin by the muscle and another myokine, which enhances energy expenditure through the process of browning white adipose tissues27, 28, and FGF21 enhances the irisin‐induced browning process29, 30. Most circulating irisin is derived from muscle in healthy conditions, but adipose tissue actively secretes irisin in body mass index (BMI)‐atypical settings, such as obesity31. In patients with type 2 diabetes, conversely, circulating levels of irisin were lower than in control individuals28. Thus, the pathophysiological significance of irisin in disease conditions remains complex.
As FGF21 and myostatin have positive and negative regulatory effects, respectively, on the irisin‐induced browning process27, 28, 29, 30, a cross‐talk might exist between these regulators. Thus, the pleiotropic effects of SGLT2i might be correlated to the functions of these endocrine/metabolic regulators. We recently reported the potential pathological implications of circulating myostatin in regulating muscle mass and glucose metabolism in obese patients, using a cohort of Japanese patients with obesity and/or diabetes32. In the present study based on this cohort, we investigated the add‐on effects of dapagliflozin, compared with existing antidiabetes treatments, on anthropometric/metabolic parameters, and on the levels of FGF21, myostatin and irisin in patients with type 2 diabetes.
Methods
Study design
This was a 24‐week, two‐arm, randomized, open‐label, active‐controlled, blinded end‐point trial. This study was registered in the University Hospital Medical Information Network Clinical Trial Registry (UMIN‐CTR) system (ID: UMIN000021479). Approval for the study was obtained from the ethics committee for human research at Kyoto Medical Center (approval number: 15‐109). The study was carried out in accordance with the principles of the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects. All participants provided written informed consent. Appendix S1 shows the CONSORT 2010 checklist for this trial.
Participants
The participants were screened from a cohort of Japanese diabetes patients enrolled in the outpatient clinic of the National Hospital Organization Kyoto Medical Center between November 2016 and June 2018. Eligible individuals were patients aged 20–79 years with type 2 diabetes; who had a hemoglobin A1c (HbA1c) level of ≥6.5% to <9.0% and a BMI of ≥22 kg/m2; and who were receiving diet and exercise therapy, and taking sulfonylurea, biguanide, α‐glucosidase inhibitor or dipeptidyl peptidase‐4 inhibitors or their combinations. The exclusion criteria were secondary obesity associated with endocrine disorders; type 1 diabetes; severe ketosis; diabetic coma or precoma; severe infectious disease; being in the period before or after surgery; external injury; severe hepatic dysfunction; serum creatinine ≥1.5 mg/dL (men) or ≥1.3 mg/dL (women); a history of severe vascular disease in the previous 6 months (including myocardial infarction and stroke); dehydration or diarrhea that would cause dehydration; taking thiazolidinediones and/or fibrate; taking SGLT2is, insulin formulations or GLP‐1 receptor agonists; pregnancy or lactation; a history of hypersensitivity to SGLT2i; and findings suggestive of ineligibility by an attending doctor.
Randomization and masking
The participants were centrally randomized at the SATISTA Co., Ltd. (Kyoto, Japan), a site external to the trial, and assigned to the control or dapagliflozin group in a 1:1 allocation ratio, through computer‐generated random number and assignment tables. Allocation was carried out using random permuted blocks of size four. Stratification factors were based on the patient’s age, HbA1c level and medications at baseline. During the randomization procedure, no content was disclosed to the clinical staff or assessors, whereas this was an open‐label trial, and the physicians and patients recognized the type of medication used.
Intervention
The dapagliflozin group received once‐daily 5 mg dapagliflozin in addition to their conventional type 2 diabetes medications. The control group simply took their conventional medications for type 2 diabetes. Combination‐restrictive medicines included biguanides, sulfonylurea drugs, α‐glucosidase inhibitors and dipeptidyl peptidase‐4 inhibitors. If there should be unfavorable effects, such as side‐effects, the medications would be reduced or stopped and shifted to more appropriate treatments, according to the attending physician’s judgment. There was no restriction on multimodal treatments, such as diet and exercise therapies. Diuretics and biguanides were to be reduced or stopped if symptoms of dehydration should be observed or expected.
Outcomes and measurements
The primary outcome was changed in the level of serum FGF21 from baseline to 12 and 24 weeks after administration. The secondary outcomes were changes from baseline to 12 and 24 weeks in anthropometric/metabolic parameters, and serum levels of myostatin and irisin.
Anthropometric and metabolic parameters were measured for all patients, and blood samples were taken between 08.30 hours and 09.30 hours after overnight fasting (baseline), and after 12 and 24 weeks of intervention, using standard procedures32, 33, 34. The anthropometric parameters included BW, BMI, waist circumference, muscle thickness, SMM, visceral fat mass and subcutaneous fat mass. The metabolic parameters included systolic and diastolic blood pressure, fasting plasma glucose, HbA1c, serum immunoreactive insulin, homeostasis model assessment of insulin resistance, triglycerides, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, aspartate aminotransferase, alanine aminotransferase, γ‐glutamyl transpeptidase (γ‐GTP) and estimated glomerular filtration rate.
We measured SMM and body water within 2 weeks of blood sample collection, using a precision body composition analyzer (InBody720; InBody Japan Inc., Tokyo, Japan)32, 35. Intra‐abdominal fat area (IFA) and subcutaneous fat area (SFA) were measured with dual bioelectrical impedance analysis using DUALSCAN HDS‐2000 (Omron Healthcare Corporation, Kyoto, Japan)32, 36 on the same day as that of SMM measurement.
Serum levels of FGF21, myostatin and irisin were measured at Health Sciences West Japan (Kyoto, Japan) using a Human FGF‐21 Quantikine ELISA Kit (DF2100; R&D Systems, Minneapolis, MN, USA), a GDF‐8/Myostatin Quantikine ELISA Kit (DGDF80; R&D Systems) and a Human FNDC5/Irisin ELISA Kit (sandwich ELISA; LS‐F38053; LifeSpan BioSciences, Seattle, WA, USA), respectively, according to the manufacturer’s instructions. We measured each serum sample in duplicate and used average values for analysis.
Statistical analysis
In a previous study, changes in serum FGF21 levels within each participant were normally distributed with a standard deviation of 6037. If the true difference in the intervention and control means is 50 (intervention group, +50 ± 60 ng/L; control group, 0 ± 60 ng/L), we need to study 24 intervention participants and 24 control participants to be able to reject the null hypothesis that the population means of the experimental and control groups are equal with probability (power) of 80%. The type I error probability associated with this test of this null hypothesis is 0.05. Finally, in anticipation of a dropout rate of 20%, the sample size was set at 60 participants.
The results are described as the mean ± standard deviation or median (interquartile range). We applied a logarithmic transformation to variables with a lognormal distribution. Two‐way repeated‐measures analysis of covariance (ancova) was applied to evaluate between‐group differences in outcomes at 12 or 24 weeks compared with 0 weeks (baseline), in which time and group, as well as the interaction between time and group, were the categorical fixed factors. Then, for a significant variable identified by ancova, a two‐tailed, paired t‐test was applied to evaluate the changes in conditions between baseline and end of treatment. The results are shown as within‐ or between‐group differences with 95% confidence intervals. Effects were evaluated on an intention‐to‐treat basis, and we considered participants who did not complete the follow‐up period as not to have had any changes in measures. If levels of regulators (FGF21, myostatin and irisin) were significantly changed in the dapagliflozin group compared with the control group, Pearson’s correlation coefficient (r) was used to investigate the correlations between the changes in the levels of the regulators and the changes in anthropometric/metabolic parameters. A two‐sided P‐value <0.05 was considered to show statistical significance. The statistical analyses were carried out using SPSS version 23.0 for Windows (IBM Japan Ltd., Tokyo, Japan).
Results
Study flow
Figure 1 outlines the trial. A total of 60 patients were screened from November 2016 to June 2018, and 54 were randomly assigned to receive either dapagliflozin 5 mg/day in addition to conventional treatment for type 2 diabetes (dapagliflozin group) or conventional treatment for type 2 diabetes without dapagliflozin (control group; n = 27 each). In the dapagliflozin arm, 26 patients completed the study, with one dropping out because of study medication‐related complications. In the control arm, 24 patients completed the study, with one withdrawing and two having a scheduling conflict. There were no differences in background characteristics between participants who completed the study and those who discontinued. None of the participants in either group required treatment modifications, including the currently used oral hypoglycemic agents, during the study period.
Figure 1.
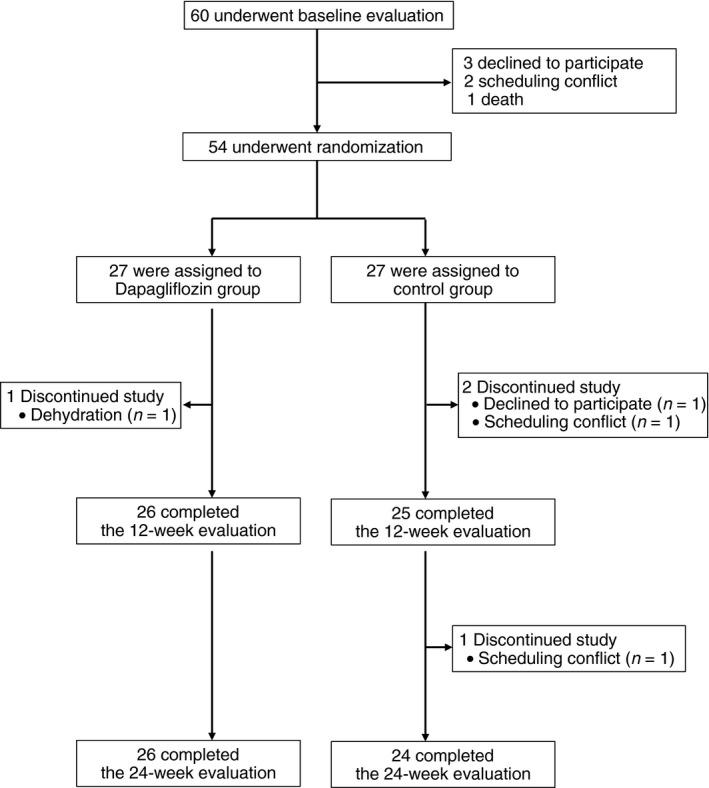
Patient flowchart for screening, randomization and completion of the 24‐week evaluation.
Baseline characteristics
Table 1 shows the baseline characteristics of the participants (n = 54). The characteristics at baseline were balanced between the dapagliflozin and control groups.
Table 1.
Characteristics of the two groups at baseline
| Characteristic | Dapagliflozin group | Control group |
|---|---|---|
| n | 27 | 27 |
| Sex, n (%) | ||
| Male | 11, 40.7 | 14, 51.9 |
| Female | 16, 59.3 | 13, 48.1 |
| Age (years) | 58.4 ± 13.0 | 60.7 ± 11.9 |
| BMI (kg/m2) | 31.3 ± 7.6 | 30.7 ± 6.2 |
| Waist circumference (cm) | 102.2 ± 17.9 | 103.2 ± 14.2 |
| IFA (cm2) | 101.4 ± 28.0 | 110.5 ± 39.8 |
| SFA (cm2) | 254.4 ± 81.0 | 223.8 ± 60.7 |
| Skeletal muscle mass (kg) | 25.9 ± 6.7 | 27.1 ± 6.3 |
| Skeletal muscle mass‐to‐BW ratio (%) | 32.7 ± 5.3 | 35.0 ± 5.3 |
| Muscle thickness (mm) | 12.2 ± 4.0 | 12.2 ± 2.6 |
| Body water (L) | 34.9 ± 8.3 | 36.8 ± 7.7 |
| SBP (mmHg) | 134.6 ± 13.2 | 132.9 ± 11.9 |
| DBP (mmHg) | 80.0 ± 9.3 | 78.6 ± 7.8 |
| FPG (mmol/L) | 8.5 ± 2.7 | 8.1 ± 2.0 |
| HbA1c (%) | 7.5 ± 0.8 | 7.4 ± 0.9 |
| HbA1c (mmol/mol) | 58.3 ± 8.5 | 57.7 ± 9.9 |
| IRI (pmol/L) | 111.3 [59.5–145.6] | 73.2 [58.3–125.5] |
| HOMA‐IR | 2.8 [2.2–4.2] | 3.4 [2.5–6.0] |
| Triglycerides (mmol/L) | 1.5 [1.1–1.9] | 1.5 [1.2–2.6] |
| HDL‐C (mmol/L) | 1.3 ± 0.2 | 1.4 ± 0.5 |
| LDL‐C (mmol/L) | 3.0 ± 0.6 | 2.8 ± 0.7 |
| AST (units/L) | 31.0 ± 16.4 | 28.9 ± 12.4 |
| ALT (units/L) | 38.3 ± 27.3 | 32.4 ± 16.5 |
| γ‐GTP (units/L) | 50.6 ± 54.3 | 58.1 ± 71.5 |
| eGFR (mL/min/1.73 m2) | 76.2 ± 18.0 | 73.0 ± 24.8 |
| Diabetes treatment, n (%) | ||
| SU | 11, 40.7 | 9, 33.3 |
| α‐GI | 1, 3.7 | 3, 11.1 |
| BG | 22, 81.5 | 17, 63.0 |
| DPP‐4 | 19, 70.4 | 19, 70.4 |
Data are expressed as the mean ± standard deviation, median [interquartile range], or the number and percentage of patients. α‐GI, α‐glucosidase inhibitor; γ‐GTP, γ‐glutamyl transpeptidase; ALT, alanine amino transferase; AST, aspartate aminotransferase; BG, biguanide; DBP, diastolic blood pressure; DPP‐4, dipeptidyl peptidase‐4; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐R, homeostasis model assessment of insulin resistance; IFA, intra‐abdominal fat area; IRI, immunoreactive insulin; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; SFA, subcutaneous fat area; SU, sulfonylurea.
Effects of dapagliflozin add‐on therapy on primary and secondary outcomes
Table 2 summarizes the changes in the primary and secondary outcomes from baseline to the middle (12 weeks) and end (24 weeks) of the intervention in the dapagliflozin and control groups.
Table 2.
Changes in primary and secondary outcomes
| Outcome | Time | Mean change from baseline | Between‐group difference | ||
|---|---|---|---|---|---|
| Dapagliflozin group | Control group | Dapagliflozin vs control | P‐value | ||
| FGF21 (pg/mL) | Baseline | 209.8 ± 101.4 | 259.4 ± 209.5 | ||
| Week 12 | 183.8 ± 85.2 | 248.0 ± 176.5 | |||
| Week 24 | 226.3 ± 230.7 | 265.1 ± 180.0 | |||
| Δ (95% CI) | 16.5 (−62.8, 95.8) | 5.7 (−46.2, 57.6) | 10.8 (−81.8, 103.3) | 0.816 | |
| Myostatin (pg/mL) | Baseline | 3,745 ± 1,970 | 3,804 ± 1,651 | ||
| Week 12 | 3,157 ± 1,493 | 3,825 ± 1,877 | |||
| Week 24 | 3,143 ± 1,546 | 4,117 ± 1,772 | |||
| Δ (95% CI) | −601 (−1,223, 21) | 314 (−8, 635) | −915 (−1,599, −231) | 0.010 | |
| Irisin (ng/mL) | Baseline | 93.3 ± 82.3 | 100.6 ± 74.8 | ||
| Week 12 | 63.2 ± 62.8 | 101.4 ± 84.4 | |||
| Week 24 | 61.1 ± 57.9 | 94.3 ± 72.2 | |||
| Δ (95% CI) | −32.3 (−55.5. −9.0) | −6.4 (−19.7, 6.9) | −25.9 (−52.1, 0.26) | 0.052 | |
| HbA1c (%) | Baseline | 7.5 ± 0.8 | 7.4 ± 0.9 | ||
| Week 12 | 7.0 ± 0.6 | 7.3 ± 0.9 | |||
| Week 24 | 6.8 ± 0.5 | 7.6 ± 1.0 | |||
| Δ (95% CI) | −0.6 (−0.9, −0.4) | 0.2 (−0.2, 0.4) | −0.8 (−1.3, −0.3) | 0.001 | |
| BW (kg) | Baseline | 80.5 ± 22.6 | 79.0 ± 16.3 | ||
| Week 12 | 78.3 ± 22.8 | 78.9 ± 16.3 | |||
| Week 24 | 77.3 ± 22.0 | 79.0 ± 15.7 | |||
| Δ (95% CI) | −3.2 (−4.1, −2.4) | 0.0 (−1.0, 1.0) | −3.2 (−4.5, −2.0) | <0.001 | |
| BMI | Baseline | 31.3 ± 7.6 | 30.7 ± 6.2 | ||
| Week 12 | 30.4 ± 7.7 | 30.7 ± 6.3 | |||
| Week 24 | 30.0 ± 7.4 | 30.7 ± 6.0 | |||
| Δ (95% CI) | −1.2 (−1.6, −0.9) | 0.0 (−0.4, 0.4) | −1.3 (−1.8, −0.8) | <0.001 | |
| IFA (cm2) | Baseline | 101.4 ± 28.0 | 110.5 ± 39.8 | ||
| Week 12 | 90.9 ± 26.3 | 112.3 ± 42.2 | |||
| Week 24 | 91.5 ± 28.7 | 114.0 ± 46.4 | |||
| Δ (95% CI) | −9.8 (−17.7, −2.0) | 3.5 (−3.5, 10.5) | −13.3 (−23.4, −3.2) | 0.009 | |
| SFA (cm2) | Baseline | 254.4 ± 81.0 | 223.8 ± 60.7 | ||
| Week 12 | 243.8 ± 79.6 | 229.0 ± 59.4 | |||
| Week 24 | 238.1 ± 74.8 | 237.1 ± 68.1 | |||
| Δ (95% CI) | −16.3 (−31.2, −1.4) | 13.3 (−7.9, 34.5) | −29.6 (−55.2, −4.0) | 0.025 | |
| SMM (kg) | Baseline | 25.9 ± 6.7 | 27.1 ± 6.3 | ||
| Week 12 | 26.1 ± 6.7 | 27.2 ± 6.4 | |||
| Week 24 | 26.0 ± 7.4 | 26.9 ± 6.1 | |||
| Δ (95% CI) | 0.1 (−1.0. 1.2) | −0.2 (−0.4, 0.1) | 0.3 (−0.9, 1.4) | 0.611 | |
| SMM‐to‐BW ratio (%) | Baseline | 32.7 ± 5.3 | 35.0 ± 5.3 | ||
| Week 12 | 34.1 ± 6.1 | 35.3 ± 5.5 | |||
| Week 24 | 34.3 ± 7.1 | 34.8 ± 5.4 | |||
| Δ (95% CI) | 1.6 (0.1, 3.1) | −0.2 (−0.7, 0.3) | 1.8 (0.2, 3.4) | 0.028 | |
| Body water (L) | Baseline | 34.9 ± 8.3 | 36.8 ± 7.7 | ||
| Week 12 | 35.2 ± 8.2 | 37.0 ± 7.8 | |||
| Week 24 | 35.0 ± 9.3 | 36.7 ± 7.5 | |||
| Δ (95% CI) | 0.1 (−1.7, 1.5) | −0.1 (−0.2, 0.5) | 0.2 (−1.4, 1.8) | 0.787 | |
Δ Represents the difference between the 24‐week and baseline values. “Between‐group difference” indicates the difference between the dapagliflozin group and the control group values. P‐values from two‐way repeated‐measures ancova (time [baseline and at 24 weeks] × group [dapagliflozin and control]). 95% CI, 95% confidence interval; α‐GI, α‐glucosidase inhibitor; γ‐GTP, γ‐glutamyl transpeptidase; ALT, alanine amino transferase; AST, aspartate aminotransferase; BG, biguanide; DBP, diastolic blood pressure; DPP‐4, dipeptidyl peptidase‐4; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment of insulin resistance; IFA, intra‐abdominal fat area; IRI, immunoreactive insulin; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; SFA, subcutaneous fat area; SU, sulfonylurea.
Serum levels of FGF21 did not differ significantly between baseline and the end of the intervention in either group (dapagliflozin group, P = 0.673; control group, P = 0.823). There were also no significant differences in the changes between the groups (P = 0.816). The serum level of myostatin decreased in the dapagliflozin group from baseline to the end of the intervention (P = 0.052), but increased to a nearly significant extent in the control group (P = 0.066). There was a significant difference between the groups in the changes in myostatin levels (P = 0.010). Serum levels of irisin were reduced in the dapagliflozin group (P = 0.039), but unchanged in the control group (P = 0.200). There was a nearly significant difference between the groups in the changes in irisin levels (P = 0.052).
The dapagliflozin group had a significant reduction in HbA1c values from baseline to the end of the intervention (P < 0.001), but the control group had no significant change (P = 0.391), leading to a significant difference between the groups in the changes in HbA1c values (P = 0.001). BW was reduced in the dapagliflozin group (P < 0.001), but not in the control group (P = 0.997), which resulted in a significant difference between the groups in BW changes (P < 0.001). BMI was decreased in the dapagliflozin group (P < 0.001), but not in the control group (P = 0.954), and there was a significant difference between the groups in BMI changes (P < 0.001). We further found a significant reduction in IFA in the dapagliflozin group (P = 0.028), but no significant change in the control group (P = 0.695); there was a significant difference between the groups in IFA changes (P = 0.009). Similarly, SFA was decreased in the dapagliflozin group (P = 0.034), but not changed in the control group (P = 0.205), and the difference in SFA changes between the groups was significant (P = 0.025). SMM remained unchanged in both groups (dapagliflozin group, P = 0.975; control group, P = 0.121), and there was no significant difference between the groups in SMM changes (P = 0.611).
We found a significant increase and no significant change in the SMM‐to‐BW ratio values from baseline to the end of the intervention in the dapagliflozin (P = 0.047) and control (P = 0.408) groups, respectively. There was a significant difference between the groups in the changes in the SMM‐to‐BW ratio values (P = 0.028).
As we observed significant changes in myostatin levels (P = 0.010) and SMM‐to‐BW ratio values (P = 0.028) between the dapagliflozin and control groups, we further analyzed the relationship between the changes in myostatin levels and the changes in SMM‐to‐BW ratio values in these groups. Among all patients, the changes in myostatin levels showed a significant negative correlation with the changes in SMM‐to‐BW ratio values (r = −0.334, P = 0.020). Furthermore, there was an almost significant negative correlation between the changes in myostatin levels and the changes in SMM‐to‐BW ratio values in the dapagliflozin group (r = −0.331, P = 0.122), whereas this was not seen in the control group (r = −0.062, P = 0.783).
Body water did not change significantly from baseline to the end of the intervention in the dapagliflozin (P = 0.900) and control (P = 0.465) groups. There was also no significant difference between the groups in the changes in body water (P = 0.787).
Table S1 shows the changes in markers of liver function. Aspartate aminotransferase values did not change significantly from baseline to the end of the intervention in both the dapagliflozin group (P = 0.183) and the control group (P = 0.218), and the changes in aspartate aminotransferase values did not significantly differ between the groups (P = 0.071). ALT values remained unchanged between baseline and the end of intervention in the dapagliflozin (P = 0.429) and control (P = 0.212) groups, and there was no significant difference between the groups in the changes in ALT values (P = 0.203). γ‐GTP values decreased to a nearly significant extent in the dapagliflozin group (P = 0.068), but did not change in the control group (P = 0.184). There was a significant difference between the groups in the changes in γ‐GTP values (P = 0.034).
Adverse events
Five adverse events were reported by two patients after randomization. No patient had a serious adverse event requiring hospitalization. One patient in the dapagliflozin group developed dehydration within 1 day of initiation of the drug. The drug was discontinued, and the symptoms improved. One patient in the control group developed hunger, dizziness and cramps within 1 day of initiation of the drug, and finger tumor deterioration within 20 weeks of initiation of the drug.
Discussion
This is the first study to examine the mechanisms underlying the pleiotropic beneficial effects of SGLT2i by investigating the regulators of the endocrine system (FGF21), SMM (myostatin) and metabolism (irisin). We showed that dapagliflozin add‐on therapy did not significantly affect the circulating levels of FGF21, but decreased those of myostatin and irisin to a significant and nearly significant extent, respectively, in patients with type 2 diabetes. Furthermore, dapagliflozin maintained SMM, in parallel with reducing HbA1c, BW, BMI, IFA and SFA, which led to the elevation of SMM‐to‐BW ratios. Accordingly, these findings suggest that dapagliflozin decreases myostatin production, thereby contributing to reducing the risk of muscle loss in type 2 diabetes patients.
In the present study, dapagliflozin did not significantly affect the circulating levels of FGF21, but markedly decreased those of myostatin. Therefore, dapagliflozin does not cause pleiotropic effects through direct modulation of the circulating levels of FGF21, and FGF21 and myostatin could function independently of each other. However, dapagliflozin improved glucose metabolism and the SMM‐to‐BW ratio; thus, it concomitantly contributed in restoring the sensitivity of FGF21 in target tissues, thereby indirectly enhancing the metabolic effects of FGF21. Notably, regarding the maintenance of SMM, a recent study that used FGF21 knockout mice reported that FGF21 induced muscle loss through mytophagy38. Although further study must be carried out to elucidate the functional significance of FGF21 in maintaining SMM in patients with type 2 diabetes, the present results showed that dapagliflozin had a beneficial effect on the SMM‐to‐BW ratio. Although FGF21 can have detrimental effects on SMM in type 2 diabetes patients, dapagliflozin could counteract these effects.
There has been a controversy about the effects of SGLT2i on muscle mass, and the effects of SGLT2i on myostatin have not been determined. Here, dapagliflozin significantly reduced circulating levels of myostatin in type 2 diabetes patients. Reportedly, diabetes/obesity‐related factors, such as hyperglycemia and palmitate, activate myostatin expression, which would cause muscle wasting39. In the present study, dapagliflozin improved glucose metabolism, fat mass and BMI, in parallel with reducing myostatin levels. Adipose tissue produces myostatin, but its level is limited compared with that of muscle17, and dapagliflozin did not significantly reduce muscle mass in the present study. Accordingly, loss of myostatin‐producing tissues would have a minor role in reducing the myostatin levels observed in this study; rather, dapagliflozin showed beneficial effects on glucose metabolism and fat mass, which would consequently reduce myostatin levels by alleviating intramuscular pathways involved in myostatin production. These results therefore imply qualitative improvements of muscle, which represent potential novel benefits of dapagliflozin in reducing the risk of muscle atrophy in type 2 diabetes patients.
Previous preclinical studies reported that myostatin inactivation led to irisin elevation27, 28. However, in the present study, irisin levels were not increased by dapagliflozin (but rather were decreased), despite the reduction in myostatin levels. Muscle is a main producer of irisin in healthy conditions, whereas adipose tissue actively elevates irisin in atypical BMI settings, such as obesity31. Accordingly, the decrease in irisin levels might be attributable to dapagliflozin‐mediated reduction of fat mass. Dapagliflozin did not significantly affect FGF21 levels. Thus, whether dapagliflozin is involved in the enhancement of the irisin‐induced browning process by FGF21 remains unclear, and further investigations must be carried out to address this issue.
SGLT2i is reportedly involved in reducing liver fat and ALT levels in patients with type 2 diabetes and non‐alcoholic fatty liver disease40, 41. Although the mechanistic details underlying the beneficial effects of SGLT2i on liver function have not been clarified, a preclinical study found deleterious effects of myostatin on hepatocytes, such as inhibition of proliferation and insulin‐stimulated glucose uptake42. The present study confirmed that dapagliflozin reduced myostatin levels and improved γ‐GTP levels compared with control treatment in type 2 diabetes patients. Accordingly, dapagliflozin‐mediated reduction in myostatin levels could contribute to improving liver function as well, in addition to maintaining muscle mass, in type 2 diabetes patients.
The present study had some limitations. First, this was an open‐label design, and the physicians and patients recognized the type of medications used, which might have caused unintentional bias. Second, the study addressed changes in muscle mass, but changes in muscle power and function remain unclear. Investigating sarcopenia‐related markers, such as grip strength, would be helpful for a comprehensive understanding of the effects of SGLT2i on muscle. Additionally, the changes before and after intervention in the activation levels of myostatin signaling or insulin signaling in muscle, liver and adipose tissue remain to be elucidated. The activation levels of FGF21 signaling in these tissues also remain unclear. Experimental studies using biopsies and model mice would be critical to address these issues.
In conclusion, the present study has provided the first evidence showing that dapagliflozin add‐on therapy maintained the levels of FGF21, and reduced those of myostatin and irisin to a significant and nearly significant extent, respectively, in patients with type 2 diabetes. These findings imply that dapagliflozin beneficially affects intramuscular signaling, potentially by improving glucose homeostasis and fat mass; this would contribute to reducing the risk of muscle loss and improving FGF21 sensitivity in type 2 diabetes patients. Future studies to elucidate the mechanistic details underlying the effects of SGLT2i on muscle would be helpful to develop novel strategies for preventing and treating muscle atrophy in type 2 diabetes.
Disclosure
NS‐A has received a research grant from AstraZeneca and ONO PHARMACEUTICAL CO., LTD. The other authors declare no conflict of interest.
Supporting information
Table S1 | Changes in markers of liver function.
Appendix S1 | CONSORT 2010 checklist of information reporting a randomized trial.
Acknowledgments
We thank Mr Kazuya Muranaka at Kyoto Medical Center for his technical assistance. We also thank Enago (http://www.enago.jp) for the English language review. This work was supported in part by Grant‐in‐Aid for Scientific Research (C) to HY (JSPS KAKENHI Grant Number JP19K07905), MT (JP19K07927), TI (JP19K11760), and TK (JP17K09827) and (B) to NS‐A (JP18H02737), and by Grant‐in‐Aid for Exploratory Research to NS‐A (JP18K19769), from Japan Society for the Promotion of Science. This work was also funded in part by AstraZeneca and ONO PHARMACEUTICAL CO., LTD. This study was also supported in part by a grant from Takeda Science Foundation to MT; a grant from Health Science University to MT; a grant from Smoking Research Foundation to NS‐A (2019T004); and a grant from the National Hospital Organization for collaborative clinical research to NS‐A (H26‐NHO‐02). The funders had no role in data collection, analysis, decision to publish or preparation of the manuscript.
J Diabetes Investig 2020; 11: 653–661
Clinical Trial Registry
University Hospital Medical Information Network
UMIN000021479
References
- 1. Kalyani RR, Corriere M, Ferrucci L. Age‐related and disease‐related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol 2014; 2: 819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nomura T, Kawae T, Kataoka H, et al Aging, physical activity, and diabetic complications related to loss of muscle strength in patients with type 2 diabetes. Phys Ther Res 2018; 21: 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kalaitzoglou E, Fowlkes JL, Popescu I, et al Diabetes pharmacotherapy and effects on the musculoskeletal system. Diabetes Metab Res Rev 2019; 35: e3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vivian EM. Dapagliflozin: a new sodium‐glucose cotransporter 2 inhibitor for treatment of type 2 diabetes. Am J Health Syst Pharm 2015; 72: 361–372. [DOI] [PubMed] [Google Scholar]
- 5. Tsimihodimos V, Filippatos TD, Elisaf MS. Effects of sodium‐glucose co‐transporter 2 inhibitors on metabolism: unanswered questions and controversies. Expert Opin Drug Metab Toxicol 2017; 13: 399–408. [DOI] [PubMed] [Google Scholar]
- 6. Yamamoto C, Miyoshi H, Ono K, et al Ipragliflozin effectively reduced visceral fat in Japanese patients with type 2 diabetes under adequate diet therapy. Endocr J 2016; 63: 589–596. [DOI] [PubMed] [Google Scholar]
- 7. Sasaki T, Sugawara M, Fukuda M. Sodium‐glucose cotransporter 2 inhibitor‐induced changes in body composition and simultaneous changes in metabolic profile: 52‐week prospective LIGHT (Luseogliflozin: the Components of Weight Loss in Japanese Patients with Type 2 Diabetes Mellitus) Study. J Diabetes Investig 2019; 10: 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sugiyama S, Jinnouchi H, Kurinami N, et al Dapagliflozin reduces fat mass without affecting muscle mass in type 2 diabetes. J Atheroscler Thromb 2018; 25: 467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Inoue H, Morino K, Ugi S, et al Ipragliflozin, a sodium‐glucose cotransporter 2 inhibitor, reduces bodyweight and fat mass, but not muscle mass, in Japanese type 2 diabetes patients treated with insulin: a randomized clinical trial. J Diabetes Investig 2019; 10: 1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Potthoff MJ. FGF21 and metabolic disease in 2016: a new frontier in FGF21 biology. Nat Rev Endocrinol 2017; 13: 74–76. [DOI] [PubMed] [Google Scholar]
- 11. Xie T, Leung PS. Fibroblast growth factor 21: a regulator of metabolic disease and health span. Am J Physiol Endocrinol Metab 2017; 313: E292– E302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mashili FL, Austin RL, Deshmukh AS, et al Direct effects of FGF21 on glucose uptake in human skeletal muscle: implications for type 2 diabetes and obesity. Diabetes Metab Res Rev 2011; 27: 286–297. [DOI] [PubMed] [Google Scholar]
- 13. Chavez AO, Molina‐Carrion M, Abdul‐Ghani MA, et al Circulating fibroblast growth factor‐21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care 2009; 32: 1542–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suomalainen A, Elo JM, Pietiläinen KH, et al FGF‐21 as a biomarker for muscle‐manifesting mitochondrial respiratory chain deficiencies: a diagnostic study. Lancet Neurol 2011; 10: 806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ji K, Zheng J, Lv J, et al Skeletal muscle increases FGF21 expression in mitochondrial disorders to compensate for energy metabolic insufficiency by activating the mTOR‐YY1‐PGC1α pathway. Free Radic Biol Med 2015; 84: 161–170. [DOI] [PubMed] [Google Scholar]
- 16. Kim KH, Lee MS. FGF21 as a mediator of adaptive responses to stress and metabolic benefits of anti‐diabetic drugs. J Endocrinol 2015; 226: R1–R16. [DOI] [PubMed] [Google Scholar]
- 17. McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF‐beta superfamily member. Nature 1997; 387: 83–90. [DOI] [PubMed] [Google Scholar]
- 18. Deng B, Zhang F, Wen J, et al The function of myostatin in the regulation of fat mass in mammals. Nutr Metab 2017; 14: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leal LG, Lopes MA, Batista ML Jr. Physical exercise‐induced myokines and muscle‐adipose tissue crosstalk: a review of current knowledge and the implications for health and metabolic diseases. Front Physiol 2018; 9: 1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gonzalez‐Cadavid NF, Taylor WE, Yarasheski K, et al Organization of the human myostatin gene and expression in healthy men and HIV‐infected men with muscle wasting. Proc Natl Acad Sci USA 1998; 95: 14938–14943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hittel DS, Berggren JR, Shearer J, et al Increased secretion and expression of myostatin in skeletal muscle from extremely obese women. Diabetes 2009; 58: 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ju CR, Chen RC. Serum myostatin levels and skeletal muscle wasting in chronic obstructive pulmonary disease. Respir Med 2012; 106: 102–108. [DOI] [PubMed] [Google Scholar]
- 23. Argilés JM, Orpí M, Busquets S, et al Myostatin: more than just a regulator of muscle mass. Drug Discov Today 2012; 17: 702–709. [DOI] [PubMed] [Google Scholar]
- 24. García‐Fontana B, Reyes‐García R, Morales‐Santana S, et al Relationship between myostatin and irisin in type 2 diabetes mellitus: a compensatory mechanism to an unfavourable metabolic state? Endocrine 2016; 52: 54–62. [DOI] [PubMed] [Google Scholar]
- 25. Brandt C, Nielsen AR, Fischer CP, et al Plasma and muscle myostatin in relation to type 2 diabetes. PLoS ONE 2012; 7: e37236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheng CF, Ku HC, Lin H. PGC‐1α as a pivotal factor in lipid and metabolic regulation. Int J Mol Sci 2018; 19: 3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shan T, Liang X, Bi P, et al Myostatin knockout drives browning of white adipose tissue through activating the AMPK‐PGC1α‐Fndc5 pathway in muscle. FASEB J 2013; 27: 1981–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perakakis N, Triantafyllou GA, Fernández‐Real JM, et al Physiology and role of irisin in glucose homeostasis. Nat Rev Endocrinol 2017; 13: 324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee P, Linderman JD, Smith S, et al Irisin and FGF21 are cold‐induced endocrine activators of brown fat function in humans. Cell Metab 2014; 19: 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rodríguez A, Becerril S, Ezquerro S, et al Crosstalk between adipokines and myokines in fat browning. Acta Physiol (Oxf) 2017; 219: 362–381. [DOI] [PubMed] [Google Scholar]
- 31. Roca‐Rivada A, Castelao C, Senin LL, et al FNDC5/irisin is not only a myokine but also an adipokine. PLoS ONE 2013; 8: e60563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tanaka M, Masuda S, Yamakage H, et al Role of serum myostatin in the association between hyperinsulinemia and muscle atrophy in Japanese obese patients. Diabetes Res Clin Pract 2018; 142: 195–202. [DOI] [PubMed] [Google Scholar]
- 33. Satoh‐Asahara N, Sasaki Y, Wada H, et al A dipeptidyl peptidase‐4 inhibitor, sitagliptin, exerts anti‐inflammatory effects in type 2 diabetic patients. Metabolism 2013; 62: 347–351. [DOI] [PubMed] [Google Scholar]
- 34. Satoh‐Asahara N, Kotani K, Yamakage H, et al Cardio‐ankle vascular index predicts for the incidence of cardiovascular events in obese patients: a multicenter prospective cohort study (Japan Obesity and Metabolic Syndrome Study: JOMS). Atherosclerosis 2015; 242: 461–468. [DOI] [PubMed] [Google Scholar]
- 35. Kaido T, Tamai Y, Hamaguchi Y, et al Effects of pretransplant sarcopenia and sequential changes in sarcopenic parameters after living donor liver transplantation. Nutrition 2017; 33: 195–198. [DOI] [PubMed] [Google Scholar]
- 36. Yamakage H, Ito R, Tochiya M, et al The utility of dual bioelectrical impedance analysis in detecting intra‐abdominal fat area in obese patients during weight reduction therapy in comparison with waist circumference and abdominal CT. Endocr J 2014; 61: 807–819. [DOI] [PubMed] [Google Scholar]
- 37. da Silveira Campos RM, Oyama LM, Masquio DCL, et al The role of insulin resistance on FGF‐21 and inflammatory markers in obese adolescents undergoing multicomponent long‐term weight loss therapy. Eur Med J 2017; 2: 97–105. [Google Scholar]
- 38. Oost LJ, Kustermann M, Armani A, et al Fibroblast growth factor 21 controls mitophagy and muscle mass. J Cachexia Sarcopenia Muscle 2019; 10: 630–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sharma M, McFarlane C, Kambadur R, et al Myostatin: expanding horizons. IUBMB Life 2015; 67: 589–600. [DOI] [PubMed] [Google Scholar]
- 40. Eriksson JW, Lundkvist P, Jansson PA, et al Effects of dapagliflozin and n‐3 carboxylic acids on non‐alcoholic fatty liver disease in people with type 2 diabetes: a double‐blind randomised placebo‐controlled study. Diabetologia 2018; 61: 1923–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kuchay MS, Krishan S, Mishra SK, et al Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E‐LIFT Trial). Diabetes Care 2018; 41: 1801–1808. [DOI] [PubMed] [Google Scholar]
- 42. Watts R, Ghozlan M, Hughey CC, et al Myostatin inhibits proliferation and insulin‐stimulated glucose uptake in mouse liver cells. Biochem Cell Biol 2014; 92: 226–234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Changes in markers of liver function.
Appendix S1 | CONSORT 2010 checklist of information reporting a randomized trial.


