Abstract
A 70‐year‐old woman with type 2 diabetes was admitted to Gifu University Hospital, Gifu, Japan, because of ketosis. She was diagnosed with type 2 diabetes at age 49 years and started insulin therapy at age 57 years, which restored glycemic control. Insulin therapy was discontinued and oral antidiabetes drugs, including sodium–glucose cotransporter 2 inhibitor dapagliflozin, were initiated at age 69 years. Thereafter, her bodyweight declined from 40.0 kg to 29.8 kg in 12 months; glycated hemoglobin remained >8.0%. On admission to our hospital, her laboratory tests and computed tomography scan showed ketosis, insulinopenia, and the presence of dehydration and bacterial pneumonia. She also lost substantial bodyweight and developed sarcopenia. The current case shows the importance of patient assessment before sodium–glucose cotransporter 2 inhibitor initiation in the elderly.
Keywords: Ketosis, Sarcopenia, Sodium–glucose cotransporter 2 inhibitor
We report a case in which the sodium–glucose cotransporter 2 inhibitor, dapagliflozin, contributed to the development of ketosis and sarcopenia in a lean elderly woman with type 2 diabetes. Sodium–glucose cotransporter 2 inhibitor use and reduced β‐cell function together resulted in insulinopenia and probably hyperglucagonemia, which enhanced proteinolysis and impaired protein synthesis in the muscle, thereby leading to the patient developing sarcopenia.
Introduction
Sarcopenia, defined as a progressive, generalized loss of skeletal muscle mass and strength, impairs activities of daily living, increasing the risk of mortality in elderly adults with diabetes1. Sodium–glucose cotransporter 2 inhibitors (SGLT2is) are widely used for diabetes patients2; there are warnings that SGLT2is should be used cautiously in elderly adults with diabetes, as the drugs can increase risks of dehydration, as well as sarcopenia3. Here, we report a lean elderly woman with type 2 diabetes in which the SGLT2i, dapagliflozin, might have contributed to the development of ketosis and sarcopenia.
Case Report
At age 49 years, the patient was diagnosed with type 2 diabetes at a neighboring clinic, where she received glibenclamide and pioglitazone that did not improve her glycated hemoglobin (HbA1c). At age 57 years, she was admitted to Gifu University Hospital, Gifu, Japan, for reconsideration of her antidiabetes medications. The patient's bodyweight, height and body mass index were 45 kg, 148.8 cm and 20.3 kg/m2, respectively; her insulin secretory capacity was relatively low (fasting blood glucose [BG] 168 mg/dL and C‐peptide 1.04 ng/mL), even in the presence of glibenclamide. She therefore started insulin therapy. After discharge from our hospital, she visited the neighboring clinic to continue her insulin therapy. At age 65 years, as her HbA1c was well controlled (HbA1c <7.0%), the patient's insulin therapy was replaced by metformin and alogliptin. Her HbA1c then gradually increased. At age 69 years, she started receiving dapagliflozin with metformin and alogliptin. Despite substantial bodyweight reduction (from 40.0 to 29.8 kg) in 12 months, her HbA1c remained high (>8.0%).
At age 70 years, she was again referred to our hospital for analysis of bodyweight reduction and chronic hyperglycemia. She had felt cold and experienced shortness of breath during the previous 3 weeks. Her body temperature, blood pressure, pulse and SpO2 were 37.8°C, 157/83 mmHg, 124/min and 93%, respectively. Bodyweight and body mass index were 29.8 kg and 13.5 kg/m2, respectively. HbA1c, BG and C‐peptide were 9.2%, 180 mg/dL and 0.26 ng/dL, respectively. Despite high blood 3‐hydroxybutyrate (3,154 μmol/L) and acetoacetate (997 μmol/L), arterial blood pH (7.42) and anion gap (9.1 mEq/L) were within normal ranges. While inferior vena cava diameter and respiratory variability were not assessed by ultrasonography, urine‐specific gravity (1.029), blood urea nitrogen (18.2 mg/dL), creatinine (0.3 mg/dL), hematocrit (43.8%) and total protein (7.8 mg/dL) were consistent with the presence of moderate dehydration. In addition to positivity for Haemophilus influenzae infection in sputum culture, air bronchograms and lobar distribution of consolidation on computed tomography scan, and white blood cell count (18.4 × 103/μL), percentage of neutrophils (87.1%) and C‐reactive protein (5.98 mg/dL) suggested the presence of bacterial pneumonia (BP), which might underlie the patient's symptoms in the previous 3 weeks (i.e., coldness and shortness of breath).
Insulin therapy, saline infusion and antibiotic therapy (3 g sulbactam/ampicillin, twice daily) were initiated for ketosis, dehydration and BP on hospitalization on day 1. By day 3, the patient recovered from dehydration, as indicated by near normalized urine‐specific gravity (1.011), blood urea nitrogen (12.2 mg/dL), creatinine (0.25 mg/dL), hematocrit (39.8%) and total protein (6.2 mg/dL; Figure 1). By day 8, she recovered from fever and dyspnea due to BP, and white blood cell count (8.6 × 103/μL), percentage of neutrophils (55.0%) and C‐reactive protein (0.24 mg/dL) were near normalized.
Figure 1.
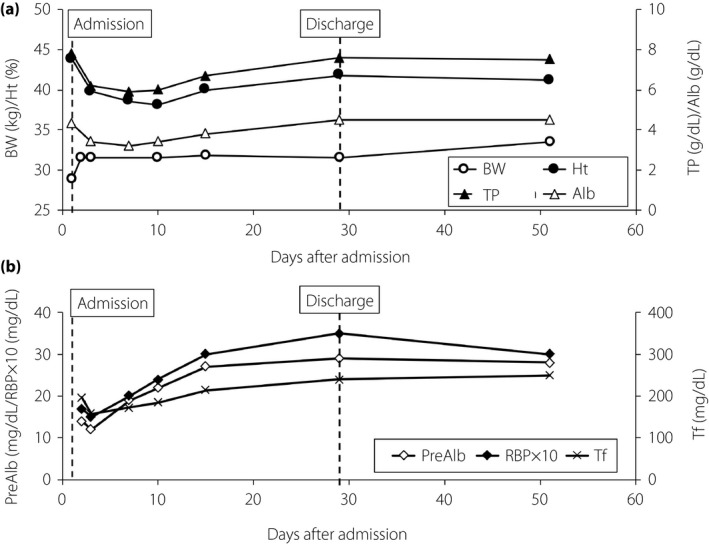
Changes in bodyweight (BW), and dehydration‐related and nutrition‐related parameters of the patient. (a) Changes in BM (open circles) and parameters of dehydration, such as hematocrit (Ht; closed circles), serum albumin (Alb; open triangles) and total protein (TP; closed triangles) are shown. (b) Changes in rapid turnover proteins, prealbumin (PreAlb; open diamonds), retinol binding protein (RBP; closed diamonds) and transferrin (Tf; crosses) are shown. Day 1, admission to Gifu University Hospital, Gifu, Japan, and day 29, discharge from hospital.
In addition to her bodyweight being severely reduced in the previous 12 months, her serum levels of albumin (3.4 mg/dL) and rapid turnover proteins, prealbumin (14 mg/dL; normal range 23–42 mg/dL), retinol‐binding protein (1.7 mg/dL; normal range 2.4–7.0 mg/dL) and transferrin (197 mg/dL; normal range 200–340 mg/dL) on day 3 were low (Figure 1), possibly due to chronic malnutrition before admission and/or BP. Grip strength (right 14 kg; left 14 kg on day 16) was lower than the female cut‐off value (<18 kg) of the Asian Working Group for Sarcopenia4, suggesting the presence of sarcopenia. Muscle mass reduction was evaluated by psoas muscle mass index, which is calculated by bilateral psoas muscle area at the L3 vertebrate (cm2) / height (m)2, and is highly correlated with lean body mass measured by bioimpedance analysis5. Psoas muscle mass index (3.7; cut‐off values for sarcopenia5 <3.92) measured on day 2 was more than twofold lower than that measured 13 years before (7.7; Figure 2). Rapid turnover proteins (Figure 1b), grip strength (right 18.5 kg; left 18.1 kg on day 51) and psoas muscle mass index (5.1 on day 29) were substantially improved by initiation of insulin injection, medical nutrition therapy supplying an appropriate amount of protein and resistance exercise after dapagliflozin withdrawal, together with a substantial increase in her subcutaneous and visceral fat areas measured at the umbilicus level (Figure 2).
Figure 2.
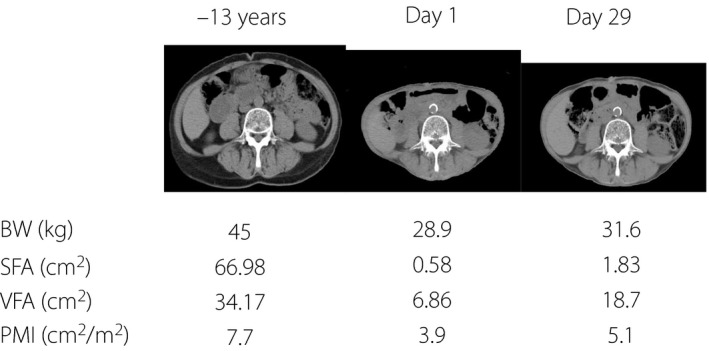
Changes of bodyweight (BW), visceral fat area (VFA), subcutaneous fat area (SFA) and psoas muscle mass index (PMI). Comparison of computer tomography at L3 vertebrae. Changes of BW, VFA, SFA and PMI at –13 years on day 1 and on day 29 are shown. PMI is calculated as the bilateral psoas muscle area at the L3 vertebrae on computed tomography (cm2) / height2 (m2).
In the current case, it would seem that dapagliflozin by itself had little effect on insulin secretion, as casual BG and C‐peptide levels before and after dapagliflozin withdrawal did not differ (day 1, BG 180 mg/dL and C‐peptide 0.26 ng/mL; day 16, BG 171 mg/dL and C‐peptide 0.42 ng/mL). Insulin secretory capacity had been relatively low since the patient started insulin therapy at age 57 years, which was confirmed by a glucagon stimulation test on day 17 (before BG, 142 mg/dL and C‐peptide 0.45 ng/mL; 6 min after glucagon administration, BG 158 mg/dL and C‐peptide 0.75 ng/mL).
Written informed consent was obtained from the patient for publication of this report. Formal ethics approval was waived, because this is a case report.
Discussion
Sarcopenia is one of the most serious health‐related problems among elderly adults with diabetes1, 4, 5. In addition to aging and poor nutrition, insulin insufficiency plays a pivotal role in the development of sarcopenia, as insulin promotes amino acid uptake and protein synthesis in muscle6. SGLT2is lower BG with a concomitant decrease in insulin levels7; SGLT2is also enhance glucagon secretion, thereby promoting muscle proteinolysis and subsequent sarcopenia3. The present patient had been insulinopenic and receiving insulin injection for years. Therefore, withdrawal of insulin injection and dapagliflozin initiation might have exacerbated insulinopenia and possibly induced hyperglucagonemia to drastically promote sarcopenia (Figure 3).
Figure 3.
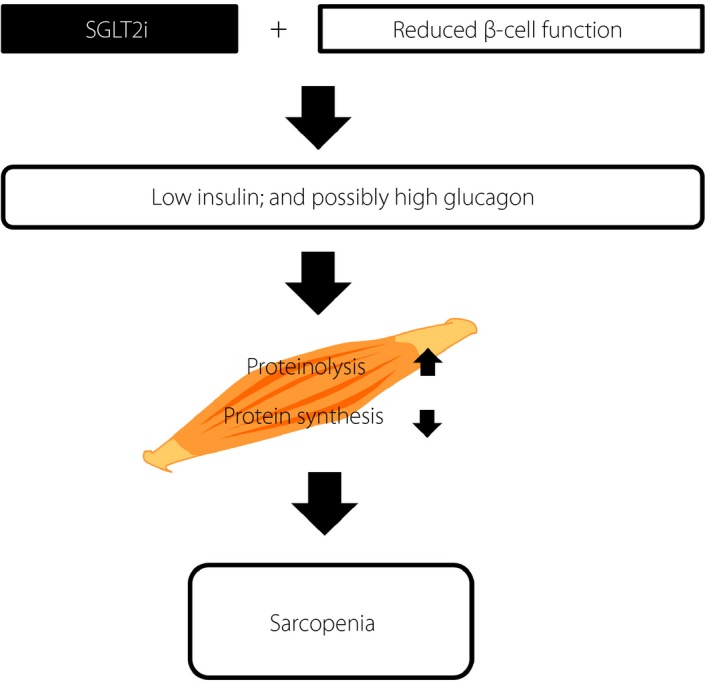
Schematic presentation of pathophysiology in the patient. Sodium–glucose cotransporter 2 inhibitor (SGLT2i) use and reduced β‐cell function together resulted in insulinopenia and, likely, hyperglucagonemia, which enhanced proteinolysis and impaired protein synthesis in the muscle, thereby promoting sarcopenia.
Both dehydration and insulinopenia are shown to play a role in the development of euglycemic ketoacidosis8, 9. The present patient had insulinopenia, dehydration due to BP and consistent ketosis. The risk of dehydration is increased due to fever, diarrhea and decreased water intake on sick days3. Therefore, patient education to avoid the use of SGLT2is on sick days is critical, especially among dehydration‐prone elderly adults with diabetes3.
In conclusion, we report a case of an elderly type 2 diabetes patient in whom dapagliflozin might have triggered sarcopenia and ketosis. The current case shows the importance of patient assessment before SGLT2i initiation to prevent sarcopenia, especially in elderly patients.
Disclosures
The authors declare no conflict of interest.
J Diabetes Investig 2020; 11: 745–747
References
- 1. Park S, Goodpaster B, Strotmeyer E, et al Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes the health, aging, and body composition study. Diabetes Care 2007; 30: 1507–1512. [DOI] [PubMed] [Google Scholar]
- 2. American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes‐2019. Diabetes Care 2019; 42(Suppl 1): S90–S102. [DOI] [PubMed] [Google Scholar]
- 3. Yabe D, Nishikino R, Kaneko M, et al Short‐term impacts of sodium/glucose co‐transporter 2 inhibitors in Japanese clinical practice: considerations for their appropriate use to avoid serious adverse events. Expert Opin Drug Saf 2015; 14: 795–800. [DOI] [PubMed] [Google Scholar]
- 4. Chen LK, Liu LK, Woo J, et al Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014; 15: 95–101. [DOI] [PubMed] [Google Scholar]
- 5. Hamaguchi Y, Kaido T, Okumura S, et al Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition 2016; 32: 1200–1205. [DOI] [PubMed] [Google Scholar]
- 6. Garlick PJ, Fern M, Preedy VR. The effect of insulin infusion and food intake on muscle protein synthesis in postabsorptive rats. Biochem J 1983; 210: 669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ogawa W, Sakaguchi K. Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: possible mechanism and contributing factors. J Diabetes Investig 2016; 7: 135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perry RJ, Rabin‐Court A, Song JD, et al Dehydration and insulinopenia are necessary and sufficient for euglycemic ketoacidosis in SGLT2 inhibitor‐treated rats. Nat Commun 2019; 10: 548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern With SGLT2 inhibitors. Diabetes Care 2015; 38: 1638–1642. [DOI] [PubMed] [Google Scholar]


