Abstract
Aims/Introduction
We compared the results of testing for glutamic acid decarboxylase antibodies (GADAb) using a radioimmunoassay (RIA) and an enzyme‐linked immunosorbent assay (ELISA) in individuals with childhood‐onset type 1 diabetes mellitus.
Materials and Methods
Serum specimens were collected from 1,024 Japanese children (426 boys and 598 girls) in 2013. The median age at diagnosis was 7 years (0–18 years). The blood specimens were obtained at a median age of 13 years (2–22 years).
Results
Among the 628 children whose serum specimens were collected within 5 years after diagnosis, the rate of GADAb positivity was 47.9% using RIA and 69.4% using ELISA. The participants were divided into four groups according to their RIA and ELISA results for GADAb as follows: group I (RIA+/ELISA+), group II (RIA+/ELISA−), group III (RIA−/ELISA+) and group IV (RIA−/ELISA−). The clinical and genetic characteristics of group I and group III were quite similar in terms of age at diagnosis, male/female ratio, relatively high positive rates for both autoantibody to protein tyrosine phosphatase IA‐2 and autoantibody to the cation efflux transporter zinc transporter 8, and human leukocyte antigen genotype. Group II contained just five patients, and was characterized by a younger age at diagnosis, low positive rates for both autoantibody to protein tyrosine phosphatase IA‐2 and autoantibody to the cation efflux transporter zinc transporter 8, and a unique human leukocyte antigen genotype. If the positive rates of either autoantibody to protein tyrosine phosphatase IA‐2 or autoantibody to the cation efflux transporter zinc transporter 8 or both were added to the GADAb results using RIA, the percentage of autoimmune type 1 diabetes increased from 47.9% to 78.5%.
Conclusions
The diagnosis of autoimmune childhood‐onset Japanese type 1 diabetes increased when GADAb results were obtained using a new ELISA method, compared with a previously utilized RIA method.
Keywords: Enzyme‐linked immunosorbent assay, Glutamic decarboxylase antibody, Radioimmunoassay
The diagnosis of autoimmune childhood‐onset Japanese type 1 diabetes was increased by the glutamic acid decarboxylase antibodies using a new enzyme‐linked immunosorbent assay in comparison with a previous radioimmunoassay.
Introduction
Type 1 diabetes mellitus is caused by the autoimmune destruction of pancreatic islet β‐cells. Autoantibodies to glutamic acid decarboxylase (GADAb) are some of the major anti‐islet autoantibodies, and a high prevalence of GADAb has been shown in patients with type 1 diabetes1, 2, 3, 4. The risk of developing type 1 diabetes in the population varies remarkably according to the country of residence and ethnicity. Japan has one of the lowest incidences of type 1 diabetes in the world1, and the incidence of type 1 diabetes in children aged 0–14 years in Japan has been reported to have remained constant over the past decade, averaging 2.25 cases per 100,000 persons5.
Until 2016, a radioimmunoassay (RIA) using the RIA kit GADAb (Cosmic, Tokyo, Japan), which has been described in detail elsewhere6, 7, 8, was used for the measurement of GADAb; however, since 2016, the method for measuring GADAb has been completely changed in Japan to an enzyme‐linked immunosorbent assay (ELISA) using the GADAb ELISA (Cosmic)9. Recently, studies have reported that sera from 8% to 15% of GADAb‐positive patients with type 1 diabetes showed discrepant results when compared using the two assays10, 11, 12. Also, 25–30% of GADAb‐positive, slowly progressive type 1 diabetes adult‐onset patients who had been originally diagnosed using RIA were later found to be negative using ELISA11, 12. Therefore, the Japan Diabetes Society has proposed that caution be exercised and made some recommendations13.
The evaluation of RIA and ELISA measurements of GADAb has been limited to Japanese patients with childhood‐onset type 1 diabetes. We carried out the present study to examine the clinical and genetic characteristics of Japanese children and adolescents with type 1 diabetes who showed discrepant GADAb measurements using RIA and ELISA.
Methods
Participants
The participants of the present study were 1,024 Japanese children and adolescents (426 boys and 598 girls) diagnosed as having type 1 diabetes when they were aged ≤16 years and who had been enrolled in the 2013 cohort (March 2013) for the Japanese Study Group of Insulin Therapy for Childhood and Adolescent Diabetes (JSGIT) from 75 institutions across Japan14. Thus, the participants were a part of the fourth cohort for the JSGIT, which is a multicenter collaborative nationwide cohort study in Japan. Type 1 diabetes was diagnosed according to the criteria of the Japan Diabetes Society and the American Diabetes Association15, 16. The participants were diagnosed as having type 1 diabetes at a median age of 7 years (range 0–18 years); they were registered in this study, and their blood specimens were collected when they were a median age of 13 years (range 2–22 years). Of the participants, 628 (252 boys and 376 girls) were recruited within 5 years of being diagnosed as having type 1 diabetes, and human leukocyte antigen (HLA) typing data were obtained from 649 participants (263 boys and 386 girls) who provided additional informed consent for genetic analysis out of the total of 1,024 type 1 diabetes patients (Table 1).
Table 1.
Positivity rates of radioimmunoassay and enzyme‐linked immunosorbent assay for glutamic acid decarboxylase antibodies in children and adolescents with type 1 diabetes
| T1D patients | n | Positivity for GADAb | Concordance rate (%) | |
|---|---|---|---|---|
| RIA (%) | ELISA (%) | |||
| All recruited patients | 1,024 | 41.4 | 62.1 | 76.6 |
| Within 5 years of diagnosis | 628 | 47.9 | 69.4 | 76.9 |
| 6~17 years after diagnosis | 396 | 31.1 | 50.5 | 76.0 |
| HLA haplotype | 649 | 41.7 | 64.7 | 75.5 |
ELISA, enzyme‐linked immunosorbent assay; GADAb, glutamic acid decarboxylase antibodies; RIA, radioimmunoassay; T1D, type 1 diabetes.
Most of the 1,024 type 1 diabetes patients in the present study were diagnosed after an acute onset. Just 14 patients (7 boys and 7 girls, aged 1–14 years at diagnosis) were considered to have fulminant‐type type 1 diabetes based on the following criteria: (i) ketoacidosis within a week after the onset of hyperglycemic symptoms; and (ii) a plasma glucose level ≥288 mg/dL and glycated hemoglobin <8.9% at the first visit17. A total of 10 of the 14 patients with fulminant type 1 diabetes tested positive for GADAb at the time of their diagnosis. Just seven patients (2 boys and 5 girls, aged 7–14 years at diagnosis) of the total of 1,024, and four out of 628 patients within 5 years after diagnosis were diagnosed as having slowly progressive type 1 diabetes based on the following criteria: (i) the presence of GADAb at some time point during the patient’s clinical course; (ii) the absence of ketosis or ketoacidosis at the onset (or diagnosis) of diabetes without the need for insulin treatment to correct hyperglycemia immediately after diagnosis; and (iii) no requirement for insulin treatment for at least 6 months after diagnosis18.
Autoantibody assay
The serum GADAb titers were measured using a commercial RIA and 125I‐labeled recombinant human GAD65 as the tracer reagent (Cosmic); patients with GADAb titers of ≥1.5 U/mL (mean in Japanese controls + 3 SD) were judged as being positive, in accordance with previous reports6, 7, 8, 19. The GADAb titers in the same serum specimens were then measured by ELISA using the GADAb ELISA kit (Cosmic), and patients with GADAb titers of ≥5.0 U/mL (99th percentile of the 300 normal controls) were judged as being positive, in accordance with previous reports9, 10, 19.
The autoantibody to protein tyrosine phosphatase IA‐2 (IA‐2Ab) level was measured by RIA using the IA‐2Ab kit (Cosmic), and patients with IA‐2Ab titers of ≥0.4 U/mL (mean in Japanese normal controls + 3 SD) were judged as being positive, in accordance with a previous report20.
The autoantibody to the cation efflux transporter zinc transporter 8 (ZnT8Ab) level was determined by a radioligand binding assay using a dimeric complementary deoxyribonucleic acid construct of the carboxy‐terminal domains (aa268–369) carrying 325Trp and 325Arg (CW‐CR)21. The cut‐off value for ZnT8A‐CW‐CR was an index of 0.007, which was based on the 99th percentile in sera obtained from 139 healthy control individuals21.
HLA typing
Genomic DNA was extracted from whole blood specimens. HLA class II typing was carried out using a Luminex Multi‐Analyte Profiling system with a WAKFlow HLA typing kit (Wakunaga, Hiroshima, Japan)22. Briefly, highly polymorphic exon 2 of the HLA‐DRB1 and ‐DQB1 genes were amplified using the primer pairs included in the kit. Each polymerase chain reaction product was hybridized using sequence‐specific oligonucleotide probes that were complementary to the allele‐specific sequences22.
Statistical analysis
The results are expressed as the mean ± standard deviation or median (range). Clinical data among the four groups were compared using the Kruskal–Wallis test. Fisher’s exact test was applied to a two‐by‐two contingency table, and the corrected P‐values, equivalent to the P‐values multiplied by the number of comparisons for each locus or haplotype of HLA DRB1 and DQB1, were determined. P‐values or corrected P‐values of <0.05 were considered to denote statistical significance. All the statistical analyses were carried out using IBM SPSS, version 24.0 (Armonk, NY, USA).
Ethical approval
This study was approved by the review board of Tokyo Women’s Medical University and each of the participating institutions, and was carried out in accordance with the ethical guidelines and regulations laid out in the Declaration of Helsinki. Written consent was obtained from each of the patients or their parents.
Results
Among the 1,024 patients with type 1 diabetes who were enrolled in the present study, a positive result for GADAb was obtained using RIA in 424 patients (41.4%), and using ELISA in 636 patients (62.1%; Table 1). Among the 628 patients in whom the assays were carried out within 5 years after diagnosis, a positive result for GADAb was obtained using RIA in 301 patients (47.9%), and by ELISA in 436 patients (69.4%). The concordance rate was as low as 77%. The positivity rate for GADAb was significantly lower (31.1% using RIA and 50.5% using ELISA) in patients with long‐standing type 1 diabetes (tested between 6 and 17 years after the diagnosis) than in those who were tested within 5 years of the diagnosis. Of note, the positivity rate using RIA to test for GADAb was approximately 20% lower than that using ELISA (Table 1).
Figure 1 shows the correlation between the GADAb titers determined using RIA and ELISA in the 628 type 1 diabetes patients who were assayed within 5 years after diagnosis. A significant positive correlation between the GADAb titers determined using RIA (0.6–100 U/mL) and ELISA (2.0–2,000 U/mL) was observed in a linear range in 387 of the 628 patients in whom the testing was carried out within 5 years after diagnosis: y = 27.807 x – 19.526 (r = 0.845, P < 0.001, y = GADAb [U/mL] using ELISA, and x = GADAb [U/mL] using RIA).
Figure 1.
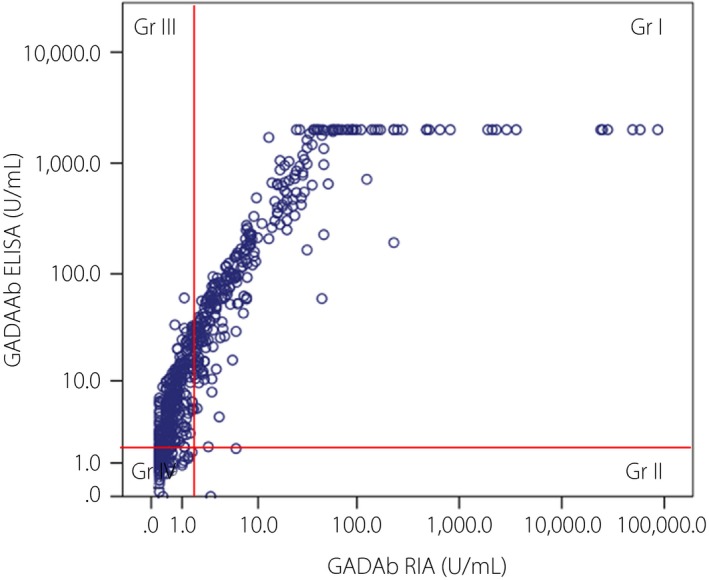
Correlation between the glutamic acid decarboxylase antibodies (GADAb) titers measured using radioimmunoassay (RIA) and enzyme‐linked immunosorbent assay (ELISA) in 628 type 1 diabetes patients within 5 years after diagnosis. Gr 1, group 1; G II, group II; Gr III, group III; Gr IV, group IV.
Table 4 shows the correlation between the positivity rates of RIA and ELISA for GADAb in the 628 patients with type 1 diabetes assayed within 5 years after diagnosis. These patients were divided into four groups according to their RIA/ELISA results for GADAb (Figure 1; Table 4), as follows: group (Gr) I (RIA+/ELISA+), n = 296 patients (47.1%); Gr II (RIA+/ELISA−), n = 5 patients (0.8%); Gr III (RIA−/ELISA+), n = 140 patients (22.3%); and Gr IV (RIA−/ELISA−), n = 187 patients (29.8%; Tables 4, 5).
Table 4.
Positivity rates of radioimmunoassay and enzyme‐linked immunosorbent assay for glutamic acid decarboxylase antibodies in 628 patients with type 1 diabetes assayed within 5 years after diagnosis
| Patient number (%) | GADAb RIA | |||
|---|---|---|---|---|
| Positive (%) | Negative (%) | Total (%) | ||
| GADAb | Positive | 296 (47.1) | 140 (22.3) | 436 (69.4) |
| ELISA | Negative | 5 (0.8) | 187 (29.8) | 192 (30.6) |
| Total | 301 (47.9) | 297 (47.3) | 628 (100) | |
ELISA, enzyme‐linked immunosorbent assay; GADAb, glutamic acid decarboxylase antibodies; RIA, radioimmunoassay.
Table 5.
Clinical characteristics of the four groups divided according to the results of radioimmunoassay/enzyme‐linked immunosorbent assay for glutamic acid decarboxylase antibodies among subjects assayed within 5 years after diagnosis
| Gr | Group I | Group II | Group III | Group IV | P |
|---|---|---|---|---|---|
| RIA+/ELISA+ | RIA+/ELISA‐ | RIA‐/ELISA+ | RIA‐/EISA‐ | ||
| GADAb RIA range (U/mL) | 1.5–86,000 | 1.5–5.7 | <1.5 | <1.5 | |
| GADAb ELISA range (U/mL) | 5.0‐>2,000 | <5.0 | 5.1–60.3 | <5.0 | |
| n | 296 | 5 | 140 | 187 | |
| % | 47.1% | 0.8% | 22.3% | 29.8% | |
| Mean age (SD) at diagnosis | 9.2 (6.9) | 3.2 (2.5) | 7.9 (3.5) | 7.9 (7.9) | P < 0.01: I vs II, I vs IV |
| Years after diagnosis mean (SD) | 2.8 (1.6) | 3.3 (1.7) | 3.0 (1.7) | 3.3 (1.6) | P < 0.05: I vs IV |
| Male/female | 115/181 | 3/2 | 47/93 | 87/100 | NS |
| Mean HbA1c (SD) % | 8.0 (1.3) | 8.9 (1.0) | 8.2 (1.4) | 8.3 (1.2) | NS |
| Positivity for IA‐2Ab (%) | 70.6 | 20.0 | 62.9 | 47.1 | P < 0.001 |
| Positivity for ZnT8Ab (%) | 36.8 | 0.0 | 32.9 | 23.5 | P < 0.01 |
ELISA, enzyme‐linked immunosorbent assay; GADAb, glutamic acid decarboxylase antibodies; HbA1c, glycated hemoglobin; IA‐2Ab, autoantibody to protein tyrosine phosphatase IA‐2; NS, not significant; RIA, radioimmunoassay; SD, standard deviation; ZnT8Ab, autoantibody to the cation efflux transporter zinc transporter 8.
Among the 396 patients with long‐standing type 1 diabetes (6–17 years after diagnosis), 114 patients (28.8%) were categorized in Gr I, nine patients (2.3%) were categorized in Gr II; 86 patients (21.7%) were categorized in Gr III; and 187 patients (47.2%) were categorized in Gr IV. There were no significant differences in the percentages of Gr II (0.8% vs 2.3%) or Gr III (29.8% vs 21.7%) according to the years after diagnosis (within 5 years vs 6–17 years).
Table 5 shows the clinical characteristics of the 628 patients who were assayed within 5 years after diagnosis and were divided into four groups according to their RIA/ELISA results for GADAb. Of note, the clinical characteristics of Gr I and Gr III were quite similar in terms of the age at diagnosis (Gr I: mean of 9.2 years, Gr III: mean of 7.9 years), the male/female ratio (Gr I: 115/181, Gr III: 47/93) and the relatively high positive rates for both IA‐2Ab (Gr I: 70.6%, Gr III: 62.9%) and ZnT8Ab (Gr I: 36.8%, Gr III: 62.9%). However, the GADAb titer in Gr III was lower (5.1–60.3 U/mL) than that in Gr I. Gr II had just five (out of the 628) patients and had unique clinical features. The age at diagnosis in this group (3.2 ± 2.5 years) was significantly lower than that in Gr I (9.2 ± 6.9 years), and the male/female ratio (3/2) in this group was higher than that in Gr I. The prevalences of both IA‐2Ab (20.0%) and ZnT8Ab (0%) were significantly lower than those in both Gr I (IA‐2Ab: 70.6%, ZnT8Ab: 36.8%) and Gr III (IA‐2Ab: 62.9%, ZnT8Ab: 32.9%; P < 0.001 for IA‐2Ab and P < 0.01 for ZnT8Ab; Table 5).
Although the positivity rate for GADAb using RIA was lower than that using ELISA by approximately 20% (Table 1), when the results for IA‐2Ab and ZnT8Ab were added to the RIA results, the total prevalence of autoantibody‐positive (type 1A) type 1 diabetes became nearly equal to that determined using ELISA in the 628 patients with type 1 diabetes assayed within 5 years after diagnosis (Figure 2). Thus, the percentage positivity for antibodies in the type 1A patients increased significantly with the addition of the IA‐2Ab and ZnT8Ab results to the RIA/ELISA results for GADAb as follows: 47.9% using RIA for GADAb alone, 76.0% using RIA for GADAb + IA‐2Ab, and 78.5% using RIA for GADAb + IA‐2Ab + ZnT8Ab; 69.4% using ELISA for GADAb alone, 83.6% using ELISA for GADAb + IA‐2Ab, and 84.9% using ELISA for GADAb + IA‐2Ab + ZnT8Ab (Figure 2). These results suggest that many type 1A (autoimmune) patients were present (false negative cases) among the patients that were determined to be GADAb‐negative using RIA.
Figure 2.
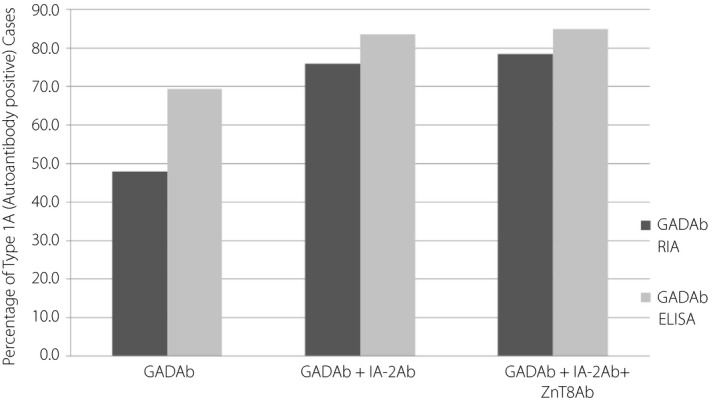
Ratio of type 1A (autoantibody‐positive) diabetes patients based on the positive detection of either autoantibody to protein tyrosine phosphatase IA‐2 (IA‐2Ab) or autoantibody to the cation efflux transporter zinc transporter 8 (ZnT8Ab) or both in addition to glutamic acid decarboxylase antibodies (GADAb) in 628 type 1 diabetes patients within 5 years after diagnosis. The percentage of type 1A patients diagnosed increased significantly with the addition of the IA‐2Ab and ZnT8Ab results to the GADAb results using radioimmunoassay (RIA): from 47.9% using the RIA results for GADAb alone to 76.0% using the RIA + IA‐2Ab results and 78.5% using the RIA + IA‐2 Ab + ZnT8 Ab results, and from 69.4% using the ELISA results for GADAb alone to 83.6% using the ELISA + IA‐2 Ab results and 84.9% using the ELISA + IA‐2Ab + ZnT8Ab results.
Table 2 shows the HLA‐DRB1, DQB1 allele frequencies in the four groups divided according to the positivity profiles for GADAb RIA and ELISA in 649 children with type 1 diabetes. In Table 2, significant differences were seen between Gr I versus the control, between Gr III versus the control, and between Gr IV versus the control in terms of the frequencies of susceptible and protective DRB1 and DQB1 alleles. In contrast, significant differences in DRB1*09:01 (P < 0.01) and DQB1*03:03 (P < 0.05) were only observed for Gr II versus the control. The other allele numbers in Gr II might have been too small to be analyzed statistically. Gr I and Gr III only had different frequencies of the protective allele DQB1*03:01 (P < 10–5), and Gr I and Gr IV had different frequencies of the susceptible allele DQB1*04:01 (P < 0.05), as well as the protective allele DQB1*03:01 (P < 0.01).
Table 2.
Human leukocyte antigen DRB1 and DQB1 allele frequencies in the four groups divided according to the positivity profiles for glutamic acid decarboxylase antibodies radioimmunoassay and enzyme‐linked immunosorbent assay in 649 children with type 1 diabetes
| HLA allele | Group I | Group II | Group III | Group IV | Control | I vs Control | II vs Control | III vs Control | IV vs Control | I vs II | I vs III | I vs IV | II vs III | II vs IV | III vs IV | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 532 | % | n = 10 | % | n = 308 | % | n = 448 | % | n = 2432 | % | |||||||||||
| DRB1 | ||||||||||||||||||||
| Susceptible | ||||||||||||||||||||
| *04:05 | 126 | 23.68 | 2 | 20.00 | 90 | 29.22 | 142 | 31.70 | 322 | 13.26 | <10−6 | NS | <10−9 | <10−16 | NS | NS | NS | NS | NS | NS |
| *08:02 | 48 | 9.02 | 0 | 0.00 | 32 | 10.39 | 29 | 6.47 | 102 | 4.18 | <10−3 | NS | <10−3 | NS | NS | NS | NS | NS | NS | NS |
| *09:01 | 169 | 31.77 | 6 | 60.00 | 102 | 33.12 | 132 | 29.46 | 342 | 14.08 | <10−17 | <0.01 | <10−12 | <10−11 | NS | NS | NS | NS | NS | NS |
| Protective | ||||||||||||||||||||
| *08:03 | 6 | 1.13 | 0 | 0.00 | 3 | 0.97 | 7 | 1.56 | 202 | 8.29 | <10−9 | NS | <10−5 | <10−6 | NS | NS | NS | NS | NS | NS |
| *15:01 | 3 | 0.56 | 0 | 0.00 | 0 | 0.00 | 7 | 1.56 | 173 | 7.11 | <10−9 | NS | <10−7 | <10−4 | NS | NS | NS | NS | NS | NS |
| *15:02 | 13 | 2.44 | 0 | 0.00 | 6 | 1.95 | 14 | 3.13 | 246 | 10.13 | <10−7 | NS | <10−5 | <10−4 | NS | NS | NS | NS | NS | NS |
| Neutral | ||||||||||||||||||||
| 13:02 | 36 | 6.77 | 0 | 0.00 | 21 | 6.82 | 30 | 6.70 | 166 | 6.83 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| *01:01 | 38 | 7.14 | 2 | 20.00 | 11 | 3.57 | 37 | 8.26 | 141 | 5.81 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Others | 93 | 17.48 | 0 | 0.00 | 43 | 13.96 | 50 | 11.16 | 738 | 30.35 | <10−7 | NS | <10−7 | <10−16 | NS | NS | NS | NS | NS | NS |
| DQB1 | ||||||||||||||||||||
| Susceptible | ||||||||||||||||||||
| *03:02 | 97 | 18.23 | 0 | 0.00 | 59 | 19.16 | 62 | 13.84 | 227 | 9.32 | <10−5 | NS | <10−4 | <0.05 | NS | NS | NS | NS | NS | NS |
| *03:03 | 172 | 32.33 | 6 | 60.00 | 103 | 33.44 | 134 | 29.91 | 361 | 14.86 | <10−16 | <0.05 | <10−11 | <10−10 | NS | NS | NS | NS | NS | NS |
| *04:01 | 111 | 20.86 | 2 | 20.00 | 85 | 27.60 | 133 | 29.69 | 317 | 13.03 | <10−3 | NS | <10−7 | <10−14 | NS | NS | <0.05 | NS | NS | NS |
| Protective | ||||||||||||||||||||
| *03:01 | 35 | 6.58 | 0 | 0.00 | 0 | 0.00 | 9 | 2.01 | 282 | 11.61 | <10−2 | NS | <10−13 | <10−9 | NS | <10−5 | <10−2 | NS | NS | NS |
| *06:01 | 18 | 3.38 | 0 | 0.00 | 6 | 1.95 | 19 | 4.24 | 440 | 18.11 | <10−19 | NS | <10−14 | <10−13 | NS | NS | NS | NS | NS | NS |
| *06:02 | 1 | 0.19 | 0 | 0.00 | 0 | 0.00 | 7 | 1.56 | 151 | 6.22 | <10−9 | NS | <10−5 | <10−3 | NS | NS | NS | NS | NS | NS |
| Neutral | ||||||||||||||||||||
| *04:02 | 6 | 1.13 | 0 | 0.00 | 6 | 1.95 | 7 | 1.56 | 97 | 3.98 | <10−2 | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| *06:04 | 36 | 6.77 | 0 | 0.00 | 21 | 6.82 | 29 | 6.47 | 167 | 6.88 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| *05:01 | 39 | 7.33 | 2 | 20.00 | 12 | 3.90 | 37 | 8.26 | 159 | 6.53 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Others | 17 | 3.20 | 0 | 0.00 | 16 | 5.19 | 11 | 2.46 | 231 | 9.50 | <10−4 | NS | NS | <10−5 | NS | NS | NS | NS | NS | NS |
Others of DRB1: *03:01, *04:01, *04:03, *04:04, *04:06, *04:07, *04:10, *07:01, *10:01, *11:01, *11:05, *11:06, *12:01, *12:02, *14:01, *14:03, *14:06, *16:02
Others of DQB1: *02:01, *05:02, *06:09
Control: from ref. 26. HLA, human leukocyte antigen; NS, not significant.
Table 3 shows the HLA‐DRB1‐DQB1 haplotype frequency in the four groups. In Table 3, Gr I, Gr III and Gr IV differed significantly from the control in terms of the major three susceptible haplotypes and three protective haplotypes, but Gr II only had a significant difference for the DRB1*09:01‐DQB1*03:03 haplotype (P < 0.01) relative to the control. Gr I and Gr IV had a significant difference in the susceptible DRB1*04:05‐DQB1*04:01 haplotype, but Gr I and Gr III did not have any differences in any of the DRB1‐DQB1 haplotypes.
Table 3.
Human leukocyte andtigen DRB1‐DQB1 haplotype frequencies in the four groups divided according to the positivity profiles for glutamic acid decarboxylase antibodies radioimmunoassay and enzyme‐linked immunosorbent assay in 649 children with type 1 diabetes
| HLA haplotype | Group I | Group II | Group III | Group IV | Control | Pc | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DRB1‐DQB1 | n = 532 | % | n = 10 | % | n = 308 | % | n = 448 | % | n = 1032 | % | I vs Control | II vs Control | III vs Control | IV vs Control | I vs II | I vs III | I vs IV | II vs III | II vs IV | III vs IV |
| Susceptible | ||||||||||||||||||||
| *09:01‐*03:03 | 164 | 30.8 | 6 | 60.0 | 100 | 32.5 | 128 | 28.6 | 138 | 13.37 | <10−13 | <0.01 | <10−10 | <10−8 | NS | NS | NS | NS | NS | NS |
| *04:05‐*04:01 | 112 | 21.1 | 2 | 20.0 | 87 | 28.2 | 133 | 29.7 | 134 | 12.98 | <10−3 | NS | <10−6 | <10−10 | NS | NS | <0.05 | NS | NS | NS |
| *08:02‐*03:02 | 48 | 9.0 | 0 | 0.0 | 31 | 10.1 | 25 | 5.6 | 20 | 1.94 | <10−7 | NS | <10−6 | <0.01 | NS | NS | NS | NS | NS | NS |
| *04:05‐*03:02 | 11 | 2.1 | 0 | 0.0 | 3 | 1.0 | 8 | 1.8 | 0 | 0.00 | <10−3 | NS | NS | <10−3 | NS | NS | NS | NS | NS | NS |
| Protective | ||||||||||||||||||||
| *08:03‐*06:01 | 5 | 0.9 | 0 | 0.0 | 1 | 0.3 | 7 | 1.6 | 62 | 6.01 | <10−4 | NS | <10−3 | <10−3 | NS | NS | NS | NS | NS | NS |
| *15:02‐*06:01 | 12 | 2.3 | 0 | 0.0 | 5 | 1.6 | 12 | 2.7 | 92 | 8.91 | <10−4 | NS | <10−3 | <10−3 | NS | NS | NS | NS | NS | NS |
| *15:01‐*06:02 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 7 | 1.6 | 118 | 11.43 | <10−18 | NS | <10−11 | <10−9 | NS | NS | NS | NS | NS | NS |
| Neutral | ||||||||||||||||||||
| *04:07‐*03:02 | 8 | 1.5 | 0 | 0.0 | 6 | 1.9 | 7 | 1.6 | 4 | 0.39 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| *01:01‐*05:01 | 38 | 7.1 | 2 | 20.0 | 11 | 3.6 | 37 | 8.3 | 40 | 3.88 | NS | NS | NS | <0.01 | NS | NS | NS | NS | NS | NS |
| *13:02‐*06:04 | 36 | 6.8 | 0 | 0.0 | 21 | 6.8 | 29 | 6.5 | 56 | 5.43 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| *15:01‐*03:01 | 2 | 0.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 0.19 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Others | 95 | ——17.9 | 0 | 0.0 | 43 | 14.0 | 55 | 12.3 | 366 | 35.47 | <10−10 | NS | <10−11 | <10−18 | NS | NS | NS | NS | NS | NS |
Control: from Ref. 27. HLA, human leukocyte antigen; NS, not significant.
Discussion
We showed that the positivity rate for GADAb increased by approximately 20%, from 50% to 70%, by changing the assay method from RIA to ELISA in Japanese children and adolescents with type 1 diabetes. The better sensitivity and specificity of the GADAb ELISA have also been shown in the Diabetes Antibody Standardization Program23.
In previous reports, Oikawa et al. showed that in 165 Japanese patients with type 1 diabetes, just 10 patients (6.1%) were RIA‐negative and ELISA‐positive for GADAb (Gr III), and 14 patients (22.2%) were RIA‐positive and ELISA‐negative (Gr II) among the 63 patients with slowly progressive type 1 diabetes10. Also, 25–30% of GADAb‐positive slowly progressive type 1 diabetes adult‐onset patients originally diagnosed using RIA were later found to be negative when tested using ELISA11, 12. In contrast to previous reports, the number of patients that were RIA‐negative and ELISA‐positive for GADAb (Gr III) was as high as 140 (22.3%) among the 628 patients with type 1 diabetes in the present study who were assayed within 5 years after diagnosis, and just five patients (0.8%) were RIA‐positive and ELISA‐negative for GADAb (Gr II; Tables 4, 5). Recently, Kawasaki et al. showed that the RSR‐RIA kit (which is the same as the RIA kit from Cosmic) identifies both high‐ and low‐affinity GADAb, whereas the RSR‐ELISA kit (which is the same as the ELISA kit from Cosmic) identifies only high‐affinity GADAb19. Thus, the patients in Gr II who were RIA‐positive and ELISA‐negative for GADAb might have only low‐affinity GADAb, and not high‐affinity GADAb.
In the present study, Gr II contained just five patients, and was unique in terms of the age at diagnosis (which was significantly lower in this group than in Gr I), being predominantly male, and showing significantly lower positivity rates for IA‐2Ab and ZnT8Ab (Table 5). Gr II was also genetically unique in our study, as four of the five cases in this group had HLA‐DRB1*09:01‐DQB1*03:03 (Table 3), which is a susceptible genotype for type 1 diabetes among Japanese type 1 diabetes patients, and has been reported to occur at a significantly higher frequency among patients with acute‐onset type 1 diabetes aged between 2 and 5 years22. In contrast to previous reports on adult‐onset type 1 diabetes, Gr II in the present study did not contain any patients with the clinical or genetic characteristics of slowly progressive type 1 diabetes24. In the present study, just four of the 628 patients within 5 years after diagnosis had slowly progressive type 1 diabetes. This relatively small number of patients with slowly progressive type 1 diabetes might be the major reason for the discrepancy between the results of the previous study examining adults and those of the present study examining children.
Gr III showed similar characteristics to Gr I in terms of the age at diagnosis, the male/female ratio, and the relatively high positivity rates for both IA‐2Ab and ZnT8Ab; however, the GADAb titers in this group were relatively low. Of note, the genetic characteristics in terms of the HLA genotypes were quite similar between Gr I and Gr III (Tables 2, 3). Gr I and Gr III showed no significant difference in DRB1‐DQB1 haplotype frequency (Table 3).
We considered it striking that there was a discrepancy in the positivity rates for GADAb between RIA and ELISA in the present study, because the prevalence of type 1A patients among Japanese childhood‐onset type 1 diabetes patients would decrease by 20% if RIA alone were used to measure GADAb. However, the percentage of type 1A patients increased significantly when the results of tests for IA‐2Ab and ZnT8Ab were added to the results of RIA for GADAb; the prevalence increased from 47.9%, as determined using RIA for GADAb alone, to 78.5% using RIA for GADAb + IA‐2Ab + ZnT8Ab. These results strongly suggest that Gr III might have contained type 1A patients who had been misdiagnosed using RIA for GADAb alone (false negative cases; Figure 2). These discordant results between the two assays might be related to the epitope specificity of the two assays, because the GAD65 molecules used in these two kits are different: a truncated GAD65 lacking amino acids 2–45 in the N‐terminal region is used in the RIA kit, and a full‐length recombinant protein is used in the ELISA kit19, 25. Another possible reason for the discordant GADAb result might be related to the cut‐off values for the two kits. The GADAb titer (y) using an ELISA kit was correlated with the GADA titer (x) using an RIA kit as follows: y = 27.807x – 19.526 (r = 0.845, P < 0.001). The cut‐off value for the ELISA kit (5.0 U/mL) was equivalent to 0.88 U/mL using the RIA kit, which is below the cut‐off value (1.5 U/mL) for the RIA kit. Therefore, the negative results using the RIA kit in our 140 ELISA‐positive sera samples might have been due to the low GADAb titers, even though they have high‐affinity antibodies. In other words, the positivity rate in cases of childhood‐onset Japanese type 1 diabetes with lower titers of GADAb might be improved using ELISA.
In conclusion, the positivity rate in Japanese patients with childhood‐onset type 1 diabetes and a relatively low GADAb titer was increased using an ELISA; the positivity rate for GADAb increased by approximately 20%, from 50% using RIA alone to 70% using ELISA, when the assay method was switched in cases assayed within 5 years after diagnosis.
Disclosure
The authors declare no conflict of interest.
Acknowledgment
This study was supported by a Grant‐in‐Aid for Scientific Research (KAKENHI) Scientific Research (C) from the Japan Society for the Promotion of Science, and by the Japan Diabetes Foundation. We thank all the patients, their families and members of the JSGIT. The members of the JSGIT are as follows: Akemi Koike, Koike Child Clinic; Aki Nishii, JR Sendai Hospital; Yusuke Tanahashi, Asahikawa Medical College; Akira Endo, Iwata City Hospital; Eishin Ogawa, Teikyo University; Emiko Tachikawa, Tokyo Women’s Medical University School of Medicine; Hanako Tajima, Nippon Medical School; Tomohiro Hori, Gifu University; Makoto Anzo, Kawasaki Municipal Hospital; Hiroki Matsuura, Shinshu University; Hiroko Kadowaki, Sanno Hospital; Katsuya Aizu, Saitama Children’s Medical Center; Hisakazu Nakajima, Kyoto Prefectural University of Medicine; Yumiko Kotani, Tokushima University School of Medicine; Ichiro Yokota, Shikoku Medical Center for Children and Adults; Ikuko Takahashi, Akita University Graduate School of Medicine; Ikuma Fujiwara, Tohoku University Hospital; Jiro Iwamoto, Aso‐Izuka Hospital; Junichi Nagaishi, Tottori Municipal Hospital; Junko Ito, Toranomon Hospital; Jyunichi Arai, Hosogi Hospital; Kanako Ishii and Kenji Ihara, Kyushu University School of Medicine; Kanshi Minamitani, Teikyo University Chiba Medical Center; Kaori Sasaki, Tokyo Women’s Medical University Yachiyo Medical Center; Kazuhiko Jinno, Hiroshima Prefectural Hospital; Keiichi Hanaki, Tottori Prefectural Kousei Hospital; Yohei Ogawa, Niigata University Graduate School of Medical and Dental Sciences; Hiroki Abe, Niigata City General Hospital; Kenichi Miyako, Fukuoka Children’s Hospital; Kentaro Shiga, Yokohama City University Medical Center; Kimitoshi Nakamura, Kumamoto University School of Medicine; Kisho Kobayashi, University of Yamanashi Faculty of Medicine; Kohei Sato, Sapporo Factory Kids Clinic; Koji Takemoto, Ehime University Graduate School of Medicine; Kosei Hasegawa, Okayama University School of Medicine; Mahoko Hurujyo, Okayama Medical Center; Masanori Adachi, Kanagawa Children’s Medical Center; Masaru Inoue, Okayama Red Cross General Hospital; Michiko Okajima, Kanazawa University School of Medicine; Hitomi Koyama, Dokkyo Medical University; Nobuyuki Kikuchi, Department of Pediatrics, Yokohama City Minato Red Cross Hospital; Kazuteru Kitsuda, Noriyuki Takubo and Shigeyuki Ohtsu, Kitasato University School of Medicine; Reiko Horikawa, National Center for Child Health and Development; Rika Kizu, Yokosuka Kyosai Hospital; Ryuzo Takaya, Osaka Medical College; Sachiko Kitanaka, The University of Tokyo School of Medicine; Shinichiro Miyagawa; National Hospital Organization Kure Medical Center; Shinji Kadoya, Nishinomiya Municipal Central Hospital; Haruo Mizuno, Nagoya City University; Shoji Nakayama, Mominoki Hospital; Shun Soneda, St. Marianna University School of Medicine; Susumu Kanzaki, Tottori University Faculty of Medicine; Susumu Konda, Konda Children’s Clinic; Tadayuki Ayabe, Dokkyo Medical University Koshigaya Hospital; Takahiro Mochizuki, Osaka Police Hospital; Takao Fujisawa, National Mie Hospital; Tokuo Mukai, Asahikawa‐Kosei General Hospital; Tomoyuki Hotubo, Sapporo Pediatric Endoclinology Clinic; Kohji Tsubouchi, Department of Pediatrics, Chuno Kosei Hospital; Toshi Tatematsu, Chubu Rosai Hospital; Toshihisa Okada, Kumamoto Hatsuiku Clinic; Toshikazu Takahashi, Takahashi Clinic; Tsutomu Ogata, Hamamatsu University School of Medicine; Utako Sato, Tokyo Hitachi Hospital; Yasusada Kawata, Kyushyu Rosai Hospital; Yoshiya Ito, Kitami Red Cross Hospital; Goro Sasaki, Department of Pediatrics, Tokyo Dental College Ichikawa General Hospital; Yukiyo Yamamoto, Department of Pediatrics, University of Occupational and Environmental Health; Tomoyuki Kawamura, Osaka City University Graduate School of Medicine; Tatsuhiko Urakami, Department of Pediatrics, Nihon University School of Medicine; Toru Kikuchi and Shin Amemiya, Department of Pediatrics, Saitama Medical University; and Shigetaka Sugihara, Department of Pediatrics, Tokyo Women’s Medical University Medical Center East.
J Diabetes Investig 2020; 11: 594–602
References
- 1. Couper JJ, Haller MJ, Greenbaum CJ, et al ISPAD Clinical Practice Consensus Guidelines 2018: stages of type 1 diabetes in children and adolescents. Pediatr Diabetes 2018; 19(Suppl. 27): 20–27. [DOI] [PubMed] [Google Scholar]
- 2. Kawasaki E, Matsuura N, Eguchi K. Type 1 diabetes in Japan. Diabetologia 2006; 49: 828–836. [DOI] [PubMed] [Google Scholar]
- 3. Kawasaki E, Eguchi K. Current aspects on the clinical immunology and genetics of autoimmune diabetes in Japan. Diabetes Res Clin Pract. 2007; 77(Suppl 1): S104–S109. [DOI] [PubMed] [Google Scholar]
- 4. Sugihara S. Genetic susceptibility of childhood type 1 diabetes mellitus in Japan. Pediatric diabetes, endocrine and metabolic diseases in Japan past, present and future. Pediatr Endocrinol Rev 2012; 10(Suppl. 1): 62–71. [PubMed] [Google Scholar]
- 5. Onda Y, Sugihara S, Ogata T, et al Incidence and prevalence of childhood‐onset Type 1 diabetes in Japan: the T1D study. Diabet Med 2017; 34: 909–915. [DOI] [PubMed] [Google Scholar]
- 6. Takase K, Kobayashi T, Nakanishi K, et al Clinical evaluation of Cosmic kit for GAD antibody assay. Clin Endocrinol 1996; 44: 123–128, 895–900 (Japanese). [Google Scholar]
- 7. Bingley PJ, Bonifacio E, Mueller PW. Diabetes Antibody Standardization Program: first assay proficiency evaluation. Diabetes 2003; 52: 1128–1136. [DOI] [PubMed] [Google Scholar]
- 8. Aoyama T, Ikeda H, Hamamoto Y, et al Clinical heterogeneity of adult Japanese diabetes depending on titers of glutamic acid decarboxylase autoantibodies. J Diabetes Investig 2012; 3: 266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kawasaki E, Miwa M, Tanaka A. Basic and clinical evaluation of ELISA assay kits (Cosmic) for GADAb and IA‐2Ab. Jpn J Med Pharm Sci 2011; 66: 345–352. (Japanese). [Google Scholar]
- 10. Oikawa Y, Tanaka M, Horie I, et al A study on the correlation between GAD antibody titers measured by ELISA kit and RIA kit. Jpn J Med Pharm Sci 2015; 72: 1551–1560. (Japanese). [Google Scholar]
- 11. Oikawa Y, Tanaka H, Uchida J, et al Slowly progressive insulin‐dependent (type 1) diabetes positive for anti‐GAD antibody ELISA test may be strongly associated with a future insulin‐dependent state. Endocr J 2017; 64: 163–170. [DOI] [PubMed] [Google Scholar]
- 12. Murata T, Tsuzaki K, Nirengi S, et al Diagnostic accuracy of the anti‐glutamic acid decarboxylase antibody in type 1 diabetes mellitus: comparison between radioimmunoassay and enzyme‐linked immunosorbent assay. J Diabetes Investig 2017; 8: 475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. http://www.fa.kyorin.co.jp/jds/uploads/jds_imp_GAD_201603.pdf (August 27, 2019).
- 14. Yamamoto Y, Kikuchi T, Urakami T, et al Status and trends in the use of insulin analogs, insulin delivery systems and their association with glycemic control: comparison of the two consecutive recent cohorts of Japanese children and adolescents with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2019; 32: 1–9. [DOI] [PubMed] [Google Scholar]
- 15. Seino Y, Nanjo K, Tajima N, et al Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig 2010; 1: 212–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2010; 33: S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Imagawa A, Hanafusa T, Uchigata Y, et al Fulminant type 1 diabetes: a nationwide survey in Japan. Diabetes Care 2003; 26: 2345–2352. [DOI] [PubMed] [Google Scholar]
- 18. Tanaka S, Ohmori M, Awata T, et al Diagnostic criteria for slowly progressive insulin‐dependent (type1) diabetes mellitus (SPIDDM) (2012): report by the Committee on Slowly Progressive Insulin‐Dependent (Type1) Diabetes Mellitus of the Japan Diabetes Society. Diabetol Int 2015; 6: 1–7. [Google Scholar]
- 19. Kawasaki E, Okada A, Uchida A, et al Discrepancy of glutamic acid decarboxylase 65 autoantibody results between RSR radioimmunoassay and enzyme‐linked immunosorbent assay in patients with type 1 diabetes is related to autoantibody affinity. J Diabetes Investig 2019; 10: 990–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matsuura N, Uchigata Y, Urakami T, et al The evaluation of IA‐2Ab in type 1 diabetes and the analysis of IA‐2Ab in the combination with GADAb. Multicenter collaboration study in Japan. Practice 1999; 16: 567–572. (Japanese). [Google Scholar]
- 21. Kawasaki E, Nakamura K, Kuriya G, et al Differences in the humoral autoreactivity to zinc transporter 8 between childhood‐ and adult‐onset type 1 diabetes in Japanese patients. Clin Immunol 2011; 138: 146–153. [DOI] [PubMed] [Google Scholar]
- 22. Sugihara S, Ogata T, Kawamura T, et al The Japanese Study Group of Insulin Therapy for Childhood and Adolescent Diabetes (JSGIT). HLA‐Class II and Class I genotypes among Japanese children with type 1A diabetes and their families. Pediatr Diabetes 2012; 13: 33–44.22128760 [Google Scholar]
- 23. Williams AJ, Lampasona V, Schlosser M, et al Participating laboratories. Detection of antibodies directed to the N‐terminal region of GAD is dependent on assay format and contributes to differences in the specificity of GAD autoantibody assays for type 1 diabetes. Diabetes 2015; 64(9): 3239–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yasui J, Kawasaki E, Tanaka S, et al Clinical and genetic characteristics of non‐insulin‐requiring glutamic acid decarboxylase (GAD) autoantibody‐positive diabetes: A nationwide survey in Japan. PLoS ONE One 2016; 11(5): e0155643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Powell M, Prentice L, Asawa T, et al Glutamic acid decarboxylase autoantibody assay using 125I‐labelled recombinant GAD65 produced in yeast. Clin Chim Acta 1996; 256(2): 175–188. [DOI] [PubMed] [Google Scholar]
- 26. Akaza T, Imanishi T, Fujiwara K, et al HLA allele and haplotype frequencies in Japanese. Transplantation Now Suppl 1994; 7: 87–101. (Japanese). [Google Scholar]
- 27. Nakajima F, Nakamura J, Yokota T. Analysis of HLA haplotypes in Japanese, using high resolution allele typing. MHC 2002; 8: 1–32. (in Japanese). [Google Scholar]


