Abstract
Aims/Introduction
Adipose‐derived mesenchymal stem cell (ASC) transplantation is a promising therapy for diabetic nephropathy (DN). However, intravascular administration of ASCs is associated with low engraftment in target organs. Therefore, we considered applying the cell sheet technology to ASCs. In this study, ASC sheets were directly transplanted into the kidneys of a DN rat model, and therapeutic consequences were analyzed.
Materials and Methods
Adipose‐derived mesenchymal stem cells were isolated from adipose tissues of 7‐week‐old enhanced green fluorescent protein rats, and ASC sheets were prepared using a temperature‐responsive culture dish. A DN rat model was established from 5‐week‐old Spontaneously Diabetic Torii fatty rats. Seven‐week‐old DN rats (n = 21) were assigned to one of the following groups: sham‐operated (n = 6); ASC suspension (6.0 × 106 cells/mL) administered intravenously (n = 7); six ASC sheets transplanted directly into the kidney (n = 8). The therapeutic effect of the cell sheets was determined based on urinary biomarker expression and histological analyses.
Results
The ASC sheets survived under the kidney capsule of the DN rat model for 14 days after transplantation. Furthermore, albuminuria and urinary tumor necrosis factor‐α levels were significantly lower in the ASC sheets transplanted directly into the kidney group than in the sham‐operated and ASC suspension administered intravenously groups (P < 0.05). Histologically, the ASC sheets transplanted directly into the kidney group presented mild atrophy of the proximal tubule and maintained the renal tubular structure.
Conclusions
Transplantation of ASC sheets directly into the kidney improved transplantation efficiency and suppressed renal injury progression. Therefore, the ASC sheet technology might be a promising novel treatment for DN.
Keywords: Adipose‐derived mesenchymal stem cell, Cell sheet, Renal injury
Adipose‐derived mesenchymal stem cell sheet transplantation enhanced the engraftment efficiency and suppressed the progression of diabetic nephropathy.
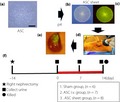
Introduction
Diabetic nephropathy (DN) is a major microvascular complication in patients with diabetes and remains the leading cause of chronic kidney disease, accounting for approximately 50% of end‐stage renal diseases worldwide1, 2. DN is triggered by sustained hyperglycemia, which drives the progression of chronic inflammation and renal injury3, 4. Dialysis is inevitable when renal injury reaches an irreversible stage5. Therefore, early‐stage detection and proper treatment of the disease are critical.
Although strict glycemic control is reported to suppress the progression of DN6, 7, there is no treatment to stop DN progression. Recently, mesenchymal stem cell (MSC) transplantation has received substantial attention as a therapy for DN. MSCs can differentiate into adipogenic and osteogenic cells8, and possess clinically useful properties, such as low antigenicity9, 10 and paracrine effects through cytokines11, 12. In particular, adipose‐derived mesenchymal stem cells (ASCs) represent an attractive cell source owing to their abundance in the body13, 14. In animal experiments on DN, intravascular administration of ASC suspensions was shown to suppress the progression of renal injury through the paracrine effect exerted by ASC‐derived cytokines15. However, intravascular administration of MSCs resulted in low engraftment to target organs, and whether the MSCs could completely adhere to the target organ remains uncertain; it also reportedly resulted in pulmonary thromboembolism and death16, 17.
To improve the engraftment in target organs, we considered preparing the cell sheet technology for transplantation on temperature‐responsive culture dishes. Cell sheets do not require enzymatic or invasive processes, hence they can maintain cell–cell junctions, cell adhesion molecules and the extracellular matrix18. Therefore, it is possible to transplant MSC sheets, both at high density and with excellent engraftment efficiency. ASC sheets showed therapeutic effects in various models, such as those for myocardial infarction19, diabetic ulcers20 and arterial injury21. However, their effect on the kidney has not yet been reported.
The purpose of the present study was to investigate the therapeutic effect of ASC sheets in DN. ASC sheets were transplanted directly into the kidneys of rats with DN, and the therapeutic effects were analyzed.
Methods
Animals
All experimental protocols were approved by the Animal Welfare Committee of Tokyo Women’s Medical University School of Medicine, Tokyo, Japan. To generate DN model rats, 5‐week‐old male Spontaneously Diabetic Torii (SDT) fatty rats (SDT.Cg‐Leprfa/JttJcl) were used. SDT fatty rats form a spontaneously obese type 2 diabetes model, established by introducing the fa allele of Zucker (fatty) rats into the genome of SDT rats22, 23. To isolate ASCs, 7‐week‐old male enhanced green fluorescent protein (EGFP) rats, strain SD‐Tg (CAG‐EGFP), were used.
Isolation and culture of ASCs
ASCs were isolated as previously described19, 20, 21. Briefly, ASCs were isolated from subcutaneous adipose tissue surrounding the epididymis of EGFP rats. The isolated ASCs were cultured in Dulbecco’s modified Eagle’s medium (Life Technologies, Carlsbad, CA, USA) with 20% fetal bovine serum (Moregate Biotech, Bulimba, QLD, Australia), 100 U/mL penicillin and 100 mg/mL streptomycin (Sigma‐Aldrich, St. Louis, MO, USA) at 37°C in a 5% CO2 incubator. ASCs were subcultured at a density of 1.0 × 105 cells/cm2 using 0.25% trypsin ethylenediaminetetraacetic acid (Life Technologies) every 3–4 days until passage 4. The isolated ASCs are shown in Figure 1a.
Figure 1.
Schematic representation of the experimental procedure for transplanting adipose‐derived mesenchymal stem cell (ASC) sheets into diabetic nephropathy rats. (a) ASCs were isolated from epididymal adipose tissue of enhanced green fluorescent protein rats (scale bar, 30 μm). Macro image of fabricated ASC sheet: (b) bright field and(c) dark field. (d) The ASC sheets were directly transplanted into the kidney of a diabetic nephropathy rat model. (e) Macro image of the kidneys immediately after transplantation. Diabetic nephropathy rats (aged 7‐weeks‐old) were randomly divided into three groups. Urine samples were collected on 0, 7 and 14 days after transplantation, and rats were killed 14 days after transplantation. (f) Red line: after transplantation.
Flow cytometry assay
To evaluate surface marker expression on the MSCs, flow cytometry was carried out, as previously described19, 20, 21. For the detection of surface markers, fluorescein isothiocyanate‐conjugated antibodies, described in Table S1, were used. For the isotype control, antibodies described in Table S2 were used. Cellular fluorescence was evaluated using a Gallios flow cytometer (Beckman Coulter, Tokyo, Japan), data were analyzed using Kaluza of the Gallios software (Beckman Coulter).
Fabrication of ASC sheets and ASC suspensions
At the fourth passage, ASCs (1.0 × 106 cells) were seeded in a temperature‐responsive 35‐mm culture dish (UpCell; CellSeed, Tokyo, Japan). The seeded ASCs were cultured in complete medium containing 82 μg/mL ascorbic acid (Wako, Osaka, Japan) for 48 h at 37°C in a CO2 incubator. To harvest the ASC sheets, the temperature in the CO2 incubator was lowered from 37°C to 20°C. Confluent ASCs were cultured for approximately 30 min and collected as ASC sheets. ASC suspension was prepared using ASCs from the fourth passage (6.0 × 106 cells/mL). The dose of ASC suspension (per mL) was approximately the same as that used in the six ASC sheets.
Measurement of cytokines in the ASC sheet supernatant
Cytokine level in the culture supernatant, during the formation of ASC sheets, was measured as follows. The culture supernatant was centrifuged at 300 g for 15 min at 4°C, and stored at −80°C. The frozen supernatant was thawed, and concentrations of bone morphogenetic protein‐7 (BMP‐7), epidermal growth factor (EGF), hepatocyte growth factor (HGF), insulin‐like growth factor‐1 (IGF‐1) and prostacyclin (PGI2) were determined using an enzyme‐linked immunosorbent assay kit (Table S3) according to the manufacturer’s recommendations. The cytokine level was expressed as the average value measured in seven culture supernatants.
Creation of DN model rats and experimental design
Unilateral nephrectomy was expected to accelerate the progression of DN pathology in the SDT fatty rats. For the establishment of a DN rat model, 5‐week‐old rats were subjected to right nephrectomy under anesthesia.
To collect urine, the rats were housed in metabolic cages (SN‐781; Shinano Manufacturing Co. Ltd., Tokyo, Japan) for 24 h. Collected urine samples were centrifuged at 300 g for 15 min at 4°C, and a part of the urine sample was stored at −80°C until further use.
The DN rats (n = 21; 7‐weeks‐old) were assigned to three groups to compare the therapeutic effects (Figure 1f) as follows:
The back of the rat was incised, the left kidney was exposed and sham‐operated (sham group, n = 6).
The groin area of the rat was incised, the femoral vein was exposed and 1 mL of the ASC suspension (6.0 × 106 cells/mL) was administered through the femoral vein (ASC i.v. group, n = 7).
The back of the rat was incised and the left kidney was exposed. Part of the renal capsule was peeled off using tweezers, under a microscope. ASC sheets harvested from temperature‐responsive culture dishes were laminated in three layers under the renal capsule using a cell sheet transfer device (the modified SWITL; Furukawa Kikou, Niigata, Japan), and the same operation was carried out by shifting the transplantation site. Six cell sheets were totally transplanted (ASC sheet group, n = 8).
At 0, 7 and 14 days after transplantation, urine samples were obtained. At 14 days after cell transplantation, the rats were killed and their kidneys excised. The excised kidneys were fixed in 4% paraformaldehyde (Muto Pure Chemicals, Tokyo, Japan) and prepared as paraffin‐embedded samples for histology.
Measurement of urinary biomarkers
For determination of albuminuria, proteinuria and urinary creatinine levels, the samples were sent to Oriental Yeast Co. (Tokyo, Japan). For analyses of podocalyxin, liver‐type fatty acid binding protein (L‐FABP), kidney injury molecule‐1 (KIM‐1), tumor necrosis factor‐α (TNF‐α) and interleukin‐6 (IL‐6) levels, the frozen urine samples were thawed and their concentrations measured using an enzyme‐linked immunosorbent assay kit according to the manufacturer’s instructions (Table S4). The levels of albumin, protein, podocalyxin, L‐FABP, KIM‐1, TNF‐α and IL‐6 were normalized to that of urinary creatinine.
Histological analysis
For histological analysis, 3‐μm thick paraffin‐embedded kidney sections were cut using a microtome (Model 2255; Leica Microsystems, Wetzlar, Germany). The sections were then deparaffinized, dehydrated and stained with the periodic acid/Schiff base. The stained sections were evaluated using an optical microscope (Eclipse E800; Nikon, Tokyo, Japan).
Immunohistochemical analysis
To carry out immunohistochemical analyses, the paraffin sections were deparaffinized and dehydrated. The sections were antigen‐activated using an activation device. The activated sections were blocked using Dako REAL peroxidase blocking solution (S2001; Dako Corporation, Carpinteria, CA, USA) and Blocking One Histo (Nacalai Tesque, Kyoto, Japan). The blocked sections were stained with an anti‐green fluorescent protein polyclonal antibody (Invitrogen, Carlsbad, CA, USA) for 2 h at 20°C. After washing, the sections were stained according to the manufacturer’s instructions using Dako REAL EnVision Detection System, Peroxidase/DAB+, Rabbit/Mouse (K5007; DAKO). The sections were then counterstained with hematoxylin, and evaluated under an optical microscope (Nikon).
Statistical analysis
All values are shown as the mean ± standard error of the mean. For comparison among multiple groups, the results were analyzed using one‐way analysis of variance, followed by Tukey’s multiple range test of JMP Pro 11.0.0 (SAS Institute, Cary, NC, USA). P < 0.05 showed statistically significant differences between groups.
Results
Characteristics of ASCs
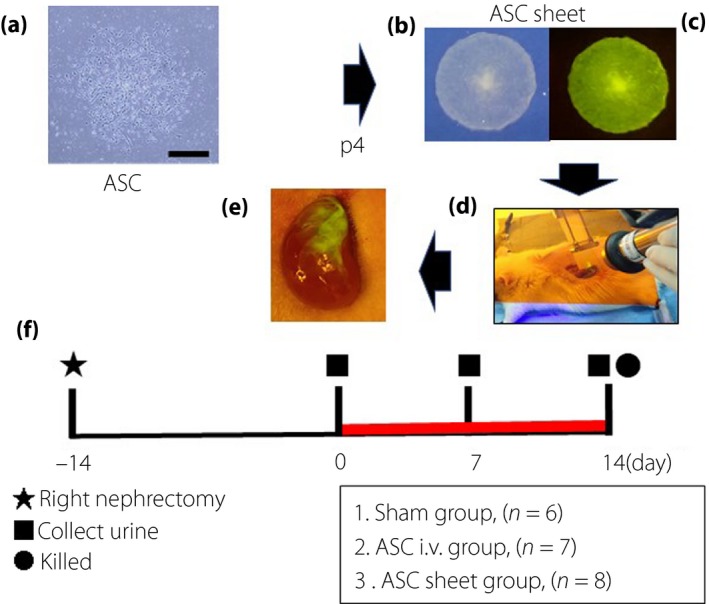
The characteristics of ASC sheets were assessed by flow cytometry after inducing differentiation. Differentiated ASCs showed colony formation (Figure 2a), adipogenesis (Figure 2b) and osteogenesis (Figure 2c). Flow cytometry, to evaluate cell surface marker expression, showed that the ASCs were positive for CD29 and CD90, and negative for CD11b, CD31 and CD45 (Figure 2d–h). These results confirmed that ASCs possessed the characteristics of MSCs.
Figure 2.
Characteristics of adipose‐derived mesenchymal stem cell (ASCs). ASCs were confirmed on the basis of colony formation, adipogenesis and osteogenesis. (a) They were stained with crystal violet to confirm colony formation. (b) The fourth passage ASCs were stained with Oil Red O to detect adipogenesis (scale bar, 50 μm). (c) They were stained with alizarin red S to detect osteogenesis (scale bar, 50 μm). (d–h) Cell surface markers of mesenchymal stem cells were evaluated using flow cytometry. The surfaces of ASCs were positive for CD29 and CD90 and negative for CD11b, CD31 and CD45
ASC sheets secreted renoprotective cytokines
Cytokine levels in the supernatants of ASC sheets were determined. Renoprotective factors, such as BMP‐7, EGF, HGF, IGF‐1 and PGI2, were detected in the supernatants (Table 1), indicating that the ASC sheets secreted renoprotective cytokines.
Table 1.
Cytokine levels in the adipose‐derived mesenchymal stem cell sheet supernatants measured using enzyme‐linked immunosorbent assay adipose‐derived mesenchymal stem cell sheets
| Cytokine | Levels |
|---|---|
| BMP‐7 (pg/mL) | 88.4 ± 33.3 |
| EGF (pg/mL) | 230.1 ± 71.0 |
| HGF (ng/mL) | 14.0 ± 2.3 |
| IGF‐1 (ng/mL) | 21.9 ± 4.7 |
| PGI2 (pg/mL) | 350.7 ± 80.5 |
Total n = 7. Data are presented as means ± standard error of the mean.
ASC sheets improved kidney engraftment
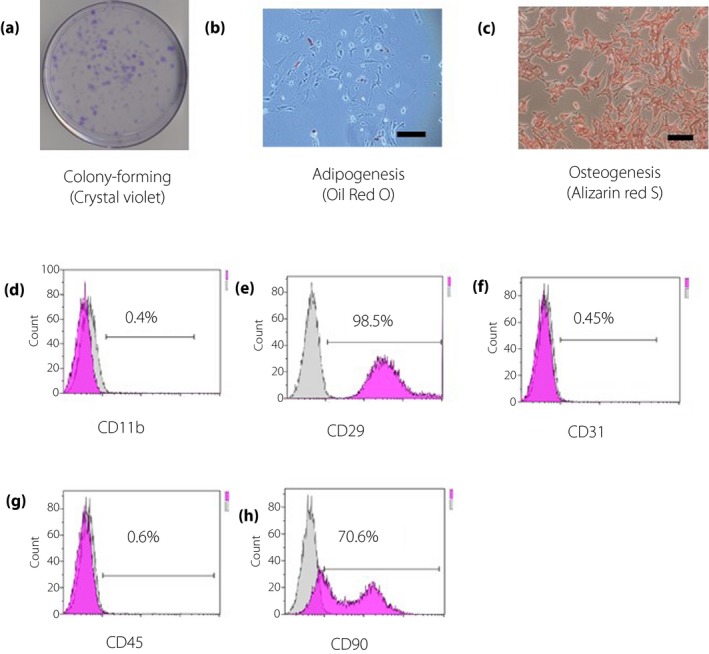
Figure 3a,b shows the kidney after 14 days of ASC sheet transplantation. EGFP and 3,3´‐diaminobenzidine immunostaining of the renal slices showed few EGFP‐positive ASCs in the kidneys of the ASC i.v. group. Compared with those in the i.v. kidneys, there were many EGFP‐positive ASCs under the renal capsule in the ASC sheet group (Figure 3c,d). The morphological analysis suggested that our cell sheet technology improved ASC engraftment.
Figure 3.
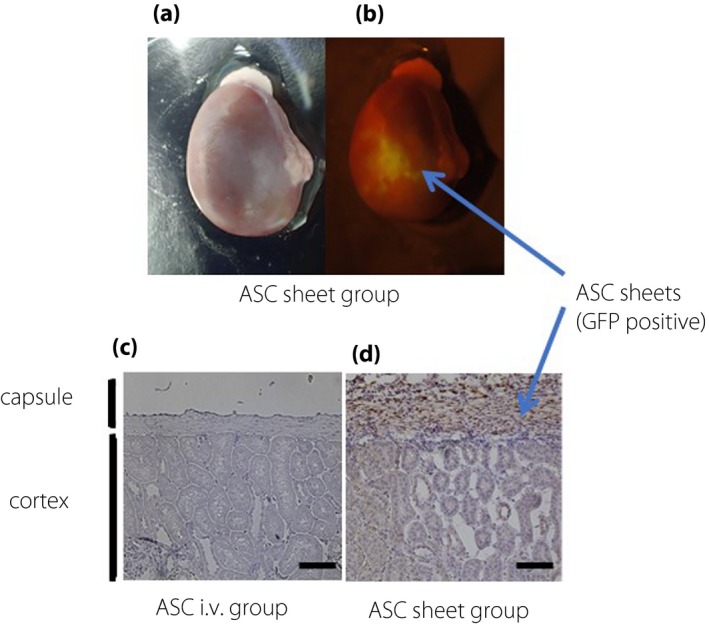
Adipose‐derived mesenchymal stem cell (ASC) sheets directly transplanted into the kidney survived for 14 days. Macro image of the kidney 14 days after transplantation: (a) bright field and (b) dark field. Immunohistochemical image of the kidney 14 days after transplantation: (c) ASC i.v. group and (d) ASC sheet group (scale bar, 50 μm). GFP, green fluorescent protein.
ASC sheets suppressed glomerular injury
Albuminuria and proteinuria were evaluated for 14 days after transplantation (Figure 4a,b). At 14 days after transplantation, the levels of albuminuria were as follows: sham group 2.7 ± 0.4 mg/mg creatinine; ASC i.v. group 2.5 ± 0.4 mg/mg creatinine; and ASC sheet group 1.2 ± 0.2 mg/mg creatinine. The protein levels were as follows: sham group 11.0 ± 1.0 mg/mg creatinine; ASC i.v. group 10.5 ± 1.3 mg/mg creatinine; and ASC sheet group 6.5 ± 0.7 mg/mg creatinine. Glomerular injury parameters were significantly lower in the ASC sheet group than in the sham and ASC i.v. groups (P < 0.05).
Figure 4.
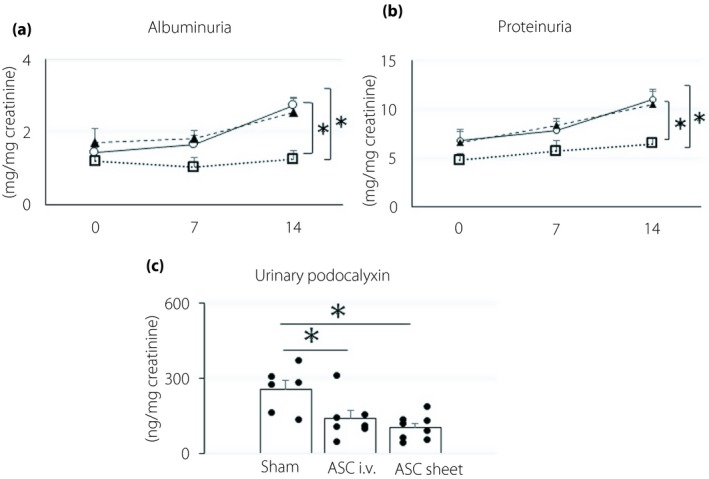
Adipose‐derived mesenchymal stem cell (ASC) sheets suppressed glomerular injury. (a,b) Change in albuminuria and proteinuria after transplantation. White circles, sham group; black triangles, ASC i.v. group; white squares, ASC sheet group. (c) Urinary podocalyxin levels 14 days after transplantation. Data are presented as the mean ± standard error of the mean (n = 6–8/group); *P < 0.05.
Levels of urinary podocalyxin, which is a standard glomerular injury marker, were as follows (Figure 4c): sham group 258.2 ± 33.2 ng/mg creatinine; ASC i.v. group 141.1 ± 29.1 ng/mg creatinine; and ASC sheet group 105.2 ± 16.6 ng/mg creatinine. The values in the ASC sheet group were significantly lower than those in the sham group (P < 0.05), showing that ASC sheets suppressed the progression of glomerular injury.
ASC sheets suppressed renal tubular injury
At 14 days after transplantation, the renal cortex of each group was stained with periodic acid Schiff base (Figure 5a–f). While the ASC sheet group maintained the renal tubular structures, the sham and ASC i.v. groups showed atrophy of the renal tubules, accompanied by a decrease in the number of renal tubular epithelial cells. At 14 days after transplantation, the levels of urinary L‐FABP and KIM‐1, general renal tubular injury markers, were measured (Figure 5g,h). The levels of urinary L‐FABP were: sham group 238.2 ± 73.9 ng/mg creatinine; ASC i.v. group 136.0 ± 14.3 ng/mg creatinine; and ASC sheet group, 78.7 ± 9.5 ng/mg creatinine. KIM‐1 excretion levels were: sham group 2.4 ± 0.3 ng/mg creatinine; ASC i.v. group 1.7 ± 0.1 ng/mg creatinine; and ASC sheet group 1.4 ± 0.2 ng/mg creatinine. L‐FABP and KIM‐1 levels were significantly lower in the ASC sheet group than in the sham group (P < 0.05), showing that ASC sheets suppressed the progression of renal tubular injury.
Figure 5.
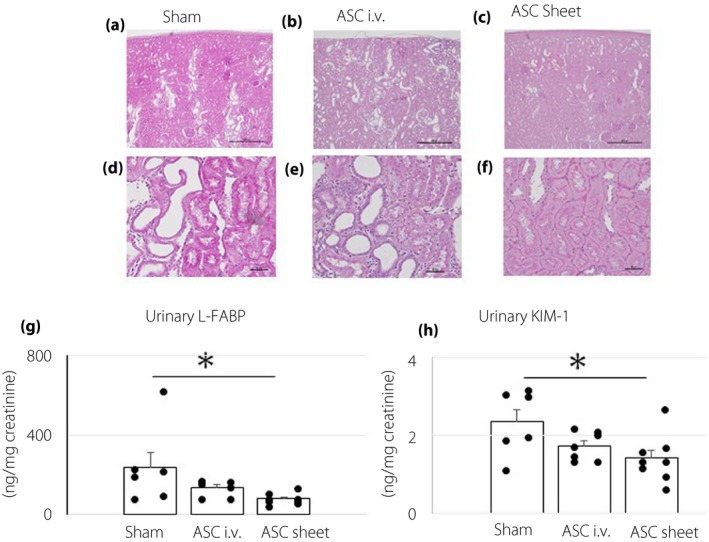
Adipose‐derived mesenchymal stem cell (ASC) sheets suppressed renal tubular injury. The renal cortex of each group, 14 days after transplantation, was stained with the periodic acid/Schiff base. Representative images are shown. (a–c) Low magnification (scale bar, 500 μm). (d–f) High magnification (scale bar, 50 μm). (g,h) Levels of urinary L‐FABP and KIM‐1 14 days after transplantation. Data are presented as the mean ± standard error of the mean (n = 6–8/group); *P < 0.05.
ASC sheets suppressed chronic inflammation in the kidney
The levels of urinary TNF‐α and IL‐6, which are general inflammatory cytokines, were estimated, as shown in Figure 6. The urinary TNF‐α levels were as follows: sham group 169.0 ± 27.7 pg/mg creatinine; ASC i.v. group 141.9 ± 31.8 pg/mg creatinine; and ASC sheet group, 47.3 ± 6.7 pg/mg creatinine. The values in the ASC sheet group were significantly lower than those in the sham and ASC i.v. groups (P < 0.01).
Figure 6.
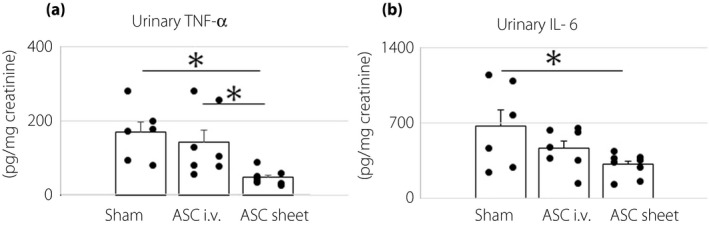
Adipose‐derived mesenchymal stem cell (ASC) sheets suppressed chronic inflammation in the kidney. (a,b) Levels of urinary tumor necrosis factor‐α (TNF‐α) and interleukin‐6 (IL‐6) 14 days after transplantation. Data are presented as mean ± standard error of the mean (n = 6–8/group); *P < 0.05.
The levels of urinary IL‐6 were as follows: sham group 672.5 ± 147.8 pg/mg creatinine; ASC i.v. group 469.7 ± 65.5 pg/mg creatinine; and ASC sheet group 313.1 ± 35.7 pg/mg creatinine. The values were significantly lower in the ASC sheet group than in the sham group (P < 0.05). Taken together, the results showed that ASC sheets suppressed the progression of chronic inflammation in the kidney.
Discussion
In the present study, we showed that the direct transplantation of an ASC sheet into the kidney of a DN rat model improved engraftment efficiency as compared with intravenous infusion therapy of ASC suspension. The significant enhancement of transplanted cell survival observed with cell sheet transplantation therapy resulted in more effective suppression of glomerular and tubular injury in the DN rat model.
Several studies have reported that MSC transplantation suppresses kidney injury in diabetic animal models through regenerative mechanisms15, 24. The primary route of renal function recovery is thought to be mediated by a paracrine effect exerted by transplanted MSCs9, 10. However, with conventional transplantation methods, such as cell suspension infusion, the rate of engraftment in target organs is insufficient due to the lack of cell–cell connection. Therefore, the most critical problem has been to develop a new cell delivery method that improves the engraftment rate of transplanted cells. Instead of cell injection therapy, here, we have developed a cell sheet transplantation strategy, which directly delivers tissues containing two‐dimensionally connected cells. To harvest cell sheets, cells are seeded in special culture dishes called temperature‐responsive culture dishes, and are cultured until confluence18. Subsequently, without using conventional enzymatic treatments, cell sheets can be collected from the surface of the dishes while retaining intercellular adhesion and extracellular matrix, only by lowering the temperature. Furthermore, the biological adhesive proteins on the bottom of the cell sheet allow the cells to adhere to the target organs and improve the engraftment rate of the transplanted cells25. Indeed, cell sheet transplantation has reportedly improved the engraftment rate of transplanted cells compared with traditional transplantation methods. In addition, they enhanced the paracrine effect and also exerted a promising therapeutic effect in many studies targeting other disease models19, 20, 21. In the present study, as shown in Figure 3, although EGFP‐positive ASCs were not detected in the ASC i.v. group 14 days after transplantation, they were detected in the ASC sheet group. This result clearly showed that the cell sheets are useful for cell delivery into the kidney, as well as into other organs, and that cell sheet transplantation therapies are widely applicable in regenerative medicine.
DN is triggered by cytotoxicity as a result of metabolic and hemodynamic effects of chronic hyperglycemia26, and progresses gradually accompanied by chronic inflammation3, 4, which leads to glomerular and renal tubular injury. Early DN shows an increase in albumin excretion, a sign of glomerular filtration barrier dysfunction. Glomerular filtration barrier is primarily constituted by podocytes, glomerular basement membrane and glomerular endothelial cells27, 28. When glomerular endothelial cells are damaged by hyperglycemia, cell adhesion factors, such as intercellular adhesion molecule 1, are expressed through an intracellular signal cascade, including nuclear factor‐κB transcription factor. They cause infiltration of blood circulating inflammatory cells, including macrophages, into glomeruli, which results in inflammatory cytokine release and renal injury progression. Furthermore, if DN progresses, not only glomerular endothelial cells, but also podocytes are injured. When severely impaired, podocytes undergo apoptosis and fallout from the glomerular filtration barrier29. Microstructural changes in the glomerular filtration barrier compromise the glomerular permeability, and in turn, more abundant quantities of albumin and protein are excreted in the urine as the disease progresses. In addition to the direct effect of hyperglycemia in renal tubules, tubular epithelial cells are injured by albumin and inflammatory cytokines leaked through the glomeruli30. In the present study, ASC sheet transplantation was capable of improving glomerular injury, decreasing urinary excretion of albumin, protein and podocalyxin31, 32, the latter of which is one of the specific markers of podocyte injury. Additionally, ASC sheets suppressed the urinary excretion of L‐FABP33 and KIM‐134, which is indicative of renal tubule injury in DN, and improved morphological atrophy of renal tubular epithelial cells. Furthermore, ASC sheet transplantation was associated with decreased excretion of inflammatory cytokines, such as TNF‐α and IL‐6, into the urine, showing protective effects against the inflammatory changes caused by hyperglycemia35, 36. Overall, ASC sheet transplantation is an exciting regenerative strategy for DN, which is thought to suppress the DN onset process through multiple mechanisms.
The renoprotective effects mentioned above are thought to be due to the paracrine effects of various trophic factors secreted from engrafted ASCs. In the present study, BMP‐7, EGF, HGF, IGF‐1 and PGI2 secretion was detected in the supernatant of ASC sheets; these factors are known to contribute to the improvement of kidney injury. BMP‐7 has been reported to suppress albuminuria and renal tubule injury by intraperitoneal administration to type 2 diabetes mice37. This study concluded that BMP‐7 exerts renoprotective effects by improving the inflammatory response in DN. Transplantation of bone marrow‐derived mesenchymal stem cells (BMMSCs) from the renal artery has been reported to suppress podocyte injury and excretion of protein in type 1 diabetes rats through secretion of BMP‐738. Li et al. reported that co‐culture of h‐ASC‐conditioned medium with mouse podocytes exposed to hyperglycemia showed a renoprotective effect that was blocked by the EGF antibody. These results showed that EGF secreted from MSC is critical for the renoprotective process39. Next, HGF has also been reported to suppress the nuclear factor‐κB signaling pathway of various cells in injured kidney and has renoprotective effects by exerting anti‐inflammatory action40. Lv et al. 41 showed that intravenous tail injection of BMMSC into type 1 diabetes rats reduced expression of inflammatory cytokines, such as IL‐6 and TNF‐α, and infiltration of macrophages in the kidney through secretion of HGF. However, when BMMSCs were intravenously injected into a cisplatin‐induced acute kidney injury mouse model, the renoprotective effect was exerted by the proliferation of renal tubule cells42. However, the protective effect disappeared when BMMSC with IGF‐1 gene suppression was used. Thus, it is suggested that the renoprotective effect exerted by MSC is mediated through IGF‐1 secretion. Furthermore, Peng et al. 43 reported that intraperitoneal administration of PGI2 in type 2 diabetes rats inhibited the p38 mitogen‐activated protein kinases signaling pathway in kidney tissue, reduced inflammatory cytokines and suppressed excretion of albumin. Thus, numerous studies reported on various cytokines that show renoprotective action. Overall, these findings indicate that the renoprotective effect is the result of multiple combined factors.
ASCs are widely used as a cell source for regenerative medicine20, 21. Many adipose tissues in the body offer ways for non‐invasive and simple collection. Although we initially considered the creation of ASC sheets using autologous ASCs, ASCs in hyperglycemic conditions have been reported to show reduced functional and therapeutic capacities compared with those under normal glucose conditions44. Furthermore, for the future industrialization, allogeneic cells are more convenient than autologous cells. Therefore, in the present study, we used allogeneic ASCs instead of autologous ASCs. Although transplantation of allogeneic cells might have a disadvantage regarding immune response, ASCs have been reported to have a high immune tolerance9, 10. In the present study, we confirmed the survival of transplanted allogeneic cells until 14 days after transplantation. Therefore, we considered that the renal protective action mediated by the paracrine effect of the ASCs was successfully accomplished during their survival period, and allogeneic ASCs seemed to have contributed to the improvement of DN.
In summary, ASC sheet transplantation enhanced the engraftment efficiency and suppressed the progression of DN through multiple pathways of paracrine effects. The therapy protected the kidney structure at both the glomerular and tubular levels. In the future, we need to confirm long‐term efficacy by large animal experiments and develop a minimally invasive transplantation method of cell sheets. Clinical application of ASC sheet transplantation for DN patients is foreseen.
Disclosure
Tatsuya Shimizu was a member of the scientific advisory board and a shareholder of CellSeed, Inc. Tokyo Women’s Medical University received research funding from CellSeed, Inc. The other authors declare no conflict of interest.
Supporting information
Table S1 | Fluorescein isothiocyanate‐conjugated antibodies used for flow cytometry
Table S2 | Isotype control antibodies used for flow cytometry
Table S3 | Enzyme‐linked immunosorbent assay kits used for measuring cytokine concentrations in adipose‐derived mesenchymal stem cell supernatant
Table S4 | Enzyme‐linked immunosorbent assay kit used for measuring biomarker concentration in urine
Acknowledgments
The authors thank Dr Aya Imafuku from the Institute of Advanced Biomedical Engineering and Science, Tokyo Women’s Medical University for valuable advice. We thank Dr Yoei Miyabe and Dr Naoko Sugiura of the Department of Medicine, Kidney Center, Tokyo Women’s Medical University for suggestions and excellent technical support in experiments. This research was partially supported by the research fund from CellSeed Inc.
J Diabetes Investig 2020; 11: 545–553
References
- 1. Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab 2008; 4: 444–452. [DOI] [PubMed] [Google Scholar]
- 2. Kanwar YS, Sun L, Xie P, et al. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol 2011; 6: 395–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mora C, Navarro JF. Inflammation and diabetic nephropathy. Curr Diab Rep 2006; 6: 463–468. [DOI] [PubMed] [Google Scholar]
- 4. Navarro‐González JF, Mora‐Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol 2008; 19: 433–442. [DOI] [PubMed] [Google Scholar]
- 5. Dressler RL. Antihypertensive agents for prevention of diabetic nephropathy. Am Fam Physician 2006; 74: 77–79. [PubMed] [Google Scholar]
- 6. Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non‐insulin‐dependent diabetes mellitus: a randomized prospective 6‐year study. Diabetes Res Clin Pract 1995; 28: 103–117. [DOI] [PubMed] [Google Scholar]
- 7. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 8. Wall ME, Bernacki SH, Loboa EG. Effects of serial passaging on the adipogenic and osteogenic differentiation potential of adipose‐derived human mesenchymal stem cells. Tissue Eng 2007; 13: 1291–1298. [DOI] [PubMed] [Google Scholar]
- 9. Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007; 110: 3499–3506. [DOI] [PubMed] [Google Scholar]
- 10. Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft‐versus‐host disease with third party haploidentical mesenchymal stem cells. Lancet 2004; 363: 1439–1441. [DOI] [PubMed] [Google Scholar]
- 11. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem 2006; 98: 1076–1084. [DOI] [PubMed] [Google Scholar]
- 12. Kinnaird T, Stabile E, Burnett MS, et al. Local delivery of marrow‐derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 2004; 109: 1543–1549. [DOI] [PubMed] [Google Scholar]
- 13. Lotfy A, Salama M, Zahran F, et al. Characterization of mesenchymal stem cells derived from rat bone marrow and adipose tissue: a comparative study. Int J Stem Cells 2014; 7: 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006; 24: 1294–1301. [DOI] [PubMed] [Google Scholar]
- 15. Fang Y, Tian X, Bai S, et al. Autologous transplantation of adipose‐derived mesenchymal stem cells ameliorates streptozotocin‐induced diabetic nephropathy in rats by inhibiting oxidative stress, pro‐inflammatory cytokines and the p38 MAPK signaling pathway. Int J Mol Med 2012; 30: 85–92. [DOI] [PubMed] [Google Scholar]
- 16. Furlani D, Ugurlucan M, Ong L, et al. Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc Res 2009; 77: 370–376. [DOI] [PubMed] [Google Scholar]
- 17. Tatsumi K, Ohashi K, Matsubara Y, et al. Tissue factor triggers procoagulation in transplanted mesenchymal stem cells leading to thromboembolism. Biochem Biophys Res Commun 2013; 431: 203–209. [DOI] [PubMed] [Google Scholar]
- 18. Okano T, Yamada N, Sakai H. A novel recovery system for cultured cells using plasma‐treated polystyrene dishes grafted with poly(N‐isopropylacrylamide). J Biomed Mater Res 1993; 27: 1243–1251. [DOI] [PubMed] [Google Scholar]
- 19. Miyahara Y, Nagaya N, Kataoka M, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med 2006; 12: 459–465. [DOI] [PubMed] [Google Scholar]
- 20. Kato Y, Iwata T, Morikawa S, et al. Allogeneic transplantation of an adipose‐derived stem cell sheet combined with artificial skin accelerates wound healing in a rat wound model of type 2 diabetes and obesity. Diabetes 2015; 64: 2723–2734. [DOI] [PubMed] [Google Scholar]
- 21. Homma J, Sekine H, Matsuura K, et al. Mesenchymal stem cell sheets exert anti‐stenotic effects in a rat arterial injury model. Tissue Eng Part A 2018; 24: 1545–1553. [DOI] [PubMed] [Google Scholar]
- 22. Ishii Y, Ohta T, Sasase T, et al. Pathophysiological analysis of female spontaneously diabetic Torii fatty rats. Exp Anim 2010; 59: 73–84. [DOI] [PubMed] [Google Scholar]
- 23. Katsuda Y, Kemmochi Y, Maki M, et al. Effects of unilateral nephrectomy on renal function in male Spontaneously Diabetic Torii fatty rats: a novel obese type 2 diabetic model. J Diabetes Res 2014; 2014: 363126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nagaishi K, Mizue Y, Chikenji T, et al. Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci Rep 2016; 6: 34842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shimizu T, Yamato M, Isoi Y, et al. Fabrication of pulsatile cardiac tissue grafts using a novel 3‐dimensional cell sheet manipulation technique and temperature‐responsive cell culture surfaces. Circ Res 2002; 90: e40. [DOI] [PubMed] [Google Scholar]
- 26. Calcutt NA, Cooper ME, Kern TS, et al. Therapies for hyperglycaemia‐induced diabetic complications: from animal models to clinical trials. Nat Rev Drug Discov 2009; 8: 417–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sakoda M, Itoh H, Ichihara A. Podocytes as a target of prorenin in diabetes. Curr Diabetes Rev 2011; 7: 17–21. [DOI] [PubMed] [Google Scholar]
- 28. Thomas MC. Pathogenesis and progression of proteinuria. Contrib Nephrol 2011; 170: 48–56. [DOI] [PubMed] [Google Scholar]
- 29. Gross ML, Dikow R, Ritz E. Diabetic nephropathy: recent insights into the pathophysiology and the progression of diabetic nephropathy. Kidney Int 2005; 94: S50–S53. [DOI] [PubMed] [Google Scholar]
- 30. Theilig F. Spread of glomerular to tubulointerstitial disease with a focus on proteinuria. Ann Anat 2010; 192: 125–132. [DOI] [PubMed] [Google Scholar]
- 31. Ye H, Bai X, Gao H, et al. Urinary podocalyxin positive‐element occurs in the early stage of diabetic nephropathy and is correlated with a clinical diagnosis of diabetic nephropathy. J Diabetes Complications 2014; 28: 96–100. [DOI] [PubMed] [Google Scholar]
- 32. Hara M, Yamagata K, Tomino Y. Urinary podocalyxin is an early marker for podocyte injury in patients with diabetes: establishment of a highly sensitive ELISA to detect urinary podocalyxin. Diabetologia 2012; 55: 2913–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suzuki K, Babazono T, Murata H, et al. Clinical significance of urinary liver‐type fatty acid‐binding protein in patients with diabetic nephropathy. Diabetes Care 2005; 28: 2038–2039. [DOI] [PubMed] [Google Scholar]
- 34. Fu WJ, Xiong SL, Fang YG, et al. Urinary tubular biomarkers in short‐term type 2 diabetes mellitus patients: a cross‐sectional study. Endocrine 2012; 41: 82–88. [DOI] [PubMed] [Google Scholar]
- 35. Wu J, Ding Y, Zhu C, et al. Urinary TNF‐α and NGAL are correlated with the progression of nephropathy in patients with type 2 diabetes. Exp Ther Med 2013; 6: 1482–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shikano M, Sobajima H, Yoshikawa H, et al. Usefulness of a highly sensitive urinary and serum IL‐6 assay in patients with diabetic nephropathy. Nephron 2000; 85: 81–85. [DOI] [PubMed] [Google Scholar]
- 37. Li RX, Yiu WH, Wu HJ, et al. BMP7 reduces inflammation and oxidative stress in diabetic tubulopathy. Clin Sci 2015; 128: 269–280. [DOI] [PubMed] [Google Scholar]
- 38. Wang S, Li Y, Zhao J, et al. Mesenchymal stem cells ameliorate podocyte injury and proteinuria in a type 1 diabetic nephropathy rat model. Biol Blood Marrow Transplant 2013; 19: 538–546. [DOI] [PubMed] [Google Scholar]
- 39. Li D, Wang N, Zhang L, et al Mesenchymal stem cells protect podocytes from apoptosis induced by high glucose via secretion of epithelial growth factor. Stem Cell Res Ther 2013; 4: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gong R, Rifai A, Tolbert EM. Hepatocyte growth factor ameliorates renal interstitial inflammation in rat remnant kidney by modulating tubular expression of macrophage chemoattractant protein‐1 and RANTES. J Am Soc Nephrol 2004; 15: 2868–2881. [DOI] [PubMed] [Google Scholar]
- 41. Lv SS, Liu G, Wang JP, et al. Mesenchymal stem cells transplantation ameliorates glomerular injury in streptozotocin‐induced diabetic nephropathy in rats via inhibiting macrophage infiltration. Int Immunopharmacol 2013; 17: 275–282. [DOI] [PubMed] [Google Scholar]
- 42. Bi B, Schmitt R, Israilova M, et al. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol 2007; 18: 2486–2496. [DOI] [PubMed] [Google Scholar]
- 43. Peng L, Li J, Xu Y, et al. The protective effect of beraprost sodium on diabetic nephropathy by inhibiting inflammation and p38 MAPK signaling pathway in high‐fat diet/streptozotocin‐induced diabetic rats. Int J Endocrinol 2015; 2016: 1690474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rennert RC, Sorkin M, Januszyk M, et al. Diabetes impairs the angiogenic potential of adipose‐derived stem cells by selectively depleting cellular subpopulations. Stem Cell Res Ther 2014; 5: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Fluorescein isothiocyanate‐conjugated antibodies used for flow cytometry
Table S2 | Isotype control antibodies used for flow cytometry
Table S3 | Enzyme‐linked immunosorbent assay kits used for measuring cytokine concentrations in adipose‐derived mesenchymal stem cell supernatant
Table S4 | Enzyme‐linked immunosorbent assay kit used for measuring biomarker concentration in urine


