Abstract
Aims/Introduction
Diabetic peripheral neuropathic pain (DPNP) affects the functionality, mood and sleep patterns of patients with diabetes. Mirogabalin, an α2δ ligand with a slower dissociation for α2δ‐1 versus α2δ‐2 subunits, showed efficacy and safety in a randomized, double‐blind, placebo‐controlled, 14‐week study in Asian patients with DPNP. This open‐label extension study evaluated the long‐term safety and efficacy of mirogabalin in Asian patients with DPNP.
Material and Methods
This 52‐week open‐label extension study was carried out in Japan, Korea and Taiwan in patients with DPNP. Patients received mirogabalin, initiated at 5 mg twice daily and increased to a flexible maintenance dosage of 10 or 15 mg twice daily. Adverse events were monitored throughout the study. Patients provided a self‐assessment of pain using the Short‐Form McGill Pain Questionnaire.
Results
Of the 214 patients who entered the study, 172 (80.4%) completed the extension study. Of 172 patients who completed the study, 149 received the highest dosage of mirogabalin (15 mg twice daily). The most common treatment‐emergent adverse events were nasopharyngitis, diabetic retinopathy, peripheral edema, somnolence, diarrhea, increased weight and dizziness. Most treatment‐emergent adverse events were mild or moderate in severity. The incidence of treatment‐emergent adverse events leading to treatment discontinuation was 13.1%. The visual analog scale and all other Short‐Form McGill Pain Questionnaire subscales (sensory score, affective score, total score and present pain intensity) generally decreased over time from baseline until week 52.
Conclusions
This extension study showed the safety and efficacy of a long‐term flexible dosing regimen of mirogabalin 10 or 15 mg twice daily in patients with DPNP.
Keywords: Diabetic peripheral neuropathic pain, Mirogabalin, Pain
Mirogabalin 30 mg/day effectively reduced pain compared with placebo and was well tolerated in a phase III, randomized, double‐blind, placebo‐controlled study in Asian patients with diabetic peripheral neuropathic pain. This open‐label extension study was carried out to evaluate the long‐term safety of flexible doses of mirogabalin 20 or 30 mg/day in Asian patients with diabetic peripheral neuropathic pain; long‐term efficacy of mirogabalin was also evaluated. This study showed the safety and efficacy of a long‐term flexible dosing regimen of mirogabalin 10 or 15 mg twice daily in patients with diabetic peripheral neuropathic pain.

Introduction
Diabetic peripheral neuropathy is present in up to half of all patients with long‐standing diabetes mellitus, and up to one‐third will develop diabetic peripheral neuropathic pain (DPNP)1, 2, 3 DPNP is a major cause of morbidity, often worsening quality of life, as well as being associated with a significant economic burden1, 4, 5. DPNP often results in sleep disturbance5, 6, and the constant tiredness from disturbed sleep and painful symptoms during the day leads to limitations in daily activities and work productivity1, 5, 7. DPNP symptoms are also frequently associated with depression and anxiety8, 9.
Mirogabalin is a novel, selective oral ligand of the α2δ calcium channel subunit. It has higher binding affinities for α2δ‐1 and α2δ‐2 subunits than pregabalin, and a slower dissociation rate for α2δ‐1 than α2δ‐210. Additionally, mirogabalin showed more potent and longer lasting analgesic effects in experimental models of neuropathic pain in rats (partial sciatic nerve ligation and streptozotocin‐induced diabetic neuropathic pain)10. Furthermore, mirogabalin (10, 30 and 100 mg/kg) and pregabalin (30, 100 and 300 mg/kg) inhibited rotarod performance and locomotor activity in rats; however, the safety indices of mirogabalin were superior to those of pregabalin10.
Mirogabalin has shown efficacy in patients with DPNP11, 12. Compared with placebo, mirogabalin significantly improved the average daily pain score in Asian patients with DPNP in a 14‐week phase III study13. However, the long‐term efficacy and safety of mirogabalin remains unclear. This 52‐week open‐label extension of the phase III study investigated the long‐term safety and efficacy of flexible‐dosage mirogabalin in Asian patients with DPNP.
Methods
Study design
The multinational, open‐label extension study of a previous phase III study (NCT02318706) was carried out between 2 May 2015 and 4 July 2017, in Asian patients with DPNP. This extension study was carried out at approximately 200 study sites in Japan, Korea and Taiwan.
The protocol for the phase III study has been described elsewhere13. Briefly, in the double‐blind, placebo‐controlled, parallel‐group study, 834 patients were randomized to receive fixed‐dosage mirogabalin 15, 20 or 30 mg/day, or placebo. The 14‐week study included a 1‐ to 2‐week titration period and a 12‐ to 13‐week fixed‐dosage period, followed by a 1‐week follow‐up period.
Eligible patients entered the open‐label extension study at the end of week 14 of the phase 3 study. There was no washout period after the phase III study. The extension study consisted of an initial 4‐week titration period, a 48‐week flexible‐dosage period and a 1‐week follow‐up period (Figure S1). Mirogabalin was administered twice daily (in the morning and at bedtime in the same manner as in the phase III study). During the titration period, mirogabalin was administered at a dosage of 5 mg twice daily for the first 2 weeks, and then 10 mg twice daily for the second 2 weeks. From the fifth week, the mirogabalin dosage was increased to 15 mg twice daily, if there were no safety issues. For the remainder of the study, the dosage could be changed to either 10 or 15 mg twice daily, depending on the safety findings at each visit.
Use of any concomitant medications or therapies during the study was documented, regardless of whether they were permitted. In particular, the concomitant use of pregabalin or gabapentin, and drugs that could cause irreversible retinal degeneration (e.g., phenothiazine antipsychotics, deferoxamine, quinine, quinidine, ethambutol, voriconazole etc.) were prohibited during the extension study.
The extension study was carried out in compliance with the Declaration of Helsinki, and was consistent with the Good Clinical Practice Guidelines of the International Council for Harmonization and applicable regulatory requirements. All participating clinical sites received institutional review board or independent ethics committee approval of the study protocol and study‐related documents before patient enrolment. All patients provided written informed consent. The independent Data Safety Monitoring Board reviewed safety data in an ongoing manner.
Study population
Asian patients with DPNP who had completed 14 weeks of administration of mirogabalin in the phase III study were eligible for inclusion in the extension study. Patients were excluded if they had experienced a critical safety issue in the phase III study or had received previous treatment with drugs that could cause irreversible retinal degeneration. In addition, the following groups of patients were excluded from the extension study: compliance <80% in the phase III study; creatinine clearance <60 mL/min; known positive hepatitis B antigen or hepatitis C antibody; pregnant or breast‐feeding women; or women who were likely to become pregnant or unwilling to take reliable contraceptive measures during the study and 4‐week follow up.
Safety assessments
Throughout the study, adverse events (AEs) were recorded and classified according to the Medical Dictionary for Regulatory Activities version 17.1. AEs were defined as any unfavorable and unintended sign (including abnormal laboratory value or vital sign), symptom or disease that developed after study entry and up to 7 days after the last dose of the study drug, regardless of the relationship to the study drug. Any symptom that the investigator regarded as associated with diabetic peripheral neuropathy was evaluated as an efficacy variable and not regarded as an AE; however, if such a symptom was considered potentially related to the study drug, it was considered an AE. Pre‐existing diseases, including diabetes mellitus, were coded as AEs if they worsened or were inadequately controlled during the study (as judged by the investigator). In addition, vital signs, physical examinations and clinical laboratory assessments were carried out. A 12‐lead electrocardiogram was carried out during the titration period visit and at the end of treatment or early termination. Other safety end‐points included bodyweight, physical examinations, evaluation of edema, a neurological examination, an ophthalmological examination, Columbia‐Suicide Severity Rating Scale14 assessment and the Hospital Anxiety and Depression Scale15 assessment.
Efficacy assessment
Patients provided a self‐assessment of pain using the Short‐Form McGill Pain Questionnaire (SF‐MPQ)16 from the titration period visit to the end of treatment/early termination visit.
Statistical analysis
Safety and efficacy analysis sets included all patients who received at least one dose of the study medication. For the SF‐MPQ (the sensory score, affective score, total score, visual analog scale [VAS] and the present pain intensity index), summary statistics were calculated for the measured value and the change from baseline at each scheduled visit (including last observation carried forward imputation and baseline observation carried forward imputation). Baseline values were defined as the last non‐missing available values before the first dose of the extension study. All statistical analyses were carried out using Statistical Analysis System® software (version 9.3 or higher; SAS Institute, Cary, NC, USA).
Results
Patients
Of the 214 patients enrolled (all of whom provided informed consent), 172 (80.4%) completed the extension study (Table 1). A total of 42 patients (19.6%) discontinued the study, with the most common reasons for withdrawal being AEs (21 patients [9.8%]) and patient withdrawal (19 patients [8.9%]).
Table 1.
Patient disposition
| Enrolled | Mirogabalin 5 mg twice daily† n = 4 | Mirogabalin 10 mg twice daily† n = 35 | Mirogabalin 15 mg twice daily† n = 175 | Total n = 214 |
|---|---|---|---|---|
| Completed | 0 | 23 (65.7) | 149 (85.1) | 172 (80.4) |
| Discontinued | 4 (100.0) | 12 (34.3) | 26 (14.9) | 42 (19.6) |
| Reason for discontinuation | ||||
| Adverse event | 2 (50.0) | 6 (17.1) | 13 (7.4) | 21 (9.8) |
| Death | 0 | 0 | 1 (0.6) | 1 (0.5) |
| Lack of efficacy | 0 | 0 | 1 (0.6) | 1 (0.5) |
| Withdrawal by subject | 2 (50.0) | 6 (17.1) | 11 (6.3) | 19 (8.9) |
Data presented as n (%). The percentage is calculated using the number of enrolled patients as the denominator.
The most frequent administered dose during the treatment period.
The demographics and baseline characteristics of the patients enrolled in the extension study are shown in Table 2. In the enrolled analysis set, the mean (standard deviation [SD]) age at informed consent was 58.9 (9.85) years. Overall, 72.0% (154/214) of the patients were male and 28.0% (60/214) were female. The mean bodyweight was 69.32 kg (SD 13.34 kg), and the mean body mass index was 25.47 kg/m2 (SD 4.16 kg/m2). The median duration of DPNP at randomization in the phase III study was 35.5 months (range 6.0–225.0 months). Overall, most of the patients (77.1%) were enrolled in Japan.
Table 2.
Patient demographics and baseline characteristics
| Parameter | Mirogabalin n = 214 |
|---|---|
| Age (years)† | 58.9 ± 9.9 |
| Sex, n (%) | |
| Male | 154 (72.0) |
| Female | 60 (28.0) |
| Height (cm)† | 164.72 ± 8.2 |
| Weight (kg) | 69.32 ± 13.3 |
| BMI (kg/m2) | 25.47 ± 4.2 |
| Creatinine clearance (mL/min) | 105.2 ± 32.7 |
| Type of diabetes mellitus, n (%) | |
| Type 1 | 12 (5.6) |
| Type 2 | 202 (94.4) |
| Duration of diabetes (years)† | |
| Median (range) | 10.0 (0–46) |
| Duration of painful DPN (months)† | |
| Median (range) | 35.5 (6–225) |
| HbA1c (%) | 7.43 (1.01) |
| History of psychiatric disease, n (%) | |
| Yes | 2 (0.9) |
| No | 212 (99.1) |
| Medical and surgical history, n (%) | 214 (100.0) |
| Country, n (%) | |
| Japan | 165 (77.1) |
| Korea | 27 (12.6) |
| Taiwan | 22 (10.3) |
Data presented as the mean ± standard deviation unless otherwise stated. Shown for the enrolled analysis set. The percentage for each categorical variable was calculated using the number of patients in a column heading as the denominator. Creatinine clearance was calculated by the Cockcroft–Gault equation.
At informed consent for extension study. BMI, body mass index; DPN, diabetic peripheral neuropathy; HbA1c, glycated hemoglobin; SD, standard deviation.
Safety
The treatment‐emergent AEs (TEAEs) occurring in ≥5% of patients during the extension study are reported in Table 3. The incidence of TEAEs (percentage of patients with at least one TEAE) was 91.1%. The most common TEAEs (reported for ≥5% of patients) were nasopharyngitis (27.1%), diabetic retinopathy (11.7%), edema peripheral (11.2%), somnolence (9.3%), diarrhea (8.4%), increased weight (7.9%), dizziness (7.5%), edema (6.1%), diabetes mellitus, hypoglycemia, constipation (5.6% each) and back pain (5.1%).
Table 3.
Treatment‐emergent adverse events occurring in ≥5% of patients during the extension study
| TEAE | Mirogabalin n = 214 |
|---|---|
| Patients with at least one TEAE | 195 (91.1) |
| Nasopharyngitis | 58 (27.1) |
| Diabetic retinopathy | 25 (11.7) |
| Edema peripheral | 24 (11.2) |
| Somnolence | 20 (9.3) |
| Diarrhea | 18 (8.4) |
| Weight increased | 17 (7.9) |
| Dizziness | 16 (7.5) |
| Edema | 13 (6.1) |
| Diabetes mellitus | 12 (5.6) |
| Hypoglycemia | 12 (5.6) |
| Constipation | 12 (5.6) |
| Back pain | 11 (5.1) |
Data presented as n (%). Coded using the Medical Dictionary for Regulatory Activities Version 17.1. TEAE, treatment‐emergent adverse event.
Most of the TEAEs resolved without any treatment, and most were mild (57.5%) or moderate (26.2%) in severity. Of patients with diabetic retinopathy, 20 cases were mild, three were moderate (one needed laser therapy) and two were severe (one experienced a clinically significant decline in visual acuity, the other did not).
The incidence of severe TEAEs was 7.5% (16/214), and the incidence of serious TEAEs was 11.2% (24/214). The incidence of TEAEs leading to treatment discontinuation was 13.1% (28/214). Overall, the incidence of AEs considered to be related to the study drug was 27.6%, with the most frequent being somnolence (7.9%), dizziness (6.1%), edema peripheral (4.7%), edema (3.7%) and increased weight (2.8%). Most were mild‐to‐moderate in severity, and most resolved without any treatment. The incidence of serious AEs related to the study drug was 1.4% (3/214). Myocardial infarction, drowning and aspartate aminotransferase increase were reported in one patient each; all were considered severe. One death was reported; a woman aged 68 years treated with mirogabalin 15 mg twice daily who drowned. This event was considered related to the study drug by the investigator. Except for reported AEs, no notable abnormalities were reported in the laboratory evaluations. No notable changes were observed in electrocardiograms, vital signs, neurological examination results, ophthalmological examination, Columbia‐Suicide Severity Rating Scale or Hospital Anxiety and Depression Scale.
Efficacy
The mean changes from baseline in the SF‐MPQ subscales at week 52 of the extension study are shown in Table 4. For VAS, the mean change from baseline was −9.8 (SD 14.06) at week 52 of the extension study using the last observation carried forward imputation method. From baseline through week 8 of the extension study, the VAS gradually decreased, with the decrease in VAS then maintained throughout the remainder of the study (Figure 1). Other SF‐MPQ subscales (total score, sensory score, affective score and present pain intensity) generally decreased from baseline to week 52 of the extension study (Table 4).
Table 4.
Short‐Form McGill Pain Questionnaire; mean change from baseline† at week 52 of the extension study
| Baseline (n = 214) | Change from baseline at week 52 (n = 214) | |
|---|---|---|
| Sensory score | 5.0 ± 5.49 | −1.2 ± 3.29 |
| Affective score | 0.9 ± 1.78 | −0.3 ± 1.30 |
| Total score | 5.9 ± 6.94 | −1.5 ± 4.31 |
| VAS (mm) | 42.1 ± 20.41 | −9.8 ± 14.06 |
| Present pain intensity | 1.5 ± 0.83 | −0.2 ± 0.69 |
Results analyzed using the last observation carried forward imputation method. Data presented as the mean ± standard deviation.
Baseline value was defined as the last non‐missing available value prior to first dose of the extension study. VAS, visual analog scale.
Figure 1.
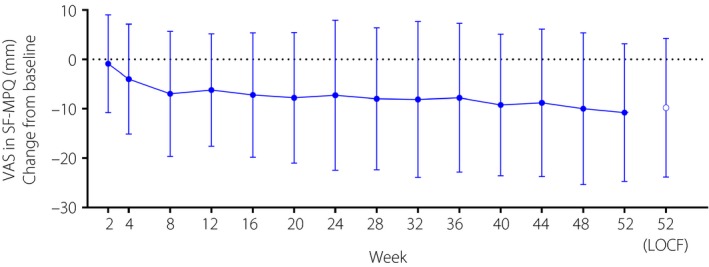
Time course of mean change (±standard deviation) in visual analog scale in the Short‐Form McGill Pain Questionnaire. Shown for the efficacy analysis set. LOCF, last observation carried forward imputation method used; SF‐MPQ, Short‐Form McGill Pain Questionnaire; VAS, visual analog scale.
Discussion
Neuropathic pain has been linked to the upregulation of the α2δ‐1 subunit of voltage‐gated Ca2+ channels in the nervous system.17, 18 The α2δ‐1 subunit has become a target of α2δ ligand analgesic drugs17. The α2δ‐1 ligands are thought to exert analgesic effects by preventing the trafficking of the α2δ‐1 unit to presynaptic terminals, decreasing presynaptic calcium influx and, consequently, reducing neurotransmitter release17.
Mirogabalin has potent and selective binding affinities for human and rat α2δ subunits, and a slower dissociation rate for the α2δ‐1 versus α2δ‐2 subunit10. It shows potent and long‐lasting analgesic effects in rat models for neuropathic pain and a wider safety margin for CNS side‐effects10. In the preceding double‐blind phase III trial in Asian patients with DPNP, mirogabalin 15–30 mg/day was well tolerated and relieved DPNP in a dose‐dependent manner for up to 14 weeks13. In the present open‐label extension study, the safety and efficacy of mirogabalin (administered using a flexible dosing of 10 or 15 mg twice daily) was shown over a longer period (52 weeks) in patients with DPNP, with >80% of the patients completing the extension study.
Mirogabalin was well tolerated in the extension study, with no notable safety concerns identified with the long‐term flexible dosing regimen. Overall, the incidence of TEAEs was 91.1%. The most common TEAEs were nasopharyngitis (27.1%), diabetic retinopathy (11.7%), peripheral edema (11.2%), somnolence (9.3%), diarrhea (8.4%), increased weight (7.9%) and dizziness (7.5%). Most of the TEAEs of somnolence, dizziness, weight gain and edema resolved without treatment. The incidence of serious TEAEs (11.2% vs 4.7%), severe TEAEs (7.5% vs 3.0%) and TEAEs leading to treatment discontinuation (13.1% vs 5.5%) remained low with long‐term (extension study) compared with short‐term (double‐blind study) administration of mirogabalin.
In terms of efficacy, improvements from baseline in SF‐MPQ subscales (sensory score, affective score, total score, VAS and present pain intensity) occurred over the 52‐week extension period, indicating the long‐term efficacy of mirogabalin for pain relief in patients with DPNP. Tachyphylaxis was not observed, because the change in VAS from baseline through the eighth week showed a gradual decrease, and the decrease in VAS was maintained throughout the study period.
With this drug class, adverse drug reactions include fatigue, dizziness, sedation, somnolence and ataxia; peripheral edema and increased weight also are frequently reported19, 20, 21, 22, 23. In a long‐term, open‐label phase III extension trial in Japanese patients with DPNP treated with pregabalin, a similar adverse event profile to that observed in the mirogabalin extension study was reported. The most common TEAEs in the pregabalin study included somnolence (22.8%), increased weight (22.0%), dizziness (20.3%) and peripheral edema (15.4%)23. Given that most TEAEs in the mirogabalin extension study resolved without treatment, it is possible that patients developed tolerance to TEAEs over time. However, further studies would be required to confirm this.
The current study had a number of limitations that should be considered. Outcomes from this open‐label extension study should be interpreted with caution due to the absence of a control arm. In addition, all enrolled patients were Asian (most were from Japan) and thus the outcomes in this study might not be transferable to other ethnicities. However, an analysis of pooled safety data from trials involving pregabalin (another α2δ ligand) found similar safety outcomes in patients from Japan and those from Western countries21. As mirogabalin is eliminated primarily as the parent drug through renal excretion, it is important to assess the efficacy and safety of mirogabalin in patients with renal impairment24. However, enrollment was restricted to patients with creatinine clearance of ≥60 mL/min at baseline in the open label phase; therefore, the impact of renal impairment on the efficacy and safety of long‐term mirogabalin could not be assessed. In addition, patients had fewer restrictions on the use of concomitant medications during the open‐label extension study than during the double‐blind study; this might have impacted the safety profile and efficacy outcomes of long‐term treatment with mirogabalin.
In conclusion, the present long‐term extension study showed the safety and efficacy of a flexible dosing regimen of mirogabalin 10 mg or 15 mg twice daily in patients with DPNP.
Disclosure
MB has received consultancy fees and speaker fees from Daiichi Sankyo Co., Ltd. NM, MK, YW and SO are employees of Daiichi Sankyo Co, Ltd.
Supporting information
Figure S1 | Study design of extension study.
Acknowledgments
This study was funded by Daiichi Sankyo Co., Ltd., Tokyo, Japan. Writing and editorial support was provided by Claire Daniele, PhD, CMPP, of AlphaBioCom, LLC, King of Prussia, PA, and supported by Daiichi Sankyo, Inc. and Daiichi Sankyo Co., Ltd., Tokyo, Japan.
J Diabetes Investig 2020; 11: 693–698
Clinical Trial Registry
DS‐5565 Phase III Study for Diabetic Peripheral Neuropathic Pain NCT02318706
References
- 1. Tesfaye S, Vileikyte L, Rayman G, et al Painful diabetic peripheral neuropathy: consensus recommendations on diagnosis, assessment and management. Diabetes Metab Res Rev 2011; 27: 629–638. [DOI] [PubMed] [Google Scholar]
- 2. Peltier A, Goutman SA, Callaghan BC. Painful diabetic neuropathy. BMJ 2014; 348: g1799. [DOI] [PubMed] [Google Scholar]
- 3. Abbott CA, Malik RA, van Ross ER, et al Prevalence and characteristics of painful diabetic neuropathy in a large community‐based diabetic population in the U.K. Diabetes Care 2011; 34: 2220–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iqbal Z, Azmi S, Yadav R, et al Diabetic peripheral neuropathy: epidemiology, diagnosis, and pharmacotherapy. Clin Ther 2018; 40: 828–849. [DOI] [PubMed] [Google Scholar]
- 5. Alleman CJ, Westerhout KY, Hensen M, et al Humanistic and economic burden of painful diabetic peripheral neuropathy in Europe: A review of the literature. Diabetes Res Clin Pract 2015; 109: 215–225. [DOI] [PubMed] [Google Scholar]
- 6. Zelman DC, Brandenburg NA, Gore M. Sleep impairment in patients with painful diabetic peripheral neuropathy. Clin J Pain 2006; 22: 681–685. [DOI] [PubMed] [Google Scholar]
- 7. Eichholz M, Alexander AH, Cappelleri JC, et al Perspectives on the impact of painful diabetic peripheral neuropathy in a multicultural population. Clin Diabetes Endocrinol 2017; 3: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vileikyte L, Leventhal H, Gonzalez JS, et al Diabetic peripheral neuropathy and depressive symptoms: the association revisited. Diabetes Care 2005; 28: 2378–2383. [DOI] [PubMed] [Google Scholar]
- 9. Gore M, Brandenburg NA, Dukes E, et al Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J Pain Symptom Manage 2005; 30: 374–385. [DOI] [PubMed] [Google Scholar]
- 10. Domon Y, Arakawa N, Inoue T, et al Binding characteristics and analgesic effects of mirogabalin, a novel ligand for the alpha2delta subunit of voltage‐gated calcium channels. J Pharmacol Exp Ther 2018; 365: 573–582. [DOI] [PubMed] [Google Scholar]
- 11. Merante D, Rosenstock J, Sharma U, et al Efficacy of Mirogabalin (DS‐5565) on patient‐reported pain and sleep interference in patients with diabetic neuropathic pain: secondary outcomes of a phase II proof‐of‐concept study. Pain Med 2017; 18: 2198–2207. [DOI] [PubMed] [Google Scholar]
- 12. Vinik A, Rosenstock J, Sharma U, et al Efficacy and safety of mirogabalin (DS‐5565) for the treatment of diabetic peripheral neuropathic pain: a randomized, double‐blind, placebo‐ and active comparator‐controlled, adaptive proof‐of‐concept phase 2 study. Diabetes Care 2014; 37: 3253–3261. [DOI] [PubMed] [Google Scholar]
- 13. Baba M, Matsui N, Kuroha M, et al Mirogabalin for the treatment of diabetic peripheral neuropathic pain: A randomized, double‐blind, placebo‐controlled phase III study in Asian patients. J Diabetes Investig 2019; 10: 1299–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oquendo MA, Halberstam B, Mann JJ.Risk factors for suicidal behavior: utility and limitations of research instruments In: First MB. (ed.). Standardized Evaluation in Clinical Practice. Arlington: American Psychiatric Publishing, Inc, 2003; 103–130. [Google Scholar]
- 15. Bjelland I, Dahl AA, Haug TT, et al The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002; 52: 69–77. [DOI] [PubMed] [Google Scholar]
- 16. Melzack R. The short‐form McGill Pain Questionnaire. Pain 1987; 30: 191–197. [DOI] [PubMed] [Google Scholar]
- 17. Bauer CS, Nieto‐Rostro M, Rahman W, et al The increased trafficking of the calcium channel subunit alpha2delta‐1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2delta ligand pregabalin. J Neurosci 2009; 29: 4076–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen J, Li L, Chen SR, et al The alpha2delta‐1‐NMDA receptor complex is critically involved in neuropathic pain development and gabapentin therapeutic actions. Cell Rep 2018; 22: 2307–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Derry S, Cording M, Wiffen PJ, et al Pregabalin for pain in fibromyalgia in adults. Cochrane Database Syst Rev 2016; 9: Cd011790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Calandre EP, Rico‐Villademoros F, Slim M. Alpha2delta ligands, gabapentin, pregabalin and mirogabalin: a review of their clinical pharmacology and therapeutic use. Expert Rev Neurother 2016; 16: 1263–1277. [DOI] [PubMed] [Google Scholar]
- 21. Ogawa S, Satoh J, Arakawa A, et al Pregabalin treatment for peripheral neuropathic pain: a review of safety data from randomized controlled trials conducted in Japan and in the west. Drug Saf 2012; 35: 793–806. [DOI] [PubMed] [Google Scholar]
- 22. Onouchi K, Koga H, Yokoyama K, et al An open‐label, long‐term study examining the safety and tolerability of pregabalin in Japanese patients with central neuropathic pain. J Pain Res 2014; 7: 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Satoh J, Yagihashi S, Baba M, et al Efficacy and safety evaluation of pregabalin treatment over 52 weeks in patients with diabetic neuropathic pain extended after a double‐blind placebo‐controlled trial. J Diabetes Investig 2011; 2: 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kato M, Tajima N, Shimizu T, et al Pharmacokinetics and Safety of a Single Oral Dose of Mirogabalin in Japanese Subjects With Varying Degrees of Renal Impairment. J Clin Pharmacol 2018; 58: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Study design of extension study.


