Abstract
Aims/Introduction
We examined the association between hemoglobin A1c (HbA1c) and anemia, which was categorized into three groups according to mean corpuscular volume (MCV), as well as the association between hemoglobin in the non‐anemic range and HbA1c.
Materials and Methods
We used the 2016 health checkup data from 36,422 workers without diabetes. Anemic people were divided into three groups based on MCV: <80, 80–90 and >90 fL. Non‐anemic people were divided into four groups based on their hemoglobin levels. We carried out multiple linear regression models to estimate the means and 95% confidence intervals (CIs) of HbA1c.
Results
For men, 0.2% had anemia with MCV <80 fL, 0.5% had anemia with MCV 80–90 fL, 0.9% had anemia with MCV >90 fL and 98.4% had no anemia. For women, the corresponding values were 6.1, 6.4, 2.8 and 84.7%, respectively. The adjusted mean HbA1c (%) values for men with anemia with MCV <80, 80–90 and >90 fL were 5.67 (95% CI 5.60–5.74), 5.58 (95% CI 5.54–5.62) and 5.41 (95% CI 5.37–5.44), respectively. Among men without anemia, HbA1c (%) increased from 5.36 (95% CI 5.34–5.39) in those with hemoglobin ≥17.5 mg/dL to 5.45 (95% CI 5.45–5.46) in those with hemoglobin 13.0 to <14.5 mg/dL (P for trend <0.001). The HbA1c values were higher in men with anemia with MCV <80 fL or MCV 80–90 fL, but lower in men with MCV >90 fL, compared with non‐anemic men with hemoglobin 13.0 to <14.5 mg/dL (All P < 0.001). Similar findings were observed in women.
Conclusions
We observed elevated HbA1c among anemic people with MCV <80 fL or MCV 80–90 fL, and decreased HbA1c among anemic people with MCV >90 fL, suggesting that different types of anemia might influence HbA1c differently. In addition, non‐anemic people with lower hemoglobin levels had higher HbA1c levels, suggesting that hemoglobin levels are in need of consideration when interpreting HbA1c values among non‐anemic people.
Keywords: Anemia, Hemoglobin, Hemoglobin A1c
We observed elevated hemoglobin A1c (HbA1c) among anemic people with MCV <80 fL or MCV 80–90 fL, and decreased HbA1c among anemic people with MCV >90 fL, suggesting that iron deficiency anemia and non‐iron deficiency anemia might influence HbA1c differently. In addition, non‐anemic people with lower hemoglobin levels had higher HbA1c levels, suggesting that hemoglobin levels are in need of consideration when interpreting HbA1c values among non‐anemic people.

Introduction
Hemoglobin A1c (HbA1c) is a glycated hemoglobin formed by the non‐enzymatic attachment of glucose to the N‐terminal valine of the β‐chain of hemoglobin1. HbA1c reflects the average blood glucose levels in the previous 2–3 months2, and is a more convenient measurement than fasting plasma glucose, as it does not require fasting. Thus, HbA1c has been increasingly used for diagnosing diabetes and monitoring blood glucose levels in patients with diabetes2, 3, 4.
Anemia can lead to falsely increased or decreased HbA1c levels, depending on the type of anemia5. A systematic review of 12 studies among people without known diabetes reported that iron deficiency anemia (IDA) was associated with a spurious increase in HbA1c6. Some types of non‐iron deficiency anemia (non‐IDA; e.g., hemolytic anemia) have been reported to falsely lower HbA1c levels5. However, just three studies assessed HbA1c in association with non‐IDA7, 8, 9; thus, the impact of non‐IDA on HbA1c remains elusive6. In addition, most studies included in the review had a small sample size (10 studies had a sample size of 50–423) and did not adjust for potential confounders (seven studies). In addition to research on anemia6, several studies have examined the association between hemoglobin in the non‐anemic range and HbA1c among non‐diabetic people7, 10, 11, 12, 13. Although some studies have reported that HbA1c values increased with decreasing hemoglobin levels10, 11, 12, others reported a positive association7 or no association13 between hemoglobin and HbA1c. The conflicting findings make it impossible to draw any conclusion about the influence of hemoglobin in the non‐anemic range on HbA1c.
To fill the evidence gap, we carried out a cross‐sectional analysis of data from a large working population in Japan. We examined the association of HbA1c with anemia, which was divided into three groups according to MCV. In addition, we tested whether hemoglobin in the non‐anemic range is negatively associated with HbA1c.
Methods
Study design
The Japan Epidemiology Collaboration on Occupational Health (J‐ECOH) Study is an ongoing multicenter, health checkup‐based cohort study among workers from several companies in Japan and has been described elsewhere14, 15. Briefly, participants in the J‐ECOH Study underwent routine physical and laboratory examinations each year. Additionally, a questionnaire that covered medical history, health‐related lifestyle factors and work environment was completed. Annual health checkup data between January 2008 and December 2016 or between April 2008 and March 2017 were collected from >100,000 employees of 11 participating companies. The study protocol, including the consent procedure, was approved by the ethics committee of the National Center for Global Health and Medicine, Japan.
In the present study, the 2016 health checkup data (between January 2016 and December 2016 or between April 2016 and March 2017) were used to assess the association of anemia and hemoglobin in the non‐anemic range with HbA1c.
Study participants
Of the 73,198 people aged ≥20 years who attended the 2016 health checkup, we excluded people with diabetes (n = 5,352, fasting glucose ≥126 mg/dL, HbA1c ≥6.5% or under treatment for diabetes). We further excluded people who had missing data on HbA1c (n = 2,257), blood glucose (n = 5) or medical treatment of diabetes (n = 146), and who had blood drawn while they were in a non‐fasted state (n = 7,900). People with missing data on hemoglobin (n = 8,093) or MCV (n = 17,561) were also excluded. Some people met two or more of the exclusion criteria. Participants with a self‐reported history of kidney disease (n = 798) or cancer (n = 438) were further excluded to eliminate the influence of these diseases on hemoglobin. We excluded those with missing data on potential confounders: body mass index (BMI; n = 6) and smoking status (n = 220). Finally, 36,422 participants (31,003 men and 5,419 women) remained for analysis.
Health checkup
Body height and weight were measured using a scale while the participants wore light clothes and no shoes. BMI was calculated as weight in kilograms divided by the square of height in meters. Smoking status was identified by a self‐administered questionnaire. Plasma glucose was measured with either the enzymatic or glucose oxidase peroxidative electrode method. HbA1c was measured using a latex agglutination immunoassay, high‐performance liquid chromatography or the enzymatic method. All of the laboratories involved in the health checkups of the participating companies received satisfactory scores (rank A or a score >95/100) from external quality control agencies.
Anemia
Anemia was defined as a hemoglobin level <13 g/dL for men and <12 g/dL for women according to the World Health Organization criteria16. We divided people with anemia into three groups based on MCV: <80, 80–90 and >90 fL, according to previous studies17, 18, 19, 20. People without anemia were divided into four groups based on their hemoglobin levels (13.0 to <14.5, 14.5 to <16.0, 16.0 to<17.5 and ≥17.5 g/dL in men; 12.0 to <13.0, 13.0 to <14.0, 14.0 to <15.0 and ≥15.0 g/dL in women).
Statistical analysis
Characteristics of the study participants are described as the means and standard deviations for continuous variables, and percentages for categorical variables. We carried out multiple linear regression models to estimate the means and 95% confidence intervals (CIs) of HbA1c according to three anemia groups (MCV <80, 80–90 and >90 fL) and hemoglobin levels (in the non‐anemic range). We first adjusted for age (years) and worksite, and then further adjusted for BMI (kg/m2) and current smoking status (yes or no)21, 22. In addition, we adjusted for fasting plasma glucose (FPG; mg/dL), which has been established as a determining factor of HbA1c23. For people without anemia, trend associations were assessed by treating the categories of hemoglobin as ordinal numbers and modeling this variable as a continuous variable.
Alcohol drinking can be a potentially important confounder, as higher alcohol consumption has been associated with lower HbA1c levels24, and heavy drinking can lead to macrocytic anemias25. In the J‐ECOH Study, questions on alcohol consumption were markedly different across the participating companies. Thus, we carried out a sensitivity analysis with an additional adjustment for alcohol drinking (non‐drinker, light drinkers [<46 g ethanol/day], heavy drinkers [≥46 g ethanol/day]) using data of one major company [n = 17,770])26. To exclude the impact of heavy drinking on the association between hemoglobin and HbA1c, we further repeated the analysis after excluding heavy drinkers (≥46 g ethanol/day). All statistical analyses were carried out using SAS version 9.3 (SAS Institute, Cary, NC, USA). A two‐sided P < 0.05 was considered statistically significant.
Results
For men, 0.2% had anemia with MCV <80 fL, 0.5% had anemia with MCV 80–90 fL, 0.9% had anemia with MCV >90 fL and 98.4% had no anemia. For women, the corresponding values were 6.1, 6.4, 2.8 and 84.7%, respectively. Table 1 shows the characteristics of the study participants. Among people without anemia, those with higher hemoglobin levels were more likely to be current smokers, and had higher mean values of BMI and FPG.
Table 1.
Characteristics of study participants according to hemoglobin category
| <13.0 | Hemoglobin (g/dL) | ||||||
|---|---|---|---|---|---|---|---|
| 13.0 to <14.5 | 14.5 to <16.0 | 16.0 to <17.5 | ≥17.5 | ||||
| MCV (fL) | |||||||
| <80 | 80–90 | >90 | |||||
| Men | |||||||
| n | 51 | 152 | 272 | 6,537 | 17,440 | 6,139 | 412 |
| Age (years) | 48.5 ± 11.7 | 51.5 ± 10.6 | 53.1 ± 8.8 | 48.1 ± 10.3 | 45.5 ± 10.4 | 44.5 ± 10.5 | 46.8 ± 9.8 |
| BMI (kg/m2) | 22.6 ± 3.4 | 22.4 ± 3.0 | 22.2 ± 3.0 | 22.8 ± 2.9 | 23.6 ± 3.2 | 24.8 ± 3.7 | 26.0 ± 4.2 |
| FPG (mg/dL) | 99.3 ± 8.5 | 98.2 ± 9.4 | 97.1 ± 10.0 | 97.2 ± 9.2 | 97.4 ± 9.5 | 98.3 ± 10.2 | 98.6 ± 11.2 |
| HbA1c (%) | 5.71 ± 0.33 | 5.63 ± 0.34 | 5.44 ± 0.38 | 5.47 ± 0.30 | 5.43 ± 0.31 | 5.42 ± 0.33 | 5.44 ± 0.36 |
| Current smoker (%) | 27.5 | 13.2 | 34.6 | 29.4 | 34.2 | 40.3 | 54.1 |
| Alcohol drinker (%)† | |||||||
| Non‐drinker | 41.9 | 40.4 | 26.0 | 28.2 | 29.0 | 30.9 | 22.5 |
| Drinker (<46 g ethanol/day) | 51.6 | 54.6 | 63.8 | 62.6 | 62.4 | 59.8 | 62.6 |
| Drinker (≥46 g ethanol/day) | 6.5 | 5.1 | 10.2 | 9.2 | 8.6 | 9.3 | 14.8 |
| <12.0 | 12.0–<13.0 | 13.0–<14.0 | 14.0–<15.0 | ≥15.0 | |||
|---|---|---|---|---|---|---|---|
| MCV (fL) | |||||||
| <80 | 80–90 | >90 | |||||
| Women | |||||||
| n | 330 | 348 | 153 | 1,437 | 2,019 | 963 | 169 |
| Age (years) | 43.4 ± 6.6 | 43.4 ± 6.6 | 46.3 ± 11.3 | 45.3 ± 11.1 | 46.2 ± 11.1 | 47.4 ± 10.5 | 48.9 ± 9.5 |
| BMI (kg/m2) | 22.2 ± 3.8 | 21.5 ± 3.4 | 21.0 ± 3.0 | 21.6 ± 3.4 | 21.9 ± 3.6 | 22.4 ± 3.9 | 23.6 ± 4.6 |
| FPG (mg/dL) | 92.5 ± 7.4 | 91.2 ± 7.7 | 90.0 ± 7.4 | 91.4 ± 8.1 | 92.1 ± 8.8 | 93.9 ± 9.6 | 95.7 ± 10.5 |
| HbA1c (%) | 5.57 ± 0.29 | 5.51 ± 0.31 | 5.31 ± 0.34 | 5.42 ± 0.32 | 5.41 ± 0.33 | 5.40 ± 0.33 | 5.41 ± 0.33 |
| Current smoker (%) | 13.0 | 8.9 | 12.4 | 9.6 | 10.0 | 13.5 | 22.5 |
| Alcohol drinker (%)† | |||||||
| Non‐drinker | 65.2 | 72.0 | 67.6 | 66.5 | 62.2 | 66.4 | 54.2 |
| Drinker (<46 g ethanol/day) | 33.8 | 28.0 | 29.4 | 31.9 | 36.4 | 32.1 | 45.8 |
| Drinker (≥46 g ethanol/day) | 1.0 | 0 | 2.9 | 1.6 | 1.4 | 1.4 | 0 |
Data were available for people in one major company (15,039 men and 2,731 women). BMI, body mass index; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; MCV, mean corpuscular volume.
People with anemia versus those without anemia
Figure 1 and Table S1 show that both men and women with anemia with MCV <80 fL or MCV 80–90 fL had higher mean values of HbA1c than people without anemia after adjusting for age, worksite, BMI, smoking and fasting plasma glucose. The findings did not change after further adjustment for alcohol consumption (Table S2). Overall, men and women with anemia with MCV >90 fL had lower adjusted HbA1c values than people without anemia. The absolute difference in HbA1c (%) between men with anemia with MCV >90 fL and those with pre‐anemia (hemoglobin 13.0 to <14.5 g/dL) was −0.05 (95% CI −0.08, −0.02). For women, the corresponding difference was −0.07 (95% CI −0.02, −0.11). People with anemia with MCV >90 fL had lower HbA1c values than people with anemia with MCV <80 fL or MCV 80–90 fL. The absolute difference in HbA1c (%) between men with anemia with MCV >90 fL and those with anemia with MCV <80 fL or MCV 80–90 fL was −0.27 (95% CI −0.34, −0.19) and −0.18 (95% CI −0.23, −0.12), respectively. For women, the corresponding values were −0.22 (95% CI −0.27, −0.17) and −0.19 (95% CI −0.23, −0.14), respectively.
Figure 1.
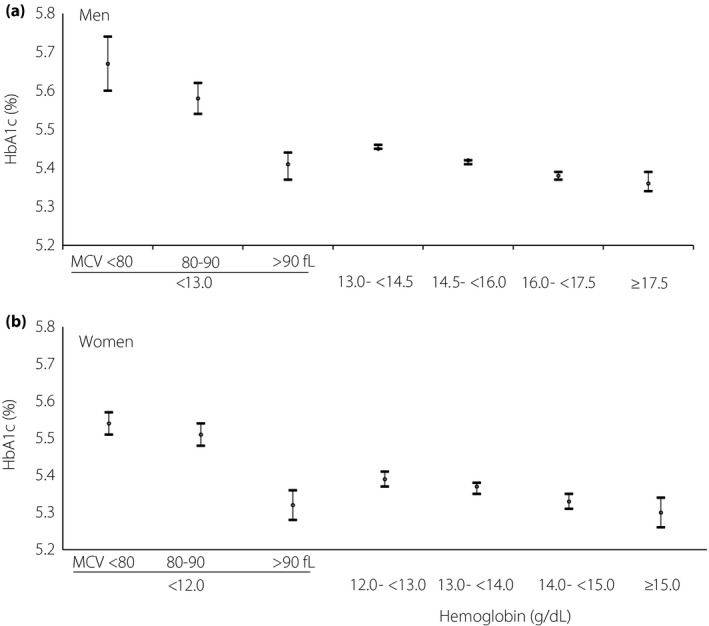
Adjusted mean (95% confidence interval) of hemoglobin A1c (HbA1c; %) according to hemoglobin category for (a) men and (b) women. Adjusted for age (years), worksite, body mass index (kg/m2), current smoker (yes or no) and fasting plasma glucose (mg/dL). MCV, mean corpuscular volume.
Hemoglobin and HbA1c among people without anemia
Among people without anemia, a higher HbA1c level was associated with lower hemoglobin levels (Figure 1). After adjusting for age, worksite, BMI, smoking status and FPG (Table S1), the values of HbA1c (%) increased from 5.36 (5.34–5.39) in men with hemoglobin ≥17.5 mg/dL to 5.45 (95% CI 5.45–5.46) in those with hemoglobin 13.0 to <14.5 mg/dL (P for trend <0.001). For women, the adjusted values of HbA1c (%) increased from 5.30 (95% CI 5.26–5.34) in those with hemoglobin ≥15.0 mg/dL to 5.39 (95% CI 5.37–5.41) in those with hemoglobin 12.0 to <13.0 mg/dL (P for trend <0.001).
Sensitivity analysis
There is no material difference in the prevalence of anemia among male non‐drinkers, light drinkers and heavy drinkers, with the prevalence rate being 1.9, 1.6 and 1.5%, respectively. For women, the corresponding values are 17.5, 15.2 and 10.8%, respectively. The associations of HbA1c with anemia and hemoglobin in the non‐anemic range in the sensitivity analysis are similar with that of the main analysis (Table S2).
Discussion
In the present study, among a large working population in Japan, we found that both men and women with anemia with MCV <80 fL or MCV 80–90 fL had higher HbA1c levels than people without anemia, especially for those with MCV <80 fL. However, both men and women with anemia with MCV >90 fL had lower HbA1c than people without anemia. We observed an inverse association between HbA1c and hemoglobin in men and women without anemia. To our knowledge, this is the first study to simultaneously investigate the association of HbA1c with anemia and hemoglobin in the non‐anemic range in Japan.
In the J‐ECOH Study, serum ferritin was not available, because it is not a mandatary item in the annual health checkup. However, as part of the J‐ECOH Study, a nutritional epidemiological survey, named the Furukawa Nutrition and Health Study27, was carried out in one of the companies participating in the J‐ECOH Study, and additional blood tests, such as serum ferritin, were carried out. We extracted the 2012–2013 Furukawa Nutrition and Health Study survey data, in which serum ferritin was measured. Among 1,977 workers who had data on serum ferritin, 36 people had anemia. It was found that all eight anemic people with MCV <80 fL had serum ferritin <15 μg/L (IDA)28, most anemic people with MCV 80–90 fL had IDA (13/16) and most anemic people with MCV > 90 fL had non‐IDA (8/12). IDA and the thalassemia trait are the commonest causes of microcytic anemia (MCV <80 fL)29. However, it was reported that the thalassemia trait is rare (<1/1,000) in the Japanese general population30. Thus, in the present study, anemic people with MCV <80 fL might consist mainly of people with IDA. Anemia with MCV 80–90 fL represents mostly IDA, and anemia with MCV >90 fL represents mostly non‐IDA.
The present finding of a higher HbA1c associated with anemia with MCV <80 fL (mainly IDA) or MCV 80–90 fL (mostly IDA) was generally in line with the findings of the previous systematic review, which showed that IDA spuriously increased HbA1c6. After the publication of the systematic review, one large study of 11,472 Korean adults also showed that people with IDA had higher HbA1c values than people without anemia (5.7 vs 5.6%)31. The present study is based on data from a large working population (n = 36, 422), controlled for potential confounders, such as smoking, BMI and alcohol consumption, and further adjusted for FPG, which is a glycemic determinant of HbA1c. Findings from the present study and previous studies6, 31 provide evidence that people with IDA had higher HbA1c values.
The exact mechanism through which IDA falsely increases HbA1c levels remains unclear. One proposed explanation is that iron deficiency might alter the quaternary structure of the hemoglobin molecule, which might result in more rapid glycation of the β‐globin chains and thus lead to higher HbA1c values32. Another suggested mechanism is that the rise in HbA1c might be a result of reduced red blood cell production in IDA, leading to a higher than normal mean age of red blood cells (exposing people with IDA to a higher glycation time than people without anemia)32. However, questions have been raised regarding this mechanism, as some studies have shown that people with IDA have normal or even shortened red blood cell lifespans33, 34. Because limited work has been carried out on the mechanism of increased HbA1c in IDA32, further studies to confirm and elucidate the effect of IDA on HbA1c are required.
We found that anemic people with MCV >90 fL (mostly non‐IDA) had lower HbA1c levels than people without anemia. Similarly, two studies from Korea and the USA observed lower HbA1c values in people with non‐IDA than in non‐anemic people (5.44 vs 5.59%, 5.16 vs 5.31%, respectively)7, 31. It was hypothesized that some subtypes of non‐IDA, such as hemolytic anemia, might decrease HbA1c as a result of a shortened red blood cell lifespan (more rapid erythrocyte turnover)35. In contrast, non‐IDA as a result of vitamin B12 or folic acid deficiencies have been associated with higher HbA1c levels32, 36. Further identification of non‐IDA subtypes might help to confirm the findings of the present study and previous studies7, 31. Given that limited data exist on the effect of non‐IDA on HbA1c, more research on this topic is required.
The present finding that hemoglobin in the non‐anemic range was inversely associated with HbA1c is consistent with several previous studies10, 11, 12. Using data from non‐anemic people who attended health checkups in the hospital setting, two cross‐sectional studies in Japan10 (n = 32,605) and Korea11 (n = 87,284) found that HbA1c (%) increased by 0.03 and 0.04 per 1‐g/dL decrease in hemoglobin, respectively. One recent study using data from the National Health and Nutrition Examination Survey (NHANES) 1999–2014 (n = 22,974) showed that HbA1c (%) increased by 0.05 when hemoglobin decreased by 1 g/dL12. In contrast, an earlier study using the NHANES 1999–2002 data (n = 8,296) reported that HbA1c (%) increased by 0.04 when hemoglobin increased by 1 g/dL7, which is the only study that reported a positive association between HbA1c and hemoglobin. The present study extended the findings of an inverse association between hemoglobin and HbA1c to an apparently healthy working population (HbA1c [%] increased by approximately 0.03 for each 1 g/dL decrease in hemoglobin; Table S1). Taken together, the findings from the present study and previous studies10, 11, 12 suggest that hemoglobin levels are in need of consideration when interpreting HbA1c values among non‐anemic people.
The present study used contemporary health checkup data from a large working population, separated people with anemia into three groups (MCV <80 [mainly IDA], 80–90 [mostly IDA] and >90 fL [mostly non‐IDA]), and adjusted for potential confounders and FPG. However, several limitations need to be considered. First, because of the lack of data on serum ferritin and other causes of microcytic anemia, such as lead toxicity and hematological disorders, we cannot further confirm the diagnosis of IDA. Second, the relatively small number of people with anemia precluded us from examining the association between the severity of anemia and HbA1c. Third, we did not have data on the duration of anemia, which might affect hemoglobin. Fourth, hemoglobin variants can interfere with HbA1c measurement37. However, we do not have data on hemoglobin variants, which makes us unable to exclude its impact on the association of HbA1c with anemia and hemoglobin in the non‐anemic range. Finally, because the present study participants were workers in Japan, caution should be exercised in generalizing the present findings to the general population or other racial/ethnic groups.
In conclusion, we observed elevated HbA1c levels among anemic people with MCV <80 fL (mainly IDA) or MCV 80–90 fL (mostly IDA) and decreased HbA1c levels among anemic people with MCV >90 fL (mostly non‐IDA), suggesting that IDA and non‐IDA might influence HbA1c differently. Caution should be exercised when assessing people with anemia when their HbA1c levels are near 5.7 or 6.5%, which are cut‐off values for prediabetes and diabetes, respectively. In addition, non‐anemic workers with lower hemoglobin levels had higher levels of HbA1c. For a more accurate interpretation of HbA1c among nonanemic people, hemoglobin levels should be taken into consideration in the future.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1 | Adjusted mean (95% confidence interval) of hemoglobin A1c (%) according to hemoglobin category.
Table S2 | Adjusted mean (95% confidence interval) of hemoglobin A1c (%) according to hemoglobin category (sensitivity analysis).
Acknowledgments
This research was supported by the Industrial Health Foundation, Industrial Disease Clinical Research Grants (grant numbers 140202‐01, 150903‐01, 170301‐01), Japan Society for the Promotion of Science KAKENHI (grant number 16H05251), and Grant of National Center for Global Health and Medicine (grant number 28‐Shi‐1206).
J Diabetes Investig 2020; 11: 719–725
References
- 1. Peterson KP, Pavlovich JG, Goldstein D, et al What is hemoglobin A1c? An analysis of glycated hemoglobins by electrospray ionization mass spectrometry. Clin Chem 1998; 44: 1951–1958. [PubMed] [Google Scholar]
- 2. American Diabetes Association . Standards of medical care in diabetes–2018. Diabetes Care 2018; 41(Suppl 1): 1–159.29263190 [Google Scholar]
- 3. World Health Organization . Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation. Geneva, 2011. [PubMed] [Google Scholar]
- 4. The Japan Diabetes Society . Evidence‐Based Practice Guideline for the Treatment of Diabetes in Japan 2013. Tokyo: Nankodo, 2013. (In Japanese). [Google Scholar]
- 5. Hellman R. When are HbA1C values misleading? AACE Clin Case Rep 2016; 2: e377–e379. [Google Scholar]
- 6. English E, Idris I, Smith G, et al The effect of anaemia and abnormalities of erythrocyte indices on HbA1c analysis: a systematic review. Diabetologia 2015; 58: 1409–1421. [DOI] [PubMed] [Google Scholar]
- 7. Ford ES, Cowie CC, Li C, et al Iron‐deficiency anemia, non‐iron‐deficiency anemia and HbA1c among adults in the US. J Diabetes 2011; 3: 67–73. [DOI] [PubMed] [Google Scholar]
- 8. Hardikar PS, Joshi SM, Bhat DS, et al Spuriously high prevalence of prediabetes diagnosed by HbA(1c) in young indians partly explained by hematological factors and iron deficiency anemia. Diabetes Care 2012; 35: 797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gram‐Hansen P, Eriksen J, Mourits‐Andersen T, et al Glycosylated haemoglobin (HbA1c) in iron‐ and vitamin B12 deficiency. J Intern Med 1990; 227: 133–136. [DOI] [PubMed] [Google Scholar]
- 10. Nakagami T, Oya J, Kasahara T, et al Effect of hemoglobin levels and sex on HbA1c levels among Japanese Population. Diabetes Endocrinol 2017; 1: 3. [Google Scholar]
- 11. Bae JC, Suh S, Jin SM, et al Hemoglobin A1c values are affected by hemoglobin level and gender in non‐anemic Koreans. J Diabetes Investig 2014; 5: 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang D, Wang Y, Madhu S, et al Total hemoglobin count has significant impact on A1C ‐ Data from National Health and Nutrition Examination Survey 1999–2014. Prim Care Diabetes 2019; 13: 316–323. [DOI] [PubMed] [Google Scholar]
- 13. Grossman A, Gafter‐Gvili A, Schmilovitz‐Weiss H, et al Association of glycated hemoglobin with hemoglobin levels in elderly nondiabetic subjects. Eur J Intern Med 2016; 36: 32–35. [DOI] [PubMed] [Google Scholar]
- 14. Hu H, Nagahama S, Nanri A, et al Duration and degree of weight change and risk of incident diabetes: Japan Epidemiology Collaboration on Occupational Health Study. Prev Med 2017; 96: 118–123. [DOI] [PubMed] [Google Scholar]
- 15. Hu H, Kurotani K, Sasaki N, et al Optimal waist circumference cut‐off points and ability of different metabolic syndrome criteria for predicting diabetes in Japanese men and women: Japan Epidemiology Collaboration on Occupational Health Study. BMC Public Health 2016; 16: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization (WHO) . Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity In Vitamin and Mineral Nutrition Information System. Geneva: WHO, 2011. [Google Scholar]
- 17. Hubbard JR, Short D. Primary Care Medicine for Psychiatrists: A Practitioner’s Guide, 1st edn New York, NY: Plenum Pub Corp, 1997. [Google Scholar]
- 18. Berdanier CD. Handbook of Nutrition and Food, 1st edn Boston, MA: CRC Press, 2002. [Google Scholar]
- 19. Hoang NTD, Orellana L, Le TD, et al Anaemia and its relation to demographic, socio‐economic and anthropometric factors in rural primary school children in Hai Phong City, Vietnam. Nutrients 2019; 11: 1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gorakshakar AC, Colah RB. Is RBC discrimination index suitable for differentiating between α‐ and β‐ thalassemias? Indian J Hum Genet 2011; 17: 115–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Skjelbakken T, Dahl IM, Wilsgaard T, et al Changes in haemoglobin levels according to changes in body mass index and smoking habits, a 20‐year follow‐up of a male cohort: the Tromsø Study 1974–1995. Eur J Epidemiol 2006; 21: 493–499. [DOI] [PubMed] [Google Scholar]
- 22. Shimakawa T, Bild DE. Relationship between hemoglobin and cardiovascular risk factors in young adults. J Clin Epidemiol 1993; 46: 1257–1266. [DOI] [PubMed] [Google Scholar]
- 23. Jansen H, Stolk RP, Nolte IM, et al Determinants of HbA1c in nondiabetic Dutch adults: genetic loci and clinical and lifestyle parameters, and their interactions in the Lifelines Cohort Study. J Intern Med 2013; 273: 283–293. [DOI] [PubMed] [Google Scholar]
- 24. Hong JW, Noh JH, Kim DJ. Association between Alcohol Intake and Hemoglobin A1c in the Korean Adults: the 2011–2013 Korea National Health and Nutrition Examination Survey. PLoS ONE ONE 2016; 11: e0167210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nagao T, Hirokawa M. Diagnosis and treatment of macrocytic anemias in adults. J Gen Fam Med 2017; 18: 200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuwahara K, Honda T, Nakagawa T, et al Associations of leisure‐time, occupational, and commuting physical activity with risk of depressive symptoms among Japanese workers: a cohort study. Int J Behav Nutr Phys Act 2015; 12: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mizoue T, Kochi T, Akter S, et al Low serum 25‐hydroxyvitamin D concentrations are associated with increased likelihood of having depressive symptoms among Japanese workers. J Nutr 2015; 145: 541–546. [DOI] [PubMed] [Google Scholar]
- 28. World Health Organization . Iron Deficiency Anaemia: assessment, Prevention and Control. [online] https://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/WHO_NHD_01.3/en/ Accessed April 5, 2019.
- 29. Hoffmann JJ, Urrechaga E, Aguirre U. Discriminant indices for distinguishing thalassemia and iron deficiency in patients with microcytic anemia: a meta‐analysis. Clin Chem Lab Med 2015; 53: 1883–1894. [DOI] [PubMed] [Google Scholar]
- 30. Yamashiro Y, Hattori Y. Hemoglobinopathies in Japan: characteristics and comparison with those of other ethnic groups. Rinsho Ketsueki 2015; 56: 752–759. [DOI] [PubMed] [Google Scholar]
- 31. Hong JW, Ku CR, Noh JH, et al Association between the presence of iron deficiency anemia and hemoglobin A1c in Korean adults: the 2011–2012 Korea National Health and Nutrition Examination Survey. Medicine 2015; 94: e825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ahmad J, Rafat D. HbA1c and iron deficiency: a review. Diabetes Metab Syndr 2013; 7: 118–122. [DOI] [PubMed] [Google Scholar]
- 33. Temperley IJ, Sharp AA. The life span of erythrocytes in iron‐deficiency anemia. J Clin Pathol 1962; 15: 346–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Verloop MC, Van der Wolk M, et al Radioactive iron studies in patients with iron deficiency anemia with concurrent abnormal hemolysis. Blood 1960; 15: 791–806. [PubMed] [Google Scholar]
- 35. Lum G. Artefactually low hemoglobin A1c in a patient with hemolytic anemia. Lab Med 2010; 4: 267–270. [Google Scholar]
- 36. Maheshwari VD, Capoor S, Chaturvedi S, et al Impact of iron and vitamin B12 anaemia at glycosylated hemoglobin level: a case control study. IOSR J Dental Med Sci 2017; 16: 1–4. [Google Scholar]
- 37. Little RR, Roberts WL. A review of variant hemoglobins interfering with hemoglobin A1c measurement. J Diabetes Sci Technol 2009; 3: 446–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Adjusted mean (95% confidence interval) of hemoglobin A1c (%) according to hemoglobin category.
Table S2 | Adjusted mean (95% confidence interval) of hemoglobin A1c (%) according to hemoglobin category (sensitivity analysis).


