Abstract
This study describes the first full genomic sequence of an mcr-9 and blaVIM-4-carrying multidrug-resistant Enterobacter hormaechei clinical isolate from Egypt. The strain was isolated in April 2015 from the sputum of a patient in Cairo, Egypt. The mcr-9 and blaVIM-4 genes were identified by PCR screening and DNA sequencing; the isolate was subjected to antimicrobial susceptibility testing, conjugation experiments, and whole genomic sequencing. mcr-9 and blaVIM-4 were carried by an IncHI2 plasmid, pAMS-38a (281,121 bp in size); the plasmid also carried genes conferring resistance against sulfonamides (sul1), quinolones (qnrA1), trimethoprim (dfrA1), β-lactams (blaTEM-1B), aminoglycosides (aac (6’)-II, aadA23, aadA2b, and ant(2’’)-Ia). The strain was susceptible to colistin (MIC, <0.25 μg/mL); this could be due to the absence of the qseC/qseB regulatory system located downstream of mcr-9 in Enterobacterales, which is involved in the induction of colistin-resistance. The genetic context of mcr-9 and blaVIM-4 was identified as IS1-mcr-9-IS903-pcoS-∆pcoE-rcnA and intI1-blaVIM-4—aac (6’)-II-dfrA1-∆aadA23-smr-ISPa21-qacE∆1, respectively. This is the first report of an mcr-9 and blaVIM-4 /IncHI2-carrying multidrug-resistant E. hormaechei clinical isolate from Africa and the Middle East. Plasmids of the IncHI2 group and the two insertion sequences (IS1, and IS903) might be the main vehicles for dissemination of mcr-9. Further screening for mcr-9 is essential for identifying its incidence and to prevent its dissemination.
Keywords: mcr-9, Egypt, VIM-4, IncHI2, WGS, Enterobacter hormaechei
1. Introduction
Problems associated with the development and spread of antimicrobial resistance (AMR) in clinical practice are increasing and are currently being viewed as a major threat to public health globally [1,2]. Carbapenem resistance in Enterobacterales is mediated through the expression of a large set of carbapenemase-encoding genes: particularly, Ambler class A β-lactamases (KPC, FRI, GES, SME), Ambler class B metallo-β-lactamases (VIM, NDM, IMP, SIM, GIM, SPM), and Ambler class D enzymes (OXA-type) [3,4]. Verona integron-mediated metallo-β-lactamase (VIM) is located as a gene cassette in a class 1 integron with genes for resistance against trimethoprim or aminoglycosides [5]. The blaVIM-4 gene encodes resistance against all β-lactams except aztreonam and was previously reported in Pseudomonas aeruginosa, Enterobacter cloacae, or other Enterobacterales from Egypt, Kuwait, and United Arab Emirates [5,6,7,8]. Recently, we reported blaVIM-2 and blaVIM-24 from clinical P. aeruginosa strains from Egypt [5].
Colistin is one of the last-resort antibiotics for treating infections caused by carbapenem resistant Gram-negative bacteria. The activity and efficacy of colistin has been challenged by the global spread of the self-transmissible mobilized colistin-resistance genes (mcr). In 2016, the first mcr-1 gene was reported from Escherichia coli and Klebsiella pneumoniae isolated from patients, food, and animals in China [9]. MCR-1 acts by modifying the lipid A part of the lipopolysaccharide in Gram-negative bacteria by adding phosphoethanolamine, which reduces the binding affinity to colistin [9]. As of 7 January 2020, 10 variants of the mcr genes are available in the Bacterial Antimicrobial Resistance Reference Gene Database (https://www.ncbi.nlm.nih.gov/pathogens/isolates#/refgene/); however, only mcr-1 has been previously reported from Egypt [10], and little is yet known about the prevalence of other mcr variants in Egypt.
Here, for the first time, we describe the complete genomic sequence of an E. hormaechei clinical isolate carrying the mcr-9 and blaVIM-4 genes on an IncHI2 plasmid from Africa and the Middle East generated using the Illumina MiniSeq and Oxford Nanopore sequencing platforms.
2. Materials and Methods
2.1. Bacterial Isolation and Identification
The E. hormaechei strain AMS-38 was isolated from the sputum swab of a patient in Cairo, Egypt in April 2015. The strain was identified by 16S ribosomal RNA gene sequencing using primers 27F and 1492R [5]. PCR and DNA sequencing were conducted to identify extended-spectrum β-lactamases, carbapenemase-encoding genes, 16S rRNA methylases, plasmid-mediated quinolone-resistance genes, and plasmid-mediated colistin-resistance genes (mcr-1–mcr-8) as previously described [5]. Additionally, mcr-9 was detected by primers mcr-9-forward: 5’-CGGTACCGCTACCGCAATAT-3’ and mcr-9-reverse: 5’-ATAACAGCGAGACACCGGTT-3’ [11].
2.2. Antimicrobial Susceptibility Testing (AST)
A standardized broth microdilution (BMD) technique was used to determine the minimum inhibitory concentration (MIC) of various antimicrobial agents according to the standards and interpretive criteria described by the Clinical and Laboratory Standards Institute (CLSI, document M100-S24) and European Committee on Antimicrobial Susceptibility Testing (EU-CAST) (for colistin and tigecycline breakpoints) (http://www.eucast.org). For all experiments, the purified powder of each antibiotic was diluted following CLSI recommendations. E. coli ATCC 25922 was used as a control.
2.3. Filter-Mating Conjugation
A Conjugation experiment was performed using the AMS-38 strain and the azide resistant E. coli J53 strain as the donor and recipient, respectively. Due to the thermo-susceptibility of IncHI2 plasmids (i.e., conjugation is impaired at 37 ˚C), we performed this experiment at 25–30 ˚C [12]. The transconjugants were selected on LB agar plates containing 100 μg/mL ampicillin and 100 μg/mL sodium azide. Colony-direct PCR was performed using VIM-F and VIM-R [5] and mcr-9-forward and mcr-9-reverse [11] primers to confirm the transfer of the plasmid carrying mcr-9 and blaVIM-4.
2.4. Whole Genome Sequencing (WGS) and Analysis
An overnight bacterial culture of the AMS-38 strain was used to extract the total genomic DNA (gDNA) using a standard proteinase K method. The quality of the isolated gDNA was assessed using a Microdrop (Thermo Scientific™, Multiskan™ Sky Microplate Spectrophotometer, Waltham, MA, USA), Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA), and a dsDNA fluorescent dye method using DeNovix (Wilmington, DE, USA). The sequencing library was constructed using a Nextera DNA Flex Library Prep Kit (Illumina, San Diego, CA, USA.) for Illumina MiniSeq. The DNA library for Oxford Nanopore sequencing was prepared using 400 ng of gDNA according to the Rapid Barcoding Sequencing kit (SQK-RBK004) (Oxford Nanopore Technologies, Oxford, UK). The sequencing libraries were purified using a Promega Size-Selective Purification System (Promega, Madison, WI, USA), and 11 μL of the sequencing library was loaded onto a FLO-MIN106 flow cell and sequenced with the GridION device (Oxford Nanopore Technologies) for 48 h. Hybrid assembly of both Illumina and Nanopore reads was performed using Flye v2.6 (https://github.com/fenderglass/Flye). Additionally, Pilon-v1.23 [13] was used to improve the genome assembly. The assembly and annotation of pAMS-38a were performed using DFAST [14]. The identification of AMS-38 was done with JSpeciesWS (http://jspecies.ribohost.com/jspeciesws/#analyse) using Tetra Correlation Search (TCS) against reference database Genome DB and the pairwise comparison using Average Nucleotide Identity calculation based on BLAST+ (ANIb). Antimicrobial resistance genes were identified by ResFinder-3.2 available at Center for Genomic Epidemiology (https://cge.cbs.dtu.dk/services/ResFinder/). The plasmid Inc groups, pMLST and multilocus sequence typing (MLST) were identified by PlasmidFinder (https://cge.cbs.dtu.dk/services/PlasmidFinder/), (https://cge.cbs.dtu.dk/services/pMLST/) and MLST 2.0 software (https://cge.cbs.dtu.dk/services/MLST/), respectively. The plasmids that were highly similar to pAMS-38a were detected by a Mash (v2.1.1) [15] distance search against the PLSDB v. 2019_10_07 [16]. The BRIG tool was used to perform a circular comparison between the complete sequence of pAMS-38 and the highly similar plasmids detected by a Mash distance search [17]. EasyFig tool was used to visualize and compare the genetic environment of mcr-9 in its harboring plasmids (http://mjsull.github.io/Easyfig/).
2.5. Nucleotide Sequence Accession Numbers
The complete genome sequence of AMS-38 and the plasmids pAMS-38a, pAMS-38b, pAMS-38c, pAMS-38d were submitted to DDBJ/ENA/GenBank under BioProject ID: PRJNA616711.
3. Results and Discussion
3.1. AST of E. hormaechei AMS-38, and Conjugation of mcr-9 and blaVIM-4-Carrying Plasmid
The sequencing of the 16S ribosomal RNA gene identified a strain belonging to the E. cloacae complex. BMD showed that the strain AMS-38 was resistant to aztreonam (MIC, 32 μg/mL), ceftazidime (MIC, >256 μg/mL), ceftriaxone (MIC, >256 μg/mL), meropenem (MIC, 64 μg/mL), ertapenem (MIC, >64 μg/mL), kanamycin (MIC, 64 μg/mL), ciprofloxacin (MIC, 2 μg/mL), fosfomycin (MIC, 128 μg/mL), tetracycline (MIC, 16 μg/mL), and tigecycline (MIC, 4 μg/mL). The strain showed intermediate resistance to chloramphenicol (MIC, 16 μg/mL) and sensitivity to colistin (MIC, < 0.25 μg/mL). PCR screening and DNA sequencing identified the presence of the metallo-β-lactamase genes, blaVIM-4, blaTEM-1, AmpC β-lactamase, the newly described inducible colistin-resistance gene mcr-9, the fosfomycin glutathione-S-transferase gene fosA, and the plasmid-mediated quinolone-resistance gene qnrA1. The results showed that the mcr-9 and blaVIM-4-carrying plasmid was successfully transferred to E. coli J53. The intact conjugative transfer locus in pAMS-38a was similar to that locus in plasmid pME-1a (accession no. CP041734), and found in two distinct regions, Tra1 and Tra2 illustrating the potential for horizontal gene transfer to other Gram-negative bacteria.
3.2. Characterization of the Genome of E. hormaechei AMS-38
By combining the short reads obtained from Illumina MiniSeq and the long reads obtained from Oxford Nanopore sequencing, we obtained assemblies with high-quality and sufficient for closing the genome and the plasmids contained in the strain. The chromosome of the AMS-38 strain was 4,914,941 bp in size with an average G + C content of 54.9%. Analyzing the average nucleotide identity (ANI) of the AMS-38 genome showed that the strain belonged to E. hormaechei subsp. steigerwaltii group (99.69% identity (ID) with 97.71% query coverage(QC) to E. hormaechei subsp. steigerwaltii (GCA 001011725) GN02001 followed by Enterobacter sp. MGH86 (99.62% ID with 95.21% QC), followed by E. hormaechei subsp. steigerwaltii 44524 (98.36% ID with 86.91% QC), followed by E. hormaechei subsp. steigerwaltii 44517 (98.36% ID with 86.92 QC)). Analysis of MLST of the strain AMS-38 showed that it belonged to ST133 (allelic profile 11-4-4-13-39-4-9).
3.3. Plasmids and Resistome Analysis of the Strain E. hormaechei AMS-38
The isolate contained four plasmids (pAMS-38a, pAMS-38b, pAMS-38c, pAMS-38d) ranging in size from 2,511 bp to 281,121 bp (Table 1). ResFinder detected that the intrinsic blaACT-7 and fosA genes were located on the chromosome. Additionally, plasmid pAMS-38a contained 10 antimicrobial resistance genes encoding resistance against colistin (mcr-9), sulphonamide (sul1), quinolones (qnrA1), trimethoprim (dfrA1), β-lactam (blaTEM-1B, and blaVIM-4), and aminoglycosides (aac (6’)-II, aadA23, aadA2b, and ant(2’’)-Ia). The remaining three plasmids did not carry any resistance genes.
Table 1.
Features of chromosome and the plasmids identified in E. hormaechei AMS-38 from Egypt.
| Sample | Size (bp) | GC% | No. of CDSs | MLST or pMLST | Inc Type * | AMR Gene |
|---|---|---|---|---|---|---|
| Chromosome | 4,914,941 | 54.9 | 6279 | ST133 | ND | fosA, blaACT-7 |
| pAMS-38a | 281,121 | 46.8 | 361 | ST1 | IncHI2 | mcr-9, sul1, qnrA1, dfrA1, blaTEM-1B, blaVIM-4, aac(6’)-Il, aadA23, aadA2b, ant(2’’)-Ia |
| pAMS-38b | 109,015 | 50.8 | 144 | ND | IncFIB(pHCM2) | ND |
| pAMS-38c | 9525 | 51.7 | 15 | ND | ND | ND |
| pAMS-38d | 2511 | 51.8 | 2 | ND | ND | ND |
* ND: not detected.
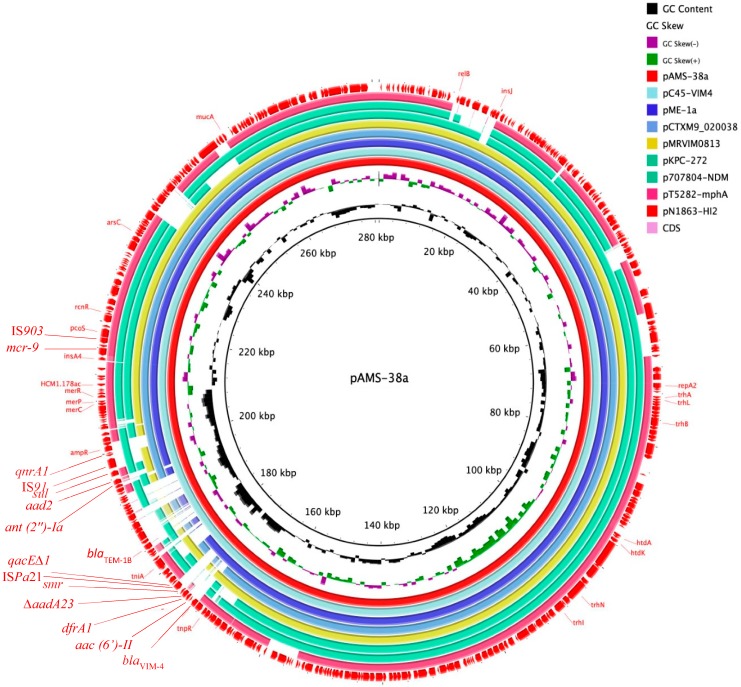
Plasmid pAMS-38a is an IncHI2 with Double Locus Sequence Type DLST1 of 281,121 bp in size encoding 361 predicted genes with an average G + C content of 46.8% (Figure 1 and Table 1). Plasmids of IncHI type are large in size (>200 kb) [12]. These plasmids have broad host range and have been commonly detected from several Enterobacterales species of clinical and environmental origin, such as Salmonella enterica serovars Typhi and Paratyphi A, E. coli, K. pneumoniae, and E. cloacae [12]. IncHI2 plasmids are characterized by carrying genes encoding heavy metal and antimicrobial resistance [12].
Figure 1.
Plasmid structure of mcr-9/IncHI2 plasmids. The whole sequence of pAMS-38a was used as the reference. Genes and open reading frames (ORFs) are shown as arrows with their transcriptional orientations indicated by arrowheads. This figure was generated using the BRIG tool [17], and the plasmids were included in the following order: pAMS-38a (identified in this study), pC45-VIM4 (LT991958), pME-1a (CP041734), pCTXM9_020038 (CP031724), pMRVIM0813 (KP975077), pKPC-272 (CP008825), p707804-NDM (MH909331), pT5282-mphA (KY270852), and pN1863-HI2 (MF344583).
A Mash distance search against the PLSDB using the whole pAMS-38a sequence identified that it has high similarity to other IncHI2 plasmids (Figure 1 and Table 2), e.g., pC45-VIM4 (accession no. LT991958), pME-1a (accession no. CP041734) [18], pCTXM9_020038 (accession no. CP031724), pMRVIM0813 (accession no. KP975077), pKPC-272 (accession no. CP008825), p707804-NDM (accession no. MH909331), pT5282-mphA (accession no. KY270852), and pN1863-HI2 (accession no. MF344583). Alarmingly, all these plasmids carried the mcr-9 gene with or without carbapenemase-encoding genes (i.e., blaVIM, blaKPC, and blaNDM) between 2012 to 2018, highlighting the early silent emergence and dissemination of mcr-9. A recent study conducted in the USA identified a similar mcr-9 and blaVIM-4/IncHI2 plasmid pME-1a (accession no. CP041734) from an E. hormaechei strain isolated from a pediatric patient with a recent history of travel to Egypt [18]. Therefore, our study raises the concern of implementing antimicrobial surveillance plans and infection control policies for mcr-9 to identify its incidence and to prevent its dissemination.
Table 2.
Features of mcr-9/IncHI2 plasmids similar to pAMS-38a.
| Plasmid | Distance To pAMS-38a * | Bacterial Species | Isolation Year ** | Country | Accession No. | |
|---|---|---|---|---|---|---|
| 1 | pC45-VIM4 | 0.0022 | E. cloacae complex | 2014 | France | LT991958 |
| 2 | pME-1a | 0.0026 | E. hormaechei subsp. steigerwaltii | 2018 | USA | CP041734 |
| 3 | pCTXM9_020038 | 0.0048 | E. hormaechei | 2016 | China | CP031724 |
| 4 | pMRVIM0813 | 0.0049 | E. cloacae | 2015 | USA | KP975077 |
| 5 | pKPC-272 | 0.0079 | E. cloacae | 2012 | USA | NZ_CP008825.1 |
| 6 | p707804-NDM | 0.0080 | Leclercia adecarboxylata | ND | China | MH909331.1 |
| 7 | pT5282-mphA | 0.0108 | E. cloacae | 2012 | China | KY270852 |
| 8 | pN1863-HI2 | 0.0120 | E. cloacae | ND | China | MF344583 |
* as detected by a Mash distance search against the PLSDB using the whole pAMS-38a sequence; ** ND: not determined.
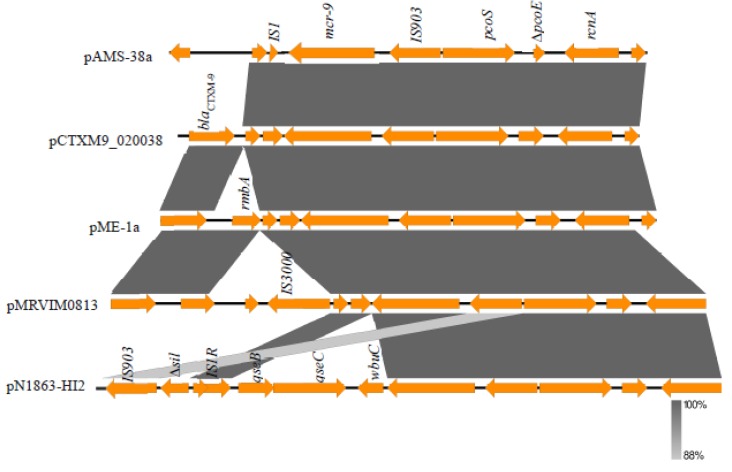
Analysis of the genetic environment of mcr-9 revealed that it was surrounded upstream by IS903 and downstream by IS1. Additionally, these two insertion sequences might be involved in the mobilization of mcr-9. This genetic environment has been reported previously from several IncHI2 plasmids (Figure 2) e.g., pME-1a (accession no. CP041734) [18], pCTXM9_020038 (accession no. CP031724), and pMRVIM0813 (accession no. KP975077). A different genetic organization, qseB-qseC-wbuC-mcr-9-IS903, has been detected from other IncHI2 plasmids e.g., pN1863-HI2 (accession no. MF344583), pT5282-mphA (accession no. KY270852), pMRVIM0813 (accession no. KP975077), and the sequenced plasmid contig from E. coli strain E68 from France [11]. In that study, Kieffer et al. showed that mcr-9 is an acquired colistin-resistance gene induced by the action of the downstream two-component regulatory system encoded by qseB/qseC using subinhibitory concentrations of colistin [11]. Another study was conducted on a clinical E. hormaechei isolate from China with the coproduction of MCR-9 and NDM-1 [19]. The investigators identified mcr-9 to be located on an IncHI2 plasmid with the downstream genes wbuC (for a cupin fold metalloprotein) and the qseB/qseC leading to colistin-resistance with an MIC of 16 μg/mL [19]. In our study, the sensitivity of the strain AMS-38 to colistin (MIC, <0.25 μg/mL) was probably due to the absence of qseB/qseC. It has been noted that mcr-9 might have originated from Buttiaxella spp. due to the presence of a gene homologous to wbuC downstream of the chromosomal mcr-9-like gene in this genus [11,19]. Of note, mcr-9 was first identified, during a routine in silico analysis, in a multidrug- resistant colistin-sensitive S. enterica serovar Typhimurium clinical isolate from the USA in 2010 [20]. Recently, mcr-9 was detected among SHV-12-producing Enterobacterales from Swedish horses with a link to IncHI2 and IncHI2A plasmids [21]. Additionally, an extensively drug-resistant blaNDM-1-carrying Klebsiella quasipneumoniae was detected to harbor mcr-9 on an IncHI2 plasmid for the first time in Latin America [22]. A novel mobile colistin resistance gene, mcr-10, was recently reported from a clinical Enterobacter roggenkampii isolate in China [23]. MCR-10 has 82.93% amino acids identity to MCR-9 [23]. Gene mcr-10 was detected to be uninducible and increases the colistin MIC by 4 folds when cloned into a colistin-susceptible E. roggenkampii strain [23].
Figure 2.
Comparison of the genetic environment of mcr-9 gene in pAMS-38a and other closely related IncHI2 plasmids. The figure was drawn using the EasyFig tool. Two different genetic environments have been detected. IS1-mcr-9-IS903 was detected from pAMS-38a (identified in this study), pME-1a (CP041734), pCTXM9_020038 (CP031724), pMRVIM0813 (KP975077), and qseB-qseC-wbuC-mcr-9-IS903 was detected from pN1863-HI2 (MF344583).
Based on the available literature, the frequency of detecting mcr-9-carrying Enterobacterales with susceptibility to polymyxins is high [18,20,21,22]. Additionally, we speculate a silent dissemination of mcr-9 since its possession confers a decreased susceptibility, not resistance, to colistin as detected in several Enterobacterales [18,20,21,22]. On the other hand, only two studies detected mcr-9 conferring clinical resistance to colistin [11,19]. Moreover, the expression of mcr-9 was shown to be inducible due to the presence of the downstream qseC/qseB regulatory system [11]. Furthermore, the lack of these two genes downstream of the mcr-9-like gene in its original progenitor, B. gaviniae, suggests that their acquisition likely has been through an independent mobilization event with respect to the acquisition of mcr-9 [11]. Similarly, that event might happen in mcr-9-carrying and colistin susceptible strains leading to the induction and colistin resistance. Until now, the clinical impact of mcr-9, in the colistin-susceptible strains lacking qseC/qseB system, is unknown.
The class B1 metallo-β-lactamase blaVIM-4 was located on an In416 class 1 integron. A BLASTn search using the whole blaVIM-4 integron sequence of pAMS-38a identified identical integrons from pC45-VIM4 (accession no. LT991958), pME-1a [18], pC309-VIM4 (accession number LT991955.1) (100% query coverage and 99.97% sequence identity). blaVIM-4 has been previously reported from E. cloacae and P. aeruginosa from Egypt, Kuwait, and United Arab Emirates [6,7,8,24]. The integron structure was blaVIM-4-aacA7-dfrA1-ΔaadA23-smr-ISPa21. Another class 1 integron was detected in pAMS-38a with the following genetic structure: intI1-ant (2’’)-Ia-aad2b-sul1-IS91-qnrA1. A BLASTn analysis identified a similar integron from pCTXM9_020038 (accession no. CP031724; 100% query coverage and 99.88% sequence identity), and pC45-VIM4 (accession no. LT991958; 100% query coverage and 99.87% sequence identity).
4. Conclusions
In conclusion, we reported the first complete genomic sequence of an mcr-9- and blaVIM-4-co-harboring E. hormaechei strain from Africa and the Middle East. Comparative genomics was used to assess the genetic environment of the mcr-9 gene. Plasmids of the IncHI2 group and the two insertion sequences (IS1, and IS903) might be the main vehicles for dissemination of mcr-9. Screening for mcr-9 and other mcr genes is essential in Egypt to determine its prevalence and to prevent its spread.
Acknowledgments
A.M.S. was supported by a fellowship from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Fellowship no. 153532). F.M. was supported by the Japan Agency for Medical Research (AMED; project number 19fk0108043h8003).
Author Contributions
Conceptualization, A.M.S. and T.S. (Tadashi Shimamoto); Data curation, A.M.S., F.M., H.O.Z., A.O. and T.S. (Toshi Shimamoto); Formal analysis, A.M.S. and F.M.; Funding acquisition, T.S. (Tadashi Shimamoto); Investigation, A.M.S. and H.N.; Methodology, A.M.S., F.M., H.O.Z., A.O., H.N. and T.S. (Toshi Shimamoto); Project administration, T.S. (Tadashi Shimamoto); Software, A.M.S. and F.M.; Validation, A.M.S.; Writing–original draft, A.M.S.; Writing–review & editing, A.M.S., F.M. and T.S. (Tadashi Shimamoto). All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.World Health Organization (WHO) Antimicrobial Resistance: Global Report on Surveillance 2014. WHO Press; Geneva, Switzerland: 2014. [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Antibiotic resistance threats in the United States, 2013. [(accessed on 24 March 2020)]; Available online: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/arthreats-2013-508.pdf.
- 3.Hsu L.Y., Apisarnthanarak A., Khan E., Suwantarat N., Ghafur A., Tambyah P.A. Carbapenem-resistant Acinetobacter baumannii and Enterobacteriaceae in South and Southeast Asia. Clin. Microbiol. Rev. 2016;30:1–22. doi: 10.1128/CMR.00042-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meunier D., Findlay J., Doumith M., Godoy D., Perry C., Pike R., Gronthoud F., Shryane T., Poirel L., Welfare W., et al. FRI-2 carbapenemase-producing Enterobacter cloacae complex in the UK. J. Antimicrob. Chemother. 2017;72:2478–2482. doi: 10.1093/jac/dkx173. [DOI] [PubMed] [Google Scholar]
- 5.Soliman A.M., Zarad H.O., Nariya H., Shimamoto T., Shimamoto T. Genetic analysis of carbapenemase-producing Gram-negative bacteria isolated from a university teaching hospital in Egypt. Infect. Genet. Evol. 2020;77:104065. doi: 10.1016/j.meegid.2019.104065. [DOI] [PubMed] [Google Scholar]
- 6.Hashem H., Hanora A., Abdalla S., Shaeky A., Saad A. Dissemination of metallo-β-lactamase in Pseudomonas aeruginosa isolates in Egypt: Mutation in blaVIM-4. APMIS. 2017;125:499–505. doi: 10.1111/apm.12669. [DOI] [PubMed] [Google Scholar]
- 7.Sonnevend A., Ghazawi A., Yahfoufi N., Al-Baloushi A., Hashmey R., Mathew M., Tariq W.Z., Pal T. VIM-4 carbapenemase-producing Enterobacter cloacae in the United Arab Emirates. Clin. Microb. Infect. 2012;18:E494–E496. doi: 10.1111/1469-0691.12051. [DOI] [PubMed] [Google Scholar]
- 8.Jamal W., Rotimi V.O., Albert M.J., Khodakhast F., Nordmann P., Poirel L. High prevalence of VIM-4 and NDM-1 metallo-β-lactamase among carbapenem-resistant Enterobacteriaceae. J. Med. Microbiol. 2013;62:1239–1244. doi: 10.1099/jmm.0.059915-0. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y.Y., Wang Y., Walsh T.R., Yi L.X., Zhang R., Spencer J., Doi Y., Tian G., Dong B., Huang X., et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 10.Elnahriry S.S., Khalifa H.O., Soliman A.M., Ahmed A.S., Hussein A.M., Shimamoto T., Shimamoto T. Emergence of plasmid-mediated colistin resistance gene mcr-1 in a clinical Escherichia coli isolate from Egypt. Antimicrob. Agents Chemother. 2016;60:3249–3250. doi: 10.1128/AAC.00269-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kieffer N., Royer G., Decousser J.W., Bourrel A.S., Palmieri M., De La Rosa J.M., Jacquier H., Denamur E., Nordmann P., Poirel L. mcr-9, an inducible gene encoding an acquired phosphoethanolamine transferase in Escherichia coli, and its origin. Antimicrob. Agents Chemother. 2019:AAC-00965. doi: 10.1128/AAC.00965-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang Q., Yin Z., Zhao Y., Liang L., Feng J., Zhan Z., Wang H., Song Y., Tong Y., Wu W., et al. Sequencing and comparative genomics analysis of the IncHI2 plasmids pT5282-mphA and p112298-catA and the IncHI5 plasmid pYNKP001-dfrA. Int. J. Antimicrob. Agents. 2017;49:709–718. doi: 10.1016/j.ijantimicag.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 13.Walker B.J., Abeel T., Shea T., Priest M., Abouelliel A., Sakthikumar S., Cuomo C.A., Zeng Q., Wortman J., Young S.K., et al. Pilon: An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE. 2014;9:e112963. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanizawa Y., Fujisawa T., Nakamura Y. DFAST: A flexible prokaryotic genome annotation pipeline for faster genome publication. Bioinformatics. 2018;34:1037–1039. doi: 10.1093/bioinformatics/btx713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ondov B.D., Treangen T.J., Melsted P., Mallonee A.B., Bergman N.H., Koren S., Phillippy A.M. Mash: Fast genome and metagenome distance estimation using MinHash. Genome Biol. 2016;17:132. doi: 10.1186/s13059-016-0997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galata V., Fehlmann T., Backes C., Keller A. PLSDB: A resource of complete bacterial plasmids. Nucleic Acids Res. 2019;47:D195–D202. doi: 10.1093/nar/gky1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alikhan N.F., Petty N.K., Zakour N.L., Beatson S.A. BLAST Ring Image Generator (BRIG): Simple prokaryote genome comparisons. BMC Genomics. 2011;12:402. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chavda K.D., Westblade L.F., Satlin M.J., Hemmert A.C., Castanheira M., Jenkins S.G., Chen L., Kreiswirth B.N. First report of blaVIM-4- and mcr-9-coharboring Enterobacter species isolated from a pediatric patient. mSphere. 2019;4:e00629-19. doi: 10.1128/mSphere.00629-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan Y., Li Y., Wang G., Li C., Xiang L., She J., Yang Y., Zhong F., Zhang L. Coproduction of MCR-9 and NDM-1 by colistin-resistant Enterobacter hormaechei isolated from bloodstream infection. Infect. Drug Resist. 2019;12:2979. doi: 10.2147/IDR.S217168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carroll L.M., Gaballa A., Guldimann C., Sullivan G., Henderson L.O., Wiedmann M. Identification of novel mobilized colistin resistance gene mcr-9 in a multidrug-resistant, colistin-susceptible Salmonella enterica serotype Typhimurium isolate. mBio. 2019;10:e00853-19. doi: 10.1128/mBio.00853-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Börjesson S., Greko C., Myrenås M., Landén A., Nilsson O., Pedersen K. A link between the newly described colistin resistance gene mcr-9 and clinical Enterobacteriaceae isolates carrying blaSHV-12 from horses in Sweden. J. Glob. Antimicrob. Resist. 2020;20:285–289. doi: 10.1016/j.jgar.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Faccone D., Martino F., Albornoz E., Gomez S., Corso A., Petroni A. Plasmid carrying mcr-9 from an extensively drug-resistant NDM-1-producing Klebsiella quasipneumoniae subsp. quasipneumoniae clinical isolate. Infect. Genet. Evol. 2020;81:104273. doi: 10.1016/j.meegid.2020.104273. [DOI] [PubMed] [Google Scholar]
- 23.Wang C., Feng Y., Liu L., Wei L., Kang M., Zong Z. Identification of novel mobile colistin resistance gene mcr-10. Emerg. Microbes Infect. 2020;9:508–516. doi: 10.1080/22221751.2020.1732231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dimude J.U., Amyes S.G.B. Molecular characterisation and diversity in Enterobacter cloacae from Edinburgh and Egypt carrying blaCTX-M-14 and blaVIM-4 β-lactamase genes. Int. J. Antimicrob. Agents. 2013;41:574–577. doi: 10.1016/j.ijantimicag.2013.02.012. [DOI] [PubMed] [Google Scholar]